Abstract
Background
The first choice for treatment of neonatal convulsions is intravenous phenobarbital, which contains propylene glycol (PG) as a solvent. Although PG is generally considered safe, the dosage can exceed safety thresholds in neonates. High PG levels can cause lactic acidosis.
Purpose/Hypothesis
To investigate a relationship between brain PG concentration and medication administered to neonates, and to study if a correlation between spectroscopically detected PG and lactate was present.
Study Type
Retrospective.
Population
Forty‐one neonates who underwent MRI/MRS.
Field Strength/Sequence
Short echo time single voxel MRS at 1.5T.
Assessment
Spectra were quantified. Concentrations of PG were correlated with medication administered, because intravenously administered phenobarbital solutions contained 10, 25, or 50 mg phenobarbital per ml, all containing 350 mg PG per ml. The interval between medication and MRI/MRS was determined.
Statistical Tests
Chi‐square test, Student's t‐test, Mann–Whitney U‐test and Spearman correlation.
Results
Eighteen neonates had brain PG >1 mM (median 3.4 mM, maximum 9.5 mM). All 18 neonates with high brain PG and 14 neonates with low brain PG (<1 mM) received phenobarbital as the only source of PG. Nine neonates did not receive any phenobarbital/PG‐containing medication. Neonates with high brain PG more often received 10 mg/ml phenobarbital, resulting in higher PG dose (high vs. low brain PG (median [interquartile range]: 1400 [595] vs. 350 [595] mg/kg, respectively, P < 0.01). In addition, the interval between the last phenobarbital dose and MRI was shorter in the high brain PG group (high vs. low brain PG: 16 [21] vs. 95 [83] hours, respectively, P < 0.001). Within neonates that received phenobarbital, there was no conclusive correlation between spectroscopically detected PG and lactate (Spearman's rho = 0.23, P = 0.10).
Data Conclusion
These MRS findings may increase awareness of potentially toxic PG concentrations in the neonatal brain due to intravenous phenobarbital administration and its dependence on the phenobarbital formulation used.
Level of Evidence: 4
Technical Efficacy: Stage 5
J. Magn. Reson. Imaging 2019;49:1062–1068.
Keywords: newborns; propylene glycol; propane‐1,2‐diol; MR spectroscopy; phenobarbital; lactate
PROPYLENE GLYCOL (PG), or propane‐1,2‐diol, is a solvent that improves solubility and stability of various intravenous drugs, including phenobarbital, which is the treatment of first choice for neonatal convulsions. PG is "generally regarded as safe" by the US Food and Drug Administration (FDA).1 The World Health Organization (WHO) recommends a dose limit of PG of 25 mg per kg body weight per day as a food additive,2 but this limit does not address its use as a drug solvent. The European Medicines Agency (EMA) guidelines adhere to a safety threshold of 50 mg per kg body weight per day in children below 5 years of age and a safety threshold of 1 mg per kg body weight per day in term and preterm neonates.3 The latter is based on the low clearance of PG in this particular age group.4, 5, 6 Indeed, when intravenous drugs containing PG as a solvent are used, daily doses of PG exceeding these upper limits are regularly administered to hospitalized neonates.5, 6, 7
The pharmacokinetics of PG in children, and even more in neonates, are largely unknown. PG is eliminated through the kidneys or metabolized in the liver through alcohol dehydrogenase to lactate and pyruvate8, 9; both elimination routes are dose‐ and concentration‐dependent.8 As a result of a lower metabolic hepatic capacity and immature renal function, clearance of PG is lower in children and neonates compared with adults.4 In more detail, PG clearance is low in neonates, especially in those born preterm and with lower birth weight, with a half‐life varying between 10.8 and 30.5 hours (mean 19.3 hours).8, 10 Therefore, neonates are at risk for PG accumulation and even adverse effects due to toxicity.
Short‐term adverse events of PG (with doses of up to 3 g per day in preterm neonates with body weight below 1500 g) have been reported and include lactic acidosis, renal failure, hepatic dysfunction, intravascular hemolysis, and seizures, as well as cardiac and respiratory problems.1, 3, 10, 11, 12 On the other hand, no short‐term biochemical impact was observed when preterm infants received 34 mg per kg body weight per day of PG for a maximum of 48 hours,7 even though this dose is above the recommended upper limits.2, 3 Nevertheless, long‐term effects due to apoptosis have been described in animal studies,13 although they are unknown in infants.
