Abstract
Objective
To clarify the clinical and laboratory characteristics of nephrolithiasis in gout by computed tomography (CT).
Methods
In 350 gout patients, unenhanced CT was performed at the 1st visit to hospital. Calculus density spots exceeding 1 mm in diameter with a CT value >120 Hounsfield units in the kidneys were defined as kidney stones. The association between laterality and the number of stones was investigated in each stone carrier. The 350 patients were classified into three groups (bilateral, unilateral and non‐stone carriers). Then serum urate (Sua), renal function, uric acid metabolism, and the prevalence of metabolic syndrome (Mets) were compared among these groups by the Tukey‐Kramer test or Fisher's exact test.
Results
Kidney stone(s) were detected in 108 (31%) of the 350 patients (bilateral in 58 and unilateral in 50). In 64 of the 108 patients (59%), there was no history of urolithiasis. Sua, serum creatinine and uric acid clearance were significantly higher (P = 0.001, P < 0.001, P = 0.043, respectively), while the estimated glomerular filtration rate was significantly lower (P = 0.039) in bilateral stone carriers than in non‐stone carriers. No significant differences of uric acid metabolism or the prevalence of Mets were noted among the three groups.
Conclusions
Approximately one‐third of gout patients had kidney stones and more than half of the patients with stones were bilateral and multiple stone carriers. Elevation of Sua might increase the stone burden in gout, leading to more severe renal dysfunction. An association between nephrolithiasis and Mets was not demonstrated in gout patients.
Keywords: computed tomography, gout, nephrolithiasis, prevalence, renal failure, urolithiasis
1. INTRODUCTION
Urolithiasis is one of the important complications of gout, and various aspects of the relation between these two conditions have already been studied. Although certain clinical consequences of the association between gout and urolithiasis have been reported, many uncertainties still remain, including the prevalence of urolithiasis in patients with gout. For example, Yu and Gutman,1 Fessel2 and Kramer et al3 reported that the prevalence of urolithiasis in gout patients was 22%, 15%, and 13.9%, respectively. The clinical profiles of the patients in each study also differed somewhat. We suspected that the main reason for the variation in prevalence and clinical profiles was that all of these studies were based on a clinical history of urolithiasis. We previously performed a computed tomography (CT) survey of 463 gout patients and found nephrolithiasis in 34% of them, while only 16% had a history of urolithiasis, that is 68% of the patients with kidney stones confirmed by CT had no clinical history of urolithiasis.4 These findings raise the question as to whether studies investigating the clinical profile of patients with a history of urolithiasis can reflect the real profile of patients who actually have kidney stones. In the present study, we performed unenhanced CT to identify gout patients with kidney stones in order to fully explore the profile of gout associated with nephrolithiasis.
2. SUBJECTS AND METHODS
2.1. Subjects
Three hundred and fifty patients were recruited for this study from among 408 patients who visited the Gout Clinic of Midorigaoka Hospital and were diagnosed as gout in a recent 4‐year period. Patients aged over 70 years and those who did not undergo CT examination and/or clearance test were excluded from the 408 patients. The remaining 350 patients were all men aged from 25 to 70 years (mean ±SD, 46.9 ± 10.4; median, 45.8 years) and diagnosed with acute gout according to American College of Rheumatology 1977 criteria.5
2.2. Methods
This study is an observational cross‐sectional study, and was approved by the ethics review board of Midorigaoka Hospital. After providing informed consent, the 350 patients underwent CT using an Aquilion 64 (Toshiba, Tokyo, Japan) helical scanner within 1 month of the 1st hospital visit. Imaging was conducted under the following conditions without contrast enhancement: collimation of 0.5 mm, a helical pitch of 23, and a beam pitch of 0.84. Images were reconstructed with a 3‐mm slice interval. To search for renal calculi, axial and coronal sections were reviewed on a monitor using the Picture Archiving and Communication System. Images were printed for further assessment, if necessary. The laterality, size, number, location, and CT value of renal calculi were examined with reference to previous reports.6, 7, 8, 9, 10, 11 A calculus density spot >1.0 mm in diameter with a CT value exceeding 120 Hounsfield units (HU) on a coronal scan was defined as a “kidney stone”. Patients with at least 1 kidney stone confirmed by CT were classified as “stone carriers”.
