Summary
Ibrutinib, a Bruton tyrosine kinase inhibitor, is approved for treatment of various B‐cell malignancies. In ibrutinib clinical studies, low‐grade haemorrhage was common, whereas major haemorrhage (MH) was infrequent. We analysed the incidence of and risk factors for MH from 15 ibrutinib clinical studies (N = 1768), including 4 randomised controlled trials (RCTs). Rates of any‐grade bleeding were similar for single‐agent ibrutinib and ibrutinib combinations (39% and 40%). Low‐grade bleeding was more common in ibrutinib‐treated than comparator‐treated patients (35% and 15%), and early low‐grade bleeding was not associated with MH. The proportion of MH in RCTs was higher with ibrutinib than comparators (4.4% vs. 2.8%), but after adjusting for longer exposure with ibrutinib (median 13 months vs. 6 months), the incidence of MH was similar (3.2 vs. 3.1 per 1000 person‐months). MH led to treatment discontinuation in 1% of all ibrutinib‐treated patients. Use of anticoagulants and/or antiplatelets (AC/AP) during the study was common (~50% of patients) and had an increased exposure‐adjusted relative risk for MH in both the total ibrutinib‐treated population (1.9; 95% confidence interval, 1.2–3.0) and RCT comparator‐treated patients (2.4; 95% confidence interval, 1.0–5.6), indicating that ibrutinib may not alter the effect of AC/AP on the risk of MH in B‐cell malignancies.
Keywords: B‐cell neoplasms, lymphoid leukaemias, signalling therapies, clinical results in lymphomas
Ibrutinib, a first‐in‐class, once‐daily inhibitor of Bruton tyrosine kinase (BTK), is approved globally for a variety of B‐cell malignancies and for graft‐versus‐host disease in the United States. Patients with chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL) and mantle cell lymphoma (MCL) are at an approximately 8‐fold increased risk of major haemorrhage (MH) compared with the age‐ and sex‐matched general population, indicating an intrinsic risk for bleeding in these patients, probably from thrombocytopenia, coagulation abnormalities or comorbidities (Gifkins et al, 2015). Clinical studies indicated that ibrutinib and other BTK inhibitors are associated with low‐grade bleeding in patients with B‐cell malignancies (Byrd et al, 2013, 2014, 2016; Wang et al, 2013; Burger et al, 2015; Treon et al, 2015; Walter et al, 2016; Seymour et al, 2017). The mechanism by which BTK inhibitors may predispose a patient to bleeding is not fully elucidated but probably depends on inhibition of collagen‐induced platelet aggregation (Levade et al, 2014; Bye et al, 2015; Kamel et al, 2015; Lipsky et al, 2015; Alberelli et al, 2016). Greater reductions in collagen‐ or ristocetin‐induced platelet aggregation have been reported to correlate with bleeding tendency in patients (Levade et al, 2014; Kamel et al, 2015; Lipsky et al, 2015; Kazianka et al, 2017).
In phase 2 and 3 studies of ibrutinib, low‐grade bleeding events were reported in approximately 50% of patients; major bleeding events (serious bleeding, grade ≥3 bleeding, or any central nervous system [CNS] haemorrhage) were observed in 1–10% of patients (Burger et al, 2015; Byrd et al, 2015, 2017a; Wang et al, 2015; Chanan‐Khan et al, 2016; Dreyling et al, 2016; Coutre et al, 2017; Jones et al, 2017). Consistent with these findings, recent systematic reviews and meta‐analyses have found a greater risk of all‐grade bleeding events, but no significant differences in MH risk in patients treated with ibrutinib compared with other therapies, with the caveat of small numbers (Caron et al, 2017; Yun et al, 2017).
Major haemorrhage can be associated with substantial morbidity and mortality, and this clinical concern is heightened by the association of atrial fibrillation (AF) with BTK inhibitors (Brown et al, 2017; Byrd et al, 2017b; Tam et al, 2017; https://www.azpicentral.com/calquence/calquence.pdf). Antiplatelet (AP) or anticoagulant (AC) therapies used to manage the risk of thromboembolism in patients with AF generally increase the risk for MH. A recent integrated analysis of AF in ibrutinib‐treated patients showed that patients with AF did not have more severe bleeding events than those without AF, with grade ≥3 bleeding events seen in 2% of ibrutinib‐treated patients who had AF and in 2.9% of all ibrutinib‐treated patients (Brown et al, 2017). Among those with an AF event on study, 47% received acetylsalicylic acid (ASA; 20% received other APs), 51% received low‐molecular‐weight heparin (LMWH), 24% received novel oral ACs, and 14% received vitamin K antagonists (VKAs) during the study. However, a recent retrospective, single‐centre study of ibrutinib‐treated patients with haematological malignancies reported a higher rate of major bleeding events, seen in 7 of the 76 patients with AF over a median follow‐up of 32 months (Wiczer et al, 2017).
To characterise the risks of MH with ibrutinib compared with other regimens, we conducted an integrated analysis of data from clinical studies with ibrutinib‐based therapy in patients with B‐cell malignancies. We further evaluated potential risk factors for MH, especially the concomitant use of AC and/or AP agents.
Methods
Data sources
This analysis included data combined from 15 completed clinical studies, including 4 randomised controlled trials (RCTs; RESONATE (Byrd et al, 2014), RESONATE‐2 (Burger et al, 2015), HELIOS (Chanan‐Khan et al, 2016), RAY(Dreyling et al, 2016)), between 3 October 2008 and 31 August 31 2016, of single‐agent or combination ibrutinib in patients with B‐cell malignancies (Table SI).
