Abstract
SIMPLICITY (NCT01244750) is an observational study exploring tyrosine kinase inhibitor (TKI) use and management patterns in patients with chronic phase‐chronic myeloid leukemia in the US and Europe in routine clinical practice. Herein we describe interruptions, discontinuations and switching of TKI therapy during the initial 2 years of treatment among 1121 patients prospectively enrolled between October 1, 2010 and March 7, 2017. Patient characteristics were broadly similar between the imatinib (n = 370), dasatinib (n = 376), and nilotinib (n = 375) cohorts. Treatment interruptions occurred in 16.4% (year 1) and 4.0% (year 2) of patients, mainly attributed to hematologic intolerances. Treatment discontinuations occurred in 21.8% (year 1) and 10.2% (year 2) of patients, with the highest rate within the first 3 months for intolerance. Switching of TKI was seen in 17.8% (year 1) and 9.5% (year 2) of patients. Significant associations were found between TKI switching and female gender (year 1), age ≥65 years at diagnosis (year 2) and treatment with imatinib (year 2). Intolerance was the most common reason given for patients discontinuing and for switching TKI therapy; however resistance was also cited. Lack of response monitoring in routine clinical practice may have resulted in lower identification of resistance in this dataset. Data from SIMPLICITY suggest that, in routine clinical practice, intolerance and resistance to TKIs influence decisions to change treatment. Changes in TKI therapy are frequent, with nearly a third of patients discontinuing their first‐line TKI.
1. INTRODUCTION
The impact of TKIs on the prognosis of patients with chronic phase‐chronic myeloid leukemia (CP‐CML) is indisputable1 and they are recommended by both the National Comprehensive Cancer Network (NCCN) and European LeukemiaNet (ELN) as first‐line therapy.2, 3 Monitoring for molecular response is recommended every 3 months until a major molecular response is achieved and after that every 3‐6 months.2, 3 If response to first‐line TKI is inadequate, switching to a different TKI is recommended.2, 3 Although imatinib (Gleevec/Glivec, Novartis), is effective in CP‐CML patients, about one‐third of patients treated with this TKI require changes in therapy for reasons including resistance (primary or acquired) and intolerance.4, 5 Dose escalation of imatinib may be able to overcome resistance in some patients with CP‐CML, however the duration of response is short‐lived.6, 7, 8, 9, 10 Therefore, second generation (2G) TKIs, which can effectively salvage these imatinib patients, are preferred. Based on the results of randomized controlled trials (RCTs) dasatinib (Sprycel, Bristol‐Myers Squibb), nilotinib (Tasigna, Novartis), and bosutinib (Bosulif, Pfizer) have also been approved as first‐line therapy.11, 12, 13 Dasatinib, nilotinib, and bosutinib are associated with higher and faster rates of cytogenetic and molecular response as well as reduced rates of progression when compared with imatinib at 400 mg in the first‐line setting, although an impact on survival has not been seen to date.14, 15, 16
Complementary to the wealth of clinical trial data supporting the efficacy and safety of imatinib, dasatinib, and nilotinib,15, 16, 17, 18, 19 observational studies contribute further insights to inform clinical decision making.20 The circumstances under which initial TKI is selected, why it is discontinued and how a second‐line TKI is chosen have been studied in the USA and Europe. However, most of these studies have a predominance of patients on first‐line imatinib,21, 22 despite the fact that use of the 2G TKIs in the first‐line setting has increased in recent years.23 Data collected from routine clinical practice indicate that up to one‐third of patients on first‐line imatinib undergo a change in treatment, including dose modification, treatment interruption, discontinuation or switch to another TKI, although this proportion may be higher.22, 23
The main objective of the ongoing observational study SIMPLICITY (NCT01244750) is to understand TKI use and management patterns in routine clinical care of patients with CP‐CML receiving imatinib, dasatinib, or nilotinib as first‐line treatment. Here we describe and provide reasons for changes in treatment patterns between the first and second year of TKI therapy in patients with ≥2 years of follow‐up.
2. METHODS
The design of SIMPLICITY has been described in detail previously.24 The TKI cohorts were closed when each had enrolled approximately 400 CP‐CML patients receiving first‐line therapy with imatinib, dasatinib, or nilotinib. The choice of TKI therapy was left to the discretion of the physician. Study sites in the USA and Europe including Russia include academic (defined as large, hospital‐based clinics or research units at universities, including specialized centers of excellence, offering care on a regional/national basis) and private/community practices (defined as small, private practices run by one or more independent physicians offering patient care on a local basis). The study protocol was approved by the relevant institutional review boards and written patient consent obtained.
Treatment interruption was defined as a gap in treatment of >1 day before restarting the same TKI, from the day the TKI was temporarily discontinued and ending the day before restarting. Treatment discontinuation was defined as cessation of TKI treatment that did not qualify as a treatment interruption; switch was defined as discontinuation of index TKI, followed by start of second‐line TKI during the follow‐up period. All patients who switched TKI treatment were considered as having discontinued therapy. Treatment interruptions and discontinuations were analyzed according to the date of TKI discontinuation and TKI switch, according to the date of second‐line TKI start. Treatment interruptions and discontinuations were grouped according to whether they occurred within 1 year of index TKI initiation (first year of therapy) or between 1 and 2 years after index TKI initiation (second year of therapy).
