Abstract
Aims
To investigate the association between day‐to‐day fasting self‐monitored blood glucose (SMBG) variability and risk of hypoglycaemia in type 1 (T1D) and type 2 diabetes (T2D), and to compare day‐to‐day fasting SMBG variability between treatments with insulin degludec (degludec) and insulin glargine 100 units/mL (glargine U100).
Materials and Methods
Data were retrieved from two double‐blind, randomized, treat‐to‐target, two‐period (32 weeks each) crossover trials of degludec vs glargine U100 in T1D (SWITCH 1, n = 501) and T2D (SWITCH 2, n = 720). Available fasting SMBGs were used to determine the standard deviation (SD) of day‐to‐day fasting SMBG variability for each patient and the treatment combination. The association between day‐to‐day fasting SMBG variability and overall symptomatic, nocturnal symptomatic and severe hypoglycaemia was analysed for the pooled population using linear regression, with fasting SMBG variability included as a three‐level factor defined by population tertiles. Finally, day‐to‐day fasting SMBG variability was compared between treatments.
Results
Linear regression showed that day‐to‐day fasting SMBG variability was significantly associated with overall symptomatic, nocturnal symptomatic and severe hypoglycaemia risk in T1D and T2D (P < 0.05). Day‐to‐day fasting SMBG variability was significantly associated (P < 0.01) with all categories of hypoglycaemia risk, with the exception of severe hypoglycaemia in T2D when analysed within tertiles. Degludec was associated with 4% lower day‐to‐day fasting SMBG variability than glargine U100 in T1D (P = 0.0082) and with 10% lower day‐to‐day fasting SMBG variability in T2D (P < 0.0001).
Conclusions
Higher day‐to‐day fasting SMBG variability is associated with an increased risk of overall symptomatic, nocturnal symptomatic and severe hypoglycaemia. Degludec has significantly lower day‐to‐day fasting SMBG variability vs glargine U100.
Keywords: basal insulin, hypoglycaemia, insulin therapy, type 1 diabetes, type 2 diabetes
1. INTRODUCTION
Diabetes, both type 1 (T1D) and type 2 (T2D), results in chronic hyperglycaemia, placing patients at risk of diabetes‐related complications1, 2, 3 that require glucose‐lowering therapies. However, with tighter glycaemic control comes an elevated risk of hypoglycaemia and its associated problems.4 Hypoglycaemia is a major concern for both patients and physicians.5 It has significant negative effects on patients' health and quality of life, and potentially increases the risk of adverse cardiovascular (CV) events.5, 6, 7 The physical and psychological effects of hypoglycaemia make it a primary barrier to establishing glycaemic control.4
Traditionally, management of diabetes has focused on HbA1c.8, 9 As an average measure of glycaemia, HbA1c values do not reflect the fluctuations in blood glucose (glycaemic variability) that more directly indicate a patient's risk of hypoglycaemia or hyperglycaemia.9
Glycaemic variability is determined by a multitude of interconnected factors, some inherent to the patient's physiology and behaviour and some inherent to the patient's diabetes, that is, remaining endogenous insulin secretion and insulin sensitivity, but also reflecting the pharmacodynamic glucose‐lowering variability of treatment.9, 10 Several studies have investigated the role of glycaemic variability on risk of complications; an association with microvascular complications has been demonstrated in patients with T2D, while conflicting results have been found in patients with T1D.11, 12 Furthermore, in patients with T2D, variability in fasting self‐monitored blood glucose (SMBG) has been linked to an increased risk of mortality.13, 14, 15
High day‐to‐day glycaemic variability exposes patients to risk of hypoglycaemia and is a frustrating issue for patients, particularly for patients treated with insulin.16 Although there may be debate about the most accurate metric for measuring glycaemic variability,9 one can expect that improved and simpler techniques will become more widely adopted when continuous glucose monitoring is more widely applied in research and clinical practice. Nonetheless, the consolidated evidence to date supports the importance of both the magnitude and duration of glucose variability9 with respect to increased risk of hypoglycaemia, regardless of the method of variability measurement.15, 17, 18, 19, 20, 21
In many cases, studies concerning glucose variability have limitations in terms of the applicability of their findings to clinical practice. For example, many of these studies were limited by the relatively small numbers of patients studied.19, 20, 21 In addition, there is heterogeneity in the definition of hypoglycaemia used across studies, with some focused on symptomatic hypoglycaemia or episodes with blood glucose (BG) ≤ 70 mg/dL (3.9 mmol/L)17 and others investigating episodes of severe15, 18 and nocturnal hypoglycaemia.18, 22
A post hoc analysis of the two double‐blind crossover trials of insulin degludec (degludec) vs insulin glargine 100 units/mL (glargine U100), in patients with T1D (SWITCH 1)23 and in those with T2D (SWITCH 2),24 provided an opportunity to further study the association between day‐to‐day fasting SMBG variability and the risk of hypoglycaemia and to analyse the difference in day‐to‐day fasting SMBG variability between degludec and glargine U100. The SWITCH trials allowed the investigation of a broader range of definitions of hypoglycaemia, including previously studied severe15, 18 and nocturnal hypoglycaemia,18, 22 but also non‐severe hypoglycaemia. Furthermore, the double‐blind, crossover design adds to the validity of data obtained during the SWITCH trials23, 24 as it reduces the influence of inter‐individual variability and investigator or patient bias on study outcomes.