Outcomes
All bleeding events (i.e. any haemorrhage) were defined with Medical Dictionary for Regulatory Activities (MedDRA; version 17.1; https://www.meddra.org/) preferred terms in the “Haemorrhage terms (excluding laboratory terms)” sub‐Standardized MedDRA Query (SMQ). MH was determined following the framework described by Schulman and Kearon (2005) and defined as the following types of adverse events (AEs) coded as MedDRA (version 17.1) preferred terms in the aforementioned sub‐SMQ: grade ≥3 non‐serious AEs, serious AEs (SAEs), or any‐grade CNS haemorrhage. Any occurrence of multiple simultaneous events or recurrence was coded as more than 1 event. Descriptive statistics were used to summarise all bleeding events and MH. Exposure‐adjusted incidence rates (EAIRs) were calculated to account for differences in treatment duration and were based on the occurrence of the first MH event per patient and should be interpreted as the average rate of the first treatment‐emergent MH event per person‐time of exposure for all patients at risk. Exposure duration was calculated from initiation of ibrutinib to onset of the first MH event or end of treatment plus 30 days, end of study, or death, whichever was the earliest. The 95% confidence interval (CI) for EAIR was calculated using approximation to a normal distribution.
Risk factors
Based on reported risk factors for MH and the availability of data in ibrutinib clinical studies, the following variables were evaluated: age (<65 years; 65–75 years; >75 years), sex, baseline platelet count (<50 × 109/l, 50–100 × 109/l, >100 × 109/l), baseline lymphocyte count (<100 × 109/l, ≥100 × 109/l), Eastern Cooperative Oncology Group (ECOG) performance status (0–1, >1), concomitant use of AP and/or AC, relevant medical history (bleeding events, excessive fall risk, neuropsychiatric disease, haemorrhagic stroke or traumatic brain injury, renal diseases, liver diseases, hypertension and alcohol abuse), use of concomitant moderate or strong CYP3A inhibitors and abnormal international normalised ratio (INR) at baseline (normal ≤1.5, abnormal >1.5). Cox proportional hazards regression models were used to perform univariate analyses to evaluate the effect of each risk factor upon MH onset. Variables with P < 0.15 and selected variables, including treatment (ibrutinib vs. comparator), AC/AP use, age and sex, were included in multivariate analyses. AC/AP use was analysed as a time‐dependent covariate in the Cox hazards model.
Concomitant AC/AP use
The crude relative risk of MH associated with concomitant AC/AP use was calculated by taking the ratio of the incidence rates of MH in the AC/AP‐exposed versus the AC/AP‐unexposed subgroups, that were defined as exposed or unexposed to AC and/or AP at any time during study drug treatment. The AC/AP‐exposure‐adjusted relative risk of MH was calculated based on the number of events per exposed versus unexposed person‐time to AC and/or AP therapy. Person‐time exposed or unexposed to AC/AP was defined as the duration with or without concomitant usage of AC/AP therapy with study drugs, respectively.
Results
Patients
This analysis included 1768 ibrutinib‐treated patients (total ibrutinib pool), composed of 1345 patients treated with single‐agent ibrutinib and 423 patients treated with ibrutinib‐based combination regimens (Table SI). In this pool, 1165 patients (65.9%) had CLL/SLL, 381 patients (21.5%) had MCL and 222 (12.6%) had other B‐cell malignancies, including Waldenström macroglobulinaemia (WM), diffuse large B‐cell lymphoma, follicular lymphoma and other non‐Hodgkin lymphomas. The RCT pools included 756 ibrutinib‐treated patients and 749 comparator‐treated patients. Most patients (82%) in the RCT pool had CLL/SLL and 18% had MCL.
Baseline characteristics were similar between ibrutinib‐ and comparator‐treated patients in the RCT pool and risk factors for MH were comparable between groups (Table 1). Characteristics between the RCT‐ibrutinib group and the total ibrutinib pool were similar, except that nearly twice the proportion of patients in the total ibrutinib pool had a history of bleeding events (8% in the RCT‐ibrutinib group and 15% in the total ibrutinib pool). History of hypertension at baseline was the most commonly reported comorbidity (44% in the RCT pool and 46% in the total ibrutinib pool). Use of AC/AP agents at any time during ibrutinib treatment was similar between the total ibrutinib pool and the RCT‐ibrutinib pool; AC agents were used in 20.1% and 21.7% and AP agents were used in 39.2% and 38.6% of patients in the total ibrutinib pool and RCT‐ibrutinib group, respectively. Approximately 10% of patients received both AC and AP during treatment in each of the total ibrutinib and RCT‐ibrutinib pools.
Table 1.
Baseline demographics*
| Characteristic, n (%) | RCT pool†: ibrutinib ± BR N = 756 | RCT pool†: comparators N = 749 | Total ibrutinib pool N = 1768 |
|---|---|---|---|
| Age | |||
| <65 years | 274 (36) | 288 (38) | 758 (43) |
| 67–75 years | 352 (47) | 331 (44) | 726 (41) |
| >75 years | 130 (17) | 130 (17) | 284 (16) |
| Male | 508 (67) | 506 (68) | 1236 (70) |
| ECOG PS >1 | 13 (2) | 13 (2) | 62 (4) |
| Baseline platelet count | |||
| <50 × 109/l | 32 (4) | 20 (3) | 102 (6) |
| 50–100 × 109/l | 176 (23) | 179 (24) | 406 (23) |
| >100 × 109/l | 545 (72) | 547 (73) | 1256 (71) |
| Lymphocyte count ≥100 × 109/l | 128 (17) | 158 (21) | 221 (13) |
| Use of AC and/or AP‡ | 373 (49) | 359 (48) | 879 (50) |
| Any use of AC | 164 (22) | 145 (19) | 356 (20) |
| Any use of AP | 292 (39) | 276 (37) | 693 (39) |
| Use of both AC and AP | 83 (11) | 62 (8) | 170 (10) |
| Use of strong/moderate CYP3A inhibitor‡ | 241 (32) | 233 (31) | 541 (31) |
| History of bleeding events | 62 (8) | 55 (7) | 261 (15) |
| History of hypertension | 332 (44) | 329 (44) | 807 (46) |
| History of haemorrhagic stroke/TBI | 0 | 0 | 0 |
| History of alcohol abuse | 0 | 3 (0.4) | 4 (0.2) |
| Baseline INR | |||
| Total INR available | 722 (96) | 704 (94) | 1224 (69) |
| Abnormal (>1.5) | 9 (1) | 12 (2) | 14 (1) |
| Normal (≤1.5) | 713 (99) | 692 (92) | 1210 (99) |
AC, anticoagulant; AP, antiplatelet; BR, bendamustine, rituximab; ECOG PS, Eastern Cooperative Oncology Group performance status; INR, international normalised ratio; MH, major haemorrhage; RCT, randomised controlled trial; TBI, traumatic brain injury.
