Abstract
Aims
To assess health‐related quality of life (HRQoL) in people with type 2 diabetes (T2D) participating in the LEADER cardiovascular outcomes trial using the five‐dimension European Quality of Life questionnaire (EQ‐5D).
Materials and methods
The EQ‐5D was administered every 12 months in a subset of patients from Canada, Denmark, Germany, Ireland, Italy, Netherlands, Spain, Sweden, the United Kingdom and the United States. We compared changes in utility index scores and visual analogue scale (VAS) scores from baseline to 36 months in participants treated with liraglutide and placebo. We also assessed which complications had the greatest impact on quality of life.
Results
At 36 months, less deterioration in EQ‐5D utility index score was seen in the liraglutide group (−0.058) than in the placebo group (−0.082; estimated treatment difference [ETD] 0.023, 95% confidence interval [CI] 0.004;0.043; P = 0.020). A smaller decrease in EQ‐5D VAS score was also demonstrated in the liraglutide group (−3.51) vs. the placebo group (−5.45; ETD 1.94, 95% CI 0.32;3.57; P = 0.019). The benefits of liraglutide treatment compared with placebo were driven primarily by shifts in the domains of mobility and self‐care. The most influential events contributing to poorer HRQoL were stroke, heart failure, malignant neoplasm and confirmed hypoglycaemia.
Conclusions
Liraglutide demonstrated a modest but significant benefit in patient‐reported health status using the EQ‐5D, compared with placebo. This benefit may be of clinical relevance and requires further study.
Keywords: EQ‐5D, health‐related quality of life, LEADER, liraglutide, patient‐reported outcomes, type 2 diabetes
1. INTRODUCTION
Health‐related quality of life (HRQoL) is an important component in the evaluation of disease burden, such as that in diabetes, and can be measured as a patient‐reported outcome. According to the American Diabetes Association guidelines, improved quality of life is a key goal of diabetes management and should be monitored as part of routine care.1 The full extent of a treatment's overall efficacy may be better gauged by considering patient‐reported outcomes such as HRQoL.2
Data relating to HRQoL are increasingly being collected during clinical trials in patients with type 2 diabetes (T2D).2, 3, 4 However, of the recent large outcome trials in diabetes, only SAVOR‐TIMI 53 has so far published HRQoL results,5 and reported no significant difference between saxagliptin and placebo in mean levels of HRQoL using the five‐dimension European Quality of Life questionnaire (EQ‐5D), which was administered to all patients at baseline, 12 and 24 months and at study completion, and at semi‐annual visits to patients who had experienced a non‐fatal myocardial infarction (MI) or ischaemic stroke since their previous visit.
The Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial was a randomized, double‐blind, placebo‐controlled trial for up to 5 years in patients with T2D at high risk of cardiovascular (CV) disease. The study showed a reduced risk for CV outcomes (hazard ratio [HR] 0.87, 95% confidence interval [CI] 0.78‐0.97), all‐cause mortality (HR 0.85, 95% CI 0.74‐0.97), hypoglycaemia and microvascular outcomes in patients treated with liraglutide vs. those treated with placebo.6, 7 To gain some insight into the HRQoL of the LEADER trial participants, assessments of the three‐level version of the EQ‐5D (EQ‐5D‐3 L) were collected from a subset of patients from 10 countries annually. The EQ‐5D utility index score and visual analogue scale (VAS) scores are reported here as a pre‐specified secondary analysis, with post hoc analyses of further HRQoL outcomes.
2. MATERIALS AND METHODS
2.1. Study design and oversight
The trial protocol of LEADER has been described elsewhere.8 LEADER was a double‐blind, placebo‐controlled trial with a follow‐up of 3.5 to 5 years. Patients with T2D and high risk of CV disease were randomly assigned (1:1 ratio) to subcutaneous liraglutide (n = 4668) or matching subcutaneous placebo (n = 4672), both in addition to standard of care. The trial was approved by institutional review boards, and all patients provided written informed consent. The disposition of trial participants has been published.6 Assessing patient‐reported outcomes measured by EQ‐5D‐3 L (hereafter referred to as EQ‐5D) was a pre‐specified secondary outcome of the LEADER trial, and EQ‐5D scores were assessed in all patients from Canada, Denmark, Germany, Ireland, Italy, Netherlands, Spain, Sweden, the United Kingdom and the United States because validated EQ‐5D questionnaires were available in the languages of these countries. The 3014 patients participating in the EQ‐5D assessments represent 32.3% of the total LEADER population. These patients completed a paper version of the EQ‐5D questionnaire while attending clinic visits at randomization and every 12 months until trial end. These data were transcribed into the electronic case report form by the site staff.
2.2. EQ‐5D‐3L
The EQ‐5D is a preferred instrument for measuring health utilities by clinical guidance bodies, is based on patient preferences and produces utility scores that can be used for cost‐effectiveness studies across diseases.9 It is one of the most widely used generic preference‐based measures of HRQoL10 and consists of two parts: a descriptive system and a VAS.