2. MATERIALS AND METHODS
2.1. SWITCH 1 and SWITCH 2 overviews
Data were retrieved from two double‐blind, randomized, two‐period (32 weeks each) crossover, multicentre, treat‐to‐target clinical trials that compared degludec (100 units/mL, Novo Nordisk, Denmark) once daily (OD) with glargine U100 (Sanofi, France) OD in patients with T1D (SWITCH 1, n = 501),23 or in insulin‐experienced patients with T2D (SWITCH 2, n = 721),24 fulfilling at least one pre‐specified risk criterion for hypoglycaemia. Detailed trial designs and methods were reported previously for SWITCH 123 (ClinicalTrials.gov number: NCT02034513) and SWITCH 224 (ClinicalTrials.gov number)
In the SWITCH 1 trial, mealtime insulin aspart (IAsp) was administered two to four times per day; in the SWITCH 2 trial, all pre‐trial oral antidiabetic drugs (OADs), including any combination of metformin, dipeptidyl peptidase‐4 inhibitor, alpha‐glucosidase inhibitor, thiazolidinediones and sodium glucose cotransporter‐2 inhibitor, were continued at the pre‐trial dose throughout the trial.
In both trials, the 64‐week trial period comprised treatment periods 1 and 2 (32 weeks each) with either degludec or glargine U100. Each treatment period comprised a 16‐week titration period (Weeks 1‐16 and Weeks 32–48) and a 16‐week maintenance period (Weeks 16‐32 and Weeks 48‐64). Consistent with pre‐specified confirmatory analyses from the primary trial results,23, 24 fasting SMBG values and hypoglycaemic episodes in this post hoc analysis were retrieved from the two 16‐week maintenance periods of both treatments (Weeks 16‐32 and Weeks 48‐64) in both trials (Figure S1, Supporting Information). During the maintenance periods, titration of basal insulin could be continued, using the same glucose target (4.0‐5.0 mmol/L [71‐90 mg/dL]) and algorithm used during the titration periods.
Trial protocols were approved according to local regulations by appropriate health authorities and by institutional review boards at all participating institutions, and were conducted in accordance with the Declaration of Helsinki25 and Good Clinical Practice guidelines.26 Written informed consent from all patients was obtained before enrolment.
2.2. Fasting SMBG
In the SWITCH 1 trial, the lowest fasting SMBG values were used for weekly titration of basal insulin, whereas in the SWITCH 2 trial, the mean of the three fasting SMBG measurements on three consecutive days before each contact were used for weekly titration of basal insulin. Therefore, up to seven fasting SMBG measurements per week were available for patients in the SWITCH 1 trial, and up to three fasting SMBG measurements per week were available for patients in the SWITCH 2 trial. Only patients with two or more fasting SMBG values within one week at least once during the maintenance periods contributed to the analyses.
2.3. Statistical analyses of day‐to‐day fasting SMBG variability and hypoglycaemia
To analyse the association between fasting SMBG variability and risk of hypoglycaemia, data were pooled, regardless of the treatment allocation, but were analysed separately for patients with T1D and those with T2D. For each patient and treatment combination, the standard deviation (SD) of fasting SMBG was determined and was used as the measure of day‐to‐day glycaemic variability. First, weekly variances were calculated based on log‐transformed fasting SMBG values. Day‐to‐day fasting SMBG SD variability, for each patient and treatment combination, was defined as the square root of the mean value of the weekly variances of fasting SMBG values across the 16 weeks of the maintenance period, thereby obtaining an accurate estimate of the SD, which is not confounded by dose adjustments.
Linear regression was initially undertaken to analyse the association between day‐to‐day fasting SMBG variability and the rate of hypoglycaemia, using a Poisson model with logarithm of the exposure time (100 years) as offset. This model was an extension of the pre‐specified confirmatory model used during the SWITCH trials,23, 24 with the addition of adjustment for variability measure. This model included treatment, treatment period, sequence and dosing time as fixed effects, day‐to‐day fasting SMBG variability, as defined above, as a covariate, and patient as a random effect.
Patients were also grouped into three tertiles, based on day‐to‐day fasting SMBG variability values, as in the previously published studies, to allow comparison of these data.15, 18 The rates of hypoglycaemia were analysed using the same model as used for the linear regression, with the exception that the day‐to‐day fasting SMBG SD variability was included as a fixed effect.
A second measure of the day‐to‐day fasting SMBG variability was calculated as the geometric mean of the weekly coefficient of variation (CV%). Patients were then grouped into three equally sized tertiles based on these values. The rates of hypoglycaemia were analysed using the same Poisson model as described above, with the exception that the day‐to‐day fasting SMBG variability (CV%) was evaluated only as a fixed effect defined by tertiles.
Hypoglycaemia episodes in the SWITCH trials were classified as overall symptomatic, nocturnal symptomatic and severe hypoglycaemia. Overall symptomatic hypoglycaemia was defined as severe or BG‐confirmed (<3.1 mmol/L [56 mg/dL]) symptomatic episodes; nocturnal symptomatic hypoglycaemia was defined as severe or BG‐confirmed episodes in the time interval of 00:01 to 05:59 am, both inclusive; severe hypoglycaemia was defined as events requiring third‐party assistance, based on the ADA definition.27 All severe episodes reported by investigators or identified via a predefined Medical Dictionary for Regulatory Activities version 18.1 search of safety data were adjudicated prospectively by an external Event Adjudication Committee; only those confirmed by adjudication were included in the analysis.
2.4. Day‐to‐day fasting SMBG variability and hypoglycaemia with degludec vs glargine U100
Available fasting SMBG values during the maintenance period were also used to calculate the day‐to‐day fasting SMBG SD variability in the two treatment arms separately. Weekly day‐to‐day fasting SMBG variability estimates (SDs) were subsequently compared between degludec and glargine U100 using a linear mixed effect model with treatment, treatment period, sex, region (only in SWITCH 1), antidiabetic therapy at screening, visit and dosing time as fixed effects, age as a covariate, and patient as a random effect. A similar treatment comparison was also undertaken using the day‐to‐day fasting SMBG variability based on the CV% values.
