Abstract
Background
This analysis of patients in a randomized population‐based health services study was done to determine the effects of faecal occult blood test (FOBT) screening of colorectal cancer (CRC) in outcomes beyond mortality, and to obtain explanations for potential sex differences in screening effectiveness.
Methods
In the Finnish FOBT screening programme (2004–2011), people aged 60–69 years were randomized into the screening and control arms. Differences in incidence, symptoms, tumour location, TNM categories, non‐vital outcomes and survival in the screening and control arms were analysed.
Results
From 321 311 individuals randomized, 743 patients with screening‐detected tumours and 617 control patients with CRC were analysed. CRC was less common in women than in men (0·34 versus 0·50 per cent; risk ratio (RR) 0·82, 95 per cent c.i. 0·74 to 0·91) and women were less often asymptomatic (16·7 versus 22·0 per cent; RR 0·76, 0·61 to 0·93). Women more often had right‐sided tumours (32·0 versus 21·3 per cent; RR 1·51, 1·26 to 1·80). Among men with left‐sided tumours, those in the screening arm had lower N (RR 1·23, 1·02 to 1·48) and M (RR 1·57, 1·14 to 2·17) categories, as well as a higher overall survival rate than those in the control arm. Furthermore among men with left‐sided tumours, non‐radical resections (26·2 versus 15·7 per cent; RR 1·67, 1·22 to 2·30) and postoperative chemotherapy sessions (61·6 versus 48·2 per cent; RR 1·28, 1·10 to 1·48) were more frequent in the control arm. Similar benefits of screening were not detected in men with right‐sided tumours or in women.
Conclusion
Biennial FOBT screening seems to be effective in terms of improving several different outcomes in men, but not in women. Differences in incidence, symptoms and tumour location may explain the differences in screening efficacy between sexes.
Short abstract
Only of benefit in men
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world, with 1·4 million new cases diagnosed annually and 0·7 million deaths attributed to the disease1. Key factors that improve the prognosis are early diagnosis and complete radical tumour resection. However, CRC is often asymptomatic in its early stages, and is not diagnosed until symptoms manifest at a later stage2. Several screening methods have been implemented to detect CRC at early stages. These include faecal occult blood testing (FOBT), endoscopy (flexible sigmoidoscopy or colonoscopy), CT colonography, and combinations thereof3.
Three population‐based prospective randomized controlled studies4, 5, 6 and one volunteer‐based trial7 found that colorectal tumours can be detected at an earlier stage and mortality from CRC reduced with biennial FOBT screening. Biennial FOBT screening reduces CRC mortality by 18 per cent on average8. Sigmoidoscopy as a screening method has been found to be more effective than FOBT in reducing CRC mortality9, but carries a small but significant risk (0·08 per cent) of major complications owing to its invasiveness10. Data regarding colonoscopy as a population‐based screening method are still lacking, and results of ongoing trials are pending.
In Finland, a prospective randomized population‐based health services programme on CRC screening was implemented in 200411, with the primary aim of determining whether CRC screening by biennial FOBT in a population‐based programme reduces CRC‐specific mortality. The target population was 60–69‐year‐old men and women, and the study expanded gradually as more regions implemented the programme. By the end of 2011, it covered 41·8 per cent of men and 40·6 per cent of women among all 60–69‐year‐old individuals living in Finland. The target age limits were chosen based on an earlier study11 showing that the incidence of CRC and mortality from the disease increase notably in Finland after the age of 60 years. Over 80 per cent of new cases were diagnosed and more than 85 per cent of CRC deaths occurred in people aged over 60 years11.
The design of the randomized public health programme and initial mortality results were published recently12. In contrast with randomized screening trials4, 5, 7, no significant difference in CRC‐specific mortality between the screening and control arms was found12. However, CRC mortality was reduced in men in the screening arm compared with controls, and was increased in women, although the difference between the sexes was not statistically significant12. Interestingly, other trials13, 14 have reported a greater reduction in CRC mortality in men undergoing biennial FOBT compared with women, whereas another15 reported no sex‐based difference. It remains unclear why men appear to benefit more than women from CRC screening.
The effects of FOBT CRC screening on many important but non‐vital outcomes remain largely unexplored. These include radical surgery, emergency surgery, and the need for chemotherapy and a stoma, which are of paramount interest to healthcare providers and patients alike. The main aims of the present study were to explore reasons for the potential sex difference in FOBT screening effectiveness and to evaluate the effects of screening on non‐vital outcomes.
Methods
This study was approved by the Ministry of Social Affairs and Health in 2004 (STM/42/07/2004) on the implementation of CRC screening in Finland, and the approval was updated in 2010 by the official authority, the National Institute of Health and Welfare (THL/619/5.05.00/2010).
The design and details of the randomized public health programme have been reported previously11. Briefly, the screening population consisted of 60–69‐year‐old individuals living in municipalities participating in the organized CRC screening programme. Data from individuals invited to participate were retrieved from the Central Population Registry, and the population was randomized 1 : 1 into the screening or control arm stratified by birth year, sex and residence. The randomized programme commenced in September 2004. Screening was performed with a biennial guaiac FOBT (Hemoccult®; Beckman Coulter, Krefeld, Germany), which was sent by mail to the screening group. If there was any blood in the stool, the person was referred for a full colonoscopy. The screening letter also contained advice to seek medical attention if any symptoms were present. All individuals in the screening group were reinvited every second year until they reached 69 years of age. The control population was not contacted at all.
The present study included patients diagnosed with CRC between the time of randomization and the end of 2011 in both the screening and control groups. Patients diagnosed with CRC from both study arms were identified from the Finnish Cancer Registry. The registry collects population‐based data on patients with cancer in Finland with high coverage (97·4 per cent) for CRCs16. Patients randomized to the screening arm were included in this group for the analyses regardless of active participation, because all individuals in the screening arm were sent the letter and FOBT test. Patients were also included in their study arms regardless of whether the tumour was found by screening or in spite of it (symptomatic patient undergoing colonoscopy even though the FOBT was negative).