Reasons for TKI changes were reported; choices were intolerance; primary resistance (failure to achieve a response)3; acquired resistance (loss of response)3; insurance/financial reasons; subject refusal; unrelated medical conditions and other. Specific definitions for reasons for TKI change were not collected. Any kinase domain sequencing undertaken was at the discretion of the treating physician.
Descriptive statistics were generated for the overall prospective population and by index TKI. In addition, statistical comparisons were made between the first and second year of treatment, regions (USA or Europe), and practice types (academic centers or community practices). Categorical data were presented as counts and percentages and continuous data as means ± SD, median, interquartile range (IQR), and range (minimum, maximum). The Kruskal‐Wallis test was used to compare differences between median values.
Multivariable logistic regression was performed to assess predictors of treatment discontinuations and switching. The model included the following predictors: gender, age at diagnosis, index TKI, region, total comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status, SOKAL score at diagnosis, and practice type. Sensitivity analyses were also performed using a subset of patients by region and index TKI.
3. RESULTS
3.1. Study population
To date, 1121 patients enrolled prospectively into SIMPLICITY between October 1, 2010 and March 7, 2017 (data download) have ≥2 years of follow‐up since initiating first‐line TKI. Patient demographics are shown in Table 1. The median age at first‐line TKI (IQR; min, max) was 56.3 years (46.2‐66.8; 17.7, 90.8). The median age (IQR; min, max) of imatinib patients was 59.3 years (47.1‐69.2; 18.4, 88.5), which was slightly higher than that of dasatinib (56.0 years [46.3‐66.5; 19.1,89.2]) and nilotinib patients (54.0 years [45.1‐65.5; 17.7, 90.8]; P = .004).
Table 1.
Patient demographics
| Characteristic | Patients with ≥2 years of follow‐up since initiating first line TKI | |||
|---|---|---|---|---|
| Imatinib | Dasatinib | Nilotinib | All patients | |
| n | 370 | 376 | 375 | 1121 |
| Male, n (%) | 211 (57.0%) | 207 (55.1%) | 201 (53.6%) | 619 (55.2%) |
| Median age at first‐line TKI, years (IQR) | 59.3 (47.1‐69.2) | 56.0 (46.3‐66.5) | 54.0 (45.1‐65.5) | 56.3 (46.2‐66.8) |
| Age at first‐line TKI, n (%) | ||||
| <50 years | 112 (30.3%) | 124 (33.0%) | 140 (37.3%) | 376 (33.5%) |
| 50‐64 years | 131 (35.4%) | 149 (39.6%) | 138 (36.8%) | 418 (37.3%) |
| ≥65 years | 127 (34.3%) | 103 (27.4%) | 97 (25.9%) | 327 (29.2%) |
| Median time from first‐line TKI to end of follow‐up, months (IQR) | 59.4 (53.2‐60.0) | 48.1 (38.8‐58.3) | 49.5 (36.7‐60.0) | 53.8 (40.4‐60.0) |
| Minimum and maximum time from first‐line TKI to end of follow‐up, months | 24.2, 75.4 | 24.8, 66.7 | 24.1, 65.8 | 24.1, 75.4 |
| Region, n (%) | ||||
| Europea | 162 (43.8%) | 101 (26.9%) | 134 (35.7%) | 397 (35.4%) |
| USA | 208 (56.2%) | 275 (73.1%) | 241 (64.3%) | 724 (64.6%) |
| Practice type, n (%) | ||||
| Academic center | 194 (52.4%) | 204 (54.3%) | 203 (54.1%) | 601 (53.6%) |
| Private or community practice | 176 (47.6%) | 172 (45.7%) | 172 (45.9%) | 520 (46.4%) |
| Mean (±SD) number of comorbiditiesb | 3.2 ± 2.6 | 3.2 ± 2.7 | 3.1 ± 2.9 | 3.2 ± 2.7 |
Demographic information according to index TKI and the total SIMPLICITY population who had ≥2 years of follow‐up since initiating TKI therapy. Abbreviations: IQR, interquartile range; TKI, tyrosine kinase inhibitor.
European countries involved in SIMPLICITY: France, Germany, Italy, The Netherlands, Russia, and Spain.
Among patients with a baseline medical history form.
Patients received first‐line treatment with imatinib (n = 370; median dose: 400 mg), dasatinib (n = 376; median dose: 100 mg) or nilotinib (n = 375; median dose: 600 mg). Median time from first‐line TKI to end of follow‐up (IQR; min, max) for imatinib was 59.4 months (53.2‐60.0; 24.2, 75.4), for dasatinib was 48.1 (38.8‐58.3; 24.8, 66.7), and for nilotinib was 49.5 months (36.7‐60.0; 24.1, 65.8). As previously described, the imatinib cohort completed recruitment before the dasatinib and nilotinib cohorts.24 Demographics of patients enrolled in the USA and Europe were similar, as were those of patients in academic centers and community practices.
