Abstract
Background
The Icatibant Outcome Survey (IOS; NCT01034969) is a Shire‐sponsored, international, observational study monitoring the safety and effectiveness of icatibant, a bradykinin B2 receptor antagonist approved for the acute treatment of adults with hereditary angioedema with C1 inhibitor deficiency (HAE‐C1‐INH).
Objective
To report IOS data comparing demographic and icatibant treatment outcomes in patients with HAE‐C1‐INH from Germany to HAE‐C1‐INH patients from 11 other IOS countries.
Methods
A descriptive, retrospective, comparative analysis of data from 685 IOS patients with HAE‐C1‐INH from seven centres in Germany (n = 93) vs. centres from Austria, Brazil, Czech Republic, Denmark, France, Greece, Israel, Italy, Spain, Sweden and the United Kingdom (n = 592, July 2009–January 2017). Icatibant treatment outcomes were retrieved from patients with complete attack outcome data for time to treatment, time to resolution and attack duration (160 attacks in 42 German patients and 1442 attacks in 251 patients from other IOS countries).
Results
German patients reported significantly fewer severe/very severe attacks (38.7% vs. 57.5%, respectively; P < 0.001). The proportion of attacks treated with a single icatibant injection was significantly higher in German patients (97.1% vs. 91.6%, P = 0.0003). The median time to treatment (0.0 h vs. 1.5 h), time to resolution (3.0 h vs. 7.0 h) and attack duration (4.3 h vs. 10.5 h) in German patients vs. other IOS countries were all significantly shorter (all P < 0.0001). No meaningful differences were identified between patients from Germany and other countries with regard to sex, median age at enrolment, median age at symptom onset and median age at diagnosis.
Conclusion
German IOS patients share similar demographic characteristics to patients from other IOS countries yet treat their attacks with icatibant significantly earlier and have markedly fewer severe or very severe attacks. Factors including regional access to and availability of icatibant may drive these outcomes and warrant further investigation.
Introduction
Hereditary angioedema due to C1 inhibitor deficiency (HAE‐C1‐INH) is a rare disease characterized by recurrent and unpredictable swellings, most commonly of the subcutaneous tissues of the skin, face and extremities and mucosa of the gastrointestinal tract.1, 2 Attacks may share a clinical presentation with a range of more common diseases, which can lead to delayed or missed diagnosis; and while the majority of untreated attacks resolve within a few days, attacks localized to the larynx can be fatal if immediate treatment is not provided.3
The recently updated and revised World Allergy Organization and European Academy of Allergy and Clinical Immunology guideline for HAE advocates the need for acute on‐demand therapies to be made available for patients with HAE‐C1‐INH.4 This is also supported by several international and regional consensus documents.5
Icatibant, a bradykinin B2 receptor antagonist, was approved in the EU in 2008 for the acute treatment of HAE attacks, based upon efficacy and safety data in adults with HAE‐CI‐INH from two phase III randomized controlled trials.6 Subsequently, in 2011, based on data from a phase IIIb open‐label study,7 approval was extended to self‐administration.
The Icatibant Outcome Survey (IOS) is an international, prospective, observational study (NCT01034969) established in 2009. Data from the IOS have clearly shown that early icatibant treatment results in earlier resolution of attacks8 and that real‐world outcomes of icatibant are comparable with the aforementioned controlled studies.9 More recently, a range of IOS publications have further described icatibant use and disease characteristics for HAE‐C1‐INH patients in the real‐world setting across the IOS countries.10, 11, 12, 13, 14, 15
A recent country‐specific IOS analysis, using 2015 data, clearly identified German patients with HAE‐C1‐INH who are enrolled in IOS administer icatibant to treat their attacks significantly earlier, following symptom onset, than similarly diagnosed IOS patients from Austria, France, Italy, Spain, and the United Kingdom.14 Here, we report data from IOS that focus on icatibant treatment outcomes in patients with HAE‐C1‐INH from seven HAE specialist centres across Germany and compares outcomes with patients from 11 other IOS countries.
Patients and methods
Study design and patients
The analyses described herein are based on IOS data collected between July 2009 and January 2017 from patients with HAE‐C1‐INH from Austria, Brazil, Czech Republic, Denmark, France, Germany, Greece, Israel, Italy, Spain, Sweden and the United Kingdom. The study methodology for the IOS has been published elsewhere.8 Retrospective data for attacks recorded in the 12 months prior to IOS enrolment were also collected, dating back to February 2008.