Several studies have reported high plasma concentrations after repeated doses of drugs containing PG as a solvent,4, 14, 15 and its presence in cerebrospinal fluid has been shown using in vitro high‐resolution magnetic resonance spectroscopy (MRS).16 In the early years of clinical in vivo MRS, detection of PG in brain of newborns who received phenobarbital has been reported17 and has been confirmed by others.18 In our hospital, neonates are referred to a clinical MRI examination for various reasons including asphyxia, infection, or stroke. This examination also includes a single‐voxel 1H MRS covering the basal ganglia and the thalamus, since this is an area vulnerable to hypoxic damage. In several neonatal spectra, we detected extremely high signals of a doublet at 1.13 ppm, suggestive of PG. Therefore, we investigated if there was a relationship between the observed concentration of PG and the type of medication that the newborns received. There had been a change in phenobarbital formulation from 50 mg/ml to 10 mg/ml due to market withdrawal by the manufacturer. All phenobarbital formulations contained 350 mg/ml PG, resulting in a 5‐fold increase in PG dose. We hypothesized that this may be an explanation for the extremely high brain PG concentrations. In addition, since high PG levels can lead to lactic acidosis,3, 19, 20 we studied if a correlation between concentrations of PG and lactate measured in neonatal MR spectra was present.
Materials and Methods
Subjects
This study was a retrospective cohort study in neonates (born at gestational age 24–42 weeks) admitted to our Neonatal Intensive Care Unit between January 2016 and January 2018. As evaluated by our Medical Ethics Review Committee, the Medical Research Involving Human Subjects Act did not apply to this study, and therefore there was a waiver of informed consent.
Neonates were only included if they received a cerebral MRI at 1.5T, including MRS, as part of standard neonatal care between birth and 4 weeks after expected date of birth. If medical records were unavailable for review, neonates were excluded. Forty‐seven neonates were eligible for inclusion; five infants were excluded because cerebral MRI was performed at 3T; one infant was excluded because of insufficient quality of the MRS due to motion. Reasons for clinical MRI/MRS examination of these 41 included neonates were perinatal asphyxia (n = 22), perinatal infection (n = 5), perinatal stroke (n = 8), metabolic disease with hyperammonemia (n = 1), and miscellaneous diagnoses (n = 5), including Prader–Willi syndrome (n = 2), neuromuscular disease (n = 1), fifth day fits (n = 1), and epilepsy due to SCN2A mutation (n = 1).
We retrospectively collected birth data (ie, gestational age [weeks], birth weight [g], mode of delivery) as well as data on medication containing PG as a solvent. In our study, the only administered medication containing PG was intravenous phenobarbital, either with a concentration of 50 mg/ml, 25 mg/ml, or 10 mg/ml. All phenobarbital formulations used contained 350 mg PG per ml. The concentration, dosage, and timing of phenobarbital were collected from medical records. Up until January 2017, a phenobarbital formulation containing 50 mg/ml phenobarbital was used. From February 2017 onwards, the supply of phenobarbital 50 mg/ml to our Neonatal Intensive Care Unit was not possible due to market withdrawal by the manufacturer and, therefore, a formulation containing 10 mg/ml phenobarbital was used. Neonates referred from other hospitals were locally treated with either 10, 25, or 50 mg/ml phenobarbital formulations. The cumulative dose of PG per kg body weight was calculated. Furthermore, the interval in hours between MRS and last dose of phenobarbital was determined and the chronological age (days) at the moment of MRI/MRS was recorded.
MRS Acquisition and Analysis
During MRI, all neonates were placed in a special cushion to maintain adequate positioning and prevent movement artifacts. Most infants were ventilated during the procedures, whereas some were spontaneously breathing. A large part of the ventilated infants received some sedation with intravenous morphine and midazolam; in some infants intravenous midazolam was also part of the treatment of neonatal convulsions. Infants who were breathing spontaneously were fed half an hour prior to MRI and did not receive additional sedation.