The descriptive system covers five dimensions of self‐reported health: mobility; self‐care; usual activities; pain/discomfort; and anxiety/depression. Each dimension of the EQ‐5D‐3L has three response category levels (no problems, some problems and extreme problems), allowing a total of 243 possible health states. Each of these health states has an accompanying utility index score or preference‐based weight, sets of which are available for different countries. In the present study, the UK value set was used, derived from a valuation exercise conducted in a large population sample.11 The resulting valuations or utilities range from −0.543 to 1, where negative values are considered worse than death, a value of 0 represents death, and a value of 1 indicates perfect health.11
The EQ‐5D also has a VAS, which consists of a 100‐point scale on which patients indicate their health score; the best state carries a score of 100 and the worst state a score of 0.12
It is also possible to analyse EQ‐5D responses in terms of answers to each question; for example, proportions selecting each level of each question, or movements when the EQ‐5D is used sequentially. In this case, nine shifts are possible, listed in order of severity, defined as: extreme problems to no problems; some problems to no problems; no problems and no change; extreme problems to some problems; some problems and no change; no problems to some problems; extreme problems and no change; some problems to extreme problems; and no problems to extreme problems.
2.3. Statistical analyses
Changes from baseline to 36 months in quality‐of‐life (EQ‐5D) utility index score and VAS were analysed using a linear mixed model for repeated measurements. In case of death, both the utility index score and VAS value were set to 0 at the first planned visit after death. Pre‐specified comparisons to estimate the treatment differences (liraglutide vs. placebo) were performed at 36 months; with a trial duration of 3.5‐5 years, this was the final visit at which EQ‐5D assessments were available for the majority of the patients included. To assess the influence of imputing values for death (0 for both utility index score and VAS), the analyses were also repeated with the imputed values for death removed.
The distribution of all possible ranked categorical shifts in individual domain states between baseline and 36 months was compared between treatments using a Kruskal‐Wallis test. The categorical shifts are presented as “worsening”, “no change” and “improvement”. Categorical shifts in overall utility index score between baseline and 36 months were also assessed. A score of 1 equated to perfect health and a score of <1 equated to problems.
Changes in EQ‐5D utility index scores associated with complications during follow‐up, that is, stroke, malignant neoplasm, severe hypoglycaemia (requiring assistance from another person to treat), confirmed hypoglycaemia (severe hypoglycaemia and/or plasma glucose <3.1 mmol/L [56 mg/dL]), MI, heart failure (HF), foot ulcer, retinopathy and nephropathy, were explored using a linear mixed model. Insulin initiation, weight reduction of 5% and a glycated haemoglobin (HbA1c) <58 mmol/mol were also included. These factors are time‐varying and were assigned a status of “no” at all time points for EQ‐5D measurements before the given event, and a status of “yes” at time points after a given event. Other factors included treatment, sex, region, CV risk at baseline and interaction between visit and age group.
Because of the exploratory nature of these analyses, no adjustments for multiple statistical comparisons were made to the P values, as defined in the prespecified statistical analysis plan for the trial.
3. RESULTS
3.1. Baseline characteristics and primary outcome of included patients
In LEADER, 9340 patients were randomized (4668 to liraglutide and 4672 to placebo), with a median follow‐up of 3.8 years. At baseline, 3014 patients were included in this sub‐study, and 2460 patients completed the EQ‐5D at 36 months. Baseline characteristics of the 3014 patients included with EQ‐5D measurements were well balanced between the randomized treatment groups (Table 1). Patients were on average older than the full trial cohort (65.6 vs. 64.3 years, respectively), had a higher body mass index (BMI; 33.2 vs. 32.5 kg/m2, respectively) and a similar mean diabetes duration (12.6 vs. 12.8 years, respectively). The proportion of insulin‐naive patients at baseline was 42.0%, vs. 55.4% in the total trial cohort. Baseline EQ‐5D index scores were similar between treatment groups (0.79 and 0.78 for liraglutide and placebo groups, respectively), as were baseline VAS scores (74.7 vs. 74.3 for liraglutide and placebo groups, respectively).
Table 1.
Baseline characteristics of patients with five‐dimension European quality of life questionnaire measurements
| Liraglutide N = 1506 | Placebo N = 1508 | |
|---|---|---|
| Age, years | 65.3 (7.3) | 65.9 (7.0) |
| BMIa, kg/m2 | 33.4 (6.3) | 33.1 (5.9) |
| Weight, kg | 97.2 (20.5) | 95.7 (19.1) |
| HbA1c, mmol/mol | 68 (15) | 68 (14) |
| Duration of diabetesa, years | 12.5 (7.7) | 12.7 (8.2) |
| Insulin‐naive, n (%) | 620 (41.2) | 645 (42.8) |
| Years of observationb, years | 3.9 (0.6) | 3.9 (0.7) |
| EQ‐5D index score | 0.79 (0.24) | 0.78 (0.24) |
| EQ‐5D VAS score | 74.7 (17.2) | 74.3 (17.9) |
Abbreviations: BMI, body mass index; EQ‐5D, five‐dimension European Quality of Life questionnaire; HbA1c, glycated haemoglobin; N, number of patients with EQ‐5D measurements; VAS, visual analogue scale.