Statistical analyses
Data for German vs. non‐German populations were compared. A mixed model analysis of repeated measures (Proc Mixed; SAS Institute, Cary, NC, USA) was used to compare time to treatment, time to resolution and duration of attack. The chi‐squared test was used for the comparison of dichotomous data, with a statistical significance level of alpha = 0.05. Data are presented as median [interquartile range (IQR)] or mean [standard deviation (SD)], unless otherwise specified.
Results
IOS patients in Germany report significantly fewer severe attacks
German patients were significantly less likely than patients from other countries to report severe or very severe attacks (38.7% vs. 57.5%, respectively; P < 0.001; Fig. 1).
Figure 1.
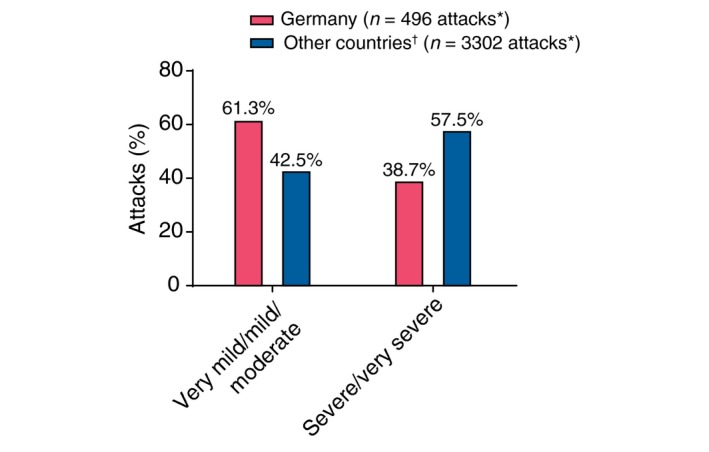
Attack severity. *All calculations exclude the ‘Unknown’ category. †Other countries include patients from Austria, Brazil, Czech Republic, Denmark, France, Greece, Israel, Italy, Spain, Sweden and the United Kingdom. Comparison based on generalized linear model for repeated measures comparing ‘(very mild + mild + moderate)’ vs. ‘(severe + very severe)’.
Patient demographics and baseline characteristics are comparable between Germany and other countries
Overall, the German IOS cohort did not differ significantly from the comparator cohort (Table 1) with respect to sex or diagnosis. Similarly, no differences were noted in the anatomic location of attacks (data not shown). The variations of several of these parameters across German centres are described in Table 2.
Table 1.
Patient demographics
| Characteristic | Germany | Other countries | Overall comparison P‐value |
|---|---|---|---|
| Patients, n | 93 | 592 | |
| Gender, n (%) | |||
| Female | 58 (62.4) | 343 (57.9) | – |
| Male | 35 (37.6) | 249 (42.1) | |
| HAE diagnosis, n (%) | |||
| HAE type I | 83 (89.2) | 551 (93.1) | – |
| HAE type II | 10 (10.8) | 41 (6.9) | |
| Age at IOS enrolment, years | |||
| n (missing) | 93 (0) | 592 (0) | 0.1076 |
| Median (IQR) | 42.8 (28.7–55.3) | 39.0 (28.4–50.6) | |
| Age at first symptoms, years | |||
| n (missing) | 79 (14) | 508 (84) | 0.5080 |
| Median (IQR) | 11.0 (7.0–19.0) | 12.0 (5.0–18.0) | |
| Age at diagnosis, years | |||
| n (missing) | 89 (4) | 550 (42) | 0.5354 |
| Median (IQR) | 21.9 (11.9–36.2) | 20.8 (13.3–32.8) | |
| Delay in diagnosis, years | |||
| n (missing) | 79 (14) | 497 (95) | 0.3694 |
| Median (IQR) | 4.5 (0.3–15.5) | 7.0 (0.4–17.7) | |
HAE, hereditary angioedema; IOS, Icatibant Outcome Survey; IQR, interquartile range.
Table 2.
