Abstract
Immune thrombocytopenia (ITP) is a rare platelet disorder that is often persistent or chronic in adults. Patient management is dependent upon physician judgment and patient preference, given both the rarity of the condition and a paucity of high‐quality clinical trial evidence to inform practice guidelines. A systematic literature review was conducted to provide an up‐to‐date summary of studies evaluating the safety and efficacy/effectiveness of therapies used to treat adults with primary ITP in the second‐line setting. Using comprehensive search strings, several medical research databases were queried. Final abstraction was performed on 186 articles. Most (75%) studies were observational in nature; nearly half were conducted in Europe. Splenectomy was the most commonly studied (n = 83, 47%), followed by rituximab (n = 49, 26%) and the thrombopoietin‐receptor agonists (TPO‐RAs) romiplostim (n = 34, 18%) and eltrombopag (n = 24, 13%). Twelve prospective, randomized controlled trials (RCTs) with a placebo or standard‐of‐care arm evaluating the safety and efficacy of either rituximab or a TPO‐RA were identified and described in detail. These trials provide important information on the safety and efficacy of these treatments, and in the absence of head‐to‐head data, offer insights on how these therapies compare with one another in treating adult ITP in the second‐line setting. This review confirms that for most second‐line ITP treatment options, there remains a lack of rigorous evidence derived from RCTs, and for many treatments, there is limited evidence of any kind. The need for additional research to guide treatment choices in this setting and greater use of standardized ITP terminology are highlighted.
1. INTRODUCTION
Primary immune thrombocytopenia (ITP) is a rare autoimmune disorder characterized by isolated thrombocytopenia that can lead to an increased tendency to bleed.1, 2 Although it typically presents as a subtle‐onset, chronic syndrome in adults, with no forewarning symptoms or illness, clinical manifestations can range from minor bruising to severe hemorrhaging.3, 4, 5 The primary goal of treatment is to achieve a safe platelet count (above which, a patient does not experience bleeding episodes), and this is determined on a case‐by‐case basis.3 Common first‐line therapies include corticosteroids, intravenous immunoglobulin (IVIg), and anti‐D (Rho[D] immune globulin intravenous).6, 7, 8 Relapse or failure to respond to these may necessitate second‐line treatment, which can include splenectomy or a variety of medical therapies, most of which have not been approved by regulatory authorities for the treatment of ITP but have been used because of efficacy demonstrated in other autoimmune diseases or as immune suppressants.9
Splenectomy has historically been considered the second‐line therapy of choice in adult ITP.10, 11 A systematic review was previously conducted to examine studies (published from 1966 to 2003) that assessed the efficacy/effectiveness of medical treatments for adult patients with ITP who have not responded to splenectomy. The review covered a total of 90 studies representing 656 patients who were splenectomized, aged >16 years, had ITP for >3 months, and a platelet count <50 × 109/L.12 Only one study13 was a randomized controlled trial (RCT) but the randomization was by dose of the same therapy; the remaining were cohort studies or uncontrolled case series. A complete response (as defined in each respective original report) was achieved in 14% of patients across the 22 treatment types, with the largest numbers of responders reported with cyclophosphamide (27% of 83 patients), rituximab (24% of 41 patients), and azathioprine (17% of 109 patients). Although partial response was achieved in 40% of patients across these three therapies, 36% to 42% had no response. This review focused on the third‐line setting and beyond, but it demonstrated that at least at that time, there was minimal evidence for the effectiveness of any medical treatment for ITP patients with persistent, severe thrombocytopenia, highlighting the need for RCTs to properly evaluate potentially effective treatments in this setting.
Since the publication of this systematic review, the management of ITP has evolved. Treatment decisions are less likely to be guided solely by platelet counts and more likely to rely on a combination of platelet levels; shared decision making between the physician and the patient; and patient factors, such as insurance coverage, lifestyle, history of bleeding, occupation, comorbidities, and expectations.9 Thrombopoietin‐receptor agonists (TPO‐RAs), including eltrombopag and romiplostim, have also entered the market after undergoing rigorous randomized trials in splenectomized and non‐splenectomized patients with persistent or chronic ITP.14, 15, 16 Additionally, the incidence of splenectomy has declined in recent years.17, 18 Despite these developments and trends, the International Consensus Report on the management of primary ITP (released in 2010) lists medical treatment options in the second‐line setting in alphabetical order to avoid indicating a preference for a specific treatment, highlighting the lack of sufficient data to rank the treatments according to efficacy.1 Similarly, shortly after the publication of this report, the American Society of Hematology (ASH) published practice guidelines for ITP, concluding that there is no evidence to guide a sequence of treatment for patients who have recurrent or persistent thrombocytopenia with bleeding after first‐line treatment with corticosteroids, IVIg, or anti‐D.19
Indeed, clinical decision‐making on optimal second‐line ITP treatment is challenging and has been described as controversial.20, 21 The lack of prescriptive clinical guidance in this setting highlights a gap in the scientific literature regarding comparisons of safety and efficacy across available treatments.2, 22 Given this and the changing treatment landscape over the past decade, we sought to systematically review published reports and provide an up‐to‐date summary of studies evaluating the safety and efficacy/effectiveness of therapies used to treat primary ITP in adults in the second‐line setting, with a particular focus on RCTs that have been conducted.
2. METHODS
The scope of this review included both interventional and observational studies that evaluated the safety, efficacy (from interventional designs), and/or effectiveness (from observational designs) of therapies used to treat primary ITP in adults in the second‐line setting. We excluded studies in which the therapy of interest was used in the first‐line setting, case series with less than 20 patients, studies published in languages other than English, and studies conducted in children, pregnant women, or patients with secondary thrombocytopenia. The therapies of interest were: splenectomy, azathioprine, cyclophosphamide, cyclosporine A, danazol, dapsone, eltrombopag, mycophenolate mofetil, rituximab, romiplostim, and vinca alkaloids, as these are the second‐line treatment options provided in the International Consensus Report and the most recent ASH guidelines on the management of primary ITP.1, 19 The outcomes of interest covered several efficacy endpoints, including any platelet‐related change (eg, platelet response, duration of platelet response, etc), rate of and/or time to splenectomy (in studies where splenectomy was not the treatment of interest), rate of rescue medication and/or other ITP therapy use, and rate of bleeding. In terms of safety endpoints, we searched for data on the rates of clinically significant bleeding, thrombotic/thromboembolic events, pulmonary hypertension, infections, respiratory tract infections, neuropathy, leukopenia, hemorrhagic cystitis, fever, and other serious adverse drug reactions. We also obtained data on mortality.
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines.23 Comprehensive literature searches were conducted by several reviewers in October 2016 in the PubMed, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews databases using multiple search strings to fully encompass all aspects of the inclusion criteria (Supplemental Table 1). Bibliographies of relevant reviews and meta‐analyses were also searched for additional pertinent publications. The flow diagram of study inclusion is presented in Figure 1. Study citations were downloaded into a database, and duplicates were removed from the search results using automated de‐duplication methods and manual screening. Studies were reviewed for relevance at the levels of title, abstract, and full text by two independent reviewers. Articles designated as eligible for inclusion were abstracted into a database and independent reviewers performed a quality control assessment for accuracy on each abstracted study. Disagreements were resolved by consensus adjudication. If more than one article from the same study population was published, data from the publication with the longest follow‐up, most recent data, and/or most specifically relevant population and/or outcomes were extracted. Data elements abstracted included study design, population characteristics, treatment description and dosage, and the aforementioned efficacy/effectiveness and safety outcomes. Several measures to assess study bias were also abstracted using the Cochrane Risk of Bias Tool,24 including evaluation of randomization, concealment, blinding, baseline comparability, follow‐up, selective reporting, and analysis.
Figure 1.