The rates of hypoglycaemia with degludec vs glargine U100 for each tertile were analysed using a Poison model with logarithm of the exposure time (100 years) as offset, and with treatment, period, sequence, dosing time, fasting SMBG variability tertiles and its interaction with treatment as fixed effects and with patient as a random effect. In addition, the interaction between fasting SMBG variability and treatment was investigated using a similar Poison model but with fasting SMBG variability on log scale as a linear regressor.
3. RESULTS
In the SWITCH 1 and 2 trials, 16 and 6 patient and treatment combinations were excluded, respectively, from statistical analysis because of too few reported SMBGs. Data sufficient to calculate the SMBG SD values for at least one week for one period were available for patients included in the analysis. Available SMBG variabilities were calculated on an average of 84 and 45 SMBG measurements per patient in the SWITCH 1 and 2 trials, respectively. Baseline characteristics of the patients in each day‐to‐day fasting SMBG variability tertile are shown in Table 1. In patients with T1D, those in the higher day‐to‐day fasting SMBG variability tertile had longer durations of diabetes, were younger and had higher HbA1c values. In patients with T2D, a similar trend was observed for duration of diabetes and HbA1c values, but there was no link between day‐to‐day fasting SMBG variability and mean age or age groups.
Table 1.
Baseline characteristics of patients grouped by low, medium and high day‐to‐day fasting SMBG variability tertiles
| Characteristics | Patients with T1D | Patients with T2D | ||||
|---|---|---|---|---|---|---|
| Low day‐to‐day fasting SMBG variability tertile | Medium day‐to‐day fasting SMBG variability tertile | High day‐to‐day fasting SMBG variability tertile | Low day‐to‐day fasting SMBG variability tertile | Medium day‐to‐day fasting SMBG variability tertile | High day‐to‐day fasting SMBG variability tertile | |
| Number of patients | 189 | 217 | 199 | 288 | 325 | 292 |
| Number of combinations of patient and treatment, n (%) | 285 (100.0) | 287 (100.0) | 285 (100.0) | 424 (100.0) | 424 (100.0) | 424 (100.0) |
| Male, n (%) | 169 (59.3) | 156 (54.4) | 139 (48.8) | 241 (56.8) | 213 (50.2) | 219 (51.7) |
| Race, n (%) | ||||||
| White | 270 (94.7) | 258 (89.9) | 266 (93.3) | 344 (81.1) | 340 (80.2) | 342 (80.7) |
| Black | 13 (4.6) | 23 (8.0) | 16 (5.6) | 55 (13.0) | 60 (14.2) | 69 (16.3) |
| Asian | 2 (0.7) | 1 (0.3) | 1 (0.4) | 19 (4.5) | 16 (3.8) | 3 (0.7) |
| Other | 0 (0.0) | 5 (1.7) | 2 (0.7) | 6 (1.3) | 8 (1.8) | 10 (2.4) |
| Ethnicity: Hispanic or Latino, n (%) | 35 (12.3) | 22 (7.7) | 28 (9.8) | 212 (50.0) | 136 (32.1) | 107 (25.2) |
| Mean age, years | 49.4 | 45.5 | 43.0 | 59.7 | 62.3 | 62.3 |
| Age group | ||||||
| 18 to 64 years, n (%) | 233 (81.8) | 265 (92.3) | 273 (95.8) | 294 (69.3) | 259 (61.1) | 247 (58.3) |
| 65 to 84 years, n (%) | 52 (18.2) | 22 (7.7) | 12 (4.2) | 126 (29.7) | 163 (38.4) | 177 (41.7) |
| >84 years, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (0.9) | 2 (0.5) | 0 (0.0) |
| Body weight, kg | 81.8 | 81.7 | 78.3 | 93.1 | 92.0 | 90.4 |
| BMI, kg/m2 | 27.8 | 27.8 | 26.7 | 32.5 | 32.3 | 31.9 |
| Duration of diabetes, years | 21.0 | 22.9 | 25.3 | 12.7 | 13.8 | 15.6 |
| HbA1c, % | 7.2 | 7.6 | 7.8 | 7.4 | 7.5 | 7.8 |
| HbA1c, mmol/mol | 55.7 | 59.5 | 62.2 | 57.9 | 58.0 | 61.6 |
| FPG, mmol/L | 9.3 | 9.5 | 9.5 | 7.9 | 7.4 | 7.4 |
| FPG, mg/dL | 166.7 | 170.7 | 170.5 | 141.9 | 133.2 | 133.2 |
| eGFR, mL/min/1.73 m2 | 87.2 | 90.8 | 91.8 | 80.8 | 78.2 | 75.5 |
| Insulin treatment at screening | ||||||
| CSII | 41 (14.4) | 59 (20.6) | 62 (21.8) | – | – | – |
| Basal | 244 (85.6) | 228 (79.4) | 223 (78.2) | 424 (100.0) | 424 (100.0) | 424 (100.0) |
| IDet, n (%) | 185 (64.9) | 172 (59.9) | 171 (60.0) | 111 (26.2) | 92 (21.7) | 83 (19.6) |
| NPH, n (%) | 58 (20.4) | 55 (19.2) | 52 (18.2) | 47 (11.1) | 31 (7.3) | 26 (6.1) |
| Glargine U100, n (%) | 1 (0.4) | 1 (0.3) | 0 (0.0) | 266 (62.7) | 301 (71.0) | 315 (74.3) |
Abbreviations: BMI, body mass index; CSII, continuous subcutaneous insulin infusion; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; IDet, insulin detemir; n, number of combinations of patient and treatment; NPH, neutral protamine Hagedorn; SMBG, self‐monitored blood glucose; T1D, type 1 diabetes; T2D, type 2 diabetes.
Data were summarized for the full analysis set. Baseline characteristics data were pooled for two treatment arms and two maintenance periods, and only patient and treatment combinations with two or more fasting SMBG measurements available within one week at least once during the maintenance periods were included in the baseline data. C‐peptide levels were not available to determine baseline endogenous insulin production. Data are given as mean values.
