Abstract
Basal insulin therapy often involves a compromise between achievement of glycaemic targets and avoidance of hypoglycaemia, dependent on how intensively insulin is titrated. In the Phase 3a EDITION 1, 2 and 3 studies, insulin glargine 300 U/mL (Gla‐300) provided glycaemic control equivalent to that of insulin glargine 100 U/mL (Gla‐100), with less hypoglycaemia in individuals with type 2 diabetes mellitus (T2DM). The current study evaluated the rates of confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia over six months of treatment with Gla‐300 or Gla‐100 in the EDITION studies, as a function of HbA1c. Analysis was performed on patient‐level data pooled from the three EDITION studies, and annualized hypoglycaemia rate as a function of HbA1c at Month 6 was fitted using a negative binomial regression model. Participants treated with Gla‐300 experienced a consistently lower rate of confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia as compared with those treated with Gla‐100, regardless of HbA1c at Month 6. Results suggest that treatment with Gla‐300 vs Gla‐100 could allow individuals with T2DM to achieve equivalent glycaemic control with less hypoglycaemia.
Keywords: basal insulin, glycaemic control, hypoglycaemia, type 2 diabetes
1. INTRODUCTION
Hypoglycaemia has been reported as the key factor limiting attainment of optimal glycaemic control in diabetes.1 Patients with diabetes are tasked with maintaining euglycaemic blood glucose levels, but those treated with insulin are at risk of developing hypoglycaemia.2 Thus, insulin therapy often involves a compromise between achievement of glycaemic control and avoidance of hypoglycaemia. Intensive insulin therapy for glycaemic control effectively reduces the risk of microvascular complications such as retinopathy, nephropathy and neuropathy in patients with diabetes,3 but there is some evidence implicating hypoglycaemia in macrovascular events.4 In addition, fear of hypoglycaemia amongst individuals with diabetes and physicians remains a major impediment to implementation of intensive therapy which can lead to sub‐optimal insulin dosing,1, 5 potentially resulting in impaired glycaemic control. Hence, an insulin that offers optimum glycaemic control with a lower risk of hypoglycaemia, compared with other treatments, could be of benefit to individuals with diabetes. This article focuses on comparing the risk of hypoglycaemia between two basal insulins.
Insulin glargine 300 U/mL (Gla‐300) has more stable and prolonged pharmacokinetic (PK) and pharmacodynamic (PD) profiles compared with insulin glargine 100 U/mL (Gla‐100), evidenced by a longer time (∼3 hours) to reach 50% of the total insulin exposure and activity during the 36‐hour clamp procedure, lower maximum exposure and activity, a shorter terminal half‐life, and tight blood glucose control that was maintained approximately five hours longer with Gla‐300 vs Gla‐100.6 This translated into comparable glycaemic control and less hypoglycaemia with Gla‐300 vs Gla‐100 in individuals with type 2 diabetes (T2DM), as demonstrated in a previous patient‐level meta‐analysis of data from the Phase 3a EDITION 1, 2 and 3 clinical trials.7 However, that meta‐analysis was unable to assess whether the reduced risk of hypoglycaemia associated with Gla‐300 vs Gla‐100 is limited to individuals with a particular HbA1c level or, rather, it is achieved irrespective of the degree of glycaemic control attained.
To address the question of whether the reduction in risk of hypoglycaemia with Gla‐300 vs Gla‐100 applies across all HbA1c levels, the current study explored the relationship between hypoglycaemia over six months and HbA1c at Month 6 in T2DM clinical trials comparing Gla‐300 with Gla‐100 using data from the EDITION 1, 2 and 3 studies.