3.2. Treatment patterns in the first year of first‐line TKI therapy
3.2.1. Treatment interruptions
Treatment interruptions in the first year of follow‐up were reported in 16.4% (n = 184) of patients; about a quarter of these patients (n = 44) had >1 interruption. Proportions of patients with treatment interruptions were consistent across TKI cohorts (Figure 1A). The median (IQR; min, max) duration of treatment interruptions in all patients was 14.0 (8.0‐27.0; 2, 1261) days. The most common events concurrent with treatment interruptions were thrombocytopenia (45 events) and neutropenia (22 events), occurring in all TKI cohorts (Table 2).
Figure 1.
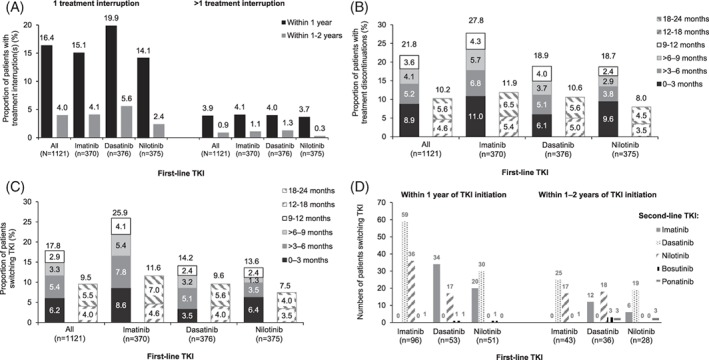
A, Proportions of patients with 1 (left hand panel) or more than 1 (right hand panel) treatment interruption for patients who had a treatment interruption within 1 year (black bars) or within 1‐2 years (gray bars) of initiating first‐line TKI. B, Proportions of patients who discontinued TKI treatment within 2 years of first‐line TKI initiation, according to the time interval within which discontinuation occurred. Totals are given above each bar. C, Proportions of patients who switched from first‐line TKI to a second‐line TKI within 2 years of TKI treatment initiation, according to the time interval within which discontinuation occurred. Totals are given above each bar. D, Numbers of patients who switched from a first‐line TKI to one of the specified second‐line TKIs
Table 2.
Patients with events concurrent with treatment interruptions within 1 year or within 1‐2 years of initiation of index TKI
| Event | Dasatinib | Nilotinib | Imatinib | All patients | ||||
|---|---|---|---|---|---|---|---|---|
| Within 1 year (n = 75) | 1‐2 years (n = 21) | Within 1 year (n = 53) | 1‐2 years (n = 9) | Within 1 year (n = 56) | 1‐2 years (n = 15) | Within 1 year (n = 184) | 1‐2 years (n = 45) | |
| Anemia, n | 8 | 2 | 2 | 0 | 8 | 0 | 18 | 2 |
| Dyspnea, n | 8 | 8 | 0 | 0 | 1 | 0 | 9 | 8 |
| Fatigue, n | 1 | 2 | 2 | 1 | 1 | 2 | 4 | 5 |
| Nausea, n | 2 | 0 | 1 | 0 | 6 | 0 | 9 | 0 |
| Neutropenia, n | 5 | 2 | 6 | 0 | 11 | 2 | 22 | 4 |
| Pleural effusion, n | 11 | 3 | 3 | 2 | 0 | 0 | 14 | 5 |
| Rash, n | 2 | 0 | 6 | 0 | 5 | 0 | 13 | 0 |
| Thrombocytopenia, n | 18 | 0 | 15 | 0 | 12 | 0 | 45 | 0 |
3.2.2. Treatment discontinuations
Treatment discontinuations were reported in 21.8% (n = 244) of patients in the first year of follow‐up (Figure 1B). The proportion of patients discontinuing varied between imatinib, dasatinib, and nilotinib (27.8% [n = 103], 18.9% [n = 71], and 18.7% [n = 70], respectively) (Figure 1B), and was greatest in the first 3 months of treatment (Figure 1B); median (IQR) time to discontinuation in the first year of treatment was 3.9 (1.6‐7.8) months.
Intolerance was the most common reason for discontinuation of first‐line TKI in the first year of follow‐up, reported in 178 (15.9%) patients; 67 receiving imatinib, 56 receiving dasatinib, and 55 receiving nilotinib. General disorders (including asthenia, chills, discomfort, edema, fatigue, mucosal inflammation, pain, pyrexia, swelling; n = 41) and gastrointestinal (GI) disorders (n = 40) were the most common types of intolerances related to treatment discontinuation (Table 3). Specific intolerances leading to TKI discontinuation were rash (n = 9) in the imatinib group, pleural effusion in the dasatinib group (n = 11), and rash (n = 8) in the nilotinib group.
Table 3.