2.3. Prevalence of nephrolithiasis in gout patients
The prevalence of nephrolithiasis was examined in the 350 patients by CT according to the above definition. The history of urolithiasis was also investigated, with patients who answered yes to either of the following questions being considered to have a history of urolithiasis. (a) “Have you ever experienced spontaneous passage of a kidney stone, gravel, or sand or had any treatment for urinary tract stones?” (b) “Have you ever noted symptoms such as flank pain and/or hematuria with confirmation of kidney stones by ultrasonography or radiography?” Then the history‐based prevalence of urolithiasis was calculated and compared with the prevalence confirmed by CT.
2.4. Association between laterality of nephrolithiasis and the number of stones
Each patient with nephrolithiasis on unenhanced CT scans was classified as a unilateral or bilateral stone carrier, and we also counted the number of stones. Then we investigated the association between the laterality of nephrolithiasis and the number of stones.
2.5. Clinical and laboratory characteristics of gout patients with and without nephrolithiasis
The 350 gout patients were divided into three groups: bilateral stone carriers, unilateral stone carriers, and non‐stone carriers. Then the associations between nephrolithiasis and the serum urate (Sua) level, renal function, uric acid metabolism, and frequency of metabolic syndrome (Mets) were investigated in these three groups. All laboratory data were measured by using serum or urine specimens obtained during a 1‐hour creatinine clearance test, which is routinely performed at our clinic to evaluate uric acid metabolism before initiation of treatment. The test was done in the morning under fasting conditions with adequate hydration and after limiting dietary intake of purine‐rich foods for the previous 2 days. If patients were taking drugs such as xanthine oxidase inhibitors, uricosuric agents, losartan, or other medications that reduce serum uric acid levels, a washout period of at least 2 weeks was set before the test.
Renal function was assessed from the serum creatinine (Scr) level, creatinine clearance (Ccr), and estimated glomerular filtration rate (eGFR). The eGFR was calculated with the equation recommended by the Japanese Society of Nephrology: eGFR (mL/min/1.73 m2) = 194 × Scr (mg/dL)−1.094 × age‐0.287.12 Uric acid metabolism was investigated by determining the uric acid clearance (Cua), Cua/Ccr ratio, and urinary excretion of uric acid (Exua). Urine pH was measured with an S2K712 pH meter (ISFETCOM Co. Ltd., Hidaka, Japan) using urine samples obtained during the 1‐hour creatinine clearance test.
The frequency of Mets was compared among the three groups to investigate its association with nephrolithiasis. According to the Japanese diagnostic criteria for obesity,13 Mets was defined as a body mass index (BMI) ≥25 kg/m2 in addition to two or more of the following: (a) triglyceride level (TG) ≥150 mg/dL and/or high‐density lipoprotein cholesterol level (HDL) ≤40 mg/dL; (b) fasting blood sugar (FBS) ≥110 mg/dL; and (c) systolic blood pressure (SBP) ≥130 mm Hg and/or diastolic blood pressure (DBP) ≥85 mm Hg.
2.6. Statistical analysis
Prevalences were expressed as percentages with 95% confidence intervals (95% CI). The mean and standard deviation were calculated for laboratory data. Differences of renal function among bilateral stone carriers, unilateral stone carriers, and non‐stone carriers were assessed by analysis of variance (ANOVA). Multiple comparisons were performed by the Tukey‐Kramer test. Differences in the frequency of Mets among the groups were determined by the Chi‐square test, and pairwise comparisons were performed by Fisher's exact test. All P values were two‐tailed and P < 0.05 was considered significant. Analyses were performed with SAS 9.3 software (SAS Institute Inc, Cary, NC, USA).
3. RESULTS
Kidney stone(s) were detected in 108 of the 350 gout patients by unenhanced helical CT at their 1st visit, so the prevalence of nephrolithiasis among gout patients was as 31% (95% CI: 26‐36) in the present cross‐sectional analysis. Only 63 of the 350 patients had a history of urolithiasis, so the historical prevalence (strictly speaking “cumulative incidence”) of urolithiasis was 18% (95% CI: 14‐22). Sixty‐four of the 108 stone carriers (59%) confirmed to have kidney stones by CT had no history of urolithiasis, that is they were silent stones (Table 1).