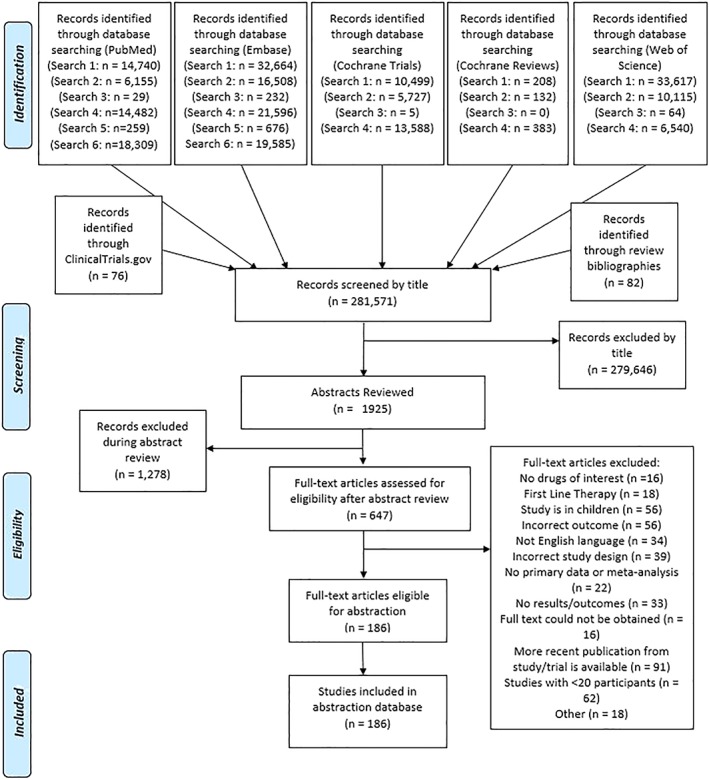
Flowchart of article selection for the systematic literature review. Comprehensive search strings were applied to the medical research databases, and studies were reviewed for relevancy at the levels of title, abstract, and full text. [Color figure can be viewed at wileyonlinelibrary.com]
Tabulated summaries were generated to explain basic characteristics of the included studies. Results for placebo‐controlled RCTs or standard‐of‐care (SOC)‐controlled RCTs were examined in detail, given their general comparability in trial design and rigor. For these studies, for endpoints/outcomes common to at least two studies (complete platelet response, overall platelet response, use of rescue therapies, and bleeding), the study definition of that endpoint and the corresponding results were described. Rate ratios and response ratios and 95% confidence intervals (CIs) were calculated to compare rate/response of the outcome of interest in patients receiving the therapy of interest and patients receiving placebo or SOC for each outcome from each study. Forest plots for each endpoint/outcome were created to display the risk/response ratios across individual studies and therapies. In addition to the measures that were formally described in rate/response ratios and forest plots, median duration of overall/complete platelet response from each study was collected and described, when available. Analyses were performed using R statistical software25 and the “metafor” package.26 A formal meta‐analysis was not performed due to the small number of studies for some treatments (n < 3).
3. RESULTS
Of nearly 300 000 publications identified through our comprehensive literature searches, 186 reports met our inclusion criteria of studies evaluating the safety and efficacy/effectiveness of the second‐line therapies of interest in adult primary ITP. An overview of the basic characteristics of these studies is summarized in Table 1. The majority (N = 139; 75%) of studies were observational in nature, with retrospective cohort studies being the most common (N = 104; 56% of total), followed by prospective cohort studies (N = 32; 17% of total). A substantial proportion (47%) of the studies were conducted solely in European countries, 14% were based on United States (US) data alone, 12% were based on data from China and Japan alone, and 3% were conducted solely in Australia. Of the therapies included in our search, splenectomy was the most commonly studied (N = 83; 47%), followed by rituximab (N = 49; 26%), romiplostim (N = 34; 18%), and eltrombopag (N = 24; 13%). All other therapies were the focus of just 1% to 4% of the studies identified, and just over 4% of the studies included a combination of at least two of the therapies of interest.
Table 1.
Studies included in the systematic review of observational and interventional studies reporting on the safety and efficacy/effectiveness of therapies used in adult immune thrombocytopenia in the second‐line setting
| Number of studies (%) | |
|---|---|
| Study design | |
| Retrospective cohort study | 104 (55.9%) |
| Prospective cohort study | 32 (17.2%) |
| Non‐single‐arm RCT | 20 (10.8%) |
| Single‐arm trial | 18 (9.7%) |
| Case series | 7 (3.8%) |
| Other observational study | 3 (1.6%) |
| Other clinical study | 2 (1.1%) |
| Case‐control study | 0 (0%) |
| Geographic region | |
| European countries only | 87 (46.8%) |
| All others | 30 (16.1%) |
| United States only | 26 (14.0%) |
| Global (involving at least 2 geographic regions above) | 15 (8.1%) |
| China only | 13 (7.0%) |
| Japan only | 10 (5.4%) |
| Australia only | 5 (2.7%) |
| Therapy studied a | |
| Splenectomy onlyb | 83 (44.6%) |
| Rituximab only | 49 (26.3%) |
| Romiplostim only | 34 (18.3%) |
| Eltrombopag only | 24 (12.9%) |
| Combination of at least 2 therapiesc | 8 (4.3%) |
| Danazol only | 7 (3.8%) |
| Vinca alkaloids only | 6 (3.2%) |
| Cyclophosphamide only | 6 (3.2%) |
| Azathioprine only | 4 (2.2%) |
| Cyclosporine A only | 4 (2.2%) |
| Mycophenolate mofetil only | 3 (1.6%) |
| Dapsone only | 2 (1.1%) |
Not mutually exclusive as studies may contain multiple arms evaluating different drugs.
Some studies included other therapies that were not of interest but all studies included splenectomy.
More than one therapy of interest was included with/without other therapies not of interest.
For most of the treatments, including azathioprine, cyclophosphamide, cyclosporine, danazol, dapsone, mycophenolate mofetil, vinblastine, and vincristine, there were limited studies of efficacy/effectiveness and safety identified. Only 22 unique studies conducted over the past four decades covered at least one of these eight therapies, the majority (n = 16) of which were observational in nature. Of note, cyclophosphamide and vincristine have not been studied in a prospective, interventional manner, and the remaining 6 therapies are supported by one such study each. However, the sole interventional study of cyclosporine evaluated this therapy in combination with oral dexamethasone and IV low‐dose rituximab in a phase 2b study.27 The other five studies are briefly described here.
In a single‐arm study of 53 adults with chronic ITP treated with azathioprine, 45% of patients had a “complete remission” after receiving azathioprine (defined as platelet counts ≥150 × 109/L for at least 3 months); median time to response was 4 months.28 In an open‐label study of dapsone among 66 adults with chronic ITP, 30.3% showed a partial response (platelet count >50 × 109/L and at least twice the initial platelet count) and 19.7% demonstrated a complete response (platelet count >150 × 109/L); median time to obtain the maximal platelet count response was 130 days.29 Similarly, in a single‐arm study of mycophenolate mofetil administered among just 21 adults with chronic ITP, 28.6% demonstrated a partial response (platelet count 50 to <100 × 109/L) and 23.8% demonstrated a complete response (platelet count ≥100 × 109/L) after 12 weeks of treatment.30 Vinblastine has been studied in a single interventional study in which 42 patients with ITP, including 17 with chronic ITP, were randomized to either receive vinblastine by IV slow infusions or vinblastine by IV bolus injections, 8 of whom had failed at least one prior therapy and thus were being treated in the second‐line setting.13 Of these, 37.5% showed a complete response (platelet count >150 × 109/L), 12.5% a partial response (platelet count 100 to 150 × 109/L), and 0 of 8 patients had a minor response (platelet count >50 × 109/L and at least a doubling of platelet counts from initial levels), which was evaluated at 6 weeks after IV vinblastine administered either through continuous infusion or bolus injection. Lastly, and most recently, a multicenter, randomized trial was conducted to assess the efficacy and safety of a recombinant human thrombopoietin (rhTPO) in patients with persistent ITP who had failed glucocorticosteroid treatment.31 A total of 140 eligible patients were randomized to receive rhTPO + danazol or danazol alone, but only short‐term (2 weeks) response with danazol alone was assessed. At 2 weeks, 36.5% of 67 patients in the danazol‐alone group had their platelet count restored to normal (≥100 × 109/L) and/or had a platelet count rise to at least 50 × 109/L or increase by 30 × 109/L above baseline with no bleeding symptoms.
Among the remaining non‐surgical therapies, we identified 12 prospective RCTs with a placebo or SOC arm evaluating the safety and efficacy of eltrombopag (5 studies14, 32, 33, 34, 35), rituximab (2 studies36, 37), or romiplostim (5 studies,15, 38, 39, 40 with one publication reporting on two studies conducted in parallel15). (No head‐to‐head RCTs directly comparing one second‐line therapy of interest to another were found.) These studies are summarized in more detail in Table 2, and study‐specific definitions of the outcomes/endpoints common to at least two of the studies are provided in Table 3. Two studies were prospective phase 2, placebo‐controlled RCTs (1 eltrombopag32 and 1 romiplostim38); one was a prospective SOC‐controlled, open label RCT (romiplostim39); one was a prospective placebo‐controlled pilot RCT (rituximab36); and the remaining eight studies were prospective phase 3, placebo‐controlled RCTs (4 eltrombopag,14, 33, 34, 35 1 rituximab,37 and 3 romiplostim39, 40).