Including common baseline risk factors for MH.
Data from RESONATE (PCYC‐1112, ibrutinib [n = 195] vs. ofatumumab [n = 191]), RESONATE‐2 (PCYC‐1115, ibrutinib [n = 135] vs. chlorambucil [n = 132]), HELIOS (CLL3001, ibrutinib + BR vs. BR alone; n = 287) and RAY (MCL3001, ibrutinib vs. temsirolimus; n = 139) (Byrd et al, 2014; Burger et al, 2015; Chanan‐Khan et al, 2016; (Dreyling et al, 2016).
Concomitant use of AC, AP, or CYP3A inhibitor with ibrutinib or comparator (at any time during safety evaluation period, including 30 days after end of ibrutinib treatment for those without MH, or before onset of first MH for those with MH) including a 7‐day grace period after end of use.
Incidence of all bleeding events
In the total ibrutinib pool, bleeding events occurred in 39% of patients, with grade ≥3 bleeding in 3%. The most common bleeding events were contusion (10.6%), epistaxis (7.9%), increased tendency to bruise (5.2%) and petechiae (6.4%), which were grade 1 or 2 in nearly all cases. The median time to onset of the first bleeding event was 49 days (range, 1–784; Table SII). Bleeding events occurred at a similar rate in patients treated with single‐agent ibrutinib or ibrutinib combinations (39% and 40%).
In the RCT pool, grade 1 or 2 bleeding events were more common in the ibrutinib (268/756, 35%) than the comparator arm (113/749, 15%), but grade ≥3 bleeding events were similarly uncommon (25/756, 3% vs. 17/749, 2%, respectively). The median time to onset of the first bleeding event was comparable between ibrutinib‐ and comparator‐treated patients (56 days [range, 1–695] and 43 days [range, 1–639], respectively; Table SII).
Patients remained on ibrutinib treatment longer than on comparators, with median treatment durations of 11.0 months in the total ibrutinib pool, 13.1 months in the RCT‐ibrutinib pool and 5.8 months in the RCT‐comparator pool that consisted largely of fixed duration therapies. The EAIR of all bleeding events was higher in ibrutinib‐treated patients than in comparator‐treated patients (53.4, 41.0 and 24.0 per 1000 person‐months for the total ibrutinib pool, RCT‐ibrutinib pool and RCT‐comparator pool, respectively; Table SII).
Incidence of MH events
In the total ibrutinib pool, the crude rate of MH was 4.1% with an EAIR of 3.6 per 1000 person‐months (Table 2). Rates of MH were higher among patients with MCL than among those with CLL (25/381, 6.6% vs. 44/1165, 3.8%; Fig 1A,B). Crude MH rates were similar between patients treated with single‐agent ibrutinib and ibrutinib combinations (4.2% and 4.0%) with EAIRs of 3.8 and 3.0 per 1000 person‐months, respectively (Fig 1A,B). Analysis of the incidence of MH by 3‐month intervals extending out to 24 months suggests the possibility of a declining rate over time, with the incidence of MH at 6 months approximately half of that at 3 months, albeit with fewer patients at risk at longer times (Figure S1).
Table 2.
Incidence and characteristics of MH
| RCT pool ibrutinib ± BR N = 756 | RCT pool comparators N = 749 | Total ibrutinib pool N = 1768 | |
|---|---|---|---|
| Patients with MH, n (%) | 33 (4.4) | 21 (2.8) | 73 (4.1) |
| Grade 3/4 | 24 (3.2) | 17 (2.3) | 53 (3.0) |
| CNS event | 7 (0.9) | 0 | 20 (1.1) |
| Serious AE | 28 (3.7) | 13 (1.7) | 59 (3.3) |
| Fatal event | 3 (0.4)* | 0 | 6 (0.3)* |
| EAIR of MH per 1000 person‐months (95% CI) | 3.2 (2.1–4.3) | 3.1 (1.8–4.5) | 3.6 (2.8–4.5) |
| Number of patients with MH events, n (%) | |||
| 1 event | 27 (3.6) | 19 (2.5) | 59 (3.3) |
| 2 events† | 6 (0.8) | 1 (0.1) | 13 (0.7) |
| >2 events† | 0 | 1 (0.1) | 1 (0.1) |
| Median time to onset of first event (range), days | 155.0 (2.0–596.0) | 27.0 (1.0–455.0) | 128.0 (1.0–678.0) |
| MH leading to dose reduction, n (%) | 1 (0.1) | 0 | 1 (0.1) |
| MH leading to treatment discontinuation, n (%) | 7 (0.9) | 2 (0.3) | 21 (1.2) |
AE, adverse event; BR, bendamustine, rituximab; CI, confidence interval; CNS, central nervous system; EAIR, exposure‐adjusted incidence rate; MH, major haemorrhage; RCT, randomised controlled trial.
The 3 fatal MH events in the randomised group occurred in patients with relapsed/refractory disease (Brown et al, 2017); 1 additional patient experienced fatal splenic rupture, which was coded as grade 4 haemorrhage.