2. METHODS
The EDITION 1, 2 and 3 trials (Table S1) were multicentre, randomized, open‐label, two‐arm, parallel‐group, treat‐to‐target Phase 3a clinical trials that compared the efficacy and safety profiles of Gla‐300 with those of Gla‐100 in different groups of individuals with T2DM over a 6‐month treatment period (NCT01499082, NCT01499095, NCT01676220).8, 9, 10 Details of these studies have been described previously.8, 9, 10 All participants from these studies were at least 18 years of age, with a diagnosis of T2DM according to World Health Organization criteria.11 In summary, in the EDITION 1 rial, participants were previously receiving basal insulin therapy (≥42 U/d of either Gla‐100 or neutral protamine Hagedorn [NPH] insulin), in conjunction with mealtime insulin, with or without metformin, for at least 1 year.8 In the EDITION 2 trial, participants had been receiving basal insulin treatment (≥42 U/d of either Gla‐100 or NPH insulin) in combination with non‐insulin antihyperglycaemic agents, excluding sulphonylureas for two months prior to randomization.9 In the EDITION 3 trial, participants were insulin‐naïve and had used non‐insulin antihyperglycaemic agents for at least six months prior to screening.10 Exclusion criteria included HbA1c < 7.0% for all three studies, HbA1c >10.0% for the EDITION 1 and 2 studies and >11.0% for the EDITION 3 study. In each study, participants were randomized (1:1) to once‐daily evening injections of Gla‐300 or Gla‐100, titrated to a fasting self‐monitored plasma glucose target of 80 to 100 mg/dL (4.4‐5.6 mmol/L). Although the EDITION 1, 2 and 3 studies were conducted in different populations, the consistent study designs and endpoints allowed a pooled analysis, as reported previously.7 To avoid excessive heterogeneity, studies in type 1 diabetes and in Japanese populations were not included in the analyses.12, 13, 14
2.1. Statistical analysis
A patient‐level pooled analysis of the EDITION 1, 2 and 3 studies was performed. A regression model for count data with negative binomial distribution was used to model the number of hypoglycaemic events up to Month 6, including treatment arm and HbA1c at Month 6 as covariates and logarithm of the observation period duration as offset. A model including a treatment‐by‐HbA1c interaction term was also implemented, which did not significantly improve the goodness‐of‐fit. Pooling the three studies, interaction P values were 0.937 and 0.829 for anytime hypoglycaemia and for nocturnal hypoglycaemia, respectively. Therefore, the model without this interaction term was considered to describe the data accurately. For each treatment group, the curve represented paired data, HbA1c at Month 6 and predicted number of events up to Month 6, across all patients in the treatment group. Goodness‐of‐fit of the model was assessed using Pearson's chi‐squared test. Analyses were performed on data from individual studies and from the pool of all three studies. Hypoglycaemia was defined as “confirmed” (≤3.9 mmol/L [≤70 mg/dL]) or “severe” based on ADA definitions,15 and including symptomatic or asymptomatic events accompanied by a blood glucose measurement of ≤3.9 mmol/L [≤70 mg/dL], as well as any events defined as severe, that is, requiring assistance by another person to administer carbohydrate, glucagon or other therapy). Annualized rates of “confirmed” (≤3.9 mmol/L [≤70 mg/dL]) or “severe” hypoglycaemia were analysed at any time of day (24 hours) and during the night (00:00‐05:59 am).
3. RESULTS
This patient‐level pooled analysis included 2496 participants, of whom 1247 and 1249 were randomized to Gla‐300 and Gla‐100, respectively. Mean baseline characteristics were comparable between treatment groups in the individual studies and in the pooled analysis population (Table S1).
As reported previously,8, 9, 10 in these treat‐to‐target trials the mean HbA1c achieved after six months was similar for Gla‐300 and Gla‐100 (EDITION 1, 7.25% and 7.28%; EDITION 2, 7.57% and 7.56%; EDITION 3, 7.08% and 7.05%, respectively).
In the pooled analysis, a significant inverse relationship between rate of hypoglycaemia and HbA1c at Month 6 (Figure 1) was documented. Importantly, a significantly lower rate of confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia during the night (00:00‐05:59 am) was observed with Gla‐300 compared with Gla‐100, regardless of HbA1c at Month 6 (P = 0.001) (Figure 1); that is, the inverse curve of hypoglycaemia risk vs HbA1c at six months was shifted down with Gla‐300 vs Gla‐100. When the individual studies were analysed for nocturnal hypoglycaemia, the only statistically significant difference was in the EDITION 2 study (P = 0.001) (Figure 2). No significant difference between treatments in rate of confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia at any time of day (24 hours) was observed in the pooled analysis (Figure 1). However, a statistically significant difference was observed in the EDITION 3 study alone (P = 0.042) (Figure 2).
Figure 1.
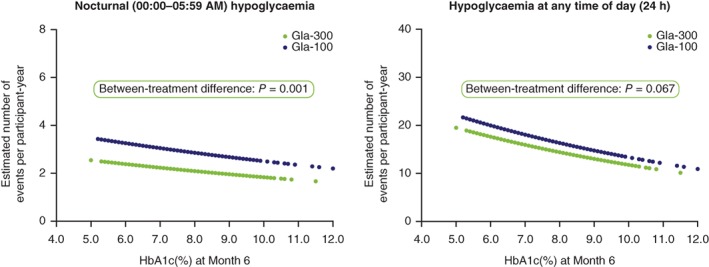
Estimated annualized rates of confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia over six months of treatment with Gla‐300 or Gla‐100 in a patient‐level pooled analysis of the T2DM EDITION 1, 2 and 3 studies, as a function of HbA1c at Month 6. Modified intent‐to‐treat population. Abbreviations: Gla‐100, insulin glargine 100 U/mL; Gla‐300, insulin glargine 300 U/mL; HbA1c, glycated haemoglobin; T2DM, type 2 diabetes mellitus
Figure 2.