Intolerances leading to discontinuation or switching of first‐line TKI therapy
| Patient group Treatment event | Dasatinib | Nilotinib | Imatinib | All patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discontinuation | Switch | Discontinuation | Switch | Discontinuation | Switch | Discontinuation | Switch | |||||||||
| Treatment year | First (n = 56) |
Second (n = 22) |
First (n = 43) |
Second (n = 25) |
First (n = 55) |
Second (n = 13) |
First (n = 24) |
Second (n = 13) |
First (n = 67) |
Second (n = 16) |
First (n = 61) |
Second (n = 16) |
First (n = 178) |
Second (n = 51) |
First (n = 146) |
Second (n = 54) |
| Blood and lymphatic system disorders, n | 11 | 0 | 7 | 2 | 7 | 1 | 5 | 2 | 5 | 3 | 3 | 3 | 23 | 4 | 15 | 7 |
| GI disorders, n | 9 | 4 | 8 | 3 | 13 | 3 | 13 | 3 | 18 | 7 | 16 | 6 | 40 | 14 | 37 | 12 |
| General disorders and administration site conditionsa, n | 11 | 5 | 8 | 6 | 8 | 2 | 6 | 3 | 22 | 4 | 22 | 3 | 41 | 11 | 36 | 12 |
| Investigations, n | 3 | 0 | 2 | 1 | 10 | 2 | 6 | 2 | 5 | 0 | 5 | 0 | 18 | 2 | 13 | 3 |
| Musculoskeletal and connective tissue disorders, n | 4 | 1 | 4 | 1 | 4 | 1 | 2 | 2 | 16 | 2 | 16 | 2 | 24 | 4 | 22 | 5 |
| Nervous system disorders, n | 5 | 1 | 4 | 2 | 3 | 1 | 2 | 2 | 6 | 1 | 5 | 2 | 14 | 3 | 11 | 6 |
| Respiratory, thoracic and mediastinal disorders, n | 17 | 16 | 14 | 16 | 1 | 1 | 1 | 0 | 4 | 1 | 4 | 1 | 22 | 18 | 19 | 17 |
| Skin and subcutaneous tissue disorders, n | 1 | 1 | 1 | 0 | 14 | 2 | 11 | 2 | 16 | 0 | 15 | 0 | 31 | 3 | 27 | 2 |
Intolerances leading to TKI discontinuation or TKI switching in the whole SIMPLICITY population who had ≥2 years of follow‐up since initiating first‐line TKI, according to index. Only intolerances where ≥20 patients discontinued TKI are included in the table; only intolerances where ≥10 patients switched TKI are included in the table.
General disorders and administration site conditions includes asthenia, chest pain, chills, discomfort, edema, fatigue, influenza‐like illness, malaise, mucosal inflammation, pain, pyrexia, and swelling.
Resistance was the second most common reason for discontinuation of first‐line TKI, reported in 22 (9.4%) patients and occurring in 16 patients receiving imatinib, 3 patients receiving dasatinib and 3 patients receiving nilotinib.
A multivariable logistic regression model identified that female patients were more likely than male patients to discontinue TKI therapy in the first year of treatment (odds ratio [OR] = 1.72, 95% CI: 1.29‐2.31; P < .001). No significant differences were found in reasons for discontinuation between male and female patients (P > .05). Patients treated with imatinib were more likely to discontinue therapy than patients treated with dasatinib (OR = 1.73, 95% CI: 1.21‐2.47; P = .002).
3.2.3. Treatment switching
Switching to second‐line TKI after discontinuing first‐line TKI in the first year of treatment was reported in 17.8% (n = 200) of patients (Figure 1C). The TKI most commonly switched from was imatinib (n = 96), followed by dasatinib (n = 53) and nilotinib (n = 51). The TKI most commonly switched to was dasatinib (n = 89), followed by imatinib (n = 54) and nilotinib (n = 53); patients also switched to bosutinib (n = 2) and ponatinib (n = 2). Of patients discontinuing dasatinib, twice as many patients switched to imatinib than nilotinib (n = 34 vs 17) (Figure 1D). Of patients discontinuing nilotinib, more switched to dasatinib than switched to imatinib (n = 30 vs 20) (Figure 1D). The overall number of patients switching from a 2G TKI to imatinib was slightly higher than the number of patients switching from a 2G TKI to another 2G TKI (n = 54 vs 47). More patients discontinuing imatinib switched to dasatinib than nilotinib (n = 59 vs 36) (Figure 1D). For all patients, and for each TKI, slightly higher proportions of patients switched therapy within 0‐3 months of treatment than in later periods (Figure 1C).
Intolerance was the most common reason for switching of first‐line TKI in the first year of treatment, reported in 146 patients. The most common types of intolerance leading to switching were GI disorders (n = 37) (Table 3). In the imatinib group, the most common types of intolerance leading to switching were GI and musculoskeletal and connective tissue disorders (n = 16 each). In the dasatinib group, the most common intolerance leading to switching were respiratory disorders (n = 14). In the nilotinib group, the most common intolerance leading to switching were GI disorders (n = 13). Specific intolerances most commonly leading to TKI switching were rash in the imatinib and nilotinib groups (n = 9 and 7, respectively) and pleural effusion in the dasatinib group (n = 8).
Primary resistance was the second most common reason for switching of first‐line TKI in the first year of follow‐up, reported in 22 patients. Most of these had been treated with imatinib (n = 16), but resistance was also reported in the dasatinib and nilotinib groups (n = 3 for each group). Mutation of ABL kinase was investigated in 200 patients in the first year, and a known ABL kinase mutation was observed in 5 patients prior to switching to second‐line TKI in the first year of therapy. Of 244 patients who discontinued treatment in the first year, 200 switched TKI within 1 year and 19 switched TKI after 1 year. A small number of patients (n = 25) did not switch to a different TKI. Reasons for this were not collected prospectively, although it is possible that these patients moved to another medical center, chose to discontinue from the study, progressed beyond CP‐CML or died.