Table 2.
Study design and population of randomized clinical trials evaluating eltrombopag, rituximab, or romiplostim vs placebo or standard of care
| Study | Study design | Location and study years | Study population | |||
|---|---|---|---|---|---|---|
| ITP | Splenectomy status | Demographic and clinical characteristics | Sample size | |||
| Eltrombopag | ||||||
| Bussel et al. (2007)32 | Prospective, multicenter, phase 2, randomized, placebo‐controlled, double‐blind | Worldwide (44 clinical sites) | ITP for at least 6 months, platelet count less than 30 × 109/L at enrollment, age 18 years or older | Non‐splenectomized (53%) and splenectomized (47%) | Placebo: Median age 42 years, 55% female, ITP duration not reported, 48% had a platelet count no higher than 15 × 109/L |
Placebo: n = 29 |
| Eltrombopag: | ||||||
| n = 30 (30 mg); | ||||||
| 2005‐2005 | n = 30 (50 mg); and | |||||
| n = 28 (75 mg) | ||||||
| Eltrombopag 30 mg: Median age 51 years, 53% female, ITP duration not reported, 50% had a platelet count no higher than 15 × 109/L | ||||||
| Eltrombopag 50 mg: Median age 45 years, 70% female, ITP duration not reported, 40% had a platelet count no higher than 15 × 109/L | ||||||
| Eltrombopag 75 mg: Median age 55 years, 71% female, ITP duration not reported, 54% had a platelet count no higher than 15 × 109/L | ||||||
| Bussel et al. (2009)14 | Prospective, multicenter, phase 3, randomized, placebo‐controlled, double‐blind | Worldwide (63 clinical sites) 2006 |
ITP for at least 6 months, pretreatment platelet count less than 30 × 109/L, age 18 years or older | Non‐splenectomized (61%) and splenectomized (39%) | Placebo: Median age 51 years, 71% female, ITP duration not reported, 45% had a platelet count no higher than 15 × 109/L |
Placebo: n = 38 |
| Eltrombopag: n = 76 | ||||||
| Eltrombopag: Median age 47 years, 57% female, ITP duration not reported, 50% had a platelet count no higher than 15 × 109/L | ||||||
| Cheng et al. (2011)33 | Prospective, phase 3, randomized, placebo‐controlled, double‐blind | Worldwide (75 clinical sites) 2006‐2007 |
ITP for at least 6 months, baseline platelet count less than 30 × 109/L, age 18 years or older | Non‐splenectomized (64%) and splenectomized (36%) | Placebo: Median age 53 years, 69% female, ITP duration not reported, median platelet count 16 × 109/L |
Placebo: n = 62 |
| Eltrombopag: n = 135 | ||||||
| Eltrombopag: Median age 47 years, 69% female, ITP duration not reported, median platelet count 16 × 109/L | ||||||
| Tomiyama et al. (2012)34 | Prospective, multicenter, phase 3, randomized, placebo‐controlled, double‐blind | Japan 2007‐2008 |
ITP for at least 6 months, platelet count less than 30 × 109/L, age 20 years or older | Non‐splenectomized (30%) and splenectomized (70%) | Placebo: Median age 61 years, 88% female, ITP duration not reported, median platelet count 9.5 × 109/L |
Placebo: n = 8 Eltrombopag: n = 15 |
| Eltrombopag: Median age 58 years, 53% female, ITP duration not reported, median platelet count 21 × 109/L | ||||||
| Yang et al. (2014)35 | Prospective, multicenter, phase 3, randomized, placebo‐controlled, double‐blind | China 2013‐2014 |
Chronic ITP with a platelet count less than 30 × 109/L, age 18 years or older | Non‐splenectomized (84%) and splenectomized (16%) | Limited data in abstract | Placebo: n = 51 |
| Eltrombopag: n = 104 | ||||||
| Placebo: Median age, gender, and median ITP duration not reported, 55% had platelet count no higher than 15 × 109/L |
||||||
| Eltrombopag: Median age, gender, and median ITP duration not reported, 52% had platelet count no higher than 15 × 109/L | ||||||
| Rituxumab | ||||||
| Arnold et al. (2012)36 | Prospective, pilot, randomized, placebo‐controlled | Canada 2006‐2010 |
Newly diagnosed or relapsed ITP with a platelet count less than 30 × 109/L, age 18 years or older | Non‐splenectomized | Placebo: Median age 40 years, 59% female, median ITP duration 0.7 years, median platelet count 14 × 109/L |
Placebo: n = 27 |
| Rituximab: n = 33 | ||||||
| Rituximab: Median age 40 years, 58% female, median ITP duration 0.3 years, median platelet count 15 × 109/L | ||||||
| Ghanima et al. (2015)37 | Prospective, multicenter, phase 3, randomized, placebo‐controlled, double‐blind | Norway, Tunisia, and France 2006‐2011 |
ITP with a platelet count less than 30 × 109/L, age 18 years or older | Non‐splenectomized | Placebo: Median age 46 years, 72% female, median ITP duration 1.0 year, median platelet count 21 × 109/L |
Placebo: n = 54 |
| Rituximab: n = 55 | ||||||
| Rituximab: Median age 46 years, 73% female, median ITP duration 0.7 years, median platelet count 16 × 109/L | ||||||
| Romiplostim | ||||||
| Bussel et al. (2006)31 | Prospective, multicenter, phase 2, randomized, placebo‐controlled, double‐blind | United States 2003‐2004 |
ITP according to ASH guidelines for at least 3 months, mean platelet count less than 30 × 109/L for patients not receiving corticosteroids or a mean platelet count less than 50 × 109/L for patients receiving corticosteroids, age 18‐65 years | Non‐splenectomized (33%) and splenectomized (67%) | All arms combined: Median age 49 years, 71% female, median ITP duration 5.2 years, median platelet count 16 × 109/L |
Placebo: n = 4 |
| Romiplostim: n = 17 | ||||||
| Kuter et al. (2008)a 15 | Prospective, multicenter, phase 3, randomized, placebo‐controlled, double‐blind | United States and Europe 2005‐2006 |
ITP according to ASH guidelines, mean platelet count less than 30 × 109/L during screening, age 18 years or older | Splenectomized | Placebo: Median age 56 years, 52% female, median ITP duration 8.5 years, median platelet count 15 × 109/L |
Placebo: n = 21 |
| Romiplostim: n = 42 | ||||||
| Romiplostim: Median age 51 years, 64% female, median ITP duration 7.8 years, median platelet count 14 × 109/L | ||||||
| Prospective, multicenter, phase 3, randomized, placebo‐controlled, double blind | United States and Europe 2005‐2006 |
ITP according to ASH guidelines, mean platelet count less than 30 × 109/L during screening, age 18 years or older | Non‐splenectomized | Placebo: Median age 46 years, 76% female, median ITP duration 1.6 years, median platelet count 19 × 109/L Romiplostim: Median age 52 years, 66% female, median ITP duration 2.2 years, median platelet count 19 × 109/L |
Placebo: n = 21 Romiplostim: n = 41 |
|
| Kuter et al. (2010)32 | Prospective, multicenter, randomized, controlled, open label | North America, Europe, and Australia 2006‐2007 |
ITP according to ASH guidelines, pre‐treatment platelet count less than 50 × 109/L, age 18 years or older | Non‐splenectomized | Standard of care: Median age 57 years, 60% female, median ITP duration 2.3 years, median platelet count 27 × 109/L |
Standard of care: n = 77 |
| Romiplostim: n = 157 | ||||||
| Romiplostim: Median age 58 years, 54% female, median ITP duration 2.1 years, median platelet count 33 × 109/L | ||||||
| Shirasugi et al. (2011)40 | Prospective, phase 3, randomized, placebo‐controlled, double blind | Japan | ITP diagnosed at least 6 months prior to enrollment, mean platelet count no higher than 30 × 109/L during screening, age 20 years or older | Non‐splenectomized (56%) and splenectomized (44%) | Placebo: Mean age 47 years, 83% female, mean ITP duration 7.6 years, mean platelet count 16 × 109/L |
Placebo: n = 12 |
| Romiplostim: n = 22 | ||||||
| 2007‐2009 | ||||||
| Romiplostim: Mean age 59 years, 64% female, mean ITP duration 9.7 years, mean platelet count 18 × 109/L | ||||||
Abbreviations: ASH, American Society of Hematology; ITP, immune thrombocytopenia; L, liter; mg, milligram.