Full analysis set. Data are mean (standard deviation, SD) unless otherwise stated.
BMI data only available for 1505 and 1506 patients in the liraglutide and placebo treatment groups, respectively; duration of diabetes data only available for 1505 and 1503 patients, respectively.
Including follow‐up years.
Of the 3014 patients included from the 10 countries in this sub‐study, 187 treated with liraglutide (12.4%) and 221 treated with placebo (14.7%) experienced a major adverse CV event. This proportion of patients with a primary endpoint in the liraglutide and placebo groups is consistent with that reported in the overall trial population (13.0% and 14.9% with liraglutide and placebo, respectively).6
3.2. Overall change in EQ‐5D utility index and VAS scores
EQ‐5D scores declined in both treatment groups during the trial. By 36 months, less deterioration in EQ‐5D utility index score was seen in the liraglutide group (−0.058) than in the placebo group (−0.082; estimated treatment difference [ETD] 0.023, 95% CI 0.004‐0.043; P = 0.020 [Figure 1A]). A smaller decrease in EQ‐5D VAS score was also demonstrated in the liraglutide (−3.51) vs. placebo group (−5.45; ETD 1.94, 95% CI 0.32‐3.57; P = 0.019 [Figure 1B]).
Figure 1.
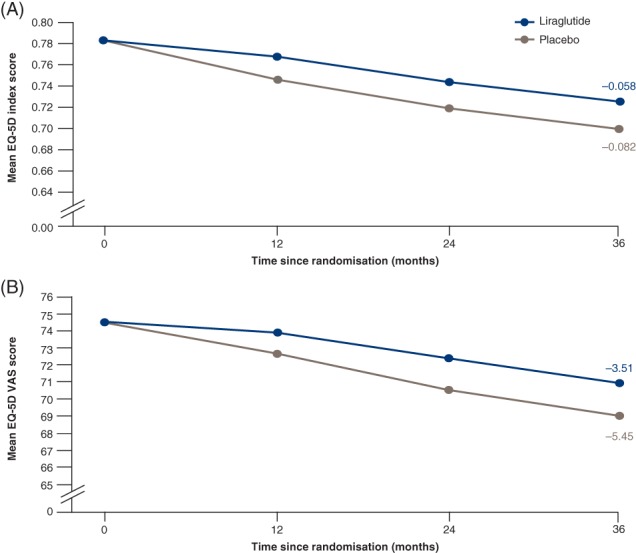
A, Change in mean five‐dimension European Quality of Life questionnaire (EQ‐5D) index score. B, Change in mean EQ‐5D visual analogue scale (VAS) score. Estimated data. Patients with an observed value contributed to the analysis. Patients who died were given a value of zero at the first planned visit after death. Change from baseline to 36‐month assessment was analysed using a linear mixed model, accounting for repeated measures within patients. Interaction between visit and treatment, sex, region and antidiabetic therapy, respectively, at baseline are included as factors, and interaction between visit and baseline EQ‐5D index/VAS score and age at baseline, respectively, are included as covariates. EOT, end of trial
Fewer patients with EQ‐5D measurements died in the liraglutide group (n = 99) than in the placebo group (n = 131), and the treatment difference seen in utility score and VAS could be attributable to the difference in the number of patients who died. However, when not including the values of 0 at the first planned visit after death, estimates for the EQ‐5D utility index at 36 months still declined less in the liraglutide (−0.027) compared with the placebo group (−0.046; ETD 0.018, 95% CI 0.001‐0.035; P = 0.034). A smaller decrease in EQ‐5D VAS score was also seen in the liraglutide (−0.146) vs. placebo group (−1.449) with imputed values of death removed (ETD 1.302, 95% CI 0.101;2.504; P = 0.034).
3.3. Categorical shifts in EQ‐5D at 36 months
The benefits of liraglutide treatment compared with placebo, seen in utility score, were driven primarily by shifts in the domains of mobility (P = 0.036) and self‐care (P = 0.041). A greater proportion of patients reported an improvement in mobility in the liraglutide (9.4%) compared with the placebo group (6.6%). Fewer liraglutide‐treated patients reported worsening in self‐care in the trial (4.6%), vs. placebo‐treated (7.4%). To a lesser degree, pain and discomfort also contributed to the shifts in utility score, whereas there were no treatment differences in the other domains (usual activities and anxiety/depression; Table 2). Analyses of categorical changes according to separation of patients into binary categories of perfect health (utility index score = 1; no problems) and any problems (utility index score < 1; problems) did not show any significant treatment differences (Table 3), indicating that the differences were mainly a consequence of quantitative changes in the patients with some level of “problems” at both baseline and 36 months.