A multivariable logistic regression model identified that female patients were more likely than male patients to switch TKI therapy in the first year of treatment (OR = 1.69, 95% CI: 1.23‐2.32; P = .001). Patients treated with imatinib were more likely to switch therapy than patients treated with dasatinib (OR = 2.28, 95% CI: 1.56‐3.35; P < .001).
3.3. Treatment patterns in the second year of first‐line TKI therapy
3.3.1. Treatment interruptions
Treatment interruptions were recorded for 4.0% (n = 45) of patients in the second year of follow‐up TKI; roughly a quarter of this number (0.9%; n = 10) had >1 treatment interruption. Proportions of patients with treatment interruptions were consistent across TKI cohorts (Figure 1A). The median (IQR; min, max) total duration of individual treatment interruptions in all patients was 16.0 (10.0‐43.0; 3, 640) days. The most common events concurrent with treatment interruptions were dyspnea (8 events), pleural effusion and fatigue (5 events each) (Table 2). Fewer treatment interruptions occurred than in the first year of follow‐up, and the events concurrent with interruptions differed.
3.3.2. Treatment discontinuations
Treatment discontinuations were recorded for 10.2% (n = 114) of patients in the second year of follow‐up (Figure 1B). The proportion of patients discontinuing treatment was greater for imatinib than dasatinib or nilotinib (11.9% [n = 44], 10.6% [n = 40], and 8.0% [n = 30], respectively) (Figure 1B), and approximately equal in the 12‐18 and 18‐24 months periods (Figure 1B).
Intolerance was the most common reason for discontinuation of first‐line TKI in the second year of follow‐up, but was reported in fewer patients than in the first year, in 51 (4.5%) of patients; 22 receiving dasatinib, 16 receiving imatinib, and 13 were receiving nilotinib (Table 3). Resistance was the second most common reason and was reported in more patients than during the first year of TKI treatment (30 vs 22 patients). Resistance was reported in 19 patients receiving imatinib, 7 patients receiving nilotinib and 4 patients receiving dasatinib.
A multivariable logistic regression model identified that female patients were less likely than male patients to discontinue TKI therapy in the second year of treatment (OR = 0.50, 95% CI: 0.33‐0.77; P = .001). No significant differences were found in reasons for discontinuation between male and female patients (P > .05). Patient age of ≥65 years at diagnosis was associated with TKI discontinuation (OR = 1.66, 95% CI: 1.06‐2.60; P = .026); this association was seen for patients treated with imatinib (OR = 2.88, 95% CI: 1.38‐5.97; P = .005), but not for those treated with dasatinib or nilotinib. Patients treated with dasatinib who had an ECOG score of 1‐4 at baseline (corresponding to restricted strenuous activity through to completely disabled) were more likely to discontinue treatment than those with an ECOG score of 0 (OR = 2.86, 95% CI: 1.21‐6.72; P = .016). This was not seen for the overall population or for other TKI groups.
3.3.3. Treatment switching
In the second year of follow‐up, switching to a new TKI after discontinuing first‐line TKI was observed in fewer patients than in the first year, with 9.5% (n = 107) of patients switching (Figure 3A). The TKI most commonly switched from was imatinib (n = 43), followed by dasatinib (n = 36) and nilotinib (n = 28). The TKI most commonly switched to was dasatinib (n = 44), followed by nilotinib (n = 35) and imatinib (n = 18); patients also switched to bosutinib (n = 3) and ponatinib (n = 7). Of patients discontinuing dasatinib, more switched to nilotinib than to imatinib (n = 18 vs n = 12) and more patients discontinuing nilotinib switched to dasatinib than to imatinib (n = 19 vs 6) (Figure 1D). The total number of patients switching from one 2G TKI to another 2G TKI was greater than the number of patients switching from a 2G TKI to imatinib (n = 37 vs 18). More patients discontinuing imatinib switched to dasatinib than nilotinib (n = 25 vs 17) (Figure 1D).
Intolerance was the most common reason for switching of first‐line TKI in the second year of treatment (n = 54). The most common types of intolerance were respiratory, thoracic and mediastinal disorders (n = 17) and GI disorders (n = 12) (Table 3). Respiratory, thoracic and mediastinal events were mainly seen with dasatinib, mostly from pleural effusion (n = 13).
Primary resistance was the second most common reason for switching of first‐line TKI (n = 30). Most patients developing resistance were treated with imatinib (n = 19), but resistance was also reported with dasatinib and nilotinib (n = 4 and 7, respectively). Mutation of ABL kinase was investigated in 107 patients in the second year, and a known ABL kinase mutation was observed in 11 patients prior to switching to second‐line TKI in the second year of therapy.
Of 114 patients who discontinued treatment in the second year, 107 switched TKI within the second year and 4 switched after 2 years. Therefore, 3 patients did not switch to a different TKI. Reasons for discontinuation without switching were not collected prospectively.