Hospitals treating the patients with CRC were identified, and copies of patients' medical records were requested. The following data were extracted manually: clinical and pathological UICC TNM stage (7th edition)17; symptoms; extent of surgery; need for emergency surgery, stoma, or chemotherapy; and histopathological diagnoses. Radical surgery was defined as complete tumour removal with tumour‐free margins of more than 1 mm after the primary surgery. Emergency surgery was defined as a colorectal operation during an emergency admission. Stomas included both loop and end stomas as well as permanent or temporary stomas. Chemotherapy included both adjuvant therapy administered after curative surgery and therapy administered for metastatic CRC. Dates of death were collected from the Central Population Registry until the end of 2015. This observational analysis was planned after the original trial had been completed.
Statistical analysis
Sex differences in incidence, histology, treatments, symptoms and laterality were estimated by risk ratios (RRs) using log‐link binomial regression, and in median age at diagnosis using quantile regression. These sex differences were adjusted for study arm. Study arm differences in non‐vital outcomes were also estimated using RRs for all patients, by sex, and by both sex and tumour laterality (right‐sided versus left‐sided). Overall study arm differences in non‐vital outcomes were estimated with adjustment for T category (T0–2 or T3–4).
Differences between the sexes in variables described above were also estimated separately by study arm. Prevalence differences between study arms were estimated with regard to histology, symptoms, laterality and TNM categories. TNM category analysis was also stratified by sex, and both sex and laterality. These estimates were adjusted for sex, laterality, or both where they were not stratified by these variables. Simple proportions of tumour locations were computed by sex and study arm. Stratifying by both sex and laterality, survival was estimated for the study arms using the Kaplan–Meier method, and differences in patient survival between the study arms were estimated by means of hazard ratios (HRs) derived using Cox regression. Follow‐up commenced at the time of diagnosis of CRC and ended upon death, emigration or on 31 December 2015, whichever was earliest.
Confidence intervals were estimated for all RRs and HRs at the 95 per cent confidence level. No estimate was adjusted for age because age adjustment had no effect. P values were estimated using two‐sided tests for the main results, unstratified sex differences and all study arm differences in non‐vital outcomes. In addition, multiple comparisons were taken into account by considering significant only the results that were accepted at a 5 per cent false detection rate based on the Benjamini–Hochberg criterion (BH+). All statistical analyses were performed using SPSS® version 24 (IBM, Armonk, New York, USA) or R version 3.4.0 (packages Epi 2.16, quantreg 5.3 and survival 2.41.3; https://www.r‐project.org/).
Results
Between 2004 and 2011, 321 311 people were randomized into the screening or control arm. Owing to a delay between population sampling from the Central Population Registry and randomization, patients who had died before the randomization date (43 in the screening arm and 41 in the control arm) were excluded from the analyses. Thus, there were 160 719 and 160 508 individuals in the screening and control arms respectively (Fig. 1). The screening participation rate (individuals who returned the FOBT) was 69·2 per cent (men, 61·9 per cent; women, 76·3 per cent), and the proportion of positive FOBT results was 2·7 per cent in women and 4·7 per cent in men. The compliance rate for further colonoscopy was 84·6 per cent (proportion of people with a positive FOBT who actually underwent colonoscopy).
Figure 1.
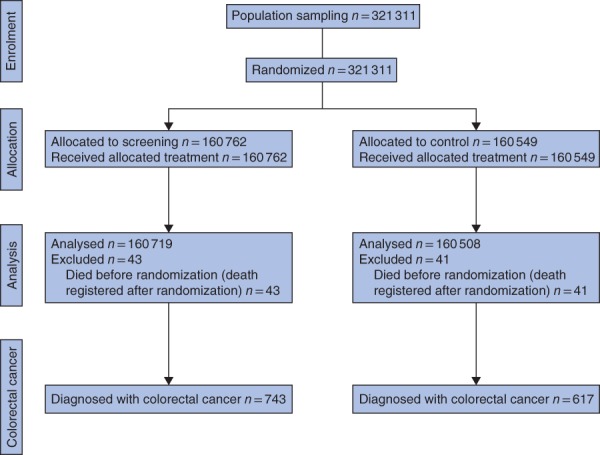
Flow chart of the original study protocol
A total of 743 and 617 CRCs were detected in the screening and control arms respectively between 2004 and the end of 2011. Women were less often diagnosed with CRC than men: prevalence 0·37 versus 0·55 per cent respectively in the screening arm and 0·32 versus 0·45 per cent in the control arm (RR 0·82, 95 per cent c.i. 0·74 to 0·91; BH+). Women had more mucinous subtype adenocarcinomas than men: 60 (11·0 per cent) versus 51 (6·5 per cent) (RR 1·70, 1·19 to 2·43; BH+). Tumours were more often located on the right side in women than in men (RR 1·51, 1·26 to 1·80; BH+). The proportion of right‐ and left‐sided tumours did not differ between the study arms (RR 1·05, 0·88 to 1·25) (Fig. 2 and Table 1).
Figure 2.