Table 2.
Categorical change in five‐dimension European Quality of Life questionnaire individual domain state scores from baseline to 36 months
| EQ‐5D domain | Liraglutide N (%) | Placebo N (%) | P* |
|---|---|---|---|
| Mobility | 0.036 | ||
| Total | 1223 (100.0) | 1170 (100.0) | |
| Worsening | 188 (15.4) | 188 (16.1) | |
| No change | 920 (75.2) | 905 (77.4) | |
| Improvement | 115 (9.4) | 77 (6.6) | |
| Self‐care | 0.041 | ||
| Total | 1221 (100.0) | 1169 (100.0) | |
| Worsening | 56 (4.6) | 87 (7.4) | |
| No change | 1140 (93.4) | 1048 (89.6) | |
| Improvement | 25 (2.0) | 34 (2.9) | |
| Usual activities | 0.987 | ||
| Total | 1223 (100.0) | 1169 (100.0) | |
| Worsening | 197 (16.1) | 168 (14.4) | |
| No change | 916 (74.9) | 896 (76.6) | |
| Improvement | 110 (9.0) | 105 (9.0) | |
| Pain/discomfort | 0.081 | ||
| Total | 1223 (100.0) | 1169 (100.0) | |
| Worsening | 256 (20.9) | 249 (21.3) | |
| No change | 783 (64.0) | 759 (64.9) | |
| Improvement | 184 (15.0) | 161 (13.8) | |
| Anxiety/depression | 0.853 | ||
| Total | 1220 (100.0) | 1169 (100.0) | |
| Worsening | 148 (12.1) | 141 (12.1) | |
| No change | 946 (77.5) | 915 (78.3) | |
| Improvement | 126 (10.3) | 113 (9.7) |
Abbreviations: EQ‐5D, five‐dimension European Quality of Life questionnaire; N, number of patients contributing to the analysis.
Full analysis set. *P value from Kruskal–Wallis test of treatment difference in all possible nine shifts ranked as: extreme problems to no problems; some problems to no problems; no problems and no change; extreme problems to some problems; some problems and no change; no problems to some problems; extreme problems and no change; some problems to extreme problems; no problems to extreme problems, by domain. No adjustment for multiplicity has been made to P values.
Table 3.
Categorical change in five‐dimension European quality of life questionnaire utility index score at 36 months
| Liraglutide | Placebo | P * | |||
|---|---|---|---|---|---|
| N (%) | Mean (SD)a | N (%) | Mean (SD)a | ||
| Total | 1256 (100.0) | −0.05 (0.25) | 1204 (100.0) | −0.07 (0.26) | 0.4156 |
| Worsening | 205 (16.3) | −0.28 (0.19) | 195 (16.2) | −0.29 (0.20) | |
| No change | 919 (73.2) | ‐ | 897 (74.5) | ‐ | |
| No problems → still no problems | 262 (20.9) | 0.00 (0.00) | 242 (20.1) | 0.00 (0.00) | |
| Problems → still problems | 657 (52.3) | −0.05 (0.28) | 655 (54.4) | −0.08 (0.28) | |
| Improvement | 132 (10.5) | 0.23 (0.11) | 112 (9.3) | 0.25 (0.17) | |
Abbreviations: EQ‐5D, five‐dimension European Quality of Life questionnaire; SD, standard deviation. Full analysis set. No adjustment for multiplicity has been made to the P value. A utility index score of 1 is classified as “no problems”, whereas scores <1 are classified as “problems”.
P value from Kruskal–Wallis test of treatment difference in four‐point shift, ranked in order of severity (worsening; problems to still problems; no problems to still no problems; improvement).
Mean (SD) change in EQ‐5D utility index score within the category.
3.4. Change in utility index score and VAS for key factors and events
The most influential events contributing to differences in HRQoL were stroke, HF, malignant neoplasm and confirmed hypoglycaemia (Figure 2).
Figure 2.