Patients were considered to have more than 1 MH event if they had a recurrence or if multiple events occurred simultaneously that were coded as different types of MH.
Figure 1.
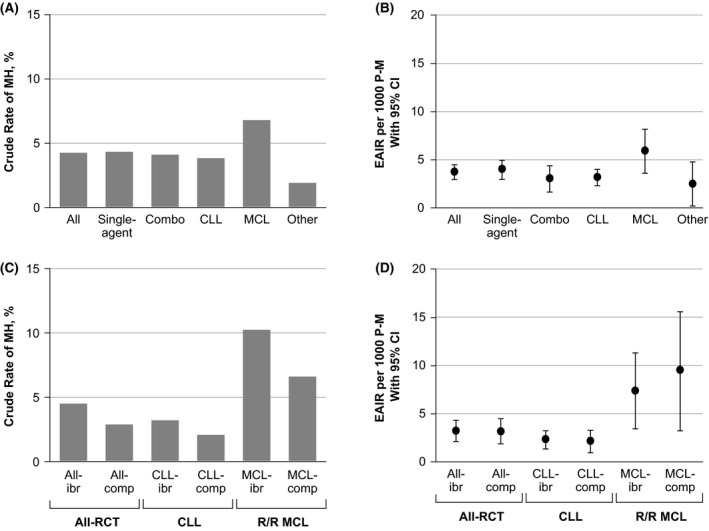
Crude rate and EAIR of MH by treatment approach and histology in the total ibrutinib pool (A, B) and in the RCT pool (C, D). CLL, chronic lymphocytic leukaemia; comp, comparator; EAIR, exposure‐adjusted incidence rate; ibr, ibrutinib; MCL, mantle cell lymphoma; MH, major haemorrhage; P‐M, person‐months; RCT, randomised controlled trial; R/R, relapsed/refractory.
In the RCT pool, the crude incidence of MH was higher in the ibrutinib‐ versus comparator‐treated patients (4.4% vs. 2.8%); however, EAIRs were similar between groups (3.2 vs. 3.1 per 1000 person‐months; Table 2, Fig 1C,D). Higher EAIRs were observed in patients with MCL than in patients with CLL in both arms. For patients with CLL, the crude rates of MH were 3.1% with ibrutinib and 2.0% with comparators (Fig 1C), with similar EAIRs between groups (2.2 vs. 2.1 per 1000 person‐months; Fig 1D). Similarly, the crude rate of MH in patients with MCL (n = 139 per arm) was 10.1% in those treated with ibrutinib and 6.5% in those treated with comparators (Fig 1C) and the EAIRs were 7.4 and 9.4 per 1000 person‐months, respectively (Fig 1D).
Analysis of early nonmajor bleeding and later MH
An exploratory analysis was performed on the total ibrutinib pool in patients who had available data for onset date of bleeding (n = 1749) to determine whether a nonmajor bleeding event increased the risk for developing a subsequent MH event. Of 631 patients with a nonmajor bleeding event, 28 (4.4%) experienced subsequent MH. Of 1118 patients without preceding nonmajor bleeding events, 44 (3.9%) experienced MH, indicating that there was no association between nonmajor bleeding events and subsequent MH events (P = 0.618).
Timing and severity of MH events
In the total ibrutinib pool, 73 patients experienced a total of 88 MH events. In both the total ibrutinib pool and the RCT pool, most patients with MH had only 1 event (Table 2). In the RCT pool, the median time to onset of the first MH event was longer among ibrutinib‐treated than comparator‐treated patients (approximately 4–6 months vs. 1 month; Table 2, Fig 2).
Figure 2.
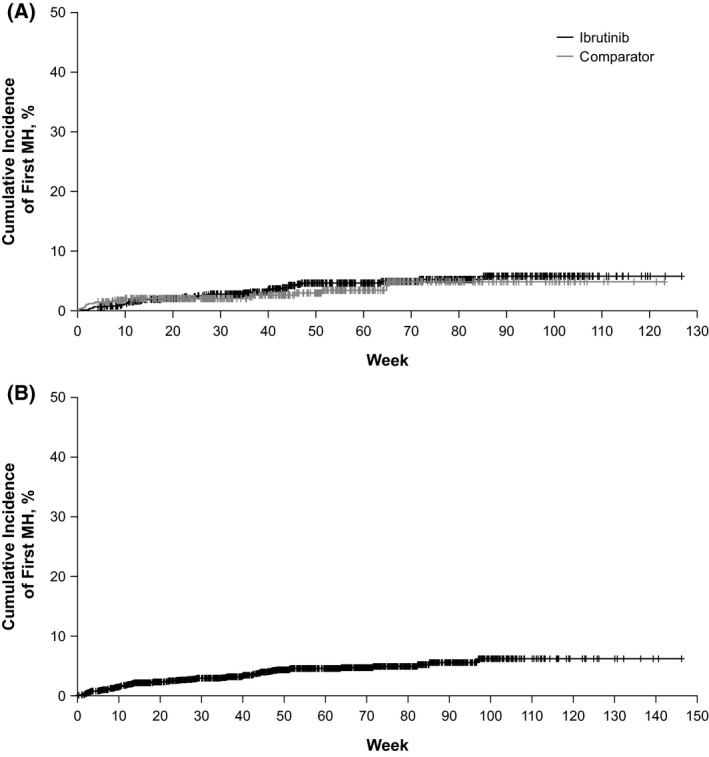
Time to onset of first major haemorrhage (MH) event in the randomised controlled trial pool (A) and total ibrutinib pool (B).
In the total ibrutinib pool, 53 patients (3.0%) reported a grade 3/4 MH event and 59 patients (3.3%) had an SAE of MH. Similar trends were reported for patients treated with single‐agent or ibrutinib combinations. In the RCT pool, grade 3/4 MH events and an SAE of MH occurred in 24 (3.2%) and 28 (3.7%) patients, respectively, in the ibrutinib group, and 17 (2.3%) and 13 (1.7%) patients, respectively, in the comparator group. With the current dataset and observation time, CNS bleeding events were reported only among ibrutinib‐treated patients, in 1.1% of the total ibrutinib pool, 0.9% of the RCT ibrutinib pool and none in the comparator pool.