Demographics by German centre
| Characteristic | Charité, Universitätsmedizin Berlin | HZRM Haemophilie Zentrum Rhein Main GmbH | Universitätsklinik Mainz | Hals‐Nasen‐Ohrenklinik und Poliklinik | Universitätsklinikum Essen | Klinikum der Johann‐Wolfgang Goethe Universität | Universitätsklinikum Carl Gustav Carus |
|---|---|---|---|---|---|---|---|
| No. of patients | 37 | 25 | 11 | 7 | 6 | 4 | 3 |
| Gender, n (%) | |||||||
| Female | 20 (54.1) | 17 (68.0) | 9 (81.8) | 5 (71.4) | 4 (66.7) | 1 (25.0) | 2 (66.7) |
| Male | 17 (45.9) | 8 (32.0) | 2 (18.2) | 2 (28.6) | 2 (33.3) | 3 (75.0) | 1 (33.3) |
| HAE diagnosis, n (%) | |||||||
| HAE type I | 35 (94.6) | 24 (96.0) | 10 (90.9) | 5 (71.4) | 4 (66.7) | 3 (75.0) | 2 (66.7) |
| HAE type II | 2 (5.4) | 1 (4.0) | 1 (9.1) | 2 (28.6) | 2 (33.3) | 1 (25.0) | 1 (33.3) |
| Age at IOS enrolment, years | |||||||
| n (missing) | 37 (0) | 25 (0) | 11 (0) | 7 (0) | 6 (0) | 4 (0) | 3 (0) |
| Median (IQR) | 37.7 (25.7–55.1) | 49.5 (30.7–54.6) | 40.7 (24.2–49.6) | 45.5 (31.8–70.3) | 55.6 (44.9–72.0) | 53.1 (38.8–63.3) | 43.8 (37.4–75.9) |
| Age at first symptoms, years | |||||||
| n (missing) | 34 (3) | 17 (8) | 11 (0) | 6 (1) | 5 (1) | 4 (0) | 2 (1) |
| Median (IQR) | 11.0 (8.0–16.0) | 7.0 (3.0–19.0) | 15.0 (8.0–22.0) | 18.0 (9.0–43.0) | 19.0 (10.0–40.0) | 8.0 (7.5–14.0) | 41.5 (11.0–72.0) |
| Age at diagnosis, years | |||||||
| n (missing) | 36 (1) | 22 (3) | 11 (0) | 7 (0) | 6 (0) | 4 (0) | 3 (0) |
| Median (IQR) | 18.1 (9.8–29.8) | 19.5 (11.5–36.5) | 18.5 (7.6–30.3) | 36.3 (26.9–68.5) | 37.5 (33.8–45.9) | 24.7 (12.4–37.9) | 43.4 (33.8–72.5) |
| Delay in diagnosis, years | |||||||
| n (missing) | 34 (3) | 17 (8) | 11 (0) | 6 (1) | 5 (1) | 4 (0) | 2 (1) |
| Median (IQR) | 3.4 (0.0–13.8) | 6.8 (0.5–15.5) | 0.5 (0.0–8.3) | 7.6 (1.7–27.3) | 23.8 (7.0–24.2) | 15.9 (4.9–23.9) | 11.6 (0.5–22.8) |
| Delay in diagnosis, years, n (%) | |||||||
| <0 | 2 (5.9) | 3 (17.6) | 1 (9.1) | 0 | 0 | 0 | 0 |
| 0–1 | 11 (32.4) | 3 (17.6) | 5 (45.5) | 1 (16.7) | 0 | 0 | 1 (50.0) |
| >1 | 21 (61.8) | 11 (64.7) | 5 (45.5) | 5 (83.3) | 5 (100.0) | 4 (100.0) | 1 (50.0) |
HAE, hereditary angioedema; IOS, Icatibant Outcome Survey; IQR, interquartile range.
IOS patients in Germany treat their attacks with icatibant significantly earlier
Overall, time to treatment with icatibant, time to resolution and duration of attack were all significantly shorter in the German group compared with other IOS countries (all P < 0.0001; Table 3). Using a generalized mixed model for repeated measures, a significant difference in attack duration was observed between patients in Germany and patients from other IOS countries (Fig. 2; P = 0.0227). The earlier treatment of German patients can be appreciated in Fig. 3, which shows duration of attack vs. timing of icatibant administration for each attack in both cohorts.
Table 3.
Icatibant treatment outcomes
| Endpoint | Patients with HAE‐C1‐INH | ||||||
|---|---|---|---|---|---|---|---|
| Germany (N = 42) | Other countriesa (N = 251) | ||||||
| n b | Mean (SD) | Median (IQR) | n b | Mean (SD) | Median (IQR) | P‐valuec | |
| Time from attack onset to treatment, hd | 160 | 1.3 (4.7) | 0.0 (0.0–0.5) | 1422 | 4.2 (7.2) | 1.5 (0.5–5.0) | <0.0001 |
| Time to complete symptom resolution, he | 160 | 7.6 (12.2) | 3.0 (1.0–8.5) | 1422 | 15.1 (19.6) | 7.0 (2.5–20.5) | <0.0001 |
| Duration of attack, hf | 160 | 8.9 (13.2) | 4.3 (1.0–10.0) | 1422 | 19.3 (22.3) | 10.5 (5.0–25.5) | <0.0001 |
Other countries include Austria, Brazil, Czech Republic, Denmark, France, Greece, Israel, Italy, Spain, Sweden and United Kingdom.