Clinical MRI/MRS was performed at 1.5T (Avanto, Siemens, Erlangen, Germany) using an 8‐channel head coil. MRI included T1‐ and T2‐weighted sequences as well as diffusion‐weighted imaging. 1H MRS was performed with point‐resolved spectroscopy localization, positioned at the level of the right basal ganglia and thalamus, volume‐of‐interest (VOI) 14 ml (20 mm RL, 35 mm AP, 20 mm SI). VOI localization is shown in Fig. 1. In four subjects, the VOI was slightly smaller (8–12 ml). In another four subjects, a VOI of 8 ml was selected in the parietal cortex. Repetition time (TR) 3000 msec, echo time (TE) 30 msec, 32 averages. For five spectra (three in basal ganglia/thalamus and two in the cortex), 64 averages were acquired. Single‐acquisition reference measurements without water suppression (TR 10,000 msec) using both head and body coil as receiving coils were obtained for quantification, using the transmitter reference amplitude of the body coil.21 Total spectroscopic acquisition time was 2 minutes (or 4 min in the case of 64 averages). Metabolite concentrations were calculated with LCModel,22 using a basis set including a model spectrum of PG18 and simulating macromolecular and lipid signals.
Figure 1.
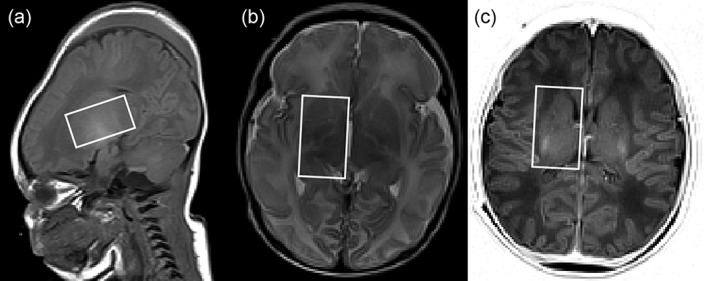
Visualization of VOI. VOI with size of 14 ml (20 mm RL, 35 mm AP, 20 mm SI) is shown in A: sagittal T1‐weighted slice at the center position; B: transverse T2‐weighted slice at most inferior position; C: transverse IR‐weighted slice at the most superior position. In this infant the contribution of CSF to the VOI was 4.9%.
Concentrations were determined in mM (ie, mmol/l VOI). Quality parameters estimated by LCModel included the Cramer–Rao lower bounds for each metabolite, estimated spectral linewidth (full‐width at half‐maximum [FWHM], expressed in Hz) and signal‐to‐noise‐ratio (SNR). Because of variability in size and number of averages, we reported a normalized SNR corresponding to the standard protocol of 14 ml and 32 averages. In addition, we checked the correlation coefficients between metabolite concentrations of PG, lactate, and lipid signals around 1.3 ppm. We also estimated the contribution of cerebrospinal fluid (CSF) within the VOI on the T2‐weighted images.
Statistical Analysis
Categorical variables were reported as frequencies (%). Normally distributed continuous variables were reported as mean with standard deviation (mean ± SD). Skewed continuous variables were reported as median with interquartile range (median, IQR). Neonates were classified in two groups: brain PG >1 mM (high brain PG) and brain PG <1 mM (low brain PG). Above the threshold of 1 mM, the doublet of PG was clearly visible in the qualitative spectra that were processed on the scanner and could also be quantified with Cramer–Rao lower bounds below 20% (see Results section). Categorical variables were compared using chi‐square tests. Normally distributed data were compared with Student's t‐test, and skewed data with Mann‐Whitney U‐tests (for comparison of two groups). Nonparametric correlations were evaluated with Spearman's rho, two‐tailed. All analyses were performed using IBM SPSS Statistics v. 22.0 (Armonk, NY). P < 0.05 was considered significant.
Results
In the group of 41 included neonates, the median gestational age was 40.0 weeks (range 29.4–41.9 weeks), median birth weight was 3190 g (range 1120–4400 g). Median chronological age at MRI/MRS was 4.5 days (range 0.8–20.4 days).