Reported on two parallel studies.
Table 3.
Study endpoint definitions used in randomized clinical trials evaluating eltrombopag, rituximab, or romiplostim vs placebo or standard of care
| Study | Therapy studied | Bleeding | Overall response | Complete response | Rescue therapy | Duration of platelet response |
|---|---|---|---|---|---|---|
| Eltrombopag | ||||||
| Bussel et al. (2007)36 | Eltrombopag vs placebo | Bleeding symptoms at day 43 of any grade according to the WHO bleeding scale (grade 0: No bleeding, grade 1: Petechiae, grade 2: Mild blood loss, grade 3: Gross blood loss, grade 4: Debilitating blood loss) | Platelet count of at least 50 × 109/L on day 43 | Not reported | Not reported | Not reported |
| Bussel et al. (2009)18 | Eltrombopag versus placebo | Definition #1: Bleeding symptoms at day 43 of any grade according to the WHO bleeding scale (grade 0: No bleeding, grade 1: Petechiae, grade 2: Mild blood loss, grade 3: Gross blood loss, grade 4: Debilitating blood loss) | Platelet count of at least 50 × 109/L on day 43 | Platelet count of at least 50 × 109/L and at least twice the baseline value at any point during treatment | Not reported | Not reported |
| Definition #2: Bleeding symptoms of any grade according to the WHO bleeding scale at any point during treatment | ||||||
| Definition #3: Bleeding of any type as an adverse event throughout treatment | ||||||
| Cheng et al. (2011)37 | Eltrombopag versus placebo | Definition #1: Bleeding symptoms of any grade according to the WHO bleeding scale (grade 0: No bleeding, grade 1: Petechiae, grade 2: Mild blood loss, grade 3: Gross blood loss, grade 4: Debilitating blood loss) | Platelet count of 50‐400 × 109/L at any assessment | Definition #1: Platelet count of 50 to 400 × 109/L at 75% or more of assessments | New treatment for chronic ITP, an increased dose of baseline treatment, platelet transfusion, or splenectomy | Mean maximum weeks of continuous response during the 6‐month treatment period |
| Definition #2: Durable response defined as achieving a platelet count of 50‐400 × 109/L in at least 6 of the last 8 weeks of treatment and never receiving rescue treatment | ||||||
| Definition #2: Bleeding symptoms of grade 2 or higher according to the WHO bleeding scale (grade 2: Mild blood loss, grade 3: Gross blood loss, grade 4: Debilitating blood loss) | ||||||
| Definition #3: Bleeding of any type as an adverse event throughout treatment | ||||||
| Definition #4: Serious bleeding as an adverse event throughout treatment | ||||||
| Tomiyama et al. (2012)38 | Eltrombopag vs placebo | Bleeding symptoms of any grade (but only reported in the eltrombopag arm) | Platelet count of 50‐400 × 109/L at 6 weeks | Platelet count of 50‐400 × 109/L at 4 or more assessments between week 2 and week 6 | Not reported | Not reported |
| Yang et al. (2014)39 | Eltrombopag versus placebo | Not reported | Platelet count of 50‐250 × 109/L at 6 weeks | Not reported | Not reported | Not reported |
| Rituximab | ||||||
| Arnold et al. (2012)34 | Rituximab versus placebo | Grade 2 or higher bleeding events according to the ITP bleeding score | Platelet count of at least 30 × 109/L plus at least a doubling of the platelet count from baseline at 6 months | Platelet count of at least 100 × 109/L at 6 months | Not explicitly defined but consisted of the following in the results: Prednisone, dexamethasone, rhesus immune globulin, azathioprine, danazol, romiplostim, and platelet transfusion | Not reported |
| Ghanima et al. (2015)35 | Rituximab versus placebo | Grade 2 or 3 bleeding events according to the WHO bleeding scale (grade 2: Mild blood loss, grade 3: Gross blood loss). | Platelet count of at least 30 × 109/L after week 4 from first study drug administration plus at least a doubling of the platelet count from baseline | Platelet count of at least 100 × 109/L after week 4 from first study drug administration plus at least a doubling of the platelet count from baseline | Not reported | Median time to relapse after achieving an overall or complete platelet response following treatment |
| Romiplostim | ||||||
| Bussel et al. (2006)31 | Romiplostim versus placebo | Bleeding as a serious adverse event | Platelet count of 50‐450 × 109/L and at least a doubling of platelet count from baseline | Not reported | Not reported | Not reported |
| Kuter et al. (2008)19 | Romiplostim versus placebo | Grade 3 or higher bleeding events (those rated as severe [grade 3], life‐threatening [grade 4], or fatal [grade 5]). | Durable + transient rates of platelet response, where: | Durable platelet response = weekly platelet response of at least 50 × 109/L during at least 6 weeks of the last 8 weeks of treatment | Increased dose of concurrent ITP therapy or use of any new drug to increase platelet counts | Not reported |
| Durable = weekly platelet response of at least 50 × 109/L during at least 6 weeks of the last 8 weeks of treatment | ||||||
| Transient = weekly platelet response of at least 50 × 109/L during at least 4 weeks without a durable platelet response from week 2 to week 25 | ||||||
| Kuter et al. (2010)32 | Romiplostim versus standard of care | Definition #1: Grade 2 or higher bleeding events (those rated as moderately severe [grade 2], severe [grade 3], life‐threatening [grade 4], or fatal [grade 5]) Definition #2: Grade 3 or higher bleeding events (those rated as severe [grade 3], life‐threatening [grade 4], or fatal [grade 5]) |
Platelet count greater than 50 × 109/L at any scheduled visit | Not reported | Not reported | Not reported |
| Shirasugi et al. (2011)33 | Romiplostim versus placebo | Definition #1: Bleeding symptoms defined as purpura/petechiae, epistaxis, oral bleeding, menorrhagia, bruising, intracranial bleeding, gastrointestinal bleeding, and/or other bleeding symptoms at week 13 | Platelet count of at least 50 × 109/L and at least a doubling of platelet count from baseline | Not reported | Any medication administered to raise platelet counts, including IVIg, platelet transfusions, corticosteroids, and an increase in dose or frequency of a concomitant oral corticosteroid, azathioprine, and/or danazol | Median duration of platelet response during the 12‐week treatment period |
| Definition #2: Grade 3 or higher bleeding events (those rated as severe [grade 3], life‐threatening [grade 4], or fatal [grade 5]) | ||||||
Abbreviations: ITP, immune thrombocytopenia; IVIg, intravenous immunoglobulin; L, liter; WHO, World Health Organization.
All 12 RCTs reported on some measure of overall platelet response, with studies of TPO‐RAs using a definition centered on a platelet count threshold of ≥50 × 109/L and rituximab studies utilizing a lower threshold of ≥30 × 109/L (Table 3). Across TPO‐RA studies, which enrolled both splenectomized and non‐splenectomized patients, the overall platelet response tended to be higher in patients receiving eltrombopag or romiplostim compared with patients in the respective placebo/SOC arms (Figure 2). The calculated response ratio comparing the rate of overall platelet response in eltrombopag vs placebo patients ranged from 1.40 (95% CI: 0.27‐7.18) to 13.24 (95% CI: 1.98‐88.62).14, 32, 33, 34, 35 For studies of romiplostim, the response ratio ranged from 1.50 (95% CI: 0.22‐10.22) to 34.28 (95% CI: 2.20‐533.41).15, 38, 39, 40 The two studies comparing rituximab to placebo in non‐splenectomized patients were generally null, demonstrating no significant effect of intervention. The response ratio comparing the overall platelet response in rituximab vs placebo patients was 0.86 (95% CI: 0.60‐1.22) calculated from the Arnold et al. study36 and 1.09 (95% CI: 0.85‐1.40) calculated from the Ghanima et al. study37 (Figure 2).
Figure 2.