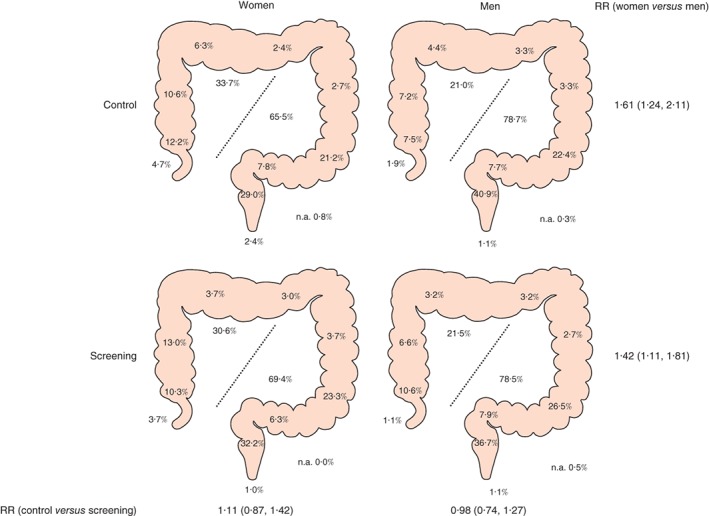
Distribution of colorectal cancer in women and in men in the screening and control arms. Percentages in the large bowel refer to the following areas: appendix, caecum, ascending colon, right transverse colon, left transverse colon, descending colon, sigmoid colon, rectosigmoid junction, rectum and anus. Percentages in the middle show proportions of right‐ and left‐sided tumours. n.a., Not available. Risk ratios (RRs), shown with 95 per cent confidence intervals, relate to the proportion of right‐sided cancers
Table 1.
Colorectal cancer diagnoses in 2004–2011 in the randomized population of the Finnish colorectal cancer screening programme: overall sex and study arm differences, and sex differences by study arm in incidence, tumour characteristics, prevalence, age at diagnosis and symptoms
| Screening | Control | Overall adjusted analyses‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | RR (women versus men)† | Men | Women | RR (women versus men)† | RR (control versus screening)†, ¶ | RR (women versus men)†, # | P (women versus men)# | |
| No. randomized | 79 871 | 80 891 | 79 882 | 80 667 | |||||
| Patients with colorectal cancer* | 442 (0·55) | 301 (0·37) | 0·67 (0·58, 0·78) | 362 (0·45) | 255 (0·32) | 0·96 (0·72, 1·28) | 0·69 (0·62, 0·77) | 0·82 (0·74, 0·91) | – |
| Right‐sided tumour | 95 (21·5) | 92 (30·6) | 1·42 (1·11, 1·81) | 76 (21·0) | 86 (33·7) | 1·61 (1·24, 2·11) | 1·05 (0·88, 1·25) | 1·51 (1·26, 1·80) | < 0·001** |
| Missing | 2 | 0 | 1 | 2 | |||||
| Median age at diagnosis (years)§ | 63·7 | 63·8 | – | 64·1 | 63·8 | –0·03 (−0·50, 0·44)§ | –0·34 (−0·80, 0·12)§ | 0·151 | |
| Cancer histology | |||||||||
| Adenocarcinoma | 387 (89·8) | 247 (84·3) | 0·94 (0·89, 0·99) | 314 (89·0) | 209 (83·3) | 0·94 (0·87, 1·00) | 0·99 (0·95, 1·03) | 0·94 (0·90, 0·98) | 0·004** |
| Mucinous adenoma | 26 (6·0) | 35 (11·9) | 1·98 (1·22, 3·25) | 25 (7·1) | 25 (10·0) | 1·41 (0·82, 2·40) | 0·97 (0·68, 1·39) | 1·70 (1·19, 2·43) | 0·004** |
| Neuroendocrine | 10 (2·3) | 3 (1·0) | 0·44 (0·10, 1·43) | 5 (1·4) | 8 (3·2) | 2·25 (0·76, 7·37) | 1·20 (0·55, 2·59) | 1·06 (0·48, 2·27) | 0·889 |
| Squamous | 5 (1·2) | 4 (1·4) | 1·18 (0·29, 4·41) | 4 (1·1) | 5 (2·0) | 1·76 (0·47, 7·04) | 1·19 (0·47, 3·04) | 1·44 (0·57, 3·66) | 0·437 |
| Other | 3 (0·7) | 4 (1·4) | 1·96 (0·44, 9·90) | 5 (1·4) | 4 (1·6) | 1·13 (0·28, 4·21) | 1·53 (0·57, 4·27) | 1·43 (0·53, 3·87) | 0·469 |
| Missing | 11 | 8 | 9 | 4 | |||||
| Symptoms | |||||||||
| Intestinal bleeding | 106 (24·3) | 84 (28·0) | 1·15 (0·90, 1·47) | 156 (43·3) | 90 (36·0) | 0·83 (0·67, 1·01) | 1·57 (1·34, 1·83) | 0·94 (0·81, 1·10) | 0·467 |
| Change in bowel habit | 104 (23·9) | 68 (22·7) | 0·95 (0·72, 1·24) | 132 (36·7) | 95 (38·0) | 1·04 (0·84, 1·27) | 1·59 (1·35, 1·88) | 1·00 (0·85, 1·18) | 0·974 |
| Total occlusion | 6 (1·4) | 8 (2·7) | 1·94 (0·68, 5·83) | 11 (3·1) | 4 (1·6) | 0·52 (0·15, 1·51) | 1·29 (0·63, 2·69) | 1·02 (0·48, 2·10) | 0·959 |
| Anaemia | 61 (14) | 36 (12·0) | 0·86 (0·58, 1·25) | 73 (20·3) | 54 (21·6) | 1·07 (0·78, 1·45) | 1·58 (1·24, 2·02) | 0·98 (0·76, 1·24) | 0·842 |
| Abdominal pain | 60 (13·8) | 68 (22·7) | 1·65 (1·20, 2·26) | 91 (25·3) | 76 (30·4) | 1·20 (0·93, 1·56) | 1·56 (1·28, 1·92) | 1·36 (1·12, 1·67) | 0·002** |
| Other | 65 (14·9) | 44 (14·7) | 0·98 (0·69, 1·40) | 75 (20·8) | 50 (20·0) | 0·96 (0·69, 1·32) | 1·38 (1·10, 1·75) | 0·97 (0·76, 1·23) | 0·805 |
| No symptoms | 163 (37·4) | 84 (28·0) | 0·75 (0·60, 0·93) | 12 (3·3) | 8 (3·2) | 0·96 (0·38, 2·29) | 0·10 (0·06, 0·15) | 0·76 (0·61, 0·93) | 0·011** |
| Missing | 6 | 1 | 2 | 5 | |||||
Values in parentheses are percentage of people with colorectal cancer, except
percentage of people randomized and
95 per cent confidence intervals.