In the total ibrutinib pool, 23/88 events were CNS MH and 65/88 events were non‐CNS MH. Twenty patients experienced the 23 events of CNS MH, with the most common events being subdural haematoma (14 events) and intracranial haemorrhage (5 events). Six of these 20 patients had MH events associated with trauma (including head injuries due to falls). Fifty‐three patients experienced the 65 events of non‐CNS MH, with the most common events (occurring in >10 patients) being injury or post‐procedural MH (13 events) and gastrointestinal MH (13 events; Table SIII). For the 11 post‐procedural MH events that occurred among 9 patients in the total ibrutinib pool, ibrutinib was not held prior to the procedure in 9 cases.
In this analysis, 6 fatal MH events were reported in the total ibrutinib pool and none were reported in the comparator pool (Table 2). Three patients with fatal MH (ruptured abdominal aortic aneurism, subdural haematoma, post‐procedural haemorrhage) from the RCT‐ibrutinib pool have previously been described (Brown et al, 2017). The 3 additional cases (intracranial haemorrhage, intraventricular haemorrhage, subdural haematoma) are described in Table SIV.
Treatment modifications
In the total ibrutinib pool, treatment was discontinued because of MH in 1% of all patients (N = 1768). Of the 88 MH events, 27 (30.7%) resulted in a temporary hold of ibrutinib, 1 (1.1%) resulted in dose reduction, 22 (25.0%) resulted in ibrutinib discontinuation and 23 (26.1%) resulted in no changes to ibrutinib treatment (Table SV). The remainder had MH events after stopping ibrutinib or had missing data. The outcomes of CNS MH events tended to be worse than non‐CNS MH events, with 4 fatalities from CNS MH events and 2 fatalities from non‐CNS MH events. Four patients with CNS MH events and 4 patients with non‐CNS MH events recovered with sequalae. Overall, most patients (69.3%) recovered without sequalae or are recovering from MH events (Table SV).
Analysis of MH and use of AC/AP agents
In the total ibrutinib pool, AC/AP use at any time during ibrutinib treatment was associated with an increased crude relative risk of MH (1.6; 95% CI, 1.0–2.6). The relative risk for MH after adjusting for AC/AP exposure was 1.9 (95% CI, 1.2–3.0). In the RCT pool, the crude relative risk of MH with AC/AP use was 1.4 (95% CI, 0.7–2.7) in the RCT‐ibrutinib pool and 1.2 (95% CI, 0.5–2.8) in the RCT‐comparator pool (Table 3). The AC/AP exposure‐adjusted relative risk for MH was 1.2 (95% CI, 0.6–2.5) in the RCT‐ibrutinib pool and 2.4 (95% CI, 1.0–5.6) in the RCT‐comparator pool.
Table 3.
The use of AC or AP agents and risk of MH in the RCT population
| RCT pool ibrutinib ± BR N = 756 | RCT pool comparators N = 749 | Total ibrutinib pool N = 1768 | ||||
|---|---|---|---|---|---|---|
| Crude rate, % (n/N) | RR (95% CI) | Crude rate, % (n/N) | RR (95% CI) | Crude rate, % (n/N) | RR (95% CI) | |
| Crude rate of MH | ||||||
| Overall | 4.4 (33/756) | – | 2.8 (21/749) | – | 4.1 (73/1768) | – |
| No use of AC or AP | 3.7 (14/383) | 1.4 (0.7–2.7) | 2.6 (10/390) | 1.2 (0.5–2.8) | 3.1 (28/889) | 1.6 (1.0–2.6) |
| Use of AC and/or AP | 5.1 (19/373) | 3.1 (11/359) | 5.1 (45/879) | |||
| Adjusted rate* (MH/P‐M) | RR (95% CI) | Adjusted rate* (MH/P‐M) | RR (95% CI) | Adjusted rate* (MH/P‐M) | RR (95% CI) | |
| AC/AP exposure‐adjusted RR | ||||||
| No use of AC or AP at time of MH event | 3.0 (23/7,666) | 1.2 (0.6–2.5) | 2.2 (11/4,942) | 2.4 (1.0–5.6) | 2.8 (40/14,410) | 1.9 (1.2–3.0) |
| Use of AC and/or AP | 3.5 (10/2,840) | 5.3 (10/1875) | 5.3 (33/6,280) | |||
AC, anticoagulants; AP, antiplatelet agents; BR, bendamustine, rituximab; CI, confidence interval; MH, major haemorrhage; P‐M, person‐months; RCT, randomised controlled trial; RR, relative risk.
MH incidence rate per 1000 person‐months.
Concurrent use of AC and/or AP at any time during the study period was observed in approximately 50% of patients in both ibrutinib and comparator arms (Table SVI). In the total ibrutinib pool, 311 patients were on AC (median duration of treatment, 16 days [range, 1–677]), and 20 (6%) experienced an MH. Of 311 patients on AC, 116 (37.3%) started AC at study entry, among whom 8 (7%) experienced an MH event (Table SVII). Among 677 patients exposed to AP agents at any time during ibrutinib treatment (median duration of AP 174 days [range, 1–982]), 30 (4%) had an MH. Of 677 patients on AP, 430 (63.5%) started AP at study entry, among whom 21 (5%) experienced an MH event (Table SVIII).