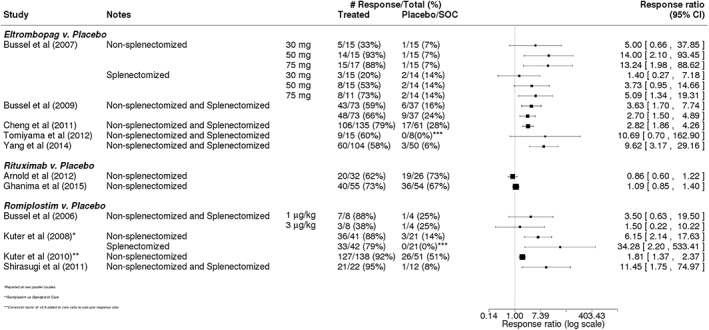
Overall platelet response in trials of eltrombopag, rituximab, or romiplostim. Calculated response ratios comparing the overall platelet response of patients receiving eltrombopag, rituximab, or romiplostim vs that in patients receiving placebo or standard of care
Complete platelet response was evaluated in seven trials.14, 15, 33, 34, 36, 37 The definition of this measure varied considerably across these studies, but most required a demonstrated minimum platelet count (eg, 50 or 100 × 109/L) over a specified period of time (Table 3). Across all three treatments evaluated, response rates tended to be higher in patients receiving one of the treatments vs those in patients receiving placebo or SOC (Figure 3). Similar to the data around overall platelet response, the differences in complete platelet response between treated and placebo/SOC patients were greatest among studies of eltrombopag (rate ratios ranging from 4.32 [95% CI: 1.87‐9.98] to 6.60 [95% CI: 0.41‐105.81])14, 33, 34 and romiplostim (rate ratios: 12.80 [95% CI: 1.86‐88.07] and 16.88 [95% CI: 1.06‐268.42]).15
Figure 3.
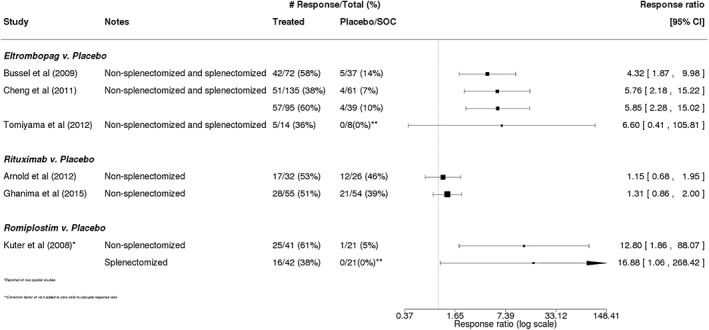
Complete platelet response in trials of eltrombopag, rituximab, or romiplostim. Calculated response ratios comparing the complete platelet response of patients receiving eltrombopag, rituximab, or romiplostim vs that in patients receiving placebo or standard of care
Duration of response during the active, blinded treatment period was reported in three of the 12 RCTs (Table 3).33, 37, 40 In a trial of eltrombopag administered daily for 6 months, the maximum continuous response was a median of 8.1 weeks among eltrombopag patients and 0 weeks among placebo patients.33, 41 A study of rituximab examined the median time to relapse after achieving overall or complete platelet response following four weekly infusions of rituximab.37 Median time to relapse over a 78‐week observation period in patients who achieved overall response was 36 weeks (IQR: 13‐not reached) in the rituximab group and 7 weeks (IQR: 5‐69 weeks) in the placebo group (P = 0.014). Similarly, median time to relapse in patients who achieved complete response was 76 weeks (IQR: 31‐not reached) and 49 weeks, respectively (P = 0.19). In a study of romiplostim given weekly for a 12‐week period, the median duration of platelet response (interquartile range [IQR]) was 11 weeks (9‐12) in the romiplostim group and 0 weeks (0‐0) in the placebo group (P < 0.0001).40
Rates of rescue therapy use were reported in four of the 12 trials,15, 33, 36, 40 and rescue therapy was generally defined as any new treatment measure (or increase in dose of current treatment) aimed at increasing platelet counts (Table 3). The rates of rescue therapy use in patients receiving a therapy of interest vs patients receiving placebo or SOC were consistently lower in the treatment arms across the studies, corresponding to a range of rate ratios of 0.28 (95% CI: 0.13‐0.59) in a study of romiplostim15 to 0.67 (95% CI: 0.41‐1.08) in a study of rituximab36 (Figure 4).
Figure 4.
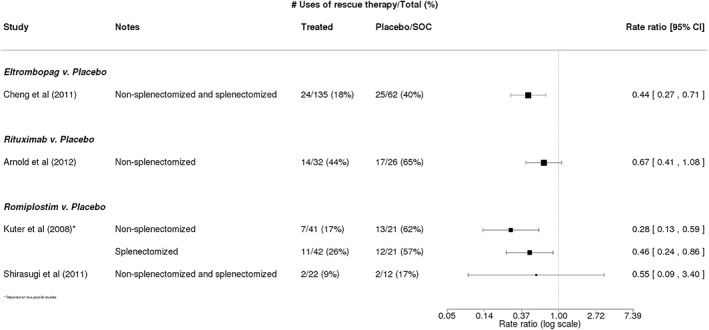
Rescue therapy use in trials of eltrombopag, rituximab, or romiplostim. Calculated rate ratios comparing the rate of rescue therapy use among patients receiving eltrombopag, rituximab, or romiplostim vs that in patients receiving placebo or standard of care
Bleeding was assessed in 10 of the 12 studies and was treated as a measure of both efficacy and safety.14, 15, 32, 33, 36, 37, 38, 39, 40 The assessment of symptoms and events of bleeding varied across the studies, utilizing a combination of the World Health Organization scale of bleeding, the Adverse Events Reporting System of bleeding events by grade, and the ITP bleeding score (Table 3). In general, bleeding rates in patients receiving one of the TPO‐RAs romiplostim or eltrombopag (among both splenectomized and non‐splenectomized patients) tended to be lower than those in the placebo or SOC arm (Figure 5), although none of the individual results were significant. For studies of eltrombopag, bleeding was up to 89% less likely in eltrombopag patients vs those receiving placebo,14, 32, 33 but in one study, the rate ratio was 1.16 (95% CI: 0.35‐3.89),32 indicating similar rates of bleeding in the eltrombopag and placebo arms. In trials of romiplostim, the occurrence of bleeding events or symptoms was 25% to 81% less likely in romiplostim patients vs those receiving placebo/SOC (range of rate ratios: 0.19 [95% CI: 0.01‐3.75] to 0.75 [95% CI: 0.39‐1.42]).15, 38, 39, 40 Both rituximab trials that reported rates of bleeding were conducted in non‐splenectomized patients and observed slightly lower but similar bleeding rates between patients receiving rituximab and placebo36, 37 (Figure 5).
Figure 5.
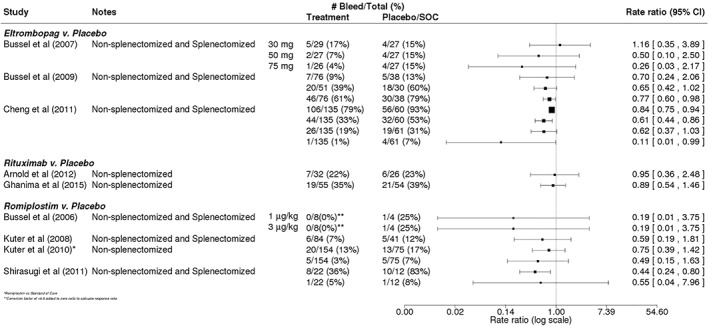
Bleeding in trials of eltrombopag, rituximab, or romiplostim. Calculated rate ratios comparing the rate of bleeding in patients receiving eltrombopag, rituximab, or romiplostim vs that in patients receiving placebo or standard of care
No studies represented prospective RCTs with splenectomy and a placebo or SOC arm, but surgical splenectomy has been well‐studied and still represents an important treatment option for ITP patients in the second‐line setting. Among the 83 studies evaluating splenectomy, the reported rates of complete response rates ranged from 37.3%42 to 100%43 with a median complete response rate of 70.5% across studies. The median partial response rate was 13.5% (range 0%43 to 88%44) and the overall response rates ranged from 63.4%45 to 100%43, 46, 47, 48, 49, with a median of 86.5%. The median duration of response was reported in five studies, ranging from 29.5 months50 to 120 months51 with a median of 81 months. The rate of relapse ranged from 0%52 to 81.8%53 with a median rate of 20%, and the median rate of significant bleeding was 4.76% (range 0%54 to 28.1%55). Mortality rates ranged from 0% among multiple studies to 28.8%56 (over a median follow‐up time of 18 years) (median 2% across studies).
4. DISCUSSION
This systematic review identified published reports of interventional and observational studies that have evaluated the efficacy/effectiveness and safety of therapies used to treat adults with primary ITP in the second‐line setting. All therapies of interest were represented in the studies identified, and the majority of studies were observational (and primarily retrospective) in nature.