RR, risk ratio.
Binomial regression with log‐link function, except
difference in medians analysed by quantile regression; adjusted for
sex and
study arm.
Significant at 5 per cent false discovery rate based on Benjamini–Hochberg criterion.
Symptoms
Almost none of the patients in the control arm were asymptomatic. There were 247 asymptomatic patients (33·6 per cent) in the screening arm and only 20 (3·3 per cent) in the control arm (RR 0·10, 95 per cent c.i. 0·06 to 0·15) (Table 1). Women were less often asymptomatic than men (RR 0·76, 0·61 to 0·93; BH+) but, of individual symptoms, only the prevalence of abdominal pain was significantly different between the sexes (RR 1·36, 1·12 to 1·67; BH+). The most common symptom in both study arms was intestinal bleeding (32·4 per cent) followed by a change in bowel habit (29·6 per cent), abdominal pain (21·9 per cent) and anaemia (16·6 per cent).
TNM stage
Cancers from the screening arm had a lower T category (RR 1·25, 95 per cent c.i. 1·16 to 1·35), N category (RR 1·14, 1·00 to 1·29) and M category (RR 1·33, 1·08 to 1·62) than those from the control arm (Table 2). In subgroup analyses, left‐sided tumours in the screening arm had a lower T category (RR 1·30, 1·18 to 1·43), N category (RR 1·16, 1·00 to 1·35) and M category (RR 1·53, 1·19 to 1·97) than those in the control arm, whereas right‐sided tumours had only a lower T category (RR 1·14, 1·02 to 1·28), but not N or M category in the screening arm. In women, the only differences between study arms were in the T and M categories of left‐sided tumours. However, men with left‐sided tumours had higher T, N and M categories in the control arm, whereas men with right‐sided tumours had only a higher T category in control arm.
Table 2.
Differences between randomization arms in T, N, and M categories by both laterality and sex, by laterality and overall
| Right | Left | |||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||
| Screening (n = 95) | Control (n = 76) | Screening (n = 92) | Control (n = 86) | Screening (n = 345) | Control (n = 285) | Screening (n = 209) | Control (n = 167) | |
| T category | ||||||||
| T ≤ 2 | 22 (26) | 9 (13) | 20 (23) | 10 (14) | 140 (43·1) | 73 (27·2) | 71 (39·4) | 36 (23·5) |
| T > 2 | 63 (74) | 59 (87) | 66 (77) | 61 (86) | 185 (56·9) | 195 (72·8) | 109 (60·6) | 117 (76·5) |
| Missing | 10 | 8 | 6 | 15 | 20 | 17 | 29 | 14 |
| RR (control versus screening)* | ||||||||
| Sex and laterality stratum† | 1·17 (1·00, 1·38) | 1·11 (0·95, 1·30) | 1·28 (1·13, 1·44) | 1·33 (1·15, 1·56) | ||||
| Laterality stratum‡ | 1·14 (1·02, 1·28) | 1·30 (1·18, 1·43) | ||||||
| Overall§ | 1·23 (1·15, 1·33) | |||||||
| N category | ||||||||
| N0 | 48 (59) | 38 (58) | 43 (52) | 32 (46) | 194 (61·0) | 138 (52·1) | 94 (54·0) | 82 (55·4) |
| N ≥ 1 | 33 (41) | 28 (42) | 39 (48) | 37 (54) | 124 (39·0) | 127 (47·9) | 80 (46·0) | 66 (44·6) |
| Missing | 14 | 10 | 10 | 17 | 27 | 20 | 35 | 19 |
| RR (control versus screening)* | ||||||||
| Sex and laterality stratum† | 1·04 (0·70, 1·53) | 1·11 (0·80, 1·53) | 1·23 (1·02, 1·48) | 1·05 (0·82, 1·35) | ||||
| Laterality stratum‡ | 1·08 (0·84, 1·38) | 1·16 (1·00, 1·35) | ||||||
| Overall§ | 1·14 (1·00, 1·29) | |||||||
| M category | ||||||||
| M0 | 70 (76) | 57 (75) | 64 (70) | 60 (71) | 289 (84·5) | 215 (75·7) | 173 (82·8) | 123 (74·5) |
| M1 | 22 (24) | 19 (25) | 28 (30) | 25 (29) | 53 (15·5) | 69 (24·3) | 36 (17·2) | 42 (25·5) |
| Missing | 3 | 0 | 0 | 1 | 3 | 1 | 0 | 2 |
| RR (control versus screening)* | ||||||||
| Sex and laterality stratum† | 1·05 (0·61, 1·78) | 0·97 (0·61, 1·52) | 1·57 (1·14, 2·17) | 1·48 (1·00, 2·21) | ||||
| Laterality stratum‡ | 1·00 (0·70, 1·41) | 1·53 (1·19, 1·97) | ||||||
| Overall§ | 1·33 (1·08, 1·62) | |||||||
Values in parentheses are percentages unless indicated otherwise;
values in parentheses are 95 per cent confidence intervals.
Risk ratio (RR) estimated separately by sex and laterality for each stage category.
RR adjusted for sex and estimated separately by laterality for each stage category.
RR adjusted for sex and laterality, and estimated separately for each stage category response.