3.1. Day‐to‐day fasting SMBG variability and hypoglycaemia
In patients with T1D, the rates of overall symptomatic, nocturnal symptomatic and severe hypoglycaemia all increased significantly, with higher day‐to‐day fasting SMBG variability (SD values) in the linear regression analysis (Figure 1). In patients with T2D, the same significant association was seen across all hypoglycaemia categories (Figure 1). With a doubling of the day‐to‐day fasting SMBG variability (SD values), the risks of overall, nocturnal symptomatic and severe hypoglycaemia increased by 2.1‐, 2.7‐ and 2.0‐fold for T1D and by 3.3‐, 3.5‐ and 1.9‐fold for T2D, respectively (Figure S2, Supporting Information).
Figure 1.
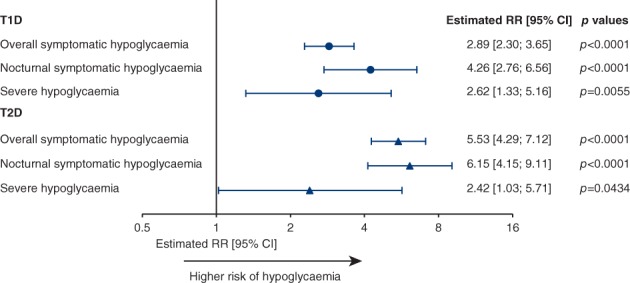
Linear regression analysis of the effect of day‐to‐day fasting SMBG variability (SDs) on rate of hypoglycaemia. Data were based on the full analysis set during the maintenance period. Abbreviations: CI, confidence interval; RR, rate ratio; SMBG, self‐monitored blood glucose; T1D, type 1 diabetes; T2D, type 2 diabetes
In patients with T1D, the cumulative number of hypoglycaemic episodes per patient in the high tertile was higher than that for patients in the low or medium tertiles during the maintenance period (Figure 2). Patients in the high day‐to‐day fasting SMBG variability tertile had a higher number of overall symptomatic, nocturnal symptomatic and severe hypoglycaemic episodes per 100 patient‐years of exposure (PYE), compared with patients in the low or medium tertiles (Figure S3, Supporting Information). In patients with T1D, the day‐to‐day fasting SMBG variability was significantly associated with the rates of overall symptomatic (P < 0.0001), nocturnal symptomatic (P < 0.0001) and severe hypoglycaemia (P = 0.0053) (Table 2 and Figure S3, Supporting Information).
Figure 2.
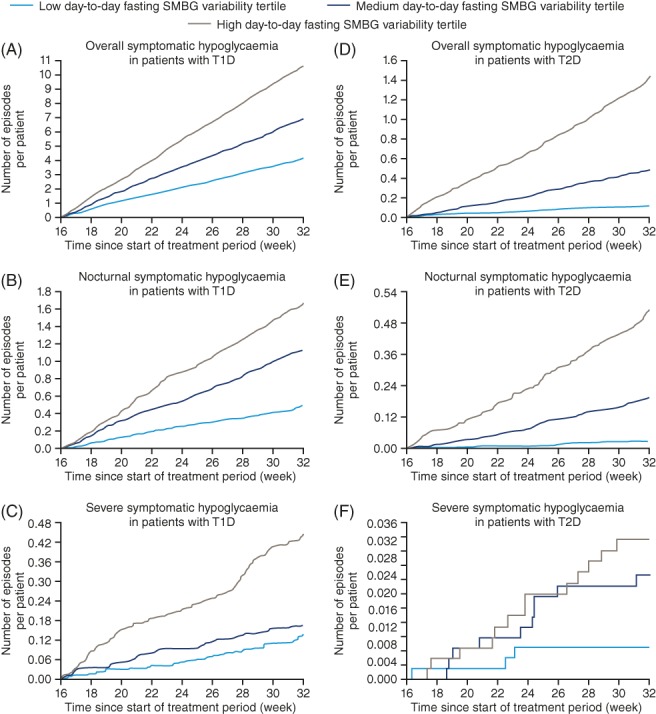
Cumulative number of hypoglycaemic episodes for patients in the low, medium or high day‐to‐day fasting SMBG variability tertile. Based on the safety analysis set. The time‐scale of Weeks 16 to 32 is included in the x‐axis, as only hypoglycaemic episodes during the maintenance periods were considered. All non‐withdrawn patients had the same duration of exposure. Abbreviations: SMBG, self‐monitored blood glucose; T1D, type 1 diabetes; T2D, type 2 diabetes
Table 2.
Effect of day‐to‐day fasting SMBG variability (SDs) on rate of hypoglycaemia by low, medium and high tertiles
| Hypoglycaemia | Day‐to‐day fasting SMBG variability tertile | Patients with T1D | Patients with T2D | ||
|---|---|---|---|---|---|
| Estimate [95% CI] | P‐value | Estimate [95% CI] | P‐value | ||
| Overall symptomatic | Low | 0.68 [0.58; 0.78] | P < 0.0001 | 0.28 [0.20; 0.40] | P < 0.0001 |
| Medium | Reference | Reference | |||
| High | 1.32 [1.19; 1.46] | 2.23 [1.79; 2.78] | |||
| Nocturnal symptomatic | Low | 0.45 [0.33; 0.62] | P < 0.0001 | 0.18 [0.09; 0.36] | P < 0.0001 |
| Medium | Reference | Reference | |||
| High | 1.59 [1.26; 2.01] | 2.18 [1.56; 3.03] | |||
| Severe | Low | 0.82 [0.49; 1.38] | P = 0.0053 | 0.33 [0.09; 1.22] | P = 0.1140 |
| Medium | Reference | Reference | |||
| High | 1.70 [1.11; 2.61] | 1.31 [0.55; 3.09] | |||
Abbreviations: CI, confidence interval; SMBG, self‐monitored blood glucose; T1D, type 1 diabetes; T2D, type 2 diabetes.