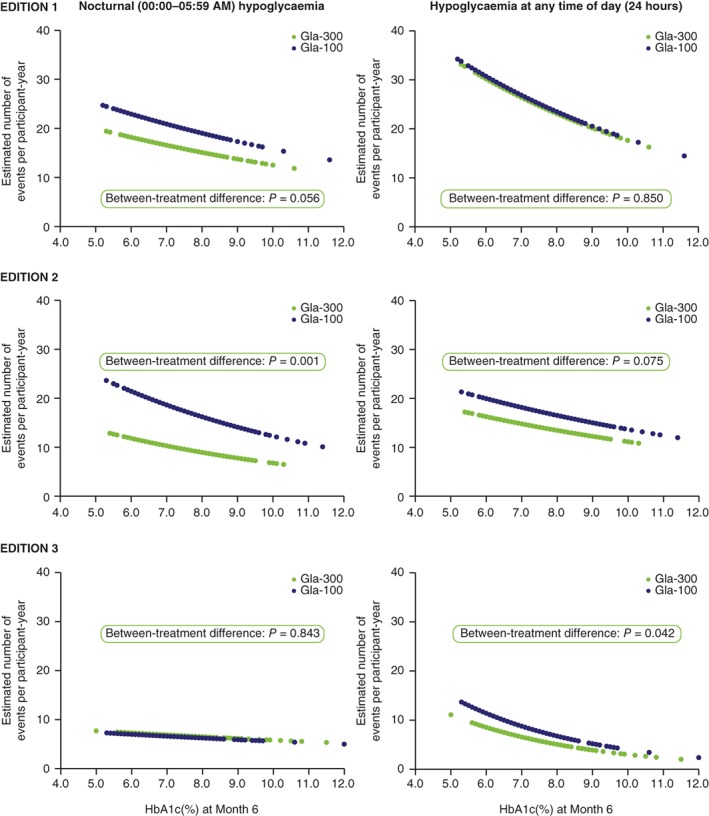
Estimated annualized rates of confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia over 6 months of treatment with Gla‐300 or Gla‐100 in the T2DM EDITION 1, 2 and 3 studies, as a function of HbA1c at Month 6. Modified intent‐to‐treat population. Glucose‐lowering therapy at screening: EDITION 1, basal insulin + mealtime insulin ± metformin; EDITION 2, basal insulin + OADs; EDITION 3, OADs (insulin‐naïve). Abbreviations: Gla‐100, insulin glargine 100 U/mL; Gla‐300, insulin glargine 300 U/mL; HbA1c, glycated haemoglobin; OAD, oral antihyperglycaemic drug; T2DM, type 2 diabetes mellitus
4. DISCUSSION
In T2DM, when evaluating hypoglycaemia, it is important to consider glycaemic control, as achieving glycaemic targets with minimal hypoglycaemia could promote adherence to therapy and reduce the risk of long‐term micro‐ and macrovascular complications.16, 17
In this patient‐level pooled analysis of individuals with T2DM, treatment with Gla‐300 resulted in a lower rate of nocturnal (00:00‐05:59 am) confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia than the rate with treatment with Gla‐100, regardless of HbA1c at Month 6. Importantly, the current analysis supports the existence of an inverse curvilinear relationship between risk of hypoglycaemia and glucose control, irrespective of the type of basal insulin used, which is in line with previous literature.18 This inverse relationship has been found with biochemical hypoglycaemia (≤3.9 mmol/L). In the EDITION studies, primary hypoglycaemia analyses were based on events confirmed by blood glucose ≤3 .9 mmol/L, in line with ADA guidelines at the time of study design.15 The newer International Hypoglycaemia Study Group guidelines highlighted the importance of blood glucose concentrations <v3.0 mmol/L,19 but the lower number of such events prevents analysis of data using the current model. It should be noted, however, that population analyses have shown no association between risk of severe hypoglycaemia and HbA1c,20 while analysis of clinical trials reveals differing hypoglycaemic rate reductions with Gla‐100 vs NPH insulin, dependent on adjustment for baseline, endpoint or change in HbA1c.18 Clearly, HbA1c is not the sole determinant of hypoglycaemia risk, which is dependent upon a number of factors including hypoglycaemia definition.