A RR above 1 indicates a greater prevalence of the higher T, N or M category in control versus screening group.
Non‐vital outcomes
Patients with CRC in the control arm experienced significantly more non‐radical surgery (26·8 versus 19·2 per cent; RR 1·39, 95 per cent c.i. 1·14 to 1·70; BH+), emergency surgery (14·1 versus 9·3 per cent; RR 1·51, 1·11 to 2·06; BH+) and administration of postoperative chemotherapy (62·8 versus 52·1 per cent; RR 1·20, 1·09 to 1·32; BH+) than those in the screening arm (Fig. 3). Controls also had more stomas, but this was not significant according to the Benjamini–Hochberg criterion (32·8 versus 27·3 per cent; RR 1·21, 1·02 to 1·43). After adjustment for T category (T0–2 versus T3–4), no significant differences were found in the rates of non‐radical surgery (RR 1·13, 0·91 to 1·41), emergency surgery (RR 1·17, 0·85 to 1·61), postoperative chemotherapy (RR 0·99, 0·92 to 1·08) or stomas (RR 1·17, 0·98 to 1·39). In separate analyses of men and women, a statistically significant improvement in these non‐vital outcomes was observed only in men.
Figure 3.
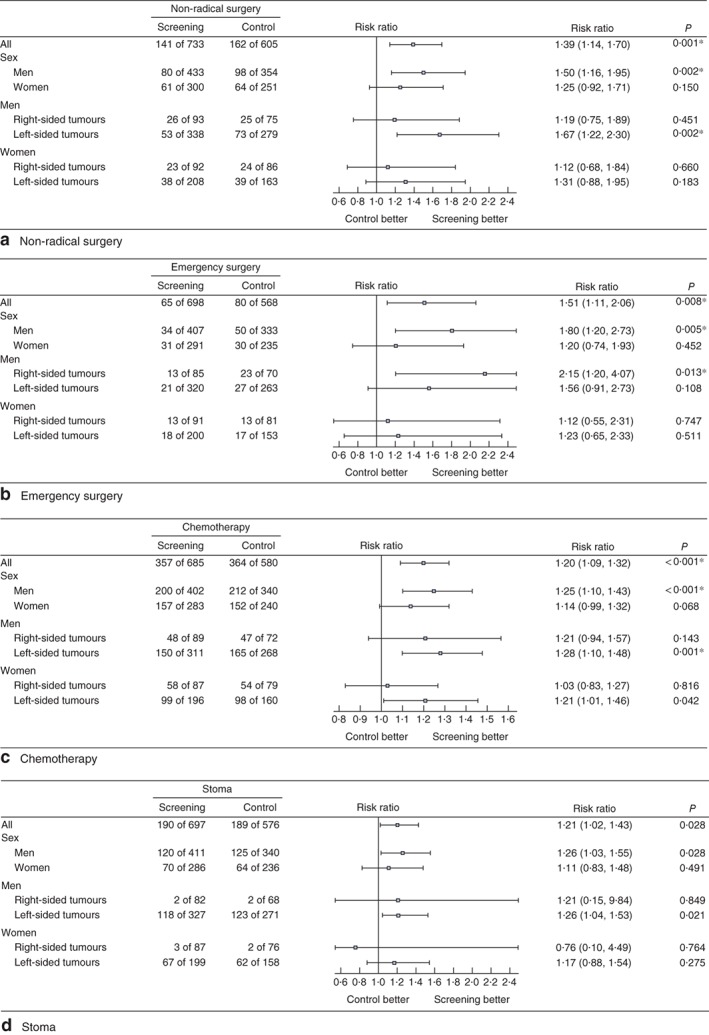
Effect of faecal occult blood test screening on non‐vital outcomes, overall, by sex, and by both sex and laterality: a non‐radical surgery, b emergency surgery, c chemotherapy and d stoma. Risk ratios are shown with 95 per cent confidence intervals for the control versus screening arms (log‐link binominal regression); *significant at 5 per cent false discovery rate based on Benjamini–Hochberg criterion
Further subgroup analyses of left‐ and right‐sided tumours indicated that the findings were restricted mainly to left‐sided cancers. An exception was observed in men with right‐sided tumours, who had emergency surgery significantly more often in the control arm compared with the screening arm (33 versus 15 per cent; RR 2·15, 1·20 to 4·07; BH+). Significantly more men with left‐sided tumours in the control group than the screening group underwent non‐radical surgery (26·2 versus 15·7 per cent; RR 1·67, 1·22 to 2·30; BH+) and postoperative chemotherapy (61·6 versus 48·2 per cent; RR 1·28, 1·10 to 1·48; BH+), and the stoma rate was increased but not significantly according to the Benjamini–Hochberg criterion (45·4 versus 36·1 per cent; RR 1·26, 1·04 to 1·53).
Survival
Survival was worse in controls than in the screening arm in men with CRC (hazard ratio (HR) 1·31, 95 per cent c.i. 1·05 to 1·64), but not in women (HR 1·07, 0·80 to 1·45). Among men, the 5‐year overall survival (OS) rates were 68·8 versus 61·5 per cent in the screening versus control arms respectively, compared with 70·7 versus 71·5 per cent in women. Among men with left‐sided tumours, survival was better in the screening arm than the control arm (HR 1·37, 1·06 to 1·77), but not in men with right‐sided tumours (HR 1·19, 0·75 to 1·89) (Fig. 4 a,b). Five‐year OS rates in men with left‐sided tumours were 70·0 per cent in the screening arm versus 62·1 per cent in the control arm; respective rates in men with right‐sided tumours were 65 versus 59 per cent. In women, survival was similar in the screening and control arms regardless of whether the tumour was located on the right side (5‐year OS rate 66 versus 67 per cent respectively; HR 1·19, 0·73 to 1·92) or left side (72·8 versus 74·9 per cent; HR 0·96, 0·65 to 1·41) (Fig. 4 c,d). Interestingly, the 5‐year OS rate in women both in the screening and control arms was similar to that of men in the screening arm (70·7, 71·5 and 68·8 per cent respectively).