Data were based on the full analysis set. Number of episodes was analysed using a Poisson Model with logarithm of exposure time (100 years) as offset. The model included treatment, period, sequence, dosing time and SMBG as fixed effects, and participant as a random effect. SMBG was incorporated as a factor with three tertiles of the fasting SMBG variability, defined by the tertiles the square root of the mean value of the weekly variances of fasting SMBG values across the 16 weeks during the maintenance period.
In patients with T2D, similarly, those in the high day‐to‐day fasting SMBG variability tertile had a higher cumulative number of hypoglycaemic episodes than those in the low or medium tertiles during the maintenance period (Figure 2). The lowest number of overall symptomatic, nocturnal symptomatic and severe hypoglycaemic episodes per 100 PYE were observed in patients in the low day‐to‐day fasting SMBG variability tertile (Figure S3, Supporting Information). Day‐to‐day fasting SMBG variability was significantly associated with overall and nocturnal symptomatic hypoglycaemia in patients with T2D (both P < 0.0001) (Table 2 and Figure S3, Supporting Information). For severe hypoglycaemia, a similar pattern of an increased number of hypoglycaemic episodes per 100 PYE, with higher day‐to‐day fasting SMBG variability,was observed; however, it did not reach statistical significance in patients with T2D (P = 0.1140) (Table 2 and Figure S3, Supporting Information).
In patients with T1D or T2D, a larger proportion in the high day‐to‐day fasting SMBG variability tertile had overall symptomatic, nocturnal symptomatic and severe hypoglycaemia than those in the low day‐to‐day fasting SMBG variability tertile (Figure S3, Supporting Information).
The second measure of day‐to‐day fasting SMBG variability using CV% values indicated the same effect on the risk of hypoglycaemia (Table 3).
Table 3.
Effect of day‐to‐day fasting SMBG variability (CV%) on rate of hypoglycaemia by low, medium and high tertiles
| Hypoglycaemia | Day‐to‐day fasting SMBG variability (CV%) tertile | Patients with T1D | Patients with T2D | ||
|---|---|---|---|---|---|
| Estimate [95% CI] | P value | Estimate [95% CI] | P value | ||
| Overall symptomatic | Low | 0.69 [0.61; 0.78] | P < 0.0001 | 0.31 [0.22; 0.44] | P < 0.0001 |
| Medium | Reference | Reference | |||
| High | 1.18 [1.07; 1.30] | 2.09 [1.67; 2.61] | |||
| Nocturnal symptomatic | Low | 0.44 [0.33; 0.59] | P < 0.0001 | 0.26 [0.14; 0.47] | P < 0.0001 |
| Medium | Reference | Reference | |||
| High | 1.34 [1.08; 1.67] | 2.05 [1.48; 2.84] | |||
| Severe | Low | 0.59 [0.35; 0.98] | P = 0.0106 | 0.68 [0.22; 2.11] | P = 0.2705 |
| Medium | Reference | Reference | |||
| High | 1.28 [0.86; 1.90] | 1.59 [0.65; 3.93] | |||
Abbreviations: CI, confidence interval; CV, coefficient of variation; SMBG, self‐monitored blood glucose; T1D, type 1 diabetes; T2D, type 2 diabetes.
Data were based on the full analysis set. Number of episodes was analysed using a Poisson Model with logarithm of the exposure time (100 years) as offset. The model included treatment, period, sequence, dosing time and SMBG as fixed effects, and participant as a random effect. SMBG was incorporated as a factor with three tertiles of the fasting SMBG variability, defined by the tertiles the geometric mean value of the weekly CV% of fasting SMBG values across the 16 weeks during the maintenance period.
When adjusting for diabetes duration and eGFR at baseline, the significant association between fasting SMBG variability and overall and nocturnal symptomatic hypoglycaemia persisted.
3.2. Day‐to‐day fasting SMBG variability and hypoglycaemia with degludec vs glargine U100
In both SWITCH trials, degludec was associated with significantly lower day‐to‐day fasting SMBG SD variability (T1D variability ratio, 0.96 [0.93; 0.99]95% CI; P = 0.0082 and T2D variability ratio, 0.90 [0.86; 0.93]95% CI; P < 0.0001) as compared to glargine U100. When using CV% values as the measure of day‐to‐day fasting SMBG variability, similar results were observed (T1D variability ratio, 0.99 [0.97; 1.00]95% CI; P = 0.0783 and T2D variability ratio, 0.95 [0.93; 0.97]95% CI; P < 0.0001) with degludec as compared to glargine U100. During treatment with degludec, there were 31% patients with T1D and 30% patients with T2D in the high day‐to‐day fasting SMBG variability tertile, whereas, during treatment with glargine U100, there were 35% patients with T1D and 37% patients with T2D in the high day‐to‐day fasting SMBG variability tertile (Figure S4, Supporting Information). Day‐to‐day fasting SMBG variability was significantly associated with hypoglycaemia for all definitions, with the exception of severe hypoglycaemia in T2D when analysed in variability tertiles. The non‐significant interaction between fasting SMBG variability and treatment in most cases (Table S1, Supporting Information) indicated that fasting SMBG variability had the same effect with the two treatments, and its overall association with the risk of hypoglycaemia remained significant. There were comparable or lower rates of hypoglycaemia with degludec vs glargine U100 within all variability tertiles (Table S1, Supporting Information).
4. DISCUSSION
In these post hoc analyses, fasting SMBG values were used to quantify day‐to‐day fasting SMBG variability and to evaluate its association with the risk of hypoglycaemia in patients with T1D or T2D.