Table 1.
Frequency of nephrolithiasis detected by computed tomography (CT) and history of urolithiasis in 350 gout patients
| CT findings | History of urolithiasis (+) | History of urolithiasis (−) | Total |
|---|---|---|---|
| Calculus/calculi (+) | 44 | 64a | 108 |
| Calculus/calculi (−) | 19 | 223 | 242 |
| Total | 63 | 287 | 350 |
Silent stone carriers.
Among the 108 stone carriers, 58 (53.7%, 95% CI: 43.8‐63.3) were bilateral stone carriers and 50 (46%, 95% CI: 36.6‐56.1) were unilateral stone carriers. Forty‐two of the 108 stone carriers (39%, 95% CI: 30‐49) had a single stone and 66 (61%, 95% CI: 51‐70) had two or more stones. Among those 66 patients, 22 (33%) had four or more stones and they were all bilateral stone carriers. The association between the laterality and number of kidney stones is shown in Figure 1.
Figure 1.
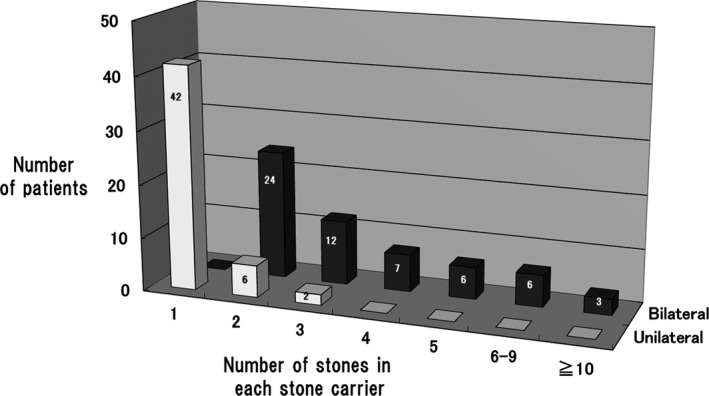
Association between stone laterality and the number of stones in 108 stone carriers
Compared with the non‐stone carriers, the bilateral stone carriers had significantly higher Sua, Scr, and Cua values (P = 0.001, P < 0.001 and P = 0.043, respectively), and eGFR was significantly lower (P = 0.039) in the bilateral carriers. There was little difference in these values between the bilateral and unilateral stone carriers. Among the three groups, there were no significant differences of Cua/Ccr, EXua, and such laboratory parameters as BMI, SBP, DBP, HDL, LDL, TG, and FBS (Table 2). The frequency of Mets also showed no significant differences among the groups according to the Chi‐square test (P = 0.3532) or pairwise comparison with Fisher's exact test (Table 3).
Table 2.
Clinical and laboratory characteristics of bilateral stone carriers, unilateral stone carriers, and non‐stone carriers
|
Bilateral N = 58 |
Unilateral N = 50 |
Non‐stone N = 242 |
ANOVA | Tukey‐Kramer test adjusted for age | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F value | P value | Bilateral vs non‐stone | Unilateral vs non‐stone | Bilateral vs unilateral | |
| Age, years | 50.54 | 10.79 | 45.71 | 8.92 | 46.27 | 10.45 | 4.4 | 0.013* | 0.014* | 0.94 | 0.042* |
| Sua, mg/dL | 9.32 | 1.46 | 8.90 | 1.20 | 8.81 | 1.15 | 6.34 | 0.002* | 0.001* | 0.89 | 0.051 |
| Scr, mg/dL | 0.98 | 0.18 | 0.91 | 0.13 | 0.89 | 0.13 | 8.64 | <0.001* | <0.001* | 0.45 | 0.07 |
| eGFR, mL/min/1.73m2 | 69.08 | 16.87 | 73.80 | 12.73 | 76.11 | 13.25 | 3.45 | 0.032* | 0.039* | 0.36 | 0.72 |
| Ccr, mL/min/1.73m2 | 104.74 | 23.73 | 109.59 | 21.10 | 114.80 | 20.16 | 3.86 | 0.022* | 0.051 | 0.14 | 0.96 |
| Cua, mL/min/1.73m2 | 4.75 | 1.38 | 5.18 | 1.10 | 5.26 | 1.43 | 2.91 | 0.055 | 0.043* | 0.93 | 0.27 |