Figure 4.
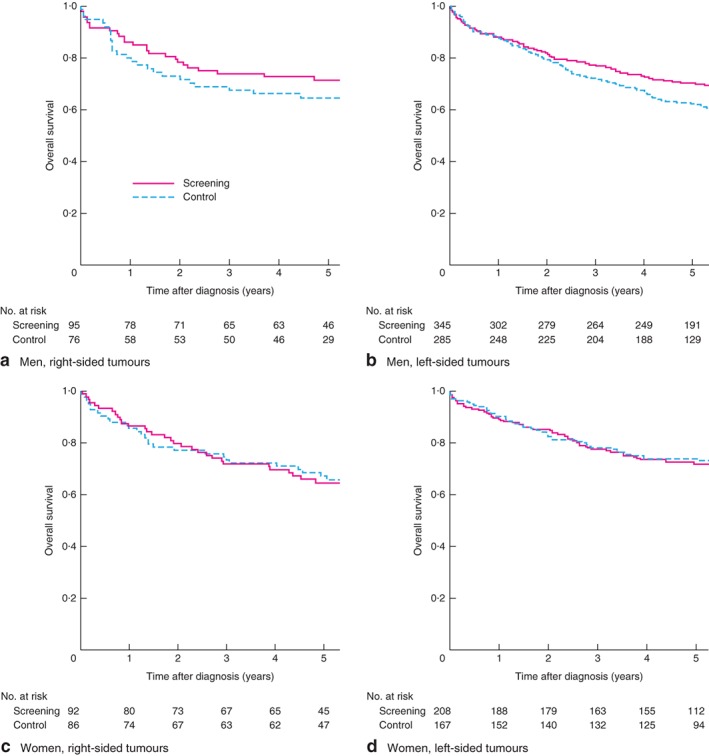
Overall survival after diagnosis of colorectal cancer in the screening and control groups: a men with right‐sided tumours, b men with left‐sided tumours, c women with right‐sided tumours and d women with left‐sided tumours analysis (follow‐up data missing for 1 patient)
Discussion
These data on biennial FOBT screening are based on the largest population‐based randomized health services study, covering over 40 per cent of 60–69‐year‐old people living in Finland. The analysis revealed several important findings. There were substantial sex differences in laterality, histology and symptoms. Improvements in non‐vital outcomes were observed in the screening group, but primarily in men with left‐sided CRC. Additional analyses showed that men with left‐sided tumours gained benefits from the screening in terms of lower TNM stage and better survival.
There are several possible explanations for the observed discrepancy according to sex. Colorectal tumours in women were more often located on the right side of the colon, as has been reported previously18, 19, 20. Longer passage of faecal blood may lower the sensitivity of the test and produce a false‐negative result; this is supported by the fact that, although screening uptake was higher in women (76·3 versus 61·9 per cent), positive results were less frequent than in men (2·7 versus 4·7 per cent). The finding that FOBT screening seemed ineffective in women could be related to the fact that women had more right‐sided tumours and that screening with FOBT can be ineffective in detecting early right‐sided colonic cancers in either sex. In addition, more women in the screening arm were symptomatic (abdominal pain), indicating that they might be more sensitive to the symptoms of CRC. Men who may not experience symptoms or who ignore them might benefit more from screening. It is interesting to note that even the higher participation rates in women did not translate into beneficial outcomes, further supporting the notion that FOBT screening is ineffective in women. It was also found that screening benefits men with left‐sided CRC across all TNM categories, but among women with left‐sided tumours only the T and M categories were lower in the screening group versus controls. This could indicate a difference in the biology of tumour dissemination between men and women. The improvements in survival and non‐vital outcomes in men with left‐sided tumours are most likely due to lower TNM stage in this subgroup. This is supported by the finding that the observed differences disappeared when outcomes were adjusted for T category. Fewer CRCs were detected in women in both study arms, which may be attributed to the fact that the incidence increases with age21 and this increase has been reported to occur later in women22. Although the set age range for screening (60–69 years) appears to be sufficient for substantial improvements for men, women might benefit from screening at an older age.
The strength of this study is its findings regarding parameters that were not investigated in earlier FOBT screening trials4, 5, 6, 7. In addition to survival benefits, it is important to know the influence of screening on other important measures, such as stomas, which are associated with reduced quality of life23 and commonly cause complications (21–70 per cent)24. These were reduced in the screening arm in men, although the reduction was not statistically significant according to the Benjamini–Hochberg criterion. From the point of view of healthcare expenditure, it is also crucial to determine the proportion of emergency surgery and the need for postoperative chemotherapy, as both increase costs and potentially influence patients' quality of life. Non‐radical and emergency surgery was rarer in the screening arm than in the control arm among men. Another retrospective study25 comparing two cohorts from different eras found a similar reduction in emergency surgery in patients undergoing CRC screening. In addition to increased costs, emergency resections have a negative impact on survival compared with elective resections26, 27.
The largest randomized FOBT trials were half the size of the present randomized health services study on CRC screening (Nottingham trial, 152 850 individuals; Göteborg trial, 68 308; Funen trial, 61 933; Minnesota trial, 46 551)4, 5, 6, 7. A meta‐analysis8 that included the latest data from these trials estimated the reduction in CRC‐specific mortality to be 18 per cent, but no reduction in overall mortality was found in an intention‐to‐treat analysis. The Nottingham trial15 reported CRC mortality ratios for men and women separately and, in contrast to the present authors' previous finding12, there was no difference between men and women (RR for screening versus control arm 0·90 in women and 0·91 in men). However, the Minnesota trial13 reported that the reduction in CRC mortality was larger for men than for women.