Attacks with complete data for time to treatment, time to complete resolution and attack duration, excluding attacks treated 100 h after attack onset.
Mixed model analysis of repeated measures comparing attacks in German vs. non‐German patients.
Time between the start of the attack and time to first icatibant injection.
Time between first injection of icatibant and complete resolution of symptoms.
Time between start of onset of attack and complete resolution of symptoms.
HAE, hereditary angioedema; IQR, interquartile range; SD, standard deviation.
Figure 2.
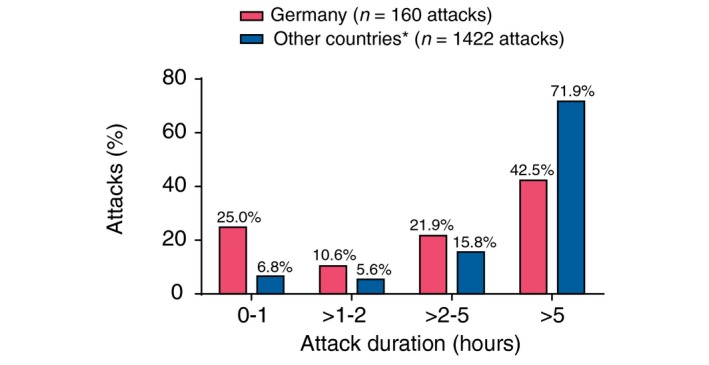
Attack duration. *Other countries include Austria, Brazil, Czech Republic, Denmark, France, Greece, Israel, Italy, Spain, Sweden and the United Kingdom.
Figure 3.
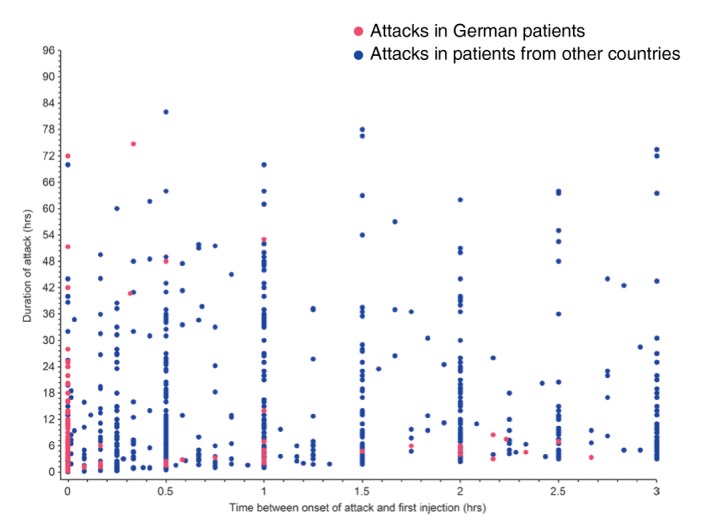
Time to treatment vs. attack duration for German vs. other IOS patients. *Other countries include Austria, Brazil, Czech Republic, Denmark, France, Greece, Israel, Italy, Spain, Sweden, and the United Kingdom. IOS, Icatibant Outcome Survey.
IOS patients in Germany self‐administer icatibant at a similar rate
Nearly all attacks treated with icatibant were treated by self‐administration in both Germany and other IOS countries (90.2% vs. 89.8%, respectively; P = 0.369).
IOS patients in Germany re‐inject icatibant at a significantly lower rate
German patients reported approximately the same number of icatibant‐treated attacks as their non‐German counterparts; however, 97.1% of attacks in Germany were treated with a single icatibant injection, compared with 91.6% of attacks in other countries (P = 0.0003; Table 4). The use of rescue medication was not significantly different between German patients (138/576 attacks, 24.0%) and patients from other countries (654/4303 attacks, 15.2%; P = 0.138). However, of those attacks treated with rescue medication, IOS patients from Germany reported a significantly greater use of C1‐INH concentrate (129/576 attacks, 22.4%) than patients from other IOS countries (325/4303 attacks; 7.6%; P < 0.001). Variation of C1‐INH concentrate rescue use was also observed between German centres (Table 5). Of patients with available prophylaxis data, German patients (n = 20) reported different patterns of short‐ and long‐term prophylaxis compared with patients in other IOS countries (n = 317; Fig. 4; overall P < 0.0001) with stanozolol, oxandrolone and tranexamic acid not utilized as prophylactic agents in IOS patients in Germany.