| Cua/Ccr, % | 4.62 | 1.25 | 4.86 | 1.20 | 4.61 | 1.12 | 1.73 | 0.18 | 0.72 | 0.28 | 0.17 |
| EXua, mg/h | 28.24 | 8.55 | 30.67 | 6.74 | 30.55 | 9.57 | 0.7 | 0.49 | 0.48 | 0.99 | 0.64 |
| Urine pH | 6.12 | 0.49 | 6.24 | 0.50 | 6.26 | 0.48 | 1.43 | 0.24 | 0.21 | 0.94 | 0.57 |
| BMI, kg/m2 | 25.82 | 3.49 | 25.88 | 3.31 | 25.46 | 3.49 | 1.27 | 0.28 | 0.28 | 0.76 | 0.82 |
| SBP, mm Hg | 135.19 | 16.22 | 137.72 | 20.04 | 133.40 | 18.04 | 1.55 | 0.21 | 0.99 | 0.20 | 0.31 |
| DBP, mm Hg | 85.52 | 9.48 | 85.82 | 12.74 | 83.03 | 13.10 | 1.31 | 0.27 | 0.79 | 0.26 | 0.73 |
| HDL, mg/dL | 51.86 | 11.96 | 55.46 | 16.25 | 54.99 | 15.30 | 1.4 | 0.24 | 0.25 | 0.97 | 0.35 |
| LDL, mg/dL | 134.79 | 37.92 | 134.52 | 30.45 | 134.58 | 34.58 | 0 | 0.99 | 0.99 | 0.99 | 0.99 |
| TG, mg/dL | 167.95 | 103.80 | 178.56 | 91.02 | 188.31 | 139.66 | 0.72 | 0.48 | 0.47 | 0.88 | 0.87 |
| FBS, mg/dL | 102.88 | 11.85 | 98.62 | 15.78 | 102.75 | 10.41 | 2.57 | 0.08 | 0.87 | 0.06 | 0.32 |
Sua, serum urate; Scr, serum creatinine; eGFR, estimated glomerular filtration rate; Ccr, creatinine clearance; Cua, uric acid clearance; EXua, urinary excretion of uric acid; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; TG, triglycerides; FBS, fasting blood sugar.
Significant (P < 0.05).
Table 3.
Prevalence of metabolic syndrome in the three groups
|
Bilateral N = 58 |
Unilateral N = 50 |
Non‐stone N = 242 |
|
|---|---|---|---|
| Number of patients with Mets | 16 | 8 | 54 |
| Prevalence of Mets | 27.6% | 16% | 22.3% |
| 95% CI | 16.7‐40.9 | 7.2‐29.1 | 17.2‐28.1 |
| Fisher's exact test | |||
|---|---|---|---|
| Bilateral vs non‐stone | Unilateral vs non‐stone | Bilateral vs unilateral | |
| P value | 0.17 | 0.44 | 0.39 |
Mets, metabolic syndrome.
4. DISCUSSION
Despite the growing array of urological and surgical techniques for treatment of urolithiasis, many issues remain unresolved, including the fundamental clinical features of gout patients with kidney stones. One reason for this may be that nearly all investigations of the association between urolithiasis and gout have been based on the clinical history of urolithiasis.
Since the original report by Smith et al,14 helical CT has made great strides and allows delineation of small stones, even radiolucent stones such as those composed of uric acid. Shorter scanning times have facilitated real‐time evaluation of stones on an outpatient basis, and CT has become the standard method for assessment of urinary tract stones (Figures 2, 3 and 2, 3). Based on our experience, 3‐dimensional CT (3DCT) was useful for confirming the distribution and number of stones, because stones in both kidneys could be easily observed on 1 screen with a high degree of accuracy (Figures 2, 3).
Figure 2.