Tumour location appeared to be the most likely explanation for the sex disparity observed in the present study. Only men with left‐sided tumours had improved survival and lower TNM categories in the screening arm compared with the control arm. The Nottingham trial15 reported similar RRs for screened versus control populations for CRC mortality in the proximal (to the sigmoid colon) and distal colorectum (RR 0·93 and 0·89 respectively), but both sexes were analysed together. The Funen trial28 revealed that FOBT screening tended to reduce mortality rates among patients with proximal compared with distal tumours, but the difference was not significant (P = 0·13). Although most CRCs are left‐sided, the present results suggest that FOBT is ineffective for screening right‐sided tumours.
In addition to the guaiac FOBT used here and in other trials, the faecal immunochemical test (FIT) has also been used for CRC screening. FIT has a high specificity, but randomized screening trials of its effectiveness are lacking29. Therefore, it is not yet possible to compare fully the effectiveness of FOBT and FIT as a screening method. Flexible sigmoidoscopy has been shown to reduce CRC mortality by 21 per cent, on average, in randomized screening trials9. Colonoscopy is recommended if polyps are discovered during sigmoidoscopy in such trials. Thus, patients with positive sigmoidoscopy findings will require a second bowel preparation and two appointments, compared with one for patients with a positive FOBT. In theory, the screening method that should improve the outcomes of patients with right‐sided CRC is colonoscopy; however, this procedure is invasive, more expensive, requires more healthcare resources and might attract fewer participants than FOBT. CT colonography might be more favourable in this regard, but has other specific disadvantages (such as radiation exposure), and its efficacy as a screening method has not yet been investigated30. Although randomized trials of colonoscopy for screening are ongoing, no results have been published to date. As the results of the Finnish randomized programme using FOBT as the primary test did not find a difference between arms in CRC‐specific or overall mortality12, CRC screening was stopped in Finland in 2014. Currently, implementation of more individualized screening programmes is under consideration.
This study has several limitations. Some of the screen‐detected cancers may have been overdiagnosed, although it is likely that the proportion of overdiagnoses is low. In the Minnesota trial31, only 6–9 per cent of screen‐detected cases were estimated to be overdiagnoses. In the Nottingham trial32, little evidence was found in support of an overdiagnosis bias. The present data suggest that roughly 30 per cent of cancers were screen‐detected in the screening arm (difference between proportions of asymptomatic cases in the study arms, 33·6 – 3·3 = 30·3 per cent). Even if 10 per cent of screen‐detected cases were overdiagnosed, this would still represent only 3 per cent of all cases in the screening arm. Therefore, any bias from overdiagnosis is likely to be small. Furthermore, estimates for sex differences were adjusted for study arm, which further reduces such bias.
Survival analyses are also affected by lead time bias, the magnitude of which remains unclear. However, as survival did not improve significantly in women, even with bias owing to overdiagnosis and lead time, it is even clearer that FOBT screening is not beneficial in women. Furthermore, the present study was a retrospective cross‐sectional analysis of patients diagnosed with CRC within the population based on a randomized health services study, where the diagnostic and treatment strategies in different hospitals were not standardized. On the other hand, the study design was pragmatic, and is thus more likely to represent real‐life clinical care scenarios.
Even though over 320 000 people were randomized and more than 1300 patients with CRC were analysed, subgroup analyses might have suffered from lack of statistical power. Many of the effects in women were in the same direction as those in men, but remained statistically non‐significant. Should there be any significant effect if larger groups were analysed, the clinical relevance would still be minor. This is also reflected in the survival curves, which are identical in the screening and control arms in women. Survival improvements occurring contemporaneously with adoption of the Finnish screening programme in the municipality were observed in patients with CRC not invited for screening33. Such an improvement in the control population would be likely to also translate to improvements in controls in non‐vital outcomes and cancer stage. Therefore, the control versus screening comparisons reported here probably understate the effect of the screening programme on such outcomes.
This investigation of patients with CRC, based on a large population‐based randomized health services study, found biennial guaiac FOBT screening to be associated with lower proportions of non‐radical surgery, emergency surgery and postoperative chemotherapy, as well as improved TNM categories and OS, in men. Improvements were observed mainly in patients with left‐sided tumours. FOBT screening did not appear to be associated with lower TNM categories or survival among 60–69‐year‐old women with CRC. Therefore, screening by different methods or at different intervals or ages should be considered among women. The sex discrepancy in outcomes may be due to factors related to the incidence, symptoms and location of colorectal tumours.
Acknowledgements
This study was supported financially by Vatsatautien Tutkimussäätiö, Helsinki University Hospital research funds, Mary and Georg Ehrnrooth's Foundation, and Cancer Foundation Finland.