Hypoglycaemia is often managed by increasing the HbA1c goal and de‐intensifying the glycaemic treatment programme,21 which can have a negative impact on rates of microvascular complications. Between‐treatment differences (Figure 1) could manifest by either a lower hypoglycaemia rate for the same HbA1c, or by a lower HbA1c for the same hypoglycaemia risk, across a wide range of HbA1c values. Therefore, a more appropriate way to limit hypoglycaemic events in a patient using Gla‐100 may be to switch to a different basal insulin that results in less hypoglycaemia, such as Gla‐300, rather than to raise the HbA1c goal, irrespective of the prevalent HbA1c level.
Thus, no matter whether the patient has poor glucose control and is at low risk of hypoglycaemia or has almost normal glucose control and is at the highest risk of hypoglycaemia, use of Gla‐300 rather than Gla‐100 is expected to convey the benefit of a sizeable reduction in risk of hypoglycaemia (Figure 1). It should be noted that the model used, without a treatment‐by‐HbA1c interaction term, does not allow the two individual treatment curves to cross each other, but addition of this interaction term did not improve the model's goodness‐of‐fit and, thus, the chosen model was appropriate for the data.
This finding of less hypoglycaemia with Gla‐300 vs Gla‐100, irrespective of HbA1c at Month 6, in this patient‐level pooled analysis was generally consistent with observations in the individual EDITION 1, 2 and 3 clinical trials, across individuals with a broad range of disease characteristics and at different stages of disease (Figure 2). For hypoglycaemia at any time of day (24 hours), the pooled results seem driven mainly by the EDITION 2 (P = 0.075) and EDITION 3 (P = 0.042) trials, and not by the EDITION 1 (P = 0.850) trial. Similarly, for nocturnal (00:00‐05:59 am) hypoglycaemia, pooled results seem driven mainly by the EDITION 1 (P = 0.056) and EDITION 2 (P = 0.001) trials, and not by the EDITION 3 (P = 0.843) trial. There are potential explanations for these apparent discrepancies. In the EDITION 1 study, participants were taking mealtime insulin in addition to basal insulin. The former is known to convey the highest risk of hypoglycaemia in patients with T2DM, which may have confounded any analysis of hypoglycaemia attributable to basal insulin,8 particularly when analysed at any time of day (24 hours). Participants in the EDITION 3 trial were insulin naïve prior to the study, and experienced a several‐fold lower rate of hypoglycaemic events than experienced by those in the EDITION 1 and 2 trials.8, 9, 10 This may have affected the ability to detect between‐treatment differences in risk of hypoglycaemia, particularly when only nocturnal events were analysed.
Limitations of this analysis include a relatively small sample size for individual studies, investigation of only hypoglycaemia rates and not incidence, as the exact methodology used cannot be applied to percentage of participants with an event, and the fact that results may not be generalizable to populations not included in these trials.
In conclusion, the results of this patient‐level pooled analysis of the EDITION 1, 2 and 3 trials document that, in patients with T2DM who are using either Gla‐100 or Gla‐300, there is an inverse relationship between HbA1c and risk of hypoglycaemia, and that this relationship is shifted towards lower rates of hypoglycaemia with Gla‐300. Thus, treatment with Gla‐300, as compared with treatment with Gla‐100, could allow individuals with T2DM to achieve comparable glycaemic control with less hypoglycaemia, or better glucose control with the same risk of hypoglycaemia, across a wide range of HbA1c levels.
CONFLICTS OF INTEREST
R. B. serves as a Board member for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck (MSD), Johnson & Johnson and Sanofi; and serves on Speakers bureaus for AstraZeneca, Eli Lilly and Sanofi.
J.‐F. Y. serves on Advisory panels for Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Medtronic, Merck, Mylan, Novo Nordisk, Sanofi and Takeda; has received research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Medtronic, Merck and Sanofi; and serves on Speakers Bureaus for Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Medtronic, Merck, Novo Nordisk, Sanofi and Takeda.
C. B.‐W. is an employee of and stock/shareholder in Sanofi.
E. B.‐Le C. is an employee of and stock/shareholder in Sanofi.
P. C. serves on Advisory panels for Abbott, Cellnovo, Eli Lilly, Medtronic, Novo Nordisk, Roche and Sanofi; has received research support from Beta‐O2 and Medtronic; and serves on Speakers bureaus for Abbott, Eli Lilly, Johnson & Johnson, Medtronic, Merck (MSD), Novo Nordisk, Roche and Sanofi.