Table 4.
Icatibant injections per attack
| Parameter | Patients with HAE‐C1‐INH | |
|---|---|---|
| Germany (N = 93) | Other countriesh (N = 592) | |
| Icatibant‐treated attacksa | ||
| Patients treated with icatibant, n | 63 | 449 |
| Attacks treated with icatibant per patient | ||
| Mean (SD) | 8.7 (12.4) | 8.8 (18.4) |
| Median (IQR) | 4.0 (1.0–10.0) | 3.0 (1.0–8.0) |
| Number of injections of icatibant per attack | ||
| Attacks (%) treated with icatibant, n | 544 | 3770 |
| 1 | 528 (97.1) | 3454 (91.6) |
| 2 | 12 (2.2) | 288 (7.6) |
| 3 | 4 (0.7) | 24 (0.6) |
| 4 | 0 (0.0) | 3 (0.1) |
| 6 | 0 (0.0) | 1 (0.0) |
| P < 0.0003g | ||
| Untreated attacks in year prior to IOS entryb | ||
| Patients with at least one untreated attack, n | 46c | 229d |
| Untreated attacks per patient | ||
| Mean (SD) | 6.2 (10.7) | 7.8 (15.0) |
| Median (IQR) | 1.0 (0.0–7.0) | 2.0 (0–8.0) |
| Untreated attacks in the IOS observation perioda | ||
| Patients with at least one untreated attack, n | 31e | 206f |
| Untreated attacks per patient | ||
| Mean (SD) | 15.0 (26.6) | 7.9 (17.1) |
| Median (IQR) | 1.0 (0.0–19.0) | 1.0 (0–8.0) |
Attacks in year prior to IOS entry and through the IOS observation period.
Untreated attacks were defined as attacks not treated with icatibant or any other treatment.
36 patients had no untreated attacks.
111 patients had no untreated attacks.
28 patients had no untreated attacks.
146 patients had no untreated attacks.
Comparison of one injection vs. more than one injection.
Other countries include Austria, Brazil, Czech Republic, Denmark, France, Greece, Israel, Italy, Spain, Sweden and United Kingdom.
HAE, hereditary angioedema; IOS, Icatibant Outcome Survey; IQR, interquartile range; SD, standard deviation.
Table 5.
C1‐INH prophylaxis and C1‐INH rescue by German centre
| Characteristic | Charité, Universitätsmedizin Berlin | HZRM Haemophilie Zentrum Rhein Main GmbH | Universitätsklinik Mainz | Hals‐Nasen‐Ohrenklinik und Poliklinik | Universitätsklinikum Essen | Klinikum der Johann‐Wolfgang Goethe Universitat | Universitätsklinikum Carl Gustav Carus |
|---|---|---|---|---|---|---|---|
| No. of patients | 37 | 25 | 11 | 7 | 6 | 4 | 3 |
| C1‐INH ongoinga long‐term or short‐term prophylaxis, n (%) | |||||||
| Yes | 9 (24.3) | 4 (16.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No | 28 (75.7) | 21 (84.0) | 11 (100.0) | 7 (100.0) | 6 (100.0) | 4 (100.0) | 3 (100.0) |
| C1‐INH as rescue medicationb, n (%) | |||||||
| Yes | 7 (18.9) | 14 (56.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 3 (75.0) | 0 (0.0) |
| No | 30 (81.1) | 11 (44.0) | 10 (90.9) | 7 (100.0) | 6 (100.0) | 1 (25.0) | 3 (100.0) |
At IOS entry and/or during the follow‐up period.
C1‐INH rescue on at least one attack at IOS entry and/or during follow‐up period.
C1‐INH, C1‐inhibitor; IOS, Icatibant Outcome Survey.
Figure 4.