Of the patients treated with AC in the total ibrutinib pool, 243/311 (78%) were on LMWH/heparin (median 9 days), 45/311(14%) on VKAs (median 57 days) and 49/311 (16%) were on direct‐acting oral anticoagulants (DOACs; median 116 days; Table SVII), including apixaban (n = 7), rivaroxaban (n = 24) and dabigatran (n = 16; Table SIX). MH occurred in 15 patients (6%) on LMWH/heparin, 5 patients (11%) on VKAs and 1 patient (2%) on a DOAC (rivaroxaban; Table SVII). Of the patients treated with AP, nonsteroidal anti‐inflammatory drugs (NSAIDs) were used in 356/677 patients (53%) and ADP inhibitors were used in 55/677 patients (8%; Table SVIII). Twenty‐five patients received both ASA and clopidogrel, and 1/25 experienced a single occurrence of MH. The types of AC and AP agents used in the randomised studies were similar between ibrutinib‐ and comparator‐based therapies (Table SVI).
Multivariate analysis of MH risk
Risk factors for MH identified at univariate analysis and pre‐selected variables (treatment, AC/AP use, age and sex) were assessed by multivariate analysis. Ibrutinib was not associated with increased risk for MH versus comparators (P = 0.7; Table 4). MCL was significantly associated with a higher risk of MH than CLL in the total ibrutinib pool (P = 0.019), the total randomised pool (P < 0.001) and both the ibrutinib and comparator arms within the randomised pool (P < 0.001). Use of AC/AP was significantly associated with an increased risk of MH in the total ibrutinib pool (hazard ratio [HR], 1.7; 95% CI, 1.0–2.7; P = 0.041) but not in the total randomised pool. An HR of 1.8 (95% CI, 0.7–4.5; P = 0.196) with AC/AP use was observed in the RCT‐comparator group but did not reach statistical significance, probably because of small sample size. Low baseline platelet count (<100x109/l) was significantly associated with increased risk of MH in comparator‐treated patients in the RCT pool (P < 0.0001) but not in ibrutinib‐treated patients in either the randomised or total pools.
Table 4.
Results of multivariate analysis of risk factors for MH
| Variable | Total ibrutinib pool N = 1768 | Total RCT pool N = 1505 | RCT pool ibrutinib ± BR N = 756 | RCT pool comparators N = 749 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Ibrutinib vs. comparator | – | NA | 1.1 (0.6–2.0) | 0.730 | – | NA | – | NA |
| RR MCL vs. CLL | 1.8 (1.1–3.0) | 0.019 | 4.2 (2.3–7.5) | <0.0001 | 3.5 (1.7–7.4) | 0.0009 | 6.3 (2.3–17.2) | 0.0003 |
| Use of AC or AP as a time‐dependent variable | 1.7 (1.0–2.7) | 0.041 | 1.3 (0.7–2.3) | 0.409 | 1.0 (0.4–2.2) | 0.967 | 1.8 (0.7–4.5) | 0.196 |
|
Baseline platelet counts ≤100 × 109/l vs. >100 × 109/l |
1.2 (0.7–2.0) | 0.458 | 2.3 (1.3–4.0) | 0.005 | 1.1 (0.5–2.5) | 0.737 | 7.1 (2.7–19.0) | <0.0001 |
AC, anticoagulant; AP, antiplatelet; BR, bendamustine, rituximab; CI, confidence interval; CLL, chronic lymphocytic leukaemia; HR, hazard ratio; MCL, mantle cell lymphoma; MH, major haemorrhage; NA, not applicable; RCT, randomised controlled trial; RR, relative risk.
An exploratory analysis showed that HAS‐BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (> 65 years), drugs/alcohol concomitantly) risk score (RS) (Pisters et al, 2010) was significantly associated with greater risk of MH in the total ibrutinib pool (HR, 1.2; 95% CI, 1.0–1.5; P = 0.02), but not in the RCT pool (HR, 1.2; 95% CI, 1.0–1.6; P = 0.099). There was no significant interaction between ibrutinib treatment and HAS‐BLED RS on MH (P = 0.52). Additional results and methodology for this analysis are presented in the Data S1 and Table SX.
Discussion
In clinical studies to date, ibrutinib has been associated with low‐grade bleeding and MH (Caron et al, 2017), thought to be due to platelet dysfunction from on‐target inhibition of BTK together with inhibition of TEC kinase (Atkinson et al, 2003; Byrd et al, 2016). In this integrated analysis of bleeding risk across 15 clinical studies of ibrutinib, including 4 RCTs, we attempted to clarify the risks of significant bleeding and of concurrent anticoagulation. Any‐grade bleeding occurred more frequently in patients in the total ibrutinib pool compared with patients treated with comparators in the randomised studies. Interestingly, prior nonmajor bleeding was not predictive of subsequent MH, suggesting that better predictors of MH would be useful.
While the crude rate of MH was higher in ibrutinib‐treated patients, EAIRs of MH were comparable between ibrutinib‐ and comparator‐treated patients in the RCT pool when considering the longer duration of ibrutinib therapy. In this current analysis with data from 4 RCTs, the severity of MH appeared to be higher in those treated with ibrutinib, in which 7 CNS MH and 3 fatal events occurred in the RCT ibrutinib pool, and no CNS or fatal events were reported in the comparator pool. Given the small numbers, emerging data and longer follow‐up from additional phase 3 randomised studies are needed to further characterise the severity of MH events with ibrutinib versus comparator treatments. Of the 20 CNS events that occurred in the total ibrutinib pool, 6 occurred in the setting of trauma, providing a reminder that many MH events with ibrutinib are provoked. Similarly, in 9 of 11 periprocedural MH events among ibrutinib‐treated patients, ibrutinib was not held prior to the procedure, underscoring the importance of holding ibrutinib for 3–7 days before and after all procedures. Overall, only 1% of all ibrutinib‐treated patients discontinued ibrutinib due to MH with this duration of observation.