VOIs had a mean CSF partial volume contribution of 4.2 ± 2.0%. The doublet at 1.13 ppm that was visually detectable in many spectra was fitted reliably by PG. In 18 of the 41 neonates, concentrations of PG were above 1 mM (high brain PG), varying between 1.3–9.5 mM, with a median of 3.4 mM. Spectra and corresponding fits of PG and lactate for a few infants are shown in Fig. 2. Whereas PG is easiest recognized by the doublet at 1.13 ppm corresponding to the methyl group, the PG spectrum also contains high signals of the methylene protons around 3.5 ppm, which partly overlap with the resonance of myo‐inositol. The spectrum of the infant with hyperammonemia showed not only high PG, but also extremely elevated glutamine (Fig. 2D). As a side note, we remark that in these neonatal spectra glutamine was determined with a median Cramer–Rao lower bound of 15%, while concentrations of glutamine and glutamate were not correlated with each other (median correlation coefficient of −0.08).
Figure 2.
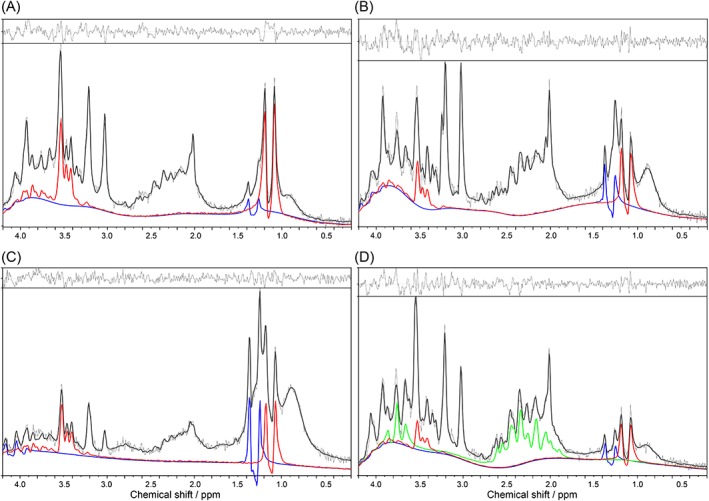
LCModel output with residuals and fits of propylene glycol (PG, red) and lactate (Lac, blue). A: Gestational age (GA) 37 weeks, birth weight (BW) 2975 g; subependymal hemorrhage; PG = 9.5 mM with Cramer–Rao lower bound (CRLB) 3%, Lac = 1.1 mM with CRLB 16%. B: GA 41 weeks, BW 4035 g; perinatal asphyxia and 72‐hour therapeutic hypothermia; PG = 3.3 mM with CRLB 8%, Lac = 1.6 mM with CRLB 14%. C: GA 38 weeks, BW 3515 g, cerebral stroke due to aortic arc thrombus; PG = 4.2 mM with CRLB 6%. Lac = 3.5 mM with CRLB 9%. Severe reduction of all other metabolites. D: GA 40 weeks, BW 3820 g, hyperammonemia; PG = 4.2 mM with CRLB 9%, Lac = 1.2 mM with CRLB 18%. The hyperammonemia in this infant also led to extremely elevated glutamine of 16.6 mM with CRLB 7% (shown in green).
As expected, Cramer–Rao lower bounds for PG decreased with increased PG concentrations, with a median value of 8.5% in the high PG spectra, and at least 26% for the low PG spectra. In individual spectra, lactate and PG were neither correlated with each other (median correlation coefficient 0.08), nor with lipid signals around 1.3 ppm (median correlation coefficients were −0.22 and 0.15 between lactate and Lip13a and Lip13b, respectively; 0.0 and 0.09 between PG and Lip13a and Lip13b, respectively).
Estimates of spectral quality did not differ between the high and low brain PG groups. Median linewidth was 1.46 Hz (1.02) and 1.97 Hz (1.46), respectively (P = 0.058) and mean normalized SNR was 16.8 ± 5.4 and 16.3 ± 3.2, respectively (P = 0.71).