A multivariable logistic regression model identified that, in the second year of follow‐up, patient age of ≥65 years at diagnosis was associated with TKI switching (OR = 1.66, 95% CI: 1.05‐2.61; P = .029). Patients treated with dasatinib who had an ECOG score of 1‐4 at baseline (corresponding to restricted strenuous activity through completely disabled) were more likely to switch treatment than those with an ECOG score of 0 (OR = 5.02, 95% CI: 1.96‐12.85; P = .001). This was not seen for the overall population or for other TKI groups.
4. DISCUSSION
Despite the availability of clinical guidelines for CP‐CML management, the patterns of use of TKIs in routine clinical practice and the reasons behind these patterns remain unclear. In this analysis, patterns of TKI treatment in the first and second years after TKI initiation vary between TKIs and over the duration of therapy.
Treatment interruptions are a known occurrence in patients receiving TKIs and are typically related to intolerances.25, 26 In SIMPLICITY, treatment interruptions were observed across all TKI cohorts, in both the first and second years after TKI initiation, that may have resulted from concurrent intolerances. Retrospective studies have found that treatment interruptions are more common with 2G TKIs, but have only a small impact on clinical response.25, 26 However, interruptions may interfere with ongoing treatment and ELN advise that they should be minimized and patients carefully monitored to ensure treatment resumes after any intolerance has resolved.27
Treatment discontinuations were twice as frequent in the first year of treatment compared with the second; patients treated with imatinib more frequently discontinued treatment. A significant association was found between imatinib treatment and both discontinuation and switching in the first year of treatment. Significant associations were also found between female gender and both switching and discontinuation of TKI therapy in the first year of treatment. The current literature does not reflect a difference in use of or response to TKIs between male and female patients, so the reason for this difference is unclear.28, 29, 30
Just over one‐sixth of patients switched TKI in their first year of treatment and just under one‐tenth in their second year of treatment. In the first year, a greater proportion of patients switched from imatinib than from a 2G TKI, but in the second year, the numbers of patients switching were similar across TKIs. The main reason for discontinuation and switching therapy in the first and the second years of treatment was intolerance, followed by primary resistance.
The intolerances observed in SIMPLICITY were in line with those known for imatinib, dasatinib, and nilotinib.15, 16, 17, 18, 19 However, TKI‐specific late intolerance was observed in some situations. In patients treated with imatinib, for example, the number of patients discontinuing for GI disorders in the second year was greater than for other TKIs (Table 3). Furthermore, in patients treated with dasatinib, the number of patients discontinuing owing to respiratory, thoracic and mediastinal disorders, especially pleural effusion, in the second year was similar to that in the first year and greater than seen for other TKIs (Tables 3 and 4). In the case of dasatinib, this is likely to be at least partly related to the fact that pleural effusion is noted to potentially occur steadily throughout exposure rather specifically within an early/late timeframe as seen with other observed intolerances. For patients treated with nilotinib, the frequency of intolerances associated with discontinuation and/or switching were comparable in the first and second years (Tables 3 and 4). The findings from SIMPLICITY indicate that management of intolerances remains an issue in clinical practice, and that these intolerances frequently result in discontinuation of TKI therapy. There is a continued need for effective management of intolerances, as well as for a range of treatment options available to switch between to suit the needs of patients.
Primary resistance played a much smaller part in dictating treatment patterns than intolerance. Consistent with previous reports, discontinuation owing to resistance in SIMPLICITY was most commonly observed with imatinib.31 Resistance may have been underestimated due to suboptimal monitoring; we have previously reported that, in the US and Europe, a significant proportion of CP‐CML patients do not undergo cytogenetic or molecular monitoring as recommended by guidelines, which if performed would identify early resistance indicating need for switching.24 The increased frequency of switching due to primary resistance in the second year is consistent with resistance occurring later in treatment, after intolerances have manifested. This suggests a shift in the predominant “driver” of switch over time, from intolerance to resistance. Clinical guidelines advocate switching of TKI if clinical response is inadequate at 3, 6, and 12 months, but are more conservative about switching due to intolerance.2, 3 However, data from SIMPLICITY suggest that in routine clinical practice a decision to switch TKI may be based on considerations beyond those specified in clinical guidance.27
The demographics of SIMPLICITY patients are similar to those of patients in clinical trials supporting imatinib, nilotinib, and dasatinib, with the exception of median patient age (56.5 years), which is older than in these trials and 3 others that studied imatinib.17, 18, 32, 33, 34 The demographics of patients in SIMPLICITY (including age) are closer to those in other observational studies, including the European Treatment and Outcome Study (EUTOS) CML registry.21 A systematic review has indicated that, in newly diagnosed and untreated elderly patients, age does not generally play a great part in determining clinical response to TKIs.35 In SIMPLICITY, significant associations were found between patient age ≥65 years and both discontinuation and switching of a TKI in the second year of treatment. Furthermore, these associations were found for patients receiving imatinib, but not dasatinib or nilotinib. The main reason for discontinuation and switching was intolerance, and it may be tempting to attribute the observed associations to increased likelihood of intolerances in older patients. It may be that primary resistance to imatinib is also an important driver in elderly patients in SIMPLICITY, although this is inconsistent with observations from other studies reporting that imatinib resistance occurs at similar rates in younger and older populations.36, 37 These observations may warrant a deeper investigation into the impact of age in the SIMPLICITY population, in which approximately one‐third were over 65 when TKI therapy was initiated.