In the present analyses, day‐to‐day fasting SMBG variability was investigated as an indicator of basal insulin action, which is not influenced by food intake or medications such as bolus insulins. The methods of statistical analyses used in this study were consistent with those used in the previous investigations of within‐subject day‐to‐day PK/PD variability of degludec and glargine U100 under clamp conditions.28
Higher day‐to‐day fasting SMBG variability was significantly associated with increased risk of overall symptomatic, nocturnal symptomatic and severe hypoglycaemia in patients with T1D or T2D. Taking day‐to‐day fasting SMBG variability tertiles into consideration, results were similar, with the exception of severe hypoglycaemia in patients with T2D, in whom event rates were relatively low. This association was not significant, although a trend was observed. Findings in the current study are supported by a previous retrospective analysis of the Diabetes Control and Complications Trial (DCCT) data in patients with T1D18 and by a study assessing the association between glycaemic variability and the risk of hypoglycaemia (glucose level < 3.9 mmol/L [70 mg/dL]) using CGM data in patients with T1D or T2D,19 although these studies did not specifically investigate the variability of fasting glucose. While these other studies focused on mean glycaemic variability, in the present analysis, fasting SMBG measurements were utilized as a measure of day‐to‐day fasting glycaemic variability that relates primarily to the effects of basal insulin.
Prior to the publication of results from the DEVOTE study,15 it was unknown whether fasting blood glucose variability confers additional risk for adverse events beyond those associated with chronic hyperglycaemia. Similar to the present study, the DEVOTE study demonstrated, in patients with T2D at high CV risk, a significant association between risk of severe hypoglycaemia and day‐to‐day glycaemic variability in fasting SMBG.15 In this secondary analysis, based on the DEVOTE study, it was also demonstrated that higher day‐to‐day fasting glycaemic variability is associated with higher risk of all‐cause mortality.
It is worth noting that the effect of fasting SMBG variability on the risk of hypoglycaemia appears to be the same for the two treatments, as indicated by the lack of interaction in most cases between treatment and fasting SMBG. The significantly lower day‐to‐day fasting SMBG variability of degludec compared with glargine U100 is consistent with results from both the PK/PD clamp trial in patients with T1D28 and a previous prospective observational study in patients with T1D.29 In the clamp study, degludec had a four‐times lower day‐to‐day variability for the parameter of area under the glucose infusion rate curve during one dosing interval (AUCGIR0‐24; CV 20%) than glargine U100 (CV 82%) under steady‐state conditions.28 The observation of lower fasting SMBG variability with degludec in the present analyses is probably explained by its lower day‐to‐day PD variability vs glargine U100.28, 30 The lower rate of hypoglycaemia with degludec, reported in the original SWITCH trial and others,23, 24, 31, 32 is also probably a consequence of its flatter and less variable action profile vs glargine U100.28 The reduced risk of hypoglycaemia, as the result of the more stable PD of degludec, may itself contribute to lower glycaemic variability by reducing the likelihood of over‐treatment of hypoglycaemia and of patients experiencing rebound post‐hypoglycaemia hyperglycaemia. Thus, the PD profile of degludec may reduce both glycaemic variability and hypoglycaemia.
Strengths of these post hoc analyses based on the SWITCH trials include the crossover design of the trials, which reduces the influence of inter‐individual variability on the obtained outcomes, and the double‐blind design, which would reduce investigator and patient bias. Furthermore, the inclusion criteria of the SWITCH trials allowed for a broader patient population, more closely resembling that encountered in clinical practice, than the cohorts typical of Phase 3 parallel‐group trials. In addition, the number of patients included in these analyses were much larger than those included in some of the previous studies in this area.19, 20, 21 In the SWITCH trials, the threshold for hypoglycaemic episodes was BG < 3.1 mmol/L (56 mg/dL). This is consistent with recent recommendations from the International Hypoglycaemia Study Group whereby hypoglycaemic episodes with BG < 3.0 mmol/L (54 mg/dL) are considered clinically important.33 Furthermore, all severe hypoglycaemic episodes in these two trials were adjudicated by an external event adjudication committee; only those confirmed by adjudication were included in these analyses.
There are limitations to this study in addition to the inherent limitation of a post hoc analysis, which is not pre‐specified. Firstly, glycaemic variability was related solely to fasting SMBG, not allowing for analysis of the patients' blood glucose levels throughout the day. Secondly, as mentioned in the Results section, in the SWITCH 1 and 2 trials, 16 and 6 patients and treatment combinations were excluded, respectively, because of too few reported SMBGs. However, given the large number of patients included and the number of SMBG measurements available per patient, it is believed that the data used were sufficient to calculate the SMBG SD values. Finally, other factors that may affect fasting SMBG variability, such as exercise, food intake and stress, were not investigated in the current study.
In conclusion, these two post hoc analyses of the SWITCH 1 and SWITCH 2 trials further establish the association between day‐to‐day fasting SMBG variability and risk of hypoglycaemia, showing that lower day‐to‐day fasting SMBG variability is significantly associated with lower risk of hypoglycaemia in patients with T1D or T2D. Clearly, treatment choices that reduce day‐to‐day fasting SMBG variability could contribute to a reduced risk of hypoglycaemia. For this reason, reducing glycaemic variability might be a useful additional clinical goal in the management of diabetes.
CONFLICTS OF INTEREST
J. H. D.: advisory panel: Novo Nordisk A/S, Sanofi; research support received: Sanofi; speakers bureau: Novo Nordisk A/S.
T. S. B.: consultant: Abbott, Astra Zeneca, Ascensia, BD Medical Diabetes Care, Calibra, Capillary Biomedical, Eli Lilly, Intarcia, Medtronic, Novo Nordisk, Sanofi; research support received: Abbott, Ambra, Ascensia, BD Medical Diabetes Care, Boehringer Ingelheim, Calibra Medical, Companion Medical, Dance Biopharm, Dexcom, Eli Lilly, Glooko, Glysens, Kowa, Lexicon Pharmaceuticals, Inc., MannKind, Medtronic, Novo Nordisk A/S, Sanofi, Senseonics, Taidoc, Versartis, Xeris; speakers bureau: Abbott, Eli Lilly, Medtronic, Novo Nordisk A/S, Sanofi.