A major question we sought to address with this study is the safety of concurrent AP or AC agents with ibrutinib. During the early clinical development of ibrutinib, MH events were observed in patients who received concomitant anticoagulation with warfarin. This led to the exclusion of concomitant use of warfarin (and other VKAs) at study entry in subsequent clinical studies and its use was discouraged during the conduct of the trials (though not prohibited). Overall, about half of the patients in this study were exposed to an AC or AP agent. In the total ibrutinib pool, the rate of MH was 6% among patients treated with an AC at any point. Most of these patients received LMWH and only for a median duration of 9 days, which may limit the interpretation of these data. DOACs have been generally advocated for use in ibrutinib‐treated patients with AF (Brown, 2017; Shatzel et al, 2017) but data have been limited. In this dataset, 49 patients received DOACs for a median of 116 days, with only 1 occurrence of an MH event. Among patients in the total ibrutinib pool who started the study on AC agents, the overall rate of MH was 6.9%, but 0 of 24 patients on DOACs at study entry had an MH. Overall, these data are reassuring but remain limited by small numbers. Patients remained on AP agents for a longer time and the rate of MH was 4%, providing further reassurance that the risk of MH is minimally affected by use of AP agents. Additionally, the incremental risk of MH associated with AC or AP agents was similar between ibrutinib and comparator arms in the RCT pool, suggesting that ibrutinib may not alter or enhance the effect of these agents on bleeding risk relative to other therapies. Concurrent use of supplements with anticoagulant properties may also increase the risk of bleeding on ibrutinib, as epistaxis resulting from fish‐oil supplements has been noted previously in a small number of patients with WM treated with ibrutinib (Treon et al, 2015).
In univariate and multivariate analyses, ibrutinib use was not associated with increased risk of MH in the randomised pool. Consistent with these findings, a recent meta‐analysis that included the 4 RCTs analysed here found that ibrutinib increased the risk of any‐grade bleeding by 2.72‐fold and MH by 1.66‐fold, albeit the latter was not statistically significant (Caron et al, 2017). Caution remains regarding bleeding risk with ibrutinib, particularly in the setting of surgical procedures or concurrent anticoagulation. Concurrent use of AC/AP was significantly associated with increased MH risk in the total ibrutinib pool, although patients in both the total ibrutinib pool and RCT‐comparator pool had a similar hazard ratio for developing MH with concurrent use of AC/AP. In both the total ibrutinib pool and the RCT pool, patients with MCL had a significantly higher risk for MH than patients with CLL. Whether the higher dose of ibrutinib used for patients with MCL contributes to this risk is not known; however, this increased risk is probably disease related, as this finding was noted in both the comparator and ibrutinib groups.
To provide additional guidance in considering individual patient risk for MH on ibrutinib, we investigated the HAS‐BLED RS as a predictor of bleeding risk in this setting. While higher scores were associated with increased risk, the overall value of this assessment was limited because most patients on this study had low scores due to the eligibility requirements of these clinical studies. This observation raises a key caveat of this review, which is that patients assessed in this aggregated review were not fully representative of the CLL patient population. Specifically, very few patients were over 75 years, very few had platelet counts <50 × 109/l and few had platelet counts <100 × 109/l. Our findings are consistent with a recent retrospective cohort study, which included patients on and off clinical studies and reported MH in 3.2% of ibrutinib‐treated patients with CLL, MCL or WM (N = 437 patients) (Pavlik et al, 2016). However, another single‐centre study of patients treated with ibrutinib (N = 71, majority receiving AC or AP) reported MH in 18% of overall patients and in 78% of patients receiving dual AP and AC therapy (Kunk et al, 2016). The variations between these studies are probably due to patient selection differences.
Interestingly, studies have shown that both in vitro and clinical effects of ibrutinib on bleeding stabilise or improve over time (Ysebaert et al, 2014; Lipsky et al, 2015). A study of bleeding risk in ibrutinib‐treated patients with CLL reported a plateau in cumulative incidence of bleeding by 6 months with a median follow‐up of 24 months, suggesting that the risk of bleeding during continued therapy may decrease (Lipsky et al, 2015). In our study, the incidence of MH on ibrutinib also appears lower over time, as evidenced by the declining frequency of MH, although the patient population at risk may also change as patients discontinue ibrutinib because of AEs (Figure S1). These observations are intriguing, and analyses from both longer‐term follow‐up and forthcoming data from additional phase 3 studies will further elucidate the frequency and characteristics of MH with ibrutinib therapy. As an essential component of patient evaluation, the risk for bleeding should continue to be evaluated when considering administering ibrutinib with AC/AP medicines.
In summary, we report on the risk of bleeding with ibrutinib across more than 1700 patients treated in prospective clinical studies. We identified low‐grade bleeding in 36% and MH in 4.1% of patients, with only 1% of patients discontinuing ibrutinib because of MH. Relative to comparators, ibrutinib did not have an increased risk for MH when corrected for treatment duration. Moderate associations between AC/AP use and the risk of MH in both ibrutinib‐ and comparator‐treated patients suggest that ibrutinib may not differentially alter the risk of MH with the use of these agents. Given the significant survival benefit for patients treated with ibrutinib, ongoing studies of bleeding risk over longer follow‐up and from additional phase 3 data, as well as in the general patient population outside of clinical studies, including in the context of specific AC agents, is warranted to optimise therapeutic management.
Authorship contributions
JRB, HY, RValentino, RVempati and TG designed the analysis and interpreted the data. JRB, JM, MSE, SMO, PG, FC, TDS, GF, SR, SEC, M‐SD, PC, UJ, MD, JCB, ST and JAB collected data. RVempati, LB, MM and VR provided input on the analysis and data interpretation. AB, EYL and SC performed statistical analyses and interpreted the data. JRB wrote the initial draft of the manuscript. All authors had access to the study data. All authors critically reviewed or edited the manuscript and approved the final version of the manuscript for submission.