One value of observational research is that it includes a broader patient population than that included in RCTs.20 However, the nonrandomized design of observational studies means there is a risk of selection bias and confounding; results should thus be interpreted in the context of clinical practice and any comparisons with results from RCTs made with caution.20, 38 Some of the specific limitations of the SIMPLICITY study around patient enrolment, availability of TKIs and monitoring have been discussed previously and remain relevant here.24 Limitations specific to this particular analysis include the fact that intolerances were not graded, so it is not possible to understand if severity of an intolerance played a role in TKI discontinuation. Also, caution must be exercised when drawing conclusions over reasons for TKI switching, as these were not prospectively recorded, which means that hypotheses on this are difficult to test. Switching patterns with TKIs may also be reflective of the changing CML treatment landscape; as reported previously, there was a shift from the use of imatinib to 2G TKIs in the SIMPLICITY population from 2009 to 2015, a shift that is exaggerated given enrollment into the imatinib cohort was completed earlier than for the dasatinib and nilotinib cohorts.24 The link between TKI discontinuations and switching is currently being explored and will be reported elsewhere.
Analysis of data from the first 2 years of treatment in SIMPLICITY indicates that intolerance, and to a lesser degree resistance, influence the TKIs that CML‐CP patients receive. Age and gender may be implicated in the ability of patients to tolerate TKIs. Further follow‐up may elucidate whether these and other variables impact the ability of patients to remain on specific TKIs long‐term or whether late side effects emerge that might influence treatment.
CONFLICT OF INTEREST
RH has received consultancy fees from BMS and grants from Novartis. JC has received grants and/or consultancy fees from Ariad, BMS, Novartis, Pfizer and Teva. TZ, SG, and ID are employees of BMS. CGP has received grants and consultancy fees from BMS, and honoraria/grants from Pfizer. SLG has received grants and/or consultancy fees from BMS, Ariad, Novartis, and Pfizer. M Mauro has received grants from Novartis Oncology and Ariad/Takeda, and consultancy fees from BMS, Ariad/Takeda and Pfizer. M Michallet reports grants from BMS, consultant fees/honoraria from BMS, Pfizer, Novartis, Astellas Pharma, MSD and Genzyme. RP, BS, and LW declare no conflict of interest. AF and GS are employees of ICON Clinical Research.
ACKNOWLEDGMENTS
We thank all SIMPLICITY study investigators, the patients who consented to be part of the study and LATITUDE (AXON Communications) who provided medical writing services on behalf of the authors and Bristol‐Myers Squibb Pharmaceuticals Ltd. The study is funded by Bristol‐Myers Squibb.
Hehlmann R, Cortes JE, Zyczynski T, et al. Tyrosine kinase inhibitor interruptions, discontinuations and switching in patients with chronic‐phase chronic myeloid leukemia in routine clinical practice: SIMPLICITY. Am J Hematol. 2019;94:46–54. 10.1002/ajh.25306
Funding information Bristol‐Myers Squibb
REFERENCES
- 1. Khoury HJ, Williams LA, Atallah E, Hehlmann R. Chronic myeloid leukemia: what every practitioner needs to know in 2017. American Society of Clinical Oncology educational book American Society of Clinical Oncology Meeting. 2017;37:468‐479. [DOI] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network (NCCN) . NCCN Guidelines Chronic Myelogenous Leukemia Version 1. J Natl Compr Canc Netw. 2016;14:1505‐1512. [DOI] [PubMed] [Google Scholar]
- 3. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hochhaus A, Larson RA, Guilhot F, et al. Long‐term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hehlmann R, Lauseker M, Saussele S, et al. Assessment of imatinib as first‐line treatment of chronic myeloid leukemia: 10‐year survival results of the randomized CML study IV and impact of non‐CML determinants. Leukemia. 2017;31:2398‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kantarjian HM, Talpaz M, O'Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard‐dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473‐475. [DOI] [PubMed] [Google Scholar]
- 7. Marin D, Goldman JM, Olavarria E, Apperley JF. Transient benefit only from increasing the imatinib dose in CML patients who do not achieve complete cytogenetic remissions on conventional doses. Blood. 2003;102:2702‐2703. author reply 2703‐2704. [DOI] [PubMed] [Google Scholar]
- 8. Jabbour E, Kantarjian HM, Jones D, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard‐dose imatinib therapy. Blood. 2009;113:2154‐2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kantarjian HM, Larson RA, Guilhot F, et al. Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer. 2009;115:551‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zonder JA, Pemberton P, Brandt H, Mohamed AN, Schiffer CA. The effect of dose increase of imatinib mesylate in patients with chronic or accelerated phase chronic myelogenous leukemia with inadequate hematologic or cytogenetic response to initial treatment. Clin Cancer Res. 2003;9:2092‐2097. [PubMed] [Google Scholar]
- 11. SPRYCEL (dasatinib), tablets for oral use . Full prescribing information. Bristol‐Myers Squibb; 2011.http://packageinserts.bms.com/pi/pi_sprycel.pdf.
- 12. TASIGNA (nilotinib) capsules, for oral use . Full prescribing information. Novartis; 2012. http://www.pharma.us.novartis.com/product/pi/pdf/tasigna.pdf.