A. B.: advisory panel: Abbott, Janssen Pharmaceuticals, Inc., Sanofi; research support recieved: Boehringer Ingelheim Pharmaceuticals, Inc., Bristol‐Myers Squibb Company, Novo Nordisk A/S, Eli Lilly and Company, Dexcom, Inc., Medtronic, Sanofi, Mylan, Duke Clinical Research Institute, Janssen Pharmaceuticals, Inc., Jaeb Center for Health Research, GlaxoSmithKline, Orexogen Therapeutics, Inc., Hygieia, University of Oxford, AbbVie Inc. Speakers bureau: Abbott, Sanofi, AstraZeneca.
G. G.: research support received: Boehringer Ingelheim Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Inc., Novo Nordisk A/S. Speaker's Bureau; AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc., Dexcom, Inc., Eli Lilly and Company, Merck, Novo Nordisk A/S, Janssen Pharmaceuticals, Inc.
J. G.: consultant: Bioton SA, Merck & Co., Inc., Eli Lilly and Company, Polpharma S.A., Astra Zeneca, Novo Nordisk A/S, Sanofi; speakers bureau: Novo Nordisk A/S Eli Lilly and Company, Servier, Merck Sharp & Dohme Corp., Bioton SA, Roche Diabetes Care, Polpharma S.A., Sanofi, Mylan, AstraZeneca.
S. H.: advisory panel: Eli Lilly and Company, Novo Nordisk A/S, Takeda, Sanofi Aventis, Merck, Boehringer Ingelheim; consultant: Eli Lilly; research support received: Medtronic; speakers bureau: Novo Nordisk A/S, Eli Lilly and Company, Merck Sharp & Dohme Corp., Takeda, Sanofi Aventis, Astra Zeneca, Boehringer Ingelheim.
W. L.: advisory panel: Intarcia, Novo Nordisk A/S, Insulet Corporation, Sanofi Aventis, Thermalin Diabetes, LLC; consultant: Novo Nordisk A/S, Insulet Corporation; research support received: Novo Nordisk A/S, Eli Lilly and Company. Speaker's Bureau; Novo Nordisk A/S, Dexcom, Insulet.
C. H. W.: advisory panel: Abbott, AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc., Eli Lilly and Company, Janssen Pharmaceuticals, Inc., Novo Nordisk, Sanofi; consultant: AstraZeneca, Janssen Pharmaceuticals, Inc., Novo Nordisk A/S, Sanofi. Research Support; AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc., Eli Lilly and Company, Janssen Pharmaceuticals, Inc., Novo Nordisk A/S, Sanofi; speakers bureau: AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc., Eli Lilly and Company, Janssen Pharmaceuticals, Inc., OmniPod, Novo Nordisk A/S, Sanofi.
B. Z.: consultant: Novo Nordisk A/S, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Janssen, Sanofi, Merck.
B. A. B.: employee: Novo Nordisk A/S.
E. H. N.: employee: Novo Nordisk A/S.
A. P. T.: advisory panel: Eli Lilly and Company, Novo Nordisk A/S, Sanofi, Dexcom, Inc., AstraZeneca, Merck & Co., Inc.; research support received: Dexcom, Inc., Novo Nordisk A/S, Sanofi, Eli Lilly and Company, Janssen Pharmaceuticals, Inc., Mylan. stock/shareholder: Ionis Pharmaceuticals, Novo Nordisk A/S, Gilead.
Author contributions
All authors confirm that they meet the International Committee of Medical Journal Editors uniform requirements for authorship. Specifically, all authors made substantial contributions to the interpretation of data for the manuscript, drafted and critically revised the manuscript, provided final approval of the version to be published and agreed to be accountable for all aspects of the manuscript. All authors had access to the final results and vouch for the fidelity of the trial to the protocol. All authors are responsible for the integrity of the work as a whole.
Supporting information
Figure S1. Trial design of SWITCH 1 and SWITCH 2.
Figure S2. Effect on rate of hypoglycaemia when doubling day‐to‐day fasting SMBG variability.
Figure S3. Hypoglycaemic episodes by low, medium and high day‐to‐day fasting SMBG variability tertile.
Figure S4. Percentage of patients in the low, medium or high day‐to‐day fasting SMBG variability tertiles during each treatment.
Table S1. Rates of overall symptomatic, nocturnal symptomatic and severe hypoglycaemia with degludec versus glargine U100 in low, medium and high fasting SMBG variability tertiles.
ACKNOWLEDGMENTS
The authors thank Charlotte Thim Hansen, Novo Nordisk A/S, for her review of and input to the manuscript. Medical writing and editorial support, under the guidance of the authors, was provided by Jin Heppell and Richard McDonald of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk.
Some of the data in this paper have been presented as a poster presentation at the American Diabetes Association's 77th Scientific Sessions, 9 to 13 June 2017, San Diego, California, USA; at the 53rd Annual Meeting of the European Association for the Study of Diabetes, 11 to 15 September 2017, Lisbon, Portugal; and the Association of British Clinical Diabetologists Autumn Meeting, 9 to 10 November 2017, London; and as an oral presentation at the Australian Diabetes Society Annual Scientific Meeting, 30 August to 1 September 2017, Western Australia.
DeVries JH, Bailey TS, Bhargava A, et al. Day‐to‐day fasting self‐monitored blood glucose variability is associated with risk of hypoglycaemia in insulin‐treated patients with type 1 and type 2 diabetes: A post hoc analysis of the SWITCH Trials. Diabetes Obes Metab. 2019;21:622–630. 10.1111/dom.13565
Funding information These secondary analyses and the SWITCH 1 and SWITCH 2 trials were funded by Novo Nordisk A/S.