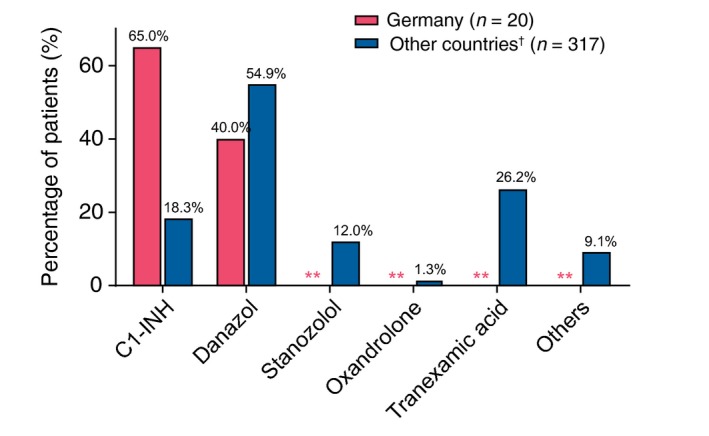
Ongoing long‐term* or short‐term* prophylaxis for patients with available prophylaxis data. *At IOS entry and/or during the follow‐up period. **Zero count. †Other countries include patients from Austria, Brazil, Denmark, France, Greece, Israel, Italy, Spain, Sweden and the United Kingdom.
Discussion
This is the first report, using real‐world IOS data, detailing the experience in Germany with icatibant for the acute treatment of HAE in adults and subsequent comparison with other IOS countries. These data build on a published report of the use of icatibant in a non‐IOS, German HAE population from a large HAE specialist centre in Frankfurt.16 That study, however, was published shortly after icatibant approval and derived from a single centre with limited data available at that time.
Overall, IOS patients in Germany were not different in terms of demographics or IOS entry characteristics from IOS patients in the other countries assessed. Delay in diagnosis for German patients, though numerically shorter than other countries, was not significantly shorter. These data suggest that while identification of HAE is certainly an improvement over the previously reported median delay in diagnosis of 8.5 years in past IOS analyses across the EU,17 this delay is still unacceptably high. Beyond the bounds of HAE specialist care, delays in diagnosis are further confounded by the presenting clinical similarities between HAE and far more common conditions (e.g. appendicitis), which may not raise clinical suspicion of non‐specialist HAE physicians.11, 18 Given the potential for fatal outcomes of laryngeal attacks,3 the continuing effort to improve physician awareness of HAE is a clear priority.
The key finding of this analysis is that German patients treat their attacks with icatibant, regardless of severity, almost immediately upon recognition of symptoms. This would affirm German patient awareness and acceptance of physician recommendations based on the various evidence‐based guidelines and consensus documents that promote treatment as early as possible. These data may even suggest growing patient confidence in management of their unpredictable disease. Treating so early may help explain why German patients reported significantly fewer severe or very severe attacks, as patients appear to treat rapidly and do not wait to see whether an attack will progress. There are, however, aspects of the German healthcare system, such as the level of reimbursement (100% for icatibant),19, 20 and easier access to icatibant, that may, in part, explain how early treatment may be a simpler proposition for a patient with HAE within Germany compared with patients in other countries, where icatibant may be more difficult to obtain and doses perhaps retained for treatment of attacks that are deemed more severe.
The comparable rate of icatibant self‐administration across IOS countries has been described in other IOS studies and represents an acceptance by the HAE patient community of the long‐established advantage to the patient of rapid access to home‐based acute therapy.10, 21 That nearly all attacks in German IOS patients were treated with only a single icatibant injection is an important observation from both a patient and healthcare economic standpoint, though the administration of icatibant so soon after symptom onset may play a role in this outcome. The icatibant reinjection rate of approximately 10% in the other countries is reflective of the reinjection rate of approximately 10% reported in the randomized controlled clinical trial (FAST‐2)22 and prior IOS analyses.10
The preference by some German patients to use C1‐INH as both long‐term and short‐term prophylaxis and as a rescue medication is not surprising when viewed in the context of the long‐standing experience of German HAE specialists with the use of C1‐INH since 1979 for acute treatment of HAE. However, the relatively low numbers of German patients reporting C1‐INH prophylaxis should be taken into account when interpreting the reduced severity of attacks in IOS patients in Germany.
Limitations of this analysis include the subjective nature of patient‐reported attack severity and the nature of reporting real‐world registry data, where data may not be complete for all outcomes in all instances. Another limitation is that standardized and validated tools to assess disease activity, impact and control were not used by all patients and not taken into account in this analysis. Instruments such as the angioedema activity score23 and the Angioedema Quality of Life Questionnaire24, 25 should be used in future studies to better understand the impact of treatment on the course of HAE and attack features.26
Conclusion
Patients enrolled in IOS in Germany share similar demographic characteristics with patients from other IOS countries yet treat their attacks with icatibant significantly earlier and have markedly fewer severe or very severe attacks. Factors including regional access to and availability of icatibant may drive these outcomes and warrant further investigation.