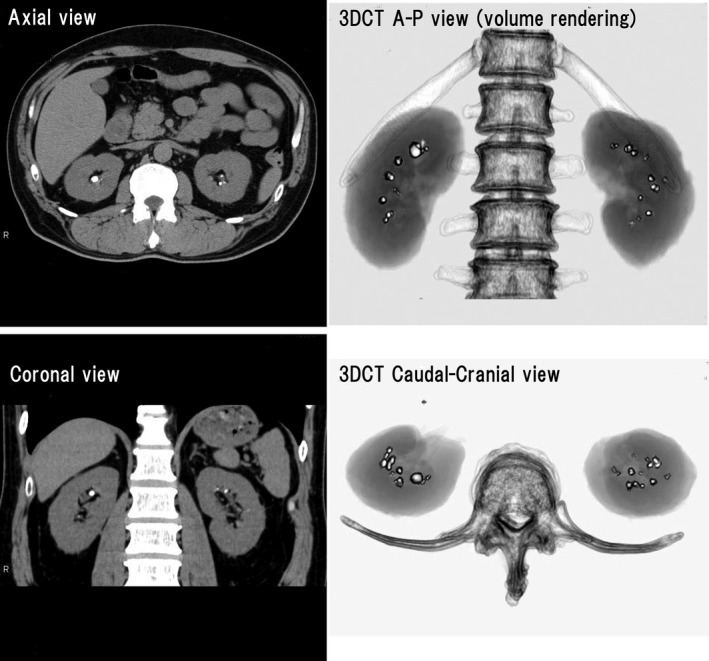
Computed tomography (CT) scans of a 55‐year‐old man with gout since the age of 30 years. Multiple bilateral stones can be seen on the axial and coronal scans. Three‐dimensional CT (3DCT) was useful for confirming the distribution and number of stones, because stones in both kidneys could be easily observed on one screen with a high degree of accuracy
Figure 3.
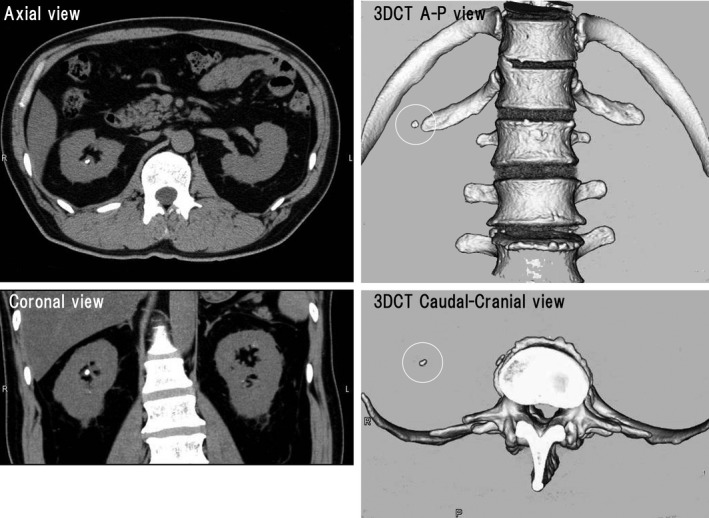
Computed tomography (CT) scans of a 57‐year‐old man with gout. There is a unilateral stone in the right kidney
Our previous CT‐based study revealed that over half of all gout patients with kidney stones had no history of urolithiasis.4 This implies that studies based on a history of urolithiasis may have missed the majority of the patients with kidney stones. Detection of all stone carriers, including those with silent stones, is required to precisely elucidate the clinical profile of nephrolithiasis in gout patients. The present CT‐based study provided several novel findings with regard to nephrolithiasis in gout patients.