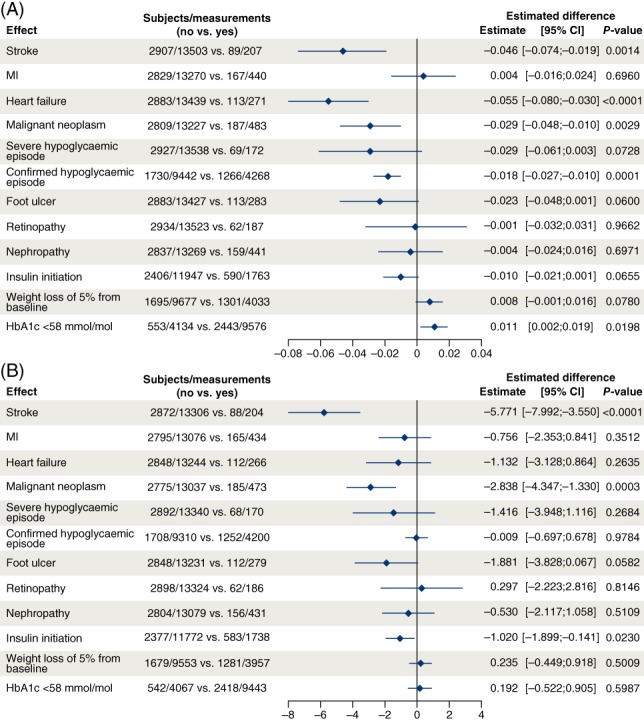
A, Change in five‐dimension European Quality of Life questionnaire (EQ‐5D) utility index score for key factors and events. B, Change in EQ‐5D visual analogue scale (VAS) score for key factors and events. Change from baseline analysed using a linear mixed model accounting for repeated measures within patients using a compound symmetry residual covariance matrix. Factors: treatment, sex, region, cardiovascular (CV) risk at baseline, stroke, cancer, severe hypoglycaemia, confirmed hypoglycaemia, myocardial infarction (MI), heart failure, foot ulcer, retinopathy, nephropathy, weight change of 5%, glycated haemoglobin (HbA1c) <58 mmol/mol, insulin initiation and interaction between visit and age group at baseline. Baseline value of analysed parameter is included as covariate. The factors of the events are given a value of “no” at time points before the given event, and a value of “yes” at time points after a given event. Patients from the following countries are included: Canada, Denmark, Ireland, Italy, Netherlands, Germany, Spain, Sweden, United Kingdom and United States
Reductions in HRQoL were seen in patients with either stroke (P = 0.001) or HF (P < 0.0001), with a greater impact of stroke than HF on HRQoL, as evidenced by the larger impact on the VAS score (P < 0.0001) than HF (P = 0.264; Figure 2).
Malignant neoplasm also had a small impact on HRQoL, seen in both the total index score (P = 0.0029) and VAS (P = 0.0003; Figure 2).
Confirmed hypoglycaemia had a small impact on HRQoL, as illustrated by the total index score (P < 0.0001) but not the VAS (P = 0.978; Figure 2). Severe hypoglycaemia also indicated an association with HRQoL reduction, although not statistically significant, possibly because of the low numbers of patients with severe hypoglycaemic events (change in total index score: −0.029; VAS: −1.416).
Achieving HbA1c <58 mmol/mol had a small positive impact on HRQoL, and initiation of insulin had a small negative impact; the former demonstrated in the total index score (P = 0.020) and not the VAS (P = 0.599), and vice versa for initiation of insulin (VAS: P = 0.023; total index score: P = 0.066 [Figure 2]).
Foot ulcers might impact HRQoL but this complication had no statistically significant influence on the total utility index score or VAS, potentially because of the low event numbers (total index score: −0.023; VAS: −1.881). Weight loss of 5% also demonstrated no impact on HRQoL (total index score: 0.008; VAS: 0.235); other events that appeared not to affect either the utility index score or VAS were non‐fatal MI, retinopathy and nephropathy (Figure 2).
Besides changes in EQ‐5D driven by the described events, the decreases in EQ‐5D utility index score were driven by time in the trial, with a mean reduction during the 36 months of −0.03, and older age (for example, at 36 months patients aged ≥75 years had a reduction of −0.043 compared with −0.013 in patients aged <65 years; data not shown).
After taking all the key factors and events into account (treatment, sex, region, CV risk at baseline, stroke, cancer, severe hypoglycaemia, confirmed hypoglycaemia, MI, HF, foot ulcer, retinopathy, nephropathy, weight change of 5%, HbA1c < 58 mmol/mol, insulin initiation and interaction between visit and age group at baseline), a small treatment difference still appears to exist in the EQ‐5D index score (ETD 0.010, 95% CI 0.001‐0.019; P = 0.0329), but not the VAS (ETD 0.587, 95% CI −0.234‐1.407; P = 0.1613), although it is possible that this difference would disappear if every effect on treatment difference could be considered.
4. DISCUSSION
LEADER is the first trial that we are aware of in patients with T2D at high risk of CV disease to report a modest but significant benefit in patient‐reported health status using the EQ‐5D with an antihyperglycaemic agent (liraglutide) compared with placebo (both in addition to standard of care). The data suggest that patients treated with liraglutide experienced less deterioration of their HRQoL, gauged by both utility index score and VAS score, over the trial duration. Because the difference in favour of liraglutide was observed both with imputation of values for death and without, the difference in HRQoL was not solely explained by fewer deaths among liraglutide‐treated patients. Specifically, patients treated with liraglutide experienced a deceleration in quality‐of‐life deterioration via the EQ‐5D domains of self‐care and mobility, indicating that those taking liraglutide were better able to retain abilities relating to washing, dressing and walking, compared with those treated with placebo. Regarding categorical shifts (from no problems at baseline to problems at 36 months, or problems at baseline to no problems at 36 months), there was no significant difference between treatment groups, as the majority of patients did not change categories during the trial. The binary categorization of “problems” vs. “no problems” does not pick up the small changes in HRQoL, which mainly seem to represent quantitative changes in the patients with “problems”.