Acknowledgements
The following IOS investigators and study staff are acknowledged for their contributions to the IOS: AUSTRIA: W. Aberer; BRAZIL: A.S. Grumach; DENMARK: A. Bygum; FRANCE: C. Blanchard Delauny, L. Bouillet, B. Coppere, O. Fain, S. Guez, P.Y. Jeandel, G. Kanny, D. Launay, L. Martin, A. Masseau, Y. Ollivier, C. Dzviga, A. Gompel, B. Goichot, H. Maillard, A. Sobel, A. Du Thanh, I. Boccon‐Gibod; GERMANY: M. Baş, K. Bork, J. Greve, M. Maurer, E. Aygören‐ Pürsün, M. Bauer, I. Martinez‐Saguer, U. Strassen, M. Magerl; GREECE: E. Papadopoulou‐Alataki, F. Psarros; ISRAEL: Y. Graif, E. Toubi, A. Reshef, S. Kivity; ITALY: M. Cicardi, F. Arcoleo, V. Montinaro, M. Triggiani, A. Zanichelli, G. Marone, P. Manconi, M. Bova; SPAIN: M.L. Baeza, T. Caballero, R. Cabañas, M. Guilarte, D. Hernandez, R. Lleonart, T. Lobera, L. Marques, C. Hernando de Larramnedi, B. Saenz de San Pedro, G. Gala Ortiz; SWEDEN: J. Bjorkander; UNITED KINGDOM: H.J. Longhurst, C. Bethune, T. Garcez. The IOS Executive Committee: W. Aberer, L. Bouillet, A. Bygum, T. Caballero, A.S. Grumach, H.J. Longhurst, M. Maurer, A. Zanichelli.
Conflicts of interest
M. Maurer has received research grant support and/or speaker/consultancy fees from BioCryst, CSL Behring, Pharming Technologies and Shire. K. Bork has received speaker fees from CSL Behring and Shire. I. Martinez‐Saguer received honoraria, research funding and travel grants from BioCryst, CSL Behring, Pharming Technologies and Shire and/or served as a consultant and/or participated in advisory boards for these companies. E. Aygören‐Pürsün received honoraria, research funding and/or travel grants from BioCryst, CSL Behring, Pharming Technologies and/or Shire and/or served as a consultant for these companies. J. Botha is a full‐time employee of Shire, Zug, Switzerland. I. Andresen is a full‐time employee of Shire, Zug, Switzerland. M. Magerl has received research grant support and/or speaker/consultancy fees from BioCryst, CSL Behring, Pharming and Shire.
Funding sources
IOS is supported by Shire GmbH, Zug, Switzerland. Under direction of the authors, David Lickorish PhD, CMPP, employee of Excel Medical Affairs, provided writing assistance for this manuscript. Editorial assistance in formatting, proofreading, and copy editing also was provided by Excel Medical Affairs. Shire Human Genetic Therapies provided funding to Excel Medical Affairs for support in editing this manuscript. The interpretation of the data was made by the authors independently.
References
- 1. Cicardi M, Aberer W, Banerji A et al Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy 2014; 69: 602–616. [DOI] [PubMed] [Google Scholar]
- 2. Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med 2006; 119: 267–274. [DOI] [PubMed] [Google Scholar]
- 3. Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1‐INH deficiency. J Allergy Clin Immunol 2012; 130: 692–697. [DOI] [PubMed] [Google Scholar]
- 4. Maurer M, Magerl M, Ansotegui I et al The international WAO/EAACI guideline for the management of hereditary angioedema‐The 2017 revision and update. Allergy 2018; 73: 1575–1596. [DOI] [PubMed] [Google Scholar]
- 5. Jose J, Zacharias J, Craig T. Review of select practice parameters, evidence‐based treatment algorithms, and international guidelines for hereditary angioedema. Clin Rev Allergy Immunol 2016; 51: 193–206. [DOI] [PubMed] [Google Scholar]
- 6. Cicardi M, Banerji A, Bracho F et al Icatibant, a new bradykinin‐receptor antagonist, in hereditary angioedema. N Engl J Med 2010; 363: 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aberer W, Maurer M, Reshef A et al Open‐label, multicenter study of self‐administered icatibant for attacks of hereditary angioedema. Allergy 2014; 69: 305–314. [DOI] [PubMed] [Google Scholar]
- 8. Maurer M, Aberer W, Bouillet L et al Hereditary angioedema attacks resolve faster and are shorter after early icatibant treatment. PLoS One 2013; 8: e53773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maurer M, Longhurst HJ, Fabien V, Li HH, Lumry WR. Treatment of hereditary angioedema with icatibant: efficacy in clinical trials versus effectiveness in the real‐world setting. Allergy Asthma Proc 2014; 35: 377–381. [DOI] [PubMed] [Google Scholar]