There were no differences in birth data between infants with high brain PG compared with those with low brain PG, except for a higher mortality in the high brain PG group (Table 1). In the latter group, causes of death were perinatal asphyxia (n = 4), perinatal infection (n = 1), perinatal stroke (n = 2), and metabolic disease (n = 1). Within the high brain PG group, there was no difference in diagnosis between those deceased and those alive (chi‐square test χ2 = 4.5, P = 0.34). In all cases, the cause of death was not directly related to PG.
Table 1.
Demographics and Clinical Data of Neonates With Brain Propylene Glycol (PG) Concentrations Above 1 mM (high PG) and Below 1 mM (low PG)
| High PG | Low PG | |||
|---|---|---|---|---|
| > 1 mM | < 1 mM | |||
| (n = 18) | (n = 23) | |||
| Malea | 12 (66.7%) | 12 (52.2%) | ||
| Mortalitya | 8 (44.4%) | 3 (13%) | P = 0.024 | |
| Gestational age (weeks)b | 38.7 (3.1) | 40.4 (3.3) | ||
| Birth weight (g)b | 3080 (844) | 3300 (1242) | ||
| Modus partusa | Vaginal | 6 (35.3%) | 7 (30.4%) | |
| Vacuum extraction | 4 (23.5%) | 7 (30.4%) | ||
| Cesarean section | 1 (5.9%) | 4 (17.4%) | ||
| Emergency cesarean | 5 (29.4%) | 4 (17.4%) | ||
| Cesarean under general anesthesia | 1 (5.9%) | 1 (4.3%) | ||
| Diagnosisa | Perinatal asphyxia | 6 (33.3%) | 16 (69.6%) | |
| Perinatal infection | 3 (16.7%) | 2 (8.7%) | ||
| Perinatal stroke | 6 (33.3%) | 2 (8.7%) | ||
| Metabolic disease | 1 (5.6%) | |||
| Other | 2 (11.1%) | 3 (13%) | ||
| Phenobarbital intravenous solutiona | 10 mg/ml | 10 (55.6%) | 7 (30.4%) | P = 0.015d |
| 25 mg/ml | 3 (16.7%) | 2 (8.7%) | ||
| 50 mg/ml | 3 (16.7%) | 5 (21.7%) | ||
| 10 and 25–50 mg/ml | 2 (11.1%) | |||
| None | 9 (39.1%) | |||
| Total PG dose (mg/kg)b,c | 1400 (595) | 350 (595) | P < 0.01 | |
| Interval last dose and MRS (hours)b,c | 16 (21) | 95 (83) | P < 0.001 | |
| Brain PG (mM)b | 3.4 (2.2) | 0.02 (0.2) | P < 0.001 | |
Parameters are reported as: afrequencies: number (%); bmedian (interquartile range); cPG < 1 mM consists of 14 infants, since 9 infants did not receive phenobarbital; dcomparison of groups by cross‐table and chi‐square test. High PG and low PG groups were compared by chi‐square test and Mann–Whitney U‐test, as appropriate.
A total of 32 neonates received at least one dose of phenobarbital before the MRS examination. There was no significant difference regarding total dose of phenobarbital administered between the high and low brain PG groups (40 [10] mg per kg vs. 30 [20] mg per kg, respectively, P = 0.065). The more frequent administration of 10 mg/ml phenobarbital to neonates with high brain PG resulted in a higher median dose of 1400 mg PG per kg body weight compared with 350 mg per kg body weight in the low brain PG group (Table 1).
The observed brain PG concentration as a function of the cumulative dose of PG administered is shown in Fig. 3. In neonates with a high cumulative dose, both high and low brain PG concentrations were observed. To evaluate the effect of the interval between the last dose of phenobarbital and the MRS examination, we distinguished short and long intervals based on the median interval of 30 hours in the total group (Fig. 3). The median interval in neonates with high PG was 16 hours and was shorter than the median interval of 95 hours in the low PG group (Table 1).
Figure 3.