The ETD of 0.023 at 36 months (P = 0.020) observed in LEADER should be considered important in comparison to published effects of pharmacotherapy; for example, a study comparing an interventional treatment strategy with a conservative treatment strategy in patients with non‐ST‐segment elevation acute coronary syndrome found a significant benefit from the interventional treatment compared with the conservative treatment, with a mean treatment difference of 0.036 (P = 0.005) at 4 months, although this was no longer significant after 1 year (ETD 0.016; P = 0.20).13 In a cross‐sectional survey of a random sample of the English population including 26 104 subjects, presence of obesity (BMI ≥30 kg/m2) has been shown to lower mean EQ‐5D index score by −0.045 compared with absence of obesity (P < 0.001), even when adjusted for confounding factors. More interestingly, the presence of diabetes was shown to lower HRQoL measured by EQ‐5D index score by −0.096, irrespective of sex, age group, ethnicity, educational achievement or socio‐economic position (P < 0.001)14; therefore, the potential for liraglutide to slow this worsening of HRQoL is relevant to this population.
The underlying causative factors for the difference in EQ‐5D score deterioration between treatment groups cannot be determined with certainty, but could be attributable to the reduction in CV events associated with liraglutide, other consequences of liraglutide treatment not related to such events, or other events or factors displayed in Figure 2. CV events have been found to play a key role in changes in utility scores and HRQoL in ADVANCE,15 SAVOR‐TIMI5 and the UK Prospective Diabetes Study (UKPDS).16 In these studies, utility scores for stroke (−0.199 to −0.099), MI (−0.051 to −0.026) and HF (−0.134 to −0.045) were similar to those reported in the present study (stroke −0.046, MI 0.004 and HF −0.055),5, 15, 16 although MI did not appear to have an effect on the utility score in the present study, possibly because the majority of MIs observed in LEADER were non‐ST‐segment elevation events.17 We also found that hypoglycaemia and initiation of insulin were associated with reductions in HRQoL, and may therefore play a role in the quality‐of‐life deterioration observed over the course of the trial as hypoglycaemia and new insulin prescription occurred more frequently in placebo‐treated patients.6 The point estimate for effects of severe hypoglycaemia also indicated an association with reduction in HRQoL, although this was not statistically significant, possibly because of the low numbers of patients with severe hypoglycaemic events.
The greatest reductions in HRQoL in the UKPDS were observed in patients who experienced amputation, with an EQ‐5D value of −0.280.16 In the present study, wounds and amputations were categorized as “foot ulcers” with no detailed differentiation, and relatively few patients experienced these (n = 113/3014). This may explain why there is only a relatively small decrease in the EQ‐5D utility index score of −0.023 (P = 0.060) after patients have a foot ulcer (including wounds and amputations) in LEADER, and a similarly small decrease was observed in the VAS (−1.881, P = 0.058; Figure 2).
The decreases in EQ‐5D utility index scores of −0.058 with liraglutide and − 0.082 with placebo at 36 months in LEADER are consistent with other datasets; for comparison, the ADVANCE trial in patients with T2D showed a reduction in EQ‐5D utility score of −0.030 independent of complications over 5 years.15 It should be noted that there were differences between patient populations in the ADVANCE and LEADER trials,6, 15 and so quantitative comparisons between the reductions in HRQoL are difficult to assess. However, it is clear that, as T2D is a progressive disease, some reduction in HRQoL can be expected over long time periods as a result of the complications associated with aging and diabetes.18
Currently, no other trials that we are aware of in patients with T2D at high risk of CV disease have reported significantly less deterioration in HRQoL with a pharmacological intervention compared with placebo; therefore, the demonstration of the ability of liraglutide to slow this decline is noteworthy. The SAVOR‐TIMI‐53 trial of saxagliptin, the only other CV outcomes trial (CVOT) in diabetes to report HRQoL outcomes, reported no CV benefit compared with placebo, and no significant differences in HRQoL between treatment groups.5 The EMPA‐REG, CANVAS, EXSCEL and SUSTAIN‐6 studies, which are the other published CVOTs of diabetes therapies to report a CV benefit with active treatment vs. placebo, have not yet reported results from HRQoL measures such as the EQ‐5D.19, 20, 21 A recent review article highlighted that EQ‐5D utility index score showed no significant change from baseline in trials with numerous antihyperglycaemic therapies, including exenatide (twice daily and once weekly), dulaglutide, liraglutide, dapagliflozin and insulin glargine.22
The only other study of liraglutide in which EQ‐5D data were collected looked at patient preferences for liraglutide compared with exenatide (twice daily). In this study, while patients indicated a preference for liraglutide based on superior efficacy and lower degree of nausea and hypoglycaemia, there was no assessment for treatment effect.23 Results from the current analysis show that liraglutide is associated with a slower decline in HRQoL, presently a novel finding.