- 13. BOSULIF (bosutinib) tablets, for oral use . Full prescribing information. Pfizer; 2017. http://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=884.
- 14. Cortes JE, Gambacorti‐Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5‐year study results of DASISION: the dasatinib versus imatinib study in treatment‐naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333‐2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hochhaus A, Saglio G, Hughes TP, et al. Long‐term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5‐year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic‐phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260‐2270. [DOI] [PubMed] [Google Scholar]
- 18. Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251‐2259. [DOI] [PubMed] [Google Scholar]
- 19. Druker BJ, Guilhot F, O'Brien SG, et al. Five‐year follow‐up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408‐2417. [DOI] [PubMed] [Google Scholar]
- 20. Mauro MJ, Davis C, Zyczynski T, Khoury HJ. The role of observational studies in optimizing the clinical management of chronic myeloid leukemia. Ther Adv Hematol. 2015;6:3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffmann VS, Baccarani M, Hasford J, et al. Treatment and outcome of 2904 CML patients from the EUTOS population‐based registry. Leukemia. 2017;31:593‐601. [DOI] [PubMed] [Google Scholar]
- 22. Vander Velde N, Chen L, Guo A, et al. Study of imatinib treatment patterns and outcomes among US veteran patients with Philadelphia chromosome‐positive chronic myeloid leukemia. J Oncol Pract. 2013;9:e212‐e219. [DOI] [PubMed] [Google Scholar]
- 23. Henk HJ, Woloj M, Shapiro M, Whiteley J. Real‐world analysis of tyrosine kinase inhibitor treatment patterns among patients with chronic myeloid leukemia in the United States. Clin Ther. 2015;37:124‐133. [DOI] [PubMed] [Google Scholar]
- 24. Goldberg SL, Cortes JE, Gambacorti‐Passerini C, et al. First‐line treatment selection and early monitoring patterns in chronic phase‐chronic myeloid leukemia in routine clinical practice: SIMPLICITY. Am J Hematol. 2017;92:1214‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Santos FPS, Kantarjian H, Fava C, et al. Clinical impact of dose reductions and interruptions of second‐generation tyrosine kinase inhibitors in patients with chronic myeloid leukaemia. Br J Haematol. 2010;150:303‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ward MA, Fang G, Richards KL, et al. Treatment interruption and regimen change in first‐generation versus second‐generation tyrosine kinase inhibitors used as first‐line therapy for chronic myeloid leukemia. J Health Econ Outcomes Res. 2015;2:181‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirji I, Gupta S, Goren A, et al. Chronic myeloid leukemia (CML): association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient's perspective. Health Qual Life Outcomes. 2013;11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rychter A, Jerzmanowski P, Hołub A, et al. Treatment adherence in chronic myeloid leukaemia patients receiving tyrosine kinase inhibitors. Med Oncol. 2017;34:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith AG, Painter D, Howell DA, et al. Determinants of survival in patients with chronic myeloid leukaemia treated in the new era of oral therapy: findings from a UK population‐based patient cohort. BMJ Open. 2014;4:e004266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jabbour EJ, Cortes JE, Kantarjian HM. Resistance to tyrosine kinase inhibition therapy for chronic myelogenous leukemia: a clinical perspective and emerging treatment options. Clin Lymphoma Myeloma Leuk. 2013;13:515‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hehlmann R, Müller MC, Lauseker M, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high‐dose imatinib: results from the randomized CML‐study IV. J Clin Oncol. 2014;32:415‐423. [DOI] [PubMed] [Google Scholar]
- 33. Preudhomme C, Guilhot J, Nicolini FE, et al. Imatinib plus peginterferon alfa‐2a in chronic myeloid leukemia. N Engl J Med. 2010;363:2511‐2521. [DOI] [PubMed] [Google Scholar]
- 34. Simonsson B, Gedde‐Dahl T, Markevarn B, et al. Combination of pegylated IFN‐alpha2b with imatinib increases molecular response rates in patients with low‐ or intermediate‐risk chronic myeloid leukemia. Blood. 2011;118:3228‐3235. [DOI] [PubMed] [Google Scholar]
- 35. Breccia M, Tiribelli M, Alimena G. Tyrosine kinase inhibitors for elderly chronic myeloid leukemia patients: a systematic review of efficacy and safety data. Crit Rev Oncol Hematol. 2012;84:93‐100. [DOI] [PubMed] [Google Scholar]
- 36. Gugliotta G, Castagnetti F, Palandri F, et al. Frontline imatinib treatment of chronic myeloid leukemia: no impact of age on outcome, a survey by the GIMEMA CML Working Party. Blood. 2011;117:5591‐5599. [DOI] [PubMed] [Google Scholar]
- 37. Latagliata R, Breccia M, Carmosino I, et al. "Real‐life" results of front‐line treatment with Imatinib in older patients (≥65 years) with newly diagnosed chronic myelogenous leukemia. Leuk Res. 2010;34:1472‐1475. [DOI] [PubMed] [Google Scholar]
- 38. Rochon PA, Gurwitz JH, Sykora K, et al. Reader's guide to critical appraisal of cohort studies: 1. Role and design. BMJ. 2005;330:895‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]