For the majority of non‐surgical treatments, including azathioprine, cyclophosphamide, cyclosporine, danazol, dapsone, mycophenolate mofetil, vinblastine, and vincristine, there were limited published reports of any kind. Just over 11% of all 186 reports meeting our inclusion criteria focused on at least one of these agents, and only 5 studies (<3% of total) investigated one of these agents as a monotherapy in a prospective, interventional manner. Of note, four of these studies were conducted over two decades ago. Therefore, it is not surprising that although these agents may have promise in treating ITP and are therefore listed as potential second‐line treatments in the International Consensus Report on the management of primary ITP,1 the 2011 ASH practice guidelines for ITP do not specify these agents in their formal recommendations, stating that research on these therapies is “inadequate to allow evidence‐based recommendations on appropriate indications or timing.”19 Our recent review of the literature suggests that this is still the case.
Although no head‐to‐head RCTs directly comparing one second‐line therapy of interest to another were found, we identified 12 prospective RCTs with a placebo or SOC arm evaluating the safety and efficacy of either eltrombopag (5 studies), rituximab (2 studies), or romiplostim (5 studies). Among endpoints/outcomes of interest, at least two of these studies measured and reported some form of overall platelet response (all 12 studies), complete platelet response (7 studies), use of rescue therapies (4 studies), and occurrence of bleeding symptoms or events (10 studies). There are an insufficient number of studies for each outcome to conduct a meta‐analysis. The rate or risk ratios calculated from each trial provide important information on the safety and efficacy of the treatment studied and offer insights on how these therapies compare with one another in treating adult ITP in the second‐line setting.
In terms of efficacy, the most compelling evidence stems from the trials of eltrombopag14, 32, 33, 34, 35 and romiplostim.15, 38, 39, 40 Without exception, across the 10 studies, patients treated with one of these therapies tended to have higher rates of overall and complete platelet responses compared with patients receiving placebo or SOC. Bleeding and use of rescue therapies were also generally lower in patients receiving one of these TPO‐RA agents. Importantly, longer‐term, open‐label studies of these treatments have demonstrated that efficacy is maintained and that the treatments are safe and well‐tolerated over longer periods of exposure.57, 58 In a study of patients treated with eltrombopag for up to 3 years, platelet counts of ≥50 × 109/L and a doubling of platelet counts from baseline were maintained for a median of 73 weeks over 104 weeks of treatment (n = 147) and 109 weeks over 156 weeks of treatment (n = 32) with no new or increased risk of safety issues.58 In a study of 291 patients treated with romiplostim for up to 5 years (representing 614 patient‐years of exposure), the median percentage of time on study with a platelet count ≥50 × 109/L was 92% (IQR: 62‐100), with a low rate of bleeding and infrequent need for rescue therapy.57 Most patients (63%) responded after just one dose of romiplostim, and the proportion of patients with a platelet ≥50 × 109/L remained between 62% and 78% through week 212. Eltrombopag and romiplostim are both agents that interact with the TPO receptor to trigger platelet production and are indicated for the treatment of thrombocytopenia in the second‐line setting and beyond in patients with chronic ITP who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.59, 60, 61, 62
Results from the two rituximab studies, which were both conducted exclusively in non‐splenectomized patients, provided less conclusive results.36, 37 In the Canadian pilot study of rituximab plus standard of care vs placebo conducted by Arnold et al.,36 no difference between rituximab and placebo groups were observed after 6 months with respect to the composite outcome of platelet response, significant bleeding, or rescue treatment. In the more recent study of rituximab vs placebo in the second‐line treatment of adult ITP patients in Norway, Tunisia, or France, Ghanima et al.37 also did not detect a difference in the rates of treatment failure (a composite endpoint of splenectomy or meeting criteria for splenectomy), response, or relapse. However, authors noted that a small benefit with rituximab cannot be ruled out based on the longer duration of response with rituximab that was observed in those who achieved an overall response (but not in those who achieved a complete response) and the numerically higher response rates observed with rituximab. This assertion is supported by data from a study of 72 adults with chronic ITP who were treated with the standard dose of rituximab of 4 weekly infusions and had demonstrated initial response to rituximab, defined as a documented ongoing platelet count of ≥50 × 109/L for 1 year after the first infusion without additional ITP treatment.57 After a median follow‐up of 3.8 years, 64% of these adults maintained their response to rituximab. Authors used these data, in combination with data from published reports, to estimate that the 1‐year and 5‐year response rates for adults treated with rituximab are 38% and 21%, respectively. Importantly, rituximab, an anti‐CD20 antibody, is currently not approved for the treatment of ITP but is widely used in this setting.63 However, as Ghanima et al.37 point out, their placebo‐controlled study of rituximab emphasizes the need for additional RCTs to assess the efficacy and safety of treatment in this setting before implementation and cautions against relying on evidence from uncontrolled studies.
Although not formally studied as a treatment arm in any prospective RCT, splenectomy is widely covered in the literature. Importantly, it is still generally recommended as the standard therapy for patients with chronic ITP, given the high probability of durable platelet response.1, 11, 19 However, this invasive surgical procedure is not without risk; perioperative and short‐term and long‐term postoperative complications, such as infections, thromboembolic events, and increased risk of certain malignancies including buccal, esophageal, colon, liver, pancreatic, lung, prostate, and hematopoietic cancers have been observed.64, 65 Additionally, there is currently no ability to reliably predict who will respond, and there is evidence that a portion of non‐splenectomized patients will experience late remissions either spontaneously or with continuing medical treatment.22, 66, 67 Due to these issues and because of the availability of medical alternatives, splenectomy may not be the “go‐to” treatment it once was for patients requiring second‐line therapy,21 as evidenced by recent temporal trends in the uptake of splenectomy.17, 18 For example, in a study conducted in Denmark, the 1‐year cumulative incidence of splenectomy among patients with ITP for a duration of at least 6 months decreased in recent years, from 10% for those diagnosed in 1996 through 2001 to 3% for those diagnosed in 2008 through 2012.18 With fewer patients undergoing splenectomy in the second‐line setting, there is a need to further investigate the potential for medical treatments to delay or obviate the need for splenectomy.
Drawing specific treatment recommendations from this review is challenging. There was a paucity of data for several of the therapies of interest, namely azathioprine, cyclophosphamide, danazol, dapsone, mycophenolate mofetil, and vinca alkaloids, making it difficult to assess the relative value of these treatments in this setting. Small sample size and rarity of events also resulted in a lack of precision for some outcomes. Additionally, comparability of data across the studies was somewhat limited by a lack of consistency in the outcomes measured and varying outcome definitions. Although recommendations on standard terminology, definitions, and outcome criteria in ITP were developed by an International Working Group nearly a decade ago,68 they have not been widely adopted, even in the clinical trial setting. For example, while some studies reported complete, partial, or overall response, others reported rates of remission and relapse or pre‐treatment and post‐treatment median platelet counts. Even when the same measure was assessed in multiple studies, the definition of that measure was not uniform across investigations. This was particularly evident in the use of bleeding scales across studies, as not all scales were validated for use in ITP and some were designed to report toxicity of chemotherapeutic agents. Of note, the International Working Group did not recommend a specific bleeding scale for use in clinical trials or cohort studies. Also, data availability on safety outcomes was particularly problematic, as it depended on the study design (eg, RCT vs retrospective cohort study), data source (eg, RCT vs electronic health record data), and therapy being investigated (eg, splenectomy vs romiplostim). Future reviews of this nature and interpretation of the data for clinical utility and potential drug development would benefit greatly from uniform use of the previously established standardized terminology and extension of standardized terminology to severity of bleeding in ITP.