The strengths of this secondary analysis include the double‐blinded nature of the LEADER trial, inclusion of a large subset of the total cohort and comprehensive collection of EQ‐5D data at multiple time points. The results show a high degree of consistency between EQ‐5D utility index scores and VAS assessments.
There are, however, some factors that may limit the interpretation of this analysis. First, EQ‐5D measurements were taken 12 months apart, so transient HRQoL changes (with a duration of <1 year) that were influenced by an event may not be captured. This analysis was not designed to investigate changes in EQ‐5D following an event, and to do so would require patients answering the EQ‐5D questionnaire before and after an event, instead of at specified time points. The analysis only included Western countries, as the EQ‐5D is only available in the language of those countries; this may have caused a patient selection bias. It is not clear whether this bias may have had an impact on the results; however, patients in the countries in this sub‐study experienced a similar reduced risk of CV outcomes compared with those in the overall trial, probably diminishing a selection bias.
Although the EQ‐5D can be sensitive to the onset of diabetes complications, such as stroke, ischaemic heart disease and neuropathy,24, 25 the scale has been criticised for being insensitive to small changes in health status in T2D.26 The EQ‐5D is considered by some to be less responsive or sensitive than disease‐specific outcome measures. However, since there is no “gold standard” for patient‐reported outcomes, questions remain as to which measure is definitively the most appropriate for this population,22 and psychometric evidence alongside these patient‐reported outcomes may be required to validate and interpret the impact of the measures. Finally, the LEADER trial did not pre‐specify any expected benefit in EQ‐5D score compared with placebo as part of a hypothesis, and the findings might be considered relevant only to those with T2D and high CV risk.
In conclusion, the results of this exploratory analysis show that the established positive safety and efficacy results observed in the LEADER trial may have a positive impact on quality of life (reported here over a period of 36 months), and this requires further confirmatory research.
CONFLICT OF INTEREST
M.A.N. reports fees for serving on advisory boards from Berlin‐Chemie, Boehringer Ingelheim, Eli Lilly, Fractyl, GlaxoSmithKline, Hanmi, Merck Sharp & Dohme, Novo Nordisk, Sanofi‐Aventis and Intarcia Therapeutics/Servier, lecture fees from Berlin‐Chemie, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novo Nordisk, Sanofi‐Aventis, AstraZeneca and Medscape, grant support from AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novo Nordisk, Novartis and travel support in conjunction with all listed activities. J.F.E.M. reports fees for serving on committees from AstraZeneca, Braun, ACI Clinical, Fresenius, Celgene, AbbVie, Novo Nordisk, Roche, Sandoz, Lanthio Pharma, Sanifit, Relypsa and ZS Pharma, lecture fees from AstraZeneca, Amgen, Braun, Fresenius, Celgene, Gambro, AbbVie, Medice, Novo Nordisk, Roche, Sandoz, Relypsa and ZS Pharma, and grant support from Celgene, AbbVie, Novo Nordisk, Roche, Sandoz. J.B.B. reports consulting fees paid to his employer and travel support for activities from Adocia, AstraZeneca, Dance Biopharm, Dexcom, Elcelyx Therapeutics, Eli Lilly, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Senseonics, Shenzhen HighTide, Takeda and vTv Therapeutics, grant support from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GI Dynamics, GlaxoSmithKline, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, Merck, Novo Nordisk, Orexigen, Sanofi, Scion NeuroStim, Takeda, Theracos and vTv Therapeutics. He holds stock options in Mellitus Health and PhaseBio, has served on the board of the AstraZeneca HealthCare Foundation, and is supported by a grant from the National Institutes of Health (UL1TR001111). A.G. reports fees for serving on advisory boards from Novo Nordisk and GlaxoSmithKline. Q.Y., H.B.‐T. and H.F.‐L. are employees of Novo Nordisk. S.P. reports honoraria from Novo Nordisk related to consultancy and LEADER Trial Steering Committee activities.
AUTHOR CONTRIBUTIONS
All authors provided input to the development of the first draft, which was subsequently revised and approved by all authors, who also assume responsibility for its content.
ACKNOWLEDGEMENTS
Medical writing and submission support were provided by Kate Booth and Izabel James of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk. All authors had access to final study results. The authors would like to thank Dr David Ørsted (Novo Nordisk) for scientific input.
Nauck MA, Buse JB, Mann JFE, et al. Health‐related quality of life in people with type 2 diabetes participating in the LEADER trial. Diabetes Obes Metab. 2019;21:525–532. 10.1111/dom.13547
Funding information Medical writing and submission support were provided by Kate Booth and Izabel James of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk.