- 10. Hernández Fernandez de Rojas D, Ibañez E, Longhurst H et al Treatment of HAE attacks in the Icatibant Outcome Survey: an analysis of icatibant self‐administration versus administration by health care professionals. Int Arch Allergy Immunol 2015; 167: 21–28. [DOI] [PubMed] [Google Scholar]
- 11. Zanichelli A, Longhurst HJ, Maurer M et al Misdiagnosis trends in patients with hereditary angioedema from the real‐world clinical setting. Ann Allergy Asthma Immunol 2016; 117: 394–398. [DOI] [PubMed] [Google Scholar]
- 12. Zanichelli A, Maurer M, Aberer W et al Long‐term safety of icatibant treatment of patients with angioedema in real‐world clinical practice. Allergy 2017; 72: 994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caballero T, Maurer M, Longhurst HJ, Aberer W, Bouillet L, Fabien V. Triggers and prodromal symptoms of angioedema attacks in patients with hereditary angioedema. J Investig Allergol Clin Immunol 2016; 26: 383–386. [DOI] [PubMed] [Google Scholar]
- 14. Caballero T, Aberer W, Longhurst HJ et al The Icatibant Outcome Survey: experience of hereditary angioedema management from six European countries. J Eur Acad Dermatol Venereol 2017; 31: 1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aberer W, Maurer M, Bouillet L et al Breakthrough attacks in patients with hereditary angioedema receiving long‐term prophylaxis are responsive to icatibant: findings from the Icatibant Outcome Survey. Allergy Asthma Clin Immunol 2017; 13: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aygören‐Pürsün E, Martinez‐Saguer I, Rusicke E, Klingebiel T, Kreuz W. On demand treatment and home therapy of hereditary angioedema in Germany – the Frankfurt experience. Allergy Asthma Clin Immunol 2010; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zanichelli A, Magerl M, Longhurst H, Fabien V, Maurer M. Hereditary angioedema with C1 inhibitor deficiency: delay in diagnosis in Europe. Allergy Asthma Clin Immunol 2013; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Otani IM, Christiansen SC, Busse P et al Emergency department management of hereditary angioedema attacks: patient perspectives. J Allergy Clin Immunol Pract 2017; 5: 128–134.e4. [DOI] [PubMed] [Google Scholar]
- 19. Blankart CR, Stargardt T, Schreyögg J. Availability of and access to orphan drugs: an international comparison of pharmaceutical treatments for pulmonary arterial hypertension, Fabry disease, hereditary angioedema and chronic myeloid leukaemia. Pharmacoeconomics 2011; 29: 63–82. [DOI] [PubMed] [Google Scholar]
- 20. Lumry WR. Pharmacoeconomics of orphan disease treatment with a focus on hereditary angioedema. Immunol Allergy Clin North Am 2017; 37: 617–628. [DOI] [PubMed] [Google Scholar]
- 21. Otani IM, Lumry WR, Hurwitz S et al Subcutaneous icatibant for the treatment of hereditary angioedema attacks: comparison of home self‐administration with administration at a medical facility. J Allergy Clin Immunol Pract 2017; 5: 442–447.e1. [DOI] [PubMed] [Google Scholar]
- 22. Baş M, Greve J, Hoffmann TK et al Repeat treatment with icatibant for multiple hereditary angioedema attacks: FAST‐2 open‐label study. Allergy 2013; 68: 1452–1459. [DOI] [PubMed] [Google Scholar]
- 23. Weller K, Groffik A, Magerl M et al Development, validation, and initial results of the Angioedema Activity Score. Allergy 2013; 68: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 24. Weller K, Groffik A, Magerl M et al Development and construct validation of the angioedema quality of life questionnaire. Allergy 2012; 67: 1289–1298. [DOI] [PubMed] [Google Scholar]
- 25. Weller K, Magerl M, Peveling‐Oberhag A, Martus P, Staubach P, Maurer M. The Angioedema Quality of Life Questionnaire (AE‐QoL) – assessment of sensitivity to change and minimal clinically important difference. Allergy 2016; 71: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 26. Bygum A, Busse P, Caballero T, Maurer M. Disease severity, activity, impact, and control and how to assess them in patients with hereditary angioedema. Front Med 2017; 4: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]