First, we identified a significant difference between the prevalence of nephrolithiasis confirmed by CT and that of urolithiasis estimated from the clinical history of stone events (31% vs 18%, P < 0.001). The “prevalence of urolithiasis” calculated from a history of stone events is not necessarily accurate and rather represents the “cumulative incidence” of events during the observation period, while CT examination at the 1st visit to hospital is warranted to determine the definitive prevalence of nephrolithiasis.15
Second, there have been no previous reports on the laterality of nephrolithiasis in gout. The present investigation clarified that more than half of all gout patients with kidney stone(s) were bilateral and multiple stone carriers. Figure 1 demonstrates that the patients with urolithiasis could generally be classified into two groups: a group with multiple bilateral stones and a group with one or a few unilateral stones. While unilateral stones may form in association with localized stagnation of urine flow caused by anatomical or passage abnormalities, bilateral kidney stones may form when the urine is lithogenic, probably in association with abnormal systemic uric acid metabolism. These two groups led us to postulate that there are at least two different mechanisms of stone formation in gout. Furthermore, these findings may help to explain why the prevalence of urolithiasis is higher in gout patients than that in the general population. According to reports from the USA and Japan, the prevalence of urolithiasis among men was 10.6% and 4.3%, respectively.16, 17
Third, comparison of clinical and laboratory findings among the three groups, that is bilateral, unilateral, and non‐stone carriers, clarified another characteristic of nephrolithiasis in gout, which was significant elevation of Sua and significantly worse renal function (evaluated from Scr and eGFR) in bilateral stone carriers than in non‐stone carriers (Table 2). This suggests that elevation of Sua in gout patients may increase the stone burden and lead to more severe renal dysfunction.
While it is generally thought that overproduction or increased urinary excretion of uric acid predispose to stone formation, we did not find a positive relation between nephrolithiasis and parameters of uric acid metabolism or urinary excretion of uric acid (Table 2). Acidic urine is also considered to promote urolithiasis. We previously investigated the circadian rhythm of urine pH in 157 gout patients, and found that both serum urate and the prevalence of urolithiasis were significantly higher in patients with a urine pH <5.8 throughout the day than in patients with a urine pH ≥5.8 throughout the day (P < 0.05).18 However, we could not confirm a positive relation between nephrolithiasis and urine pH in the present study (Table 2). One reason for this discrepancy may be the use of different methods for collecting urine samples. In our study of the circadian rhythm, urine pH was measured 3 times a day, including the 1st morning urine specimen and diurnal and nocturnal urine specimens for about 2 weeks. In the present study, urine specimens for pH measurement were obtained during the 1‐hour creatinine clearance test, which was performed with sufficient oral hydration to achieve the required urine volume. Hydration could have altered the urine pH, or improved acidic urine.
Several studies have shown that both gout and urolithiasis are associated with comorbidities such as obesity, and insulin resistance, or Mets,19, 20, 21, 22, 23 but we found no significant difference in the frequency of Mets or its components among the three groups in this study (Tables 2 and 3).
One limitation of the present study is the CT definition of kidney stones. Until now, CT‐based analysis of nephrolithiasis has rarely been performed in either gout patients or the general population, and a clear definition of the CT criteria for kidney stones has not been established. More studies will be required for better identification of the CT features of nephrolithiasis.
In conclusion, this CT‐based study provided several novel findings about gout patients with urolithiasis. Approximately one‐third of gout patients had kidney stones and more than half of those patients were bilateral and multiple stone carriers. Elevation of Sua in gout might increase the stone burden, leading to more severe renal dysfunction. An association between nephrolithiasis and Mets was not demonstrated in our gout patients.
DISCLOSURES
The authors declare they have no conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by the Gout Research Foundation of Japan.
The authors wish to thank T. Kobayashi, Eri Miyayama, and N. Fujimoto for technical assistance with helical CT.
Shimizu T, Hori H, Umeyama M, Shimizu K. Characteristics of gout patients according to the laterality of nephrolithiasis: A cross‐sectional study using helical computed tomography. Int J Rheum Dis. 2019;22:567–573. 10.1111/1756-185X.13443
Registration: This observational study was registered with UMIN‐CTR (https://www.umin.ac.jp/ctr/index-j.htm) as UMIN000006973.