REFERENCES
- 1. American Diabetes Association . 4. Lifestyle management. Diabetes Care. 2017;40(suppl 1):S33‐S43. [DOI] [PubMed] [Google Scholar]
- 2. Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15:205‐218. [DOI] [PubMed] [Google Scholar]
- 3. Lewis EF, Pfeffer MA, Feng A, et al. for the TREAT Investigators; Darbepoetin alfa impact on health status in diabetes patients with kidney disease: a randomized trial. Clin J Am Soc Nephrol. 2011;6:845‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogawa K, Fujikoshi S, Montgomery W, Alev L. Correlation between pain response and improvements in patient‐reported outcomes and health‐related quality of life in duloxetine‐treated patients with diabetic peripheral neuropathic pain. Neuropsychiatr Dis Treat. 2015;11:2101‐2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Briggs AH, Bhatt DL, Scirica BM, et al. Health‐related quality‐of‐life implications of cardiovascular events in individuals with type 2 diabetes mellitus: a subanalysis from the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)‐TIMI 53 trial. Diabetes Res Clin Pract. 2017;130:24‐33. [DOI] [PubMed] [Google Scholar]
- 6. Marso SP, Daniels GH, Brown‐Frandsen K, et al. LEADER Steering Committee, LEADER Trial Investigators; Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mann JFE, Ørsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839‐848. [DOI] [PubMed] [Google Scholar]
- 8. Marso SP, Poulter NR, Nissen SE, et al. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166:823‐830. e5. [DOI] [PubMed] [Google Scholar]
- 9. NICE . Guide to the methods of technology appraisal. 2013. https://www.nice.org.uk/guidance/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed May 10, 2018.
- 10. Brazier JE, Tsuchiya A, Roberts J, Busschbach J. A comparison of the EQ‐5D and the SF‐36 across seven patient groups. Health Econ. 2004;13:873‐884. [DOI] [PubMed] [Google Scholar]
- 11. Dolan P. Modelling valuations for EuroQol health states. Med Care. 1997;35:1095‐1108. [DOI] [PubMed] [Google Scholar]
- 12. Gudex C, Dolan P, Kind P, Williams A. Health state valuations from the general public using the visual analogue scale. Qual Life Res. 1996;5:521‐531. [DOI] [PubMed] [Google Scholar]
- 13. Kim J, Henderson RA, Pocock SJ, et al. RITA‐3 Trial Investigators; Health‐related quality of life after interventional or conservative strategy in patients with unstable angina or non‐ST‐segment elevation myocardial infarction: one‐year results of the third Randomized Intervention Trial of unstable Angina (RITA‐3). J Am Coll Cardiol. 2005;45:221‐228. [DOI] [PubMed] [Google Scholar]
- 14. Stafford M, Soljak M, Pledge V, Mindell J. Socio‐economic differences in the health‐related quality of life impact of cardiovascular conditions. Eur J Public Health. 2012;22:301‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayes A, Arima H, Woodward M, et al. Changes in quality of life associated with complications of diabetes: Results from the ADVANCE study. Value Health. 2016;19:36‐41. [DOI] [PubMed] [Google Scholar]
- 16. Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ‐5D (UKPDS 62). Med Decis Making. 2002;22:340‐349. [DOI] [PubMed] [Google Scholar]
- 17. Marso SP, Nauck MA, Monk Fries T, et al. Myocardial infarction subtypes in patients with type 2 diabetes mellitus and the effect of liraglutide therapy (from the LEADER Trial). Am J Cardiol. 2018. 10.1016/j.amjcard.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 18. Grandy S, Fox KM, SHIELD Study Group . Change in health status (EQ‐5D) over 5 years among individuals with and without type 2 diabetes mellitus in the SHIELD longitudinal study. Health Qual Life Outcomes. 2012;10:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zinman B, Wanner C, Lachin JM, et al. EMPA‐REG OUTCOME Investigators; Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 20. Neal B, Perkovic V, Mahaffey KW, et al. CANVAS Program Collaborative Group; Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 21. Marso SP, Bain SC, Consoli A, et al. SUSTAIN‐6 Investigators; Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 22. Reaney M, Elash CA, Litcher‐Kelly L. Patient Reported Outcomes (PROs) used in recent phase 3 trials for type 2 diabetes: a review of concepts assessed by these PROs and factors to consider when choosing a PRO for future trials. Diabetes Res Clin Pract. 2016;116:54‐67. [DOI] [PubMed] [Google Scholar]
- 23. Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP‐1 products–liraglutide and exenatide–for the treatment of type 2 diabetes. J Med Econ. 2010;13:655‐661. [DOI] [PubMed] [Google Scholar]
- 24. Solli O, Stavem K, Kristiansen IS. Health‐related quality of life in diabetes: the associations of complications with EQ‐5D scores. Health Qual Life Outcomes. 2010;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. U.K. Prospective Diabetes Study Group . Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). Diabetes Care. 1999;22:1125‐1136. [DOI] [PubMed] [Google Scholar]
- 26. Bradley C. Importance of differentiating health status from quality of life. Lancet. 2001;357:7‐8. [DOI] [PubMed] [Google Scholar]