T. S. B. has received research support from Abbott, Ambra, Ascensia, BD, Boehringer Ingelheim, Calibra Medical, Companion Medical, Dance Biopharm, Dexcom, Eli Lilly, Glooko, Glysens, Kowa, Lexicon, MannKind, Medtronic, Novo Nordisk, Sanofi, Senseonics, Taidoc, Versartis and Xeris; has received honoraria for consulting from Abbott, AstraZeneca, Ascensia, BD, Calibra, Capillary Biomedical, Eli Lilly, Intarcia, Medtronic, Novo Nordisk and Sanofi; and has received honoraris for speaking from Abbott, Eli Lilly, Medtronic, Novo Nordisk and Sanofi.
Author contributions
C. B.‐W. and E. B.‐Le C. contributed to the concept and design of this pooled analysis. All authors contributed to data analysis/interpretation and to drafting and critically revising the publication, and all authors approved the final version and accept accountability for the accuracy and integrity of the data.
Supporting information
Table S1. Summary of studies (including baseline characteristics) in patient‐level pooled analysis of people with T2DM.
ACKNOWLEDGMENTS
Editorial and writing assistance was provided by Simon Rees, PhD, of Fishawack Communications Ltd and was funded by Sanofi.
Bonadonna RC, Yale J‐F, Brulle‐Wohlhueter C, Boëlle‐Le Corfec E, Choudhary P, Bailey TS. Hypoglycaemia as a function of HbA1c in type 2 diabetes: Insulin glargine 300 U/mL in a patient‐level pooled analysis of EDITION 1, 2 and 3. Diabetes Obes Metab. 2019;21:715–719. 10.1111/dom.13578
Funding information The EDITION 1, 2 and 3 studies were sponsored by Sanofi.
REFERENCES
- 1. Leiter LA, Yale JF, Chiasson JL, Harris S, Kleinstiver P, Sauriol L. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemia management. Can J Diabetes. 2005;29:186‐192. [Google Scholar]
- 2. Perlmuter LC, Flanagan BP, Shah PH, Singh SP. Glycemic control and hypoglycemia: is the loser the winner? Diabetes Care. 2008;31:2072‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977‐986. [DOI] [PubMed] [Google Scholar]
- 4. Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peyrot M, Barnett AH, Meneghini LF, Schumm‐Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29:682‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units mL−1 . Diabetes Care. 2015;38:37‐643. [DOI] [PubMed] [Google Scholar]
- 7. Ritzel R, Roussel R, Bolli GB, et al. Patient‐level meta‐analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755‐2762. [DOI] [PubMed] [Google Scholar]
- 9. Yki‐Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235‐3243. [DOI] [PubMed] [Google Scholar]
- 10. Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin‐naive people with type 2 diabetes on oral glucose‐lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus. Geneva, Switzerland: World Health Organization; 1999. http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf. Accessed October, 2018. [Google Scholar]
- 12. Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 Units/mL versus glargine 100 Units/mL in people with type 1 diabetes: a randomized, Phase 3a, open‐label clinical trial (EDITION 4). Diabetes Care. 2015;38:2217‐2225. [DOI] [PubMed] [Google Scholar]
- 13. Matsuhisa M, Koyama M, Cheng X, et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 1). Diabetes Obes Metab. 2016;18:375‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Terauchi Y, Koyama M, Cheng X, et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 2). Diabetes Obes Metab. 2016;18:366‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Workgroup on Hypoglycemia ADA . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245‐1249. [DOI] [PubMed] [Google Scholar]
- 16. Donnelly LA, Morris AD, Evans JM, DARTS/MEMO collaboration . Adherence to insulin and its association with glycaemic control in patients with type 2 diabetes. QJM. 2007;100:345‐350. [DOI] [PubMed] [Google Scholar]
- 17. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mullins P, Sharplin P, Yki‐Jarvinen H, Riddle MC, Haring HU. Negative binomial meta‐regression analysis of combined glycosylated hemoglobin and hypoglycemia outcomes across eleven Phase III and IV studies of insulin glargine compared with neutral protamine Hagedorn insulin in type 1 and type 2 diabetes mellitus. Clin Ther. 2007;29:1607‐1619. [DOI] [PubMed] [Google Scholar]
- 19. International Hypoglycaemia Study Group . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155‐157. [DOI] [PubMed] [Google Scholar]
- 20. Lipska KJ, Warton EM, Huang ES, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes: the Diabetes and Aging Study. Diabetes Care. 2013;36:3535‐3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seaquist ER, Chow LS. Hypoglycemia in diabetes: does insulin type matter? JAMA. 2017;318:31‐32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of studies (including baseline characteristics) in patient‐level pooled analysis of people with T2DM.