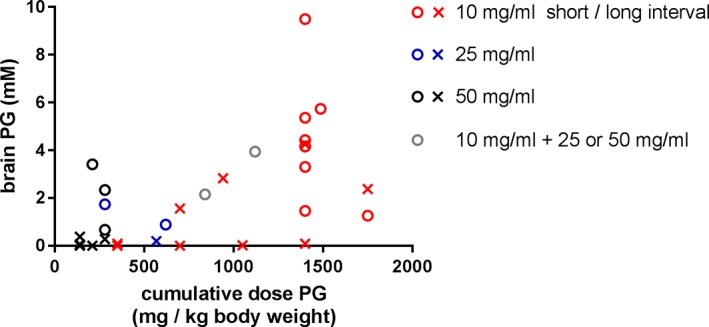
Scatterplot of estimated brain concentration of propylene glycol (PG) vs. total dose PG per kg body weight. Colors indicate the formulation of phenobarbital administered (red: 10 mg phenobarbital/ml, blue: 25 mg phenobarbital/ml, black: 50 mg phenobarbital/ml, gray: combination of 10 mg phenobarbital/ml and either 25 or 50 mg phenobarbital/ml). The interval between the last dose of phenobarbital and MR examination is indicated with circles (<30 hours) and crosses (>30 hours).
The concentration of brain lactate was highly variable between newborns, but did not differ between the infants with high and low brain PG (0.9 mM [1.4], range 0–3.5 mM vs. 0.6 [0.3], range 0.1–2.3 mM, respectively, P = 0.07) (Fig. 4). Within the group of newborns who received phenobarbital (n = 32), we did not observe a conclusive correlation between PG and lactate (Spearman's rho = 0.29, P = 0.10). Figure 4 shows that data from two infants with high PG and low lactate influence the absence of this correlation. However, within the whole group of 41 infants, there was a positive correlation (Spearman's rho = 0.38, P = 0.015). In none of the spectra did we detect pyruvate, ie, the median concentration was 0, and Cramer–Rao lower bounds were well above 50%.
Figure 4.
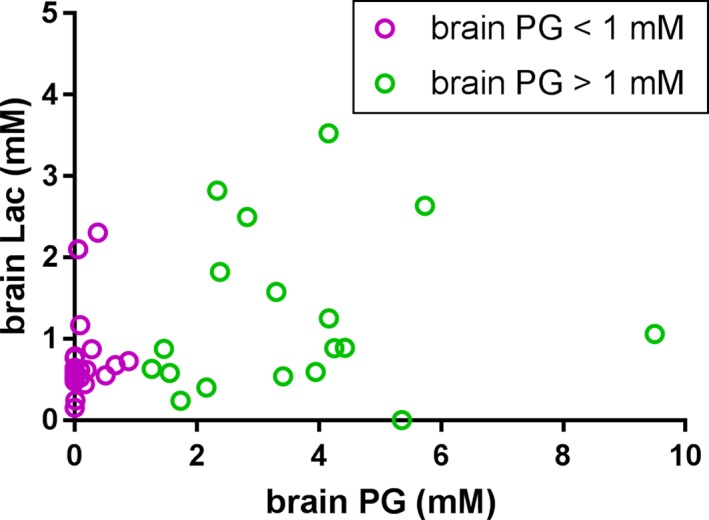
Observed concentrations of lactate (Lac) and propylene glycol (PG). There was no conclusive correlation between concentrations of PG and Lac within the group of newborns who received phenobarbital (n = 32, Spearman's rho = 0.29, P = 0.10). However, within the whole group there was a positive correlation (n = 41, Spearman's rho = 0.38, P = 0.015).
Discussion
This study demonstrated a clear relationship between brain concentration of PG and the pharmaceutical formulation of phenobarbital administered to neonates, in particular with regard to the concentration of PG used. This relationship was guided and discovered by quantitative MRS. Although it has been described decades ago that accumulated PG can be detected by MRS,17 we were surprised to detect PG concentrations as high as 9.5 mM in one infant. The high median PG level of 3.4 mM in a group of 18 neonates suggests that peak concentrations have been even higher, since the median interval between the last phenobarbital administration and MRS in this group was 16 hours.