REFERENCES
- 1. Yu TF, Gutman AB. Uric acid nephrolithiasis in gout. Ann Intern Med. 1967;67:1133‐1148. [DOI] [PubMed] [Google Scholar]
- 2. Fessel WJ. Renal outcomes of gout and hyperuricemia. Am J Med. 1979;67:74‐82. [DOI] [PubMed] [Google Scholar]
- 3. Kramer HJ, Choi HK, Atkinson K, Stampfer M. Curhan GC. The association between gout and nephrolithiasis in men. The health professionals’ Follow‐up study. Kidney Int. 2003;64(3):1022‐1026. [DOI] [PubMed] [Google Scholar]
- 4. Shimizu T, Kitada H, Umeyama M, Hori H, Takasaki N. Novel evaluation of nephrolithiasis as a complication of gout: A cross‐sectional study using helical computerized tomography. J Urol. 2013;189:1747‐1752. [DOI] [PubMed] [Google Scholar]
- 5. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895‐900. [DOI] [PubMed] [Google Scholar]
- 6. Metser U, Ghai S, Ong YY, Radomski SB. Assessment of urinary tract calculi with 64‐MDCT: the axial versus coronal plane. Am J Roentogenol. 2009;192:1509‐1513. [DOI] [PubMed] [Google Scholar]
- 7. Nadler RB, Stern JA, Kimm S, Hoff F, Rademaker AW. Coronal imaging to assess urinary tract stone size. J Urol. 2004;172:962‐964. [DOI] [PubMed] [Google Scholar]
- 8. Liden M, Thunberg P, Broxvall M, Geijer H. Two‐ and three‐dimensional CT measurements of urinary calculi length and width: a comparative study. Acta Radiol. 2015;19:487‐492. [DOI] [PubMed] [Google Scholar]
- 9. Hubert J, Blum A, Cormier L, Claudon M, Regent D, Mangin P. Three‐dimensional CT‐scan reconstruction of renal calculi. A new tool for mapping‐out staghorn calculi and follow‐up of radiolucent stones. Eur Urol. 1997;31:297‐301. [DOI] [PubMed] [Google Scholar]
- 10. Van Appledorn S, Ball AJ, Patel VR, Kim S, Leveilee RJ. Limitation of non‐contrast CT for measuring ureteral stones. J Endourol. 2003;17:851‐854. [DOI] [PubMed] [Google Scholar]
- 11. Dalla Palma L, Morra A, Grotto M. CT‐urography. Radio Med. 2005;110:170‐178. [PubMed] [Google Scholar]
- 12. Clinical practice guidebook for diagnosis and treatment of chronic kidney disease. Tokyo: Japanese Society of Nephrology 2009 (in Japanese).
- 13. Examination Committee of Criteria for “Obesity Disease” in Japan . Japan society for the study of obesity. New criteria for obesity disease in Japan. Circ J. 2002;66:987‐992. [DOI] [PubMed] [Google Scholar]
- 14. Smith RC, Rosenfield AT, ChoeKA E, et al. Acute flankpain: Comparison of non‐contrast‐enhanced CT and intravenous urography. Radiology. 1995;194:789‐794. [DOI] [PubMed] [Google Scholar]
- 15. Shimizu T, Hori H. The prevalence of nephrolithiasis in patients with primary gout: A cross‐sectional study using helical computed tomography. J Rheumatol 2009;36:1958–1962. [DOI] [PubMed] [Google Scholar]
- 16. Scales CD, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yasui T, Iguchi M, Suzuki S, Kohri K. Prevalence and epidemiological characteristics of urolithiasis in Japan: national trends between 1965and 2005. Urology. 2008;71:209–213. [DOI] [PubMed] [Google Scholar]
- 18. Shimizu T, Matsushige H, Nishikawa M, Ohya K, Honda M. Studies on urine pH in patients with gout: Circadian changes of urine PH. Purine and Pyrimidine Metabolism. 1996;20:9–15. (in Japanese). [Google Scholar]
- 19. Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta‐analysis of observational studies. Arthritis Res Ther. 2015;17:90 10.1186/s13075-015-0610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong YV, Cook P, Somani BK. The association of metabolic syndrome and urolithiasis. Int J Endocrinol. 2015; 2015:567–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abate N, Chandalia M, Cabo‐Chan AV Jr, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. [DOI] [PubMed] [Google Scholar]
- 22. Jeong IG, Kang T, Bang JK, et al. Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis. 2011;58:383–388. [DOI] [PubMed] [Google Scholar]
- 23. Negri AL, Spivacow FR, Del Valle EE, Forrester M, Rosende G, Pinduli I. Role of overweight and obesity on the urinary excretion of promoters and inhibitors of stone formation in stone formers. Urol Res. 2008;36:303–307. [DOI] [PubMed] [Google Scholar]