Disclosure: The authors declare no conflict of interest.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Alexiusdottir KK, Möller PH, Snaebjornsson P, Jonasson L, Olafsdottir EJ, Björnsson ES et al Association of symptoms of colon cancer patients with tumor location and TNM tumor stage. Scand J Gastroenterol 2012; 47: 795–801. [DOI] [PubMed] [Google Scholar]
- 3. Inadomi JM. Screening for colorectal neoplasia. N Engl J Med 2017; 376: 149–156. [DOI] [PubMed] [Google Scholar]
- 4. Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW et al Randomised controlled trial of faecal‐occult‐blood screening for colorectal cancer. Lancet 1996; 348: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 5. Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal‐occult‐blood test. Lancet 1996; 348: 1467–1471. [DOI] [PubMed] [Google Scholar]
- 6. Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg 2008; 95: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 7. Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM et al Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 8. Fitzpatrick‐Lewis D, Ali MU, Warren R, Kenny M, Sherifali D, Raina P. Screening for colorectal cancer: a systematic review and meta‐analysis. Clin Colorectal Cancer 2016; 15: 298–313. [DOI] [PubMed] [Google Scholar]
- 9. Holme Ø, Schoen RE, Senore C, Segnan N, Hoff G, Løberg M et al Effectiveness of flexible sigmoidoscopy screening in men and women and different age groups: pooled analysis of randomised trials. BMJ 2017; 356: i6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holme Ø, Bretthauer M, Fretheim A, Odgaard‐Jensen J, Hoff G. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database Syst Rev 2013: (9)CD009259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malila N, Anttila A, Hakama M. Colorectal cancer screening in Finland: details of the national screening programme implemented in Autumn 2004. J Med Screen 2005; 12: 28–32. [DOI] [PubMed] [Google Scholar]
- 12. Pitkäniemi J, Seppä K, Hakama M, Malminiemi O, Palva T, Vuoristo MS et al Effectiveness of screening for colorectal cancer with a faecal occult‐blood test, in Finland. BMJ Open Gastroenterol 2015; 2: e000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS et al Long‐term mortality after screening for colorectal cancer. N Engl J Med 2013; 369: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 14. McClements PL, Madurasinghe V, Thomson CS, Fraser CG, Carey FA, Steele RJ et al Impact of the UK colorectal cancer screening pilot studies on incidence, stage distribution and mortality trends. Cancer Epidemiol 2012; 36: e232–e242. [DOI] [PubMed] [Google Scholar]
- 15. Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20‐year follow‐up. Gut 2012; 61: 1036–1040. [DOI] [PubMed] [Google Scholar]
- 16. Leinonen MK, Miettinen J, Heikkinen S, Pitkäniemi J, Malila N. Quality measures of the population‐based Finnish Cancer Registry indicate sound data quality for solid malignant tumours. Eur J Cancer 2017; 77: 31–39. [DOI] [PubMed] [Google Scholar]
- 17. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. (eds). AJCC Cancer Staging Manual (7th edn). Springer: New York, 2010. [Google Scholar]
- 18. McCashland TM, Brand R, Lyden E, de Garmo P; CORI Research Project . Gender differences in colorectal polyps and tumors. Am J Gastroenterol 2001; 96: 882–886. [DOI] [PubMed] [Google Scholar]
- 19. Forsberg AM, Kjellström L, Agréus L, Nixon Andreasson A, Nyhlin H, Talley NJ et al Prevalence of colonic neoplasia and advanced lesions in the normal population: a prospective population‐based colonoscopy study. Scand J Gastroenterol 2012; 47: 184–190. [DOI] [PubMed] [Google Scholar]
- 20. Gaitonde SG, Nissan A, Protić M, Stojadinovic A, Wainberg ZA, Chen DC et al Sex‐specific differences in colon cancer when quality measures are adhered to: results from international, prospective, multicenter clinical trials. J Am Coll Surg 2017; 225: 85–92. [DOI] [PubMed] [Google Scholar]
- 21. Brændegaard Winther S, Baatrup G, Pfeiffer P, Qvortrup C. Academy of Geriatric Cancer Research (AgeCare) . Trends in colorectal cancer in the elderly in Denmark, 1980–2012. Acta Oncol 2016; 55(Suppl 1): 29–39. [DOI] [PubMed] [Google Scholar]
- 22. Brenner H, Hoffmeister M, Arndt V, Haug U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer 2007; 96: 828–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vonk‐Klaassen SM, de Vocht HM, den Ouden ME, Eddes EH, Schuurmans MJ. Ostomy‐related problems and their impact on quality of life of colorectal cancer ostomates: a systematic review. Qual Life Res 2016; 25: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shabbir J, Britton DC. Stoma complications: a literature overview. Colorectal Dis 2010; 12: 958–964. [DOI] [PubMed] [Google Scholar]
- 25. Askari A, Nachiappan S, Currie A, Bottle A, Abercrombie J, Athanasiou T et al Who requires emergency surgery for colorectal cancer and can national screening programmes reduce this need? Int J Surg 2017; 42: 60–68. [DOI] [PubMed] [Google Scholar]
- 26. Bass G, Fleming C, Conneely J, Martin Z, Mealy K. Emergency first presentation of colorectal cancer predicts significantly poorer outcomes: a review of 356 consecutive Irish patients. Dis Colon Rectum 2009; 52: 678–684. [DOI] [PubMed] [Google Scholar]
- 27. McArdle CS, Hole DJ. Emergency presentation of colorectal cancer is associated with poor 5‐year survival. Br J Surg 2004; 91: 605–609. [DOI] [PubMed] [Google Scholar]
- 28. Kronborg O, Jørgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol 2004; 39: 846–851. [DOI] [PubMed] [Google Scholar]
- 29. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta‐analysis. Ann Intern Med 2014; 160: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lieberman D, Ladabaum U, Cruz‐Correa M, Ginsburg C, Inadomi JM, Kim LS et al Screening for colorectal cancer and evolving issues for physicians and patients: a review. JAMA 2016; 316: 2135–2145. [DOI] [PubMed] [Google Scholar]
- 31. Luo D, Cambon AC, Wu D. Evaluating the long‐term effect of FOBT in colorectal cancer screening. Cancer Epidemiol 2012; 36: e54–e60. [DOI] [PubMed] [Google Scholar]
- 32. Robinson MH, Hardcastle JD, Moss SM, Amar SS, Chamberlain JO, Armitage NC et al The risks of screening: data from the Nottingham randomised controlled trial of faecal occult blood screening for colorectal cancer. Gut 1999; 45: 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miettinen J, Malila N, Hakama M, Pitkäniemi J. Spillover improved survival in non‐invited patients of the colorectal cancer screening programme. J Med Screen 2018; 25: 134–140. [DOI] [PubMed] [Google Scholar]