Despite these limitations, this systematic review of the literature provides a comprehensive and updated view of the evidence around the safety and efficacy/effectiveness of second‐line treatments for adult primary ITP based on nearly 200 studies conducted in several populations worldwide. It confirms that a gap remains: outside of the TPO‐RAs eltrombopag and romiplostim, the majority of treatment options for managing recurrent or persistent thrombocytopenia are still without rigorous evidence from RCTs to demonstrate safety and efficacy in this setting, and many treatments have limited supportive evidence of any kind. These findings are echoed in a recent review, although not systematic in nature but based on extensive clinical experience, where Lambert and Gernsheimer10 conclude that for the second‐line treatment of adult ITP patients with persistently low platelet counts and bleeding, evidence to date supports medical alternatives to splenectomy, specifically in the context of both TPO‐RAs, which are now backed by long‐term follow‐up data on efficacy and safety.69, 70
It is worth noting that since the publication of the most recent International Consensus Report and ASH guidelines on the management of primary ITP,1, 19 fostamatinib, a spleen tyrosine kinase (Syk) inhibitor, was approved in April 2018 by the US Food and Drug Administration for the treatment of chronic ITP in adults who have had an insufficient response to prior therapy. This was based on two parallel phase 3 placebo‐controlled RCTs conducted in Australia, Europe, and North America that studied a total of 150 patients with persistent or chronic ITP, of whom 101 received fostamatinib.71 In the pooled analysis of the two studies, 18% of patients who received fostamatinib achieved a stable response by week 24 (vs 2% in the placebo group, P = 0.0003), defined as platelet counts ≥50 × 109/L without rescue medication on at least 4 of the 6 clinic visits occurring every 2 weeks during weeks 14 through 24. Overall response, defined as at least one platelet count ≥50 × 109/L within the first 12 weeks, was assessed as a post hoc endpoint and was achieved in 43% of fostamatinib patients (vs 14% in the placebo group, P = 0.0006). Of note, these studies included patients with long‐standing ITP (median duration of 8.5 years), a median of 3 unique prior ITP treatments, and an average baseline platelet count <20 × 109/L. This may represent a promising new treatment for ITP with a unique mechanism of action, but additional research is needed to further assess its long‐term clinical efficacy and safety and identify those most likely to respond. It remains to be seen if and how this therapy will be incorporated into future ITP management guidelines.
Ideally, more randomized and controlled clinical studies would be conducted to properly assess the risk: benefit profile of any existing or new treatment by itself or against other options in this setting. Alternatively, given the rarity of both ITP and any potential adverse events associated with a given therapy, well‐designed non‐interventional studies using large population‐based sources of data, such as those from administrative claims databases, electronic health record databases, or disease‐specific or treatment‐specific registries, could offer valuable evidence on the real‐world effectiveness and safety and comparative effectiveness and safety of available treatments.72 In the absence of such studies, clinical expertise, patient preference and shared decision making will continue to be the primary drivers of treatment decisions rather than high‐quality clinical trial evidence or robust observational studies.
CONFLICT OF INTERESTS
LCB and JPF are employees of EpidStat Institute and CB consults for EpidStat Institute. EpidStat Institute received funding from Amgen Inc for this research and from Amgen Inc, Merck, Genentech, Sanofi, and AstraZeneca for other research.
KC, FC, and BM are employees of Amgen Inc and own stock in Amgen Inc.
JSW receives research support from Amgen and is a consultant and advisory board member for Amgen. JSW has consulted for Novartis and is on the Speakers Bureau for Novartis. JSW also receives research funding from Merck, Incyte, and Pfizer.
AUTHOR CONTRIBUTIONS
LCB, JPF, KC, FC, CB, BM, and JSW contributed to the design and execution of the systematic literature review, including data collection and organization. FC performed the statistical analysis of the data. LCB, JPF, KC, FC, CB, BM, and JSW assisted in analyzing and interpreting the data. LCB developed the first draft of the manuscript, and JPF, KC, FC, CB, BM, and JSW made significant contributions to the final version of the manuscript.
Supporting information
Supplemental Table 1. Search strings used in systematic literature review.
ACKNOWLEDGMENT
This study was supported in part by research funding from Amgen Inc to EpidStat Institute.
Bylsma LC, Fryzek JP, Cetin K, et al. Systematic literature review of treatments used for adult immune thrombocytopenia in the second‐line setting. Am J Hematol. 2019;94:118–132. 10.1002/ajh.25301
Funding information This study was supported in part by research funding from Amgen Inc to EpidStat Institute.
REFERENCES
- 1. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168‐186. [DOI] [PubMed] [Google Scholar]
- 2. Rodeghiero F, Ruggeri M. ITP and international guidelines: what do we know, what do we need? Presse Med (Paris, France: 1983). 2014;43:e61‐e67. [DOI] [PubMed] [Google Scholar]
- 3. Provan D. Characteristics of immune thrombocytopenic purpura: a guide for clinical practice. Eur J Haematol Suppl. 2009;82:8‐12. [DOI] [PubMed] [Google Scholar]
- 4. Neylon AJ, Saunders PW, Howard MR, et al. Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population‐based cohort of 245 patients. Br J Haematol. 2003;122:966‐974. [DOI] [PubMed] [Google Scholar]
- 5. Nomura S. Advances in diagnosis and treatments for immune thrombocytopenia. Clin Med Insights Blood Disord. 2016;9:15‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper N. Intravenous immunoglobulin and anti‐RhD therapy in the management of immune thrombocytopenia. Hematol Oncol Clin N Am. 2009;23:1317‐1327. [DOI] [PubMed] [Google Scholar]
- 7. Kitchens CS, Pendergast JF. Human thrombocytopenia is associated with structural abnormalities of the endothelium that are ameliorated by glucocorticosteroid administration. Blood. 1986;67:203‐206. [PubMed] [Google Scholar]
- 8. Newland AC, Treleaven JG, Minchinton RM, et al. High‐dose intravenous IgG in adults with autoimmune thrombocytopenia. Lancet (London, England). 1983;1:84‐87. [DOI] [PubMed] [Google Scholar]
- 9. Provan D, Newland AC. Current management of primary immune thrombocytopenia. Adv Ther. 2015;32:875‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129:2829‐2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaturvedi S, Arnold DM, McCrae KR. Splenectomy for immune thrombocytopenia: down but not out. Blood. 2018;131:1172‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vesely SK, Perdue JJ, Rizvi MA, Terrell DR, George JN. Management of adult patients with persistent idiopathic thrombocytopenic purpura following splenectomy: a systematic review. Ann Intern Med. 2004;140:112‐120. [DOI] [PubMed] [Google Scholar]
- 13. Facon T, Caulier MT, Wattel E, Jouet JP, Bauters F, Fenaux P. A randomized trial comparing vinblastine in slow infusion and by bolus i.v. injection in idiopathic thrombocytopenic purpura: a report on 42 patients. Br J Haematol. 1994;86:678‐680. [DOI] [PubMed] [Google Scholar]
- 14. Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double‐blind, placebo‐controlled trial. Lancet (London, England). 2009;373:641‐648. [DOI] [PubMed] [Google Scholar]
- 15. Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomised controlled trial. Lancet (London, England). 2008;371:395‐403. [DOI] [PubMed] [Google Scholar]
- 16. Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long‐term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113:2161‐2171. [DOI] [PubMed] [Google Scholar]
- 17. Palandri F, Polverelli N, Sollazzo D, et al. Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years. Am J Hematol. 2016;91:E267‐E272. [DOI] [PubMed] [Google Scholar]
- 18. Cetin K, Wetten S, Christiansen C, et al. Recent time trends in the uptake of splenectomy in adults diagnosed with chronic immune thrombocytopenia: a nationwide historical cohort study in Denmark, 1996‐2012. Haematologica. 2015;100:566. [Google Scholar]
- 19. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190‐4207. [DOI] [PubMed] [Google Scholar]
- 20. Lakshmanan S, Cuker A. Contemporary management of primary immune thrombocytopenia in adults. J Thromb Haemostasis: JTH. 2012;10:1988‐1998. [DOI] [PubMed] [Google Scholar]
- 21. Cuker A, Cines DB, Neunert CE. Controversies in the treatment of immune thrombocytopenia. Curr Opin Hematol. 2016;23:479‐485. [DOI] [PubMed] [Google Scholar]
- 22. Ghanima W, Godeau B, Cines DB, Bussel JB. How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second‐line treatment. Blood. 2012;120:960‐969. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ (Clin Res Ed). 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. JPT Higgins, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Collaboration 2011.
- 25. R Core Team . R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2015.