The time interval between phenobarbital administration and MRS examination affected the observed brain PG concentrations. In addition, the observed brain PG concentrations were related to the dose and formulation of phenobarbital used. All phenobarbital solutions that were used in this study contained 350 mg PG per ml; however, the concentration of phenobarbital itself varied between 10–50 mg/ml. As a consequence, the use of 10 mg/ml formulations resulted more often in higher brain PG concentrations. With a cumulative dose of up to 40 mg/kg phenobarbital, usually administered during 1 or 2 consecutive days, the median cumulative dose of PG was 350 and 1400 mg per kg body weight in neonates with low brain PG and high brain PG, respectively. These doses clearly exceed the recommended safety thresholds,2, 3 and are also above the median 34 mg PG per kg/day for which no short‐term biochemical adverse effects were observed.7 Neonates receiving these higher doses of PG may be at higher risk for short‐term adverse effects.1, 3, 10, 11, 12
In the current study, the lack of short‐term effects does not exclude potential adverse effects later in life. To date, the safe upper limit of intravenous PG remains unknown.
During hepatic elimination, PG is degraded to pyruvate and lactate.19 We did not observe any pyruvate in the spectra, and the observed lactate was primarily present in brain tissue, since the estimated contribution of CSF to the VOI was limited to a few percent. However, a correlation between brain lactate and PG was not present in the group of infants who received phenobarbital as the only source of PG, which is the only relevant group to test when assuming lactic acidosis due to PG degradation. This seems to be in contrast to the findings by Kelner and Bailey, who reported a correlation between PG and lactate in CSF and serum in five patients (two adults and three infants between 2 and 5 months of age).20 However, the fact that we also observed high brain lactate in neonates who did not receive PG at all shows that there are other explanations for lactate elevation in our cohort, such as perinatal asphyxia with hypoxic ischemic injury.23 Currently, we can only speculate that there might be situations with increased lactate metabolism that may explain why we do not observe high lactate in all high PG cases. Although mortality was higher in the high brain PG group, this could not be attributed to a higher frequency of specific medical disease nor to PG administration.
The current study has several limitations. First, we acknowledge the limitations of a retrospective study. Nonetheless, this study is of critical importance in showing that high brain PG concentrations are reached and are related to the type of phenobarbital formulation used. Second, the sample size was limited and the data were limited by data available from medical records due to the retrospective design of this study. As a consequence, a power analysis could not be performed. Further, there was some variability in localization, volume, and number of averages. Still, spectral quality was high in all cases, and it may be assumed that the accumulation of PG will be similar in cortex and in deep cortical gray matter structures. Lastly, unfortunately, our study was not designed to evaluate either short‐ or long‐term consequences of PG use. No serum concentrations of PG were available during the study period. Also, data on biochemical effects of PG, such as metabolic acidosis, liver enzymes and function, or renal function were not consistently available, and therefore could not be analyzed.
In conclusion, this MRS study showed that brain PG concentrations are directly related to PG used as a solvent in phenobarbital intravenous solutions. Although the long‐term effects of PG accumulation are not exactly known, serious adverse short‐term effects are reason enough to select the best possible pharmaceutical formulation to keep PG levels as low as possible.
Acknowledgments
We thank Prof. Dr. M.S. van der Knaap, Dr. N.I. Wolf, and Dr. D.P. Bakker, all pediatrics neurologists at VU University Medical Center, Amsterdam, The Netherlands, for their expert evaluation of MRI/MRS of all included subjects.
The first two authors contributed equally to this work.