REFERENCES
- 1. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977‐986. [DOI] [PubMed] [Google Scholar]
- 2. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643‐2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cryer PE. Glycemic goals in diabetes: trade‐off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63:2188‐2195. [DOI] [PubMed] [Google Scholar]
- 5. Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10:711‐722. [DOI] [PubMed] [Google Scholar]
- 6. Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanon VP, Sanon S, Kanakia R, et al. Hypoglycemia from a cardiologist's perspective. Clin Cardiol. 2014;37:499‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirsch IB. Glycemic variability and diabetes complications: does it matter? Of course it does! Diabetes Care. 2015;38:1610‐1614. [DOI] [PubMed] [Google Scholar]
- 9. Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vora J, Heise T. Variability of glucose‐lowering effect as a limiting factor in optimizing basal insulin therapy: a review. Diabetes Obes Metab. 2013;15:701‐712. [DOI] [PubMed] [Google Scholar]
- 11. Smith‐Palmer J, Brandle M, Trevisan R, Orsini Federici M, Liabat S, Valentine W. Assessment of the association between glycemic variability and diabetes‐related complications in type 1 and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:273‐284. [DOI] [PubMed] [Google Scholar]
- 12. Lachin JA‐O, Bebu I, Bergenstal RM, et al. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes Care 2017;40:777‐783. [DOI] [PMC free article] [PubMed]
- 13. Lin C‐C, Li C‐I, Yang S‐Y, et al. Variation of fasting plasma glucose: a predictor of mortality in patients with type 2 diabetes. Am J Med. 2012;125:416.e9‐18. [DOI] [PubMed] [Google Scholar]
- 14. Muggeo M, Zoppini G, Bonora E, et al. Fasting plasma glucose variability predicts 10‐year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care. 2000;23:45‐50. [DOI] [PubMed] [Google Scholar]
- 15. Zinman B, Marso SP, Poulter NR, et al. Day‐to‐day fasting glycaemic variability in DEVOTE: Associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2). Diabetologia. 2018;61:48‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rayman G. Glycaemic control, glucsoe variability and the triangle of diabetes care. Br J Diabetes. 2016;16(suppl 1):S3‐S6. [Google Scholar]
- 17. Qu Y, Jacober SJ, Zhang Q, Wolka LL, DeVries JH. Rate of hypoglycemia in insulin‐treated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther. 2012;14(11):1008‐1012. [DOI] [PubMed] [Google Scholar]
- 18. Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50(12):2553‐2561. [DOI] [PubMed] [Google Scholar]
- 19. Saisho Y, Tanaka C, Tanaka K, et al. Relationships among different glycemic variability indices obtained by continuous glucose monitoring. Prim Care Diabetes. 2015;9:290‐296. [DOI] [PubMed] [Google Scholar]
- 20. Crenier L, Abou‐Elias C, Corvilain B. Glucose variability assessed by low blood glucose index is predictive of hypoglycemic events in patients with type 1 diabetes switched to pump therapy. Diabetes Care. 2013;36:2148‐2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cox DJ, Kovatchev BP, Julian DM, et al. Frequency of severe hypoglycemia in insulin‐dependent diabetes mellitus can be predicted from self‐monitoring blood glucose data. J Clin Endocrinol Metab. 1994;79:1659‐1662. [DOI] [PubMed] [Google Scholar]
- 22. Niskanen L, Virkamäki A, Hansen JB, Saukkonen T. Fasting plasma glucose variability as a marker of nocturnal hypoglycemia in diabetes: evidence from the PREDICTIVE™ study. Diabetes Res Clin Pract. 2009;86:e15‐e18. [DOI] [PubMed] [Google Scholar]
- 23. Lane W, Bailey TS, Gerety G, et al. Effect of insulin degludec vs insulin glargine u100 on hypoglycemia in patients with type 1 diabetes: the switch 1 randomized clinical trial. JAMA. 2017;318:33‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wysham C, Bhargava A, Chaykin L, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: the switch 2 randomized clinical trial. JAMA. 2017;318:45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Medical Association . Declaration of Helsinki ‐ ethical principles for medical research involving human subjects. Last Amended by the 59th WMA Gemeral Assembly, Seoul, Republic of Korea, October 2008. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (Last accessed 01 October 2018).
- 26. International Conference on Harmonisation . ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Pratice E6(R1), Step 4 June 10, 1996. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (Last accessed 01 October 2018).
- 27. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady‐state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859‐864. [DOI] [PubMed] [Google Scholar]
- 29. Yamamoto C, Miyoshi H, Fujiwara Y, et al. Degludec is superior to glargine in terms of daily glycemic variability in people with type 1 diabetes mellitus. Endocr J. 2016;63:53‐60. [DOI] [PubMed] [Google Scholar]
- 30. Birkeland KI, Home PD, Wendisch U, et al. Insulin degludec in type 1 diabetes. Diabetes Care. 2011;34:661‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra‐longacting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal–Bolus Type 1): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet. 2012;379:1489‐1497. [DOI] [PubMed] [Google Scholar]
- 32. Garber AJ, King AB, Prato SD, et al. Insulin degludec, an ultra‐longacting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal‐Bolus Type 2): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet. 2012;379:1498‐1507. [DOI] [PubMed] [Google Scholar]
- 33. International Hypoglycaemia Study Group . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155‐157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Trial design of SWITCH 1 and SWITCH 2.
Figure S2. Effect on rate of hypoglycaemia when doubling day‐to‐day fasting SMBG variability.
Figure S3. Hypoglycaemic episodes by low, medium and high day‐to‐day fasting SMBG variability tertile.
Figure S4. Percentage of patients in the low, medium or high day‐to‐day fasting SMBG variability tertiles during each treatment.
Table S1. Rates of overall symptomatic, nocturnal symptomatic and severe hypoglycaemia with degludec versus glargine U100 in low, medium and high fasting SMBG variability tertiles.