- 26. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;36:1‐48. [Google Scholar]
- 27. Choi PY, Roncolato F, Badoux X, et al. A novel triple therapy for ITP using high‐dose dexamethasone, low‐dose rituximab, and cyclosporine (TT4). Blood. 2015;126:500‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quiquandon I, Fenaux P, Caulier MT, Pagniez D, Huart JJ, Bauters F. Re‐evaluation of the role of azathioprine in the treatment of adult chronic idiopathic thrombocytopenic purpura: a report on 53 cases. Br J Haematol. 1990;74:223‐228. [DOI] [PubMed] [Google Scholar]
- 29. Godeau B, Durand JM, Roudot‐Thoraval F, et al. Dapsone for chronic autoimmune thrombocytopenic purpura: a report of 66 cases. Br J Haematol. 1997;97:336‐339. [DOI] [PubMed] [Google Scholar]
- 30. Hou M, Peng J, Shi Y, et al. Mycophenolate mofetil (MMF) for the treatment of steroid‐resistant idiopathic thrombocytopenic purpura. Eur J Haematol. 2003;70:353‐357. [DOI] [PubMed] [Google Scholar]
- 31. Wang S, Yang R, Zou P, et al. A multicenter randomized controlled trial of recombinant human thrombopoietin treatment in patients with primary immune thrombocytopenia. Int J Hematol. 2012;96:222‐228. [DOI] [PubMed] [Google Scholar]
- 32. Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357:2237‐2247. [DOI] [PubMed] [Google Scholar]
- 33. Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6‐month, randomised, phase 3 study. Lancet (London, England). 2011;377:393‐402. [DOI] [PubMed] [Google Scholar]
- 34. Tomiyama Y, Miyakawa Y, Okamoto S, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemostasis: JTH. 2012;10:799‐806. [DOI] [PubMed] [Google Scholar]
- 35. Yang R, Hou M, Li J, et al. Effect of eltrombopag on platelet response and safety results in Chinese adults with chronic ITP‐primary result of a phase III study. Blood. 2014;124 Abstract 1464. [Google Scholar]
- 36. Arnold DM, Heddle NM, Carruthers J, et al. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood. 2012;119:1356‐1362. [DOI] [PubMed] [Google Scholar]
- 37. Ghanima W, Khelif A, Waage A, et al. Rituximab as second‐line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet (London, England). 2015;385:1653‐1661. [DOI] [PubMed] [Google Scholar]
- 38. Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis‐stimulating protein, for chronic ITP. N Engl J Med. 2006;355:1672‐1681. [DOI] [PubMed] [Google Scholar]
- 39. Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363:1889‐1899. [DOI] [PubMed] [Google Scholar]
- 40. Shirasugi Y, Ando K, Miyazaki K, et al. Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double‐blind, randomized phase III clinical trial. Int J Hematol. 2011;94:71‐80. [DOI] [PubMed] [Google Scholar]
- 41. ClinicalTrials.gov. RAISE: randomized placebo‐controlled idiopathic thrombocytopenic purpura (ITP) study with eltrombopag (RAISE) (NCT00370331); 2017.
- 42. Li HQ, Zhang L, Zhao H, Ji LX, Yang RC. Chronic idiopathic thrombocytopenic purpura in adult Chinese patients: a retrospective single‐centered analysis of 1791 cases. Chin Med J (Engl). 2005;118:34‐37. [PubMed] [Google Scholar]
- 43. Watson DI, Coventry BJ, Chin T, Gill PG, Malycha P. Laparoscopic versus open splenectomy for immune thrombocytopenic purpura. Surgery. 1997;121:18‐22. [DOI] [PubMed] [Google Scholar]
- 44. Lipsic T, Schnorrer M. Long‐term results of splenectomy (SE) in idiopathic thrombycytopenic purpura (ITP). Br J Haematol. 1994;87:250. [Google Scholar]
- 45. den Ottolander GJ, Gratama JW, de Koning J, et al. Long‐term follow‐up study of 168 patients with immune thrombocytopenia. Implications for therapy. Scand J Haematol. 1984;32:101‐110. [DOI] [PubMed] [Google Scholar]
- 46. Bresler L, Guerci A, Brunaud L, et al. Laparoscopic splenectomy for idiopathic thrombocytopenic purpura: outcome and long‐term results. World J Surg. 2002;26:111‐114. [DOI] [PubMed] [Google Scholar]
- 47. Novitzky N, Seth Y, Mothupi R. Long term outcome of patients undergoing splenectomy for immune thrombocytopenic purpura. Blood 2004;104 abstract 3936. [Google Scholar]
- 48. Wong GC, Lee LH. A study of idiopathic thrombocytopenic purpura (ITP) patients over a ten‐year period. Ann Acad Med Singapore. 1998;27:789‐793. [PubMed] [Google Scholar]
- 49. Gibson M, Sehon JK, White S, et al. Splenectomy for idiopathic thrombocytopenic purpura: a five‐year retrospective review. Am Surg. 2000;66:952‐954. discussion 955. [PubMed] [Google Scholar]
- 50. Ismet A, Irfan K, Emin K, et al. Splenectomy results in patients with idiopathic thrombocytopenic purpura: 10 years of experience in Turgut Ozal medical center. Clin Lab Haematol. 2004;26:211‐214. [DOI] [PubMed] [Google Scholar]
- 51. Lalayanni C, Tsimperis S, Papalexandri A, et al. Management of relapsed adult immune thrombocytopenic purpura: long‐term results. Haematologica. 2010;95:78‐79.20851862 [Google Scholar]
- 52. Fass SM, Hui TT, Lefor A, Maestroni U, Phillips EH. Safety of laparoscopic splenectomy in elderly patients with idiopathic thrombocytopenic purpura. Am Surg. 2000;66:844‐847. [PubMed] [Google Scholar]
- 53. Berends FJ, Schep N, Cuesta MA, et al. Hematological long‐term results of laparoscopic splenectomy for patients with idiopathic thrombocytopenic purpura: a case control study. Surg Endosc. 2004;18:766‐770. [DOI] [PubMed] [Google Scholar]
- 54. Pavkovic M, Trpkovska‐Terzieva S, Latifi A, Karanfilski O, Cevreska L, Stojanovic A. Long‐term follow‐up of adult patients with idiopathic thrombocytopenic purpura after splenectomy. Macedonian J Med Sci. 2011;4:285‐289. [Google Scholar]
- 55. Gonzalez‐Porras JR, Escalante F, Pardal E, et al. Safety and efficacy of splenectomy in over 65‐yrs‐old patients with immune thrombocytopenia. Eur J Haematol. 2013;91:236‐241. [DOI] [PubMed] [Google Scholar]
- 56. Johansson E, Engervall P, Landgren O, et al. Response to splenectomy is durable after a certain point in time in adult patients with chronic immune thrombocytopenic purpura. Eur J Haematol. 2006;77:61‐66. [DOI] [PubMed] [Google Scholar]
- 57. Kuter DJ, Bussel JB, Newland A, et al. Long‐term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161:411‐423. [DOI] [PubMed] [Google Scholar]
- 58. Saleh MN, Bussel JB, Cheng G, et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long‐term, open‐label EXTEND study. Blood. 2013;121:537‐545. [DOI] [PubMed] [Google Scholar]
- 59. FDA package insert . NPLATE Prescribing Information; 2008.
- 60. PRomacta Package insert FDA . 2015.
- 61. European Medicines Agency . United Kingdom Revolade, INN‐eltrombopag; 2015.
- 62. European Medicines Agency . WC500039537; 2013.
- 63. Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25‐33. [DOI] [PubMed] [Google Scholar]
- 64. Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long‐term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104:2623‐2634. [DOI] [PubMed] [Google Scholar]
- 65. Kristinsson SY, Gridley G, Hoover RN, Check D, Landgren O. Long‐term risks after splenectomy among 8,149 cancer‐free American veterans: a cohort study with up to 27 years follow‐up. Haematologica. 2014;99:392‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sailer T, Lechner K, Panzer S, Kyrle PA, Pabinger I. The course of severe autoimmune thrombocytopenia in patients not undergoing splenectomy. Haematologica. 2006;91:1041‐1045. [PubMed] [Google Scholar]
- 67. George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88:3‐40. [PubMed] [Google Scholar]
- 68. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386‐2393. [DOI] [PubMed] [Google Scholar]
- 69. Saleh MN, Bussel JB, Wong RS, et al. Hepatobiliary and thromboembolic events during long‐term EXTEN Ded treatment with etrombopag in adult patients with chronic immune thrombocytopenia (ITP). American Society of Hematology Blood. 2016;128(12). abstract 1368. [Google Scholar]
- 70. Cines DB, Gernsheimer T, Wasser J, et al. Integrated analysis of long‐term safety in patients with chronic immune thrombocytopaenia (ITP) treated with the thrombopoietin (TPO) receptor agonist romiplostim. Int J Hematol. 2015;102:259‐270. [DOI] [PubMed] [Google Scholar]
- 71. Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo‐controlled trials. Am J Hematol. 2018;93:921‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Frieden TR. Evidence for health decision making ‐ beyond randomized, controlled trials. N Engl J Med. 2017;377:465‐475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Search strings used in systematic literature review.