References
- 1. Lim TY, Poole RL, Pageler NM. Propylene glycol toxicity in children. J Pediatr Pharmacol Ther 2014;19:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seventeenth Report of Joint Food and Agriculture Organization/WHO Expert Committee on Food additives: toxicological of certain food additives with a review of general principles and of specifications, technical report series No. 539, Geneva, 1974. [PubMed]
- 3. European Medicines Agency . Questions and answers on propylene glycol used as an excipient in medicinal products for human use In: Committee for Human Medicinal Products, editor, 2017. [Google Scholar]
- 4. De Cock RF, Knibbe CA, Kulo A, et al. Developmental pharmacokinetics of propylene glycol in preterm and term neonates. Br J Clin Pharmacol 2013;75:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shehab N, Lewis CL, Streetman DD, Donn SM. Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates. Pediatr Crit Care Med 2009;10:256–259. [DOI] [PubMed] [Google Scholar]
- 6. Whittaker A, Currie AE, Turner MA, Field DJ, Mulla H, Pandya HC. Toxic additives in medication for preterm infants. Arch Dis Child Fetal Neonatal Ed 2009;94:F236–240. [DOI] [PubMed] [Google Scholar]
- 7. Allegaert K, Vanhaesebrouck S, Kulo A, et al. Prospective assessment of short‐term propylene glycol tolerance in neonates. Arch Dis Child 2010;95:1054–1058. [DOI] [PubMed] [Google Scholar]
- 8. Speth PA, Vree TB, Neilen NF, et al. Propylene glycol pharmacokinetics and effects after intravenous infusion in humans. Ther Drug Monit 1987;9:255–258. [DOI] [PubMed] [Google Scholar]
- 9. Wilson KC, Reardon C, Theodore AC, Farber HW. Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: a case series and prospective, observational pilot study. Chest 2005;128:1674–1681. [DOI] [PubMed] [Google Scholar]
- 10. Glasgow AM, Boeckx RL, Miller MK, MacDonald MG, August GP, Goodman SI. Hyperosmolality in small infants due to propylene glycol. Pediatrics 1983;72:353–355. [PubMed] [Google Scholar]
- 11. MacDonald MG, Fletcher AB, Johnson EL, Boeckx RL, Getson PR, Miller MK. The potential toxicity to neonates of multivitamin preparations used in parenteral nutrition. JPEN J Parenter Enteral Nutr 1987;11:169–171. [DOI] [PubMed] [Google Scholar]
- 12. MacDonald MG, Getson PR, Glasgow AM, Miller MK, Boeckx RL, Johnson EL. Propylene glycol: increased incidence of seizures in low birth weight infants. Pediatrics 1987;79:622–625. [PubMed] [Google Scholar]
- 13. Lau K, Swiney BS, Reeves N, Noguchi KK, Farber NB. Propylene glycol produces excessive apoptosis in the developing mouse brain, alone and in combination with phenobarbital. Pediatr Res 2012;71:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Cock RF, Allegaert K, Vanhaesebrouck S, et al. Low but inducible contribution of renal elimination to clearance of propylene glycol in preterm and term neonates. Ther Drug Monit 2014;36:278–287. [DOI] [PubMed] [Google Scholar]
- 15. Chicella M, Jansen P, Parthiban A, et al. Propylene glycol accumulation associated with continuous infusion of lorazepam in pediatric intensive care patients. Crit Care Med 2002;30:2752–2756. [DOI] [PubMed] [Google Scholar]
- 16. Petroff OA, Yu RK, Ogino T. High‐resolution proton magnetic resonance analysis of human cerebrospinal fluid. J Neurochem 1986;47:1270–1276. [DOI] [PubMed] [Google Scholar]
- 17. Cady EB, Lorek A, Penrice J, et al. Detection of propan‐1,2‐diol in neonatal brain by in vivo proton magnetic resonance spectroscopy. Magn Reson Med 1994;32:764–767. [DOI] [PubMed] [Google Scholar]
- 18. Oakden WK, Noseworthy MD. Propylene glycol is essential in the LCModel basis set for pediatric 1H‐MRS. J Comput Assist Tomogr 2005;29:136–139. [DOI] [PubMed] [Google Scholar]
- 19. Huff E. The metabolism of 1,2‐propanediol. Biochim Biophys Acta 1961;48:506–517. [DOI] [PubMed] [Google Scholar]
- 20. Kelner MJ, Bailey DN. Propylene glycol as a cause of lactic acidosis. J Anal Toxicol 1985;9:40–42. [DOI] [PubMed] [Google Scholar]
- 21. Natt O, Bezkorovaynyy V, Michaelis T, Frahm J. Use of phased array coils for a determination of absolute metabolite concentrations. Magn Reson Med 2005;53:3–8. [DOI] [PubMed] [Google Scholar]
- 22. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–679. [DOI] [PubMed] [Google Scholar]
- 23. Robertson NJ, Thayyil S, Cady EB, Raivich G. Magnetic resonance spectroscopy biomarkers in term perinatal asphyxial encephalopathy: from neuropathological correlates to future clinical applications. Curr Pediatr Rev 2014;10:37–47. [DOI] [PubMed] [Google Scholar]