Disclosures of conflicts of interest
JRB: consultancy/advisory role with Gilead, Verastem, Pharmacyclics LLC, an AbbVie Company, Janssen, Sun Biopharma, AstraZeneca, Genentech, Loxo, Sunesis, AbbVie, BeiGene, Kite Pharma, TG Therapeutics and Pfizer; research funding from Gilead, Loxo Oncology, Verastem and Sun; data safety monitoring board service for MorphoSys and Invectys; JM: consultancy/advisory role with Novartis, Pfizer, Bristol‐Myers Squibb, Takeda/Millennium and Regeneron; research funding from Bristol‐Myers Squibb and Pfizer; MSE: stock or other ownership in AstraZeneca and Bristol‐Myers Squibb; patents with Elsevier; consultancy/advisory role with AstraZeneca, Bayer and Pharmacyclics LLC, an AbbVie Company; travel expenses from Bayer and Pharmacyclics LLC, an AbbVie Company; SOB: honoraria from and consultancy/advisory role with, Janssen, AbbVie and Pharmacyclics LLC, an AbbVie Company; research funding from Pharmacyclics LLC, an AbbVie Company; PG: consultancy/advisory role with AbbVie, Acerta, BeiGene, Gilead, Janssen, Pharmacyclics LLC, an AbbVie Company, and Sunesis; research funding from AbbVie, Gilead, Janssen and Novartis; speakers bureau for Gilead; FC: honoraria from Janssen, Gilead, AbbVie, Sunesis and Roche; consultancy/advisory role with AbbVie; research funding from Sunesis; travel expenses from Roche, Gilead and AbbVie; TS: research funding from Genentech, AbbVie, GlaxoSmithKline, Pharmacyclics LLC, an AbbVie Company, Janssen, Celgene, Hospira and Cephalon; patents, royalties, other intellectual property from Mayo Clinic; GF: honoraria from Janssen and AbbVie; research funding from Janssen and Celgene; consulting or advisory role with Janssen and AbbVie; speakers bureau for Janssen and Lundbeck; SR: consultancy/advisory role with Roche, Janssen, Celgene, Napp, Kite, AstraZeneca and Celltrion; research funding and travel expenses from Roche and Janssen; SC: consultancy/advisory role with AbbVie, Gilead, Novartis, Celgene, Janssen and Pharmacyclics LLC, an AbbVie Company; research funding from AbbVie, Pharmacyclics LLC, an AbbVie Company, Gilead, Celgene and Novartis; M‐SD: honoraria and travel expenses from and consulting/advisory role with Janssen, Gilead and AbbVie; PC: honoraria from, Janssen‐Cilag, AbbVie and AstraZeneca; consulting/advisory role with AbbVie, AstraZeneca, Janssen‐Cilag and Roche; research funding from Roche, Gilead, Janssen‐Cilag, AbbVie and Acerta; travel expenses from Roche, Janssen‐Cilag and AbbVie; speakers’ bureau for Janssen‐Cilag and AbbVie; UJ: honoraria and travel expenses from and consultancy/advisory role with Gilead, Novartis and AbbVie; MD: honoraria from Bayer, Celgene, Gilead, Janssen and Roche; consultancy/advisory role with Bayer, Celgene, Gilead, Janssen, Mundipharma, Roche and Sandoz; research funding from Celgene, Janssen, Mundipharma and Roche; travel expenses from Janssen and Roche; JCB: research funding from Genentech, Acerta, Pharmacyclics LLC, an AbbVie Company and Janssen; ST: consultancy/advisory role with and travel expenses from Pharmacyclics LLC, an AbbVie Company and Janssen; research funding from Pharmacyclics LLC, an AbbVie Company; expert testimony for Johnson & Johnson; EL: employment with Pharmacyclics LLC, an AbbVie Company; equity ownership in AbbVie; SChang: employment with Pharmacyclics LLC, an AbbVie Company; equity ownership in AbbVie, Johnson & Johnson, Portola, Abbott and Ipsen; ARB: employment with Pharmacyclics LLC, an AbbVie Company; equity ownership in AbbVie; RVempati: employment with and travel expenses from Pharmacyclics LLC, an AbbVie Company; equity ownership in AbbVie; LB: employment with Pharmacyclics, an AbbVie Company, GlaxoSmithKline and AstraZeneca; equity ownership in AstraZeneca, GlaxoSmithKline, AbbVie and OncoGenex; RValentino: employment and leadership with Pharmacyclics LLC, an AbbVie Company; equity ownership in AbbVie and Gilead; VR: employment and leadership with Pharmacyclics LLC, an AbbVie Company; travel expenses from Pharmacyclics LLC, an AbbVie Company, and Janssen; equity ownership in AbbVie; MM: employment with and equity ownership in Johnson & Johnson; HY: employment with Pharmacyclics LLC, an AbbVie Company; equity ownership in AbbVie; TG: employment and leadership with Pharmacyclics LLC, an AbbVie Company; equity ownership in AbbVie; patents/royalties/other intellectual property with Pharmacyclics LLC, an AbbVie Company, and AbbVie; JAB: honoraria and travel expenses from Janssen; consultancy/advisory role with Gilead, Pharmacyclics LLC, an AbbVie Company and Janssen; research funding from Pharmacyclics LLC, an AbbVie Company.
Supporting information
Data S1. Exploratory analysis by HAS‐BLED risk score.
Table SI. Clinical studies included in the integrated analysis.
Table SII. Incidence of all bleeding events by severity.
Table SIII. Types of MH in the total ibrutinib pool by preferred term.
Table SIV. Brief narratives of patients treated with ibrutinib with MH events leading to death from non‐RCT studies.
Table SV. Summary of ibrutinib treatment modifications and outcomes due to MH in the total ibrutinib pool.
Table SVI. AC and AP agents used concomitantly with ibrutinib.
Table SVII. Use of AC agents and occurrence of MH during treatment with ibrutinib.
Table SVIII. Use of AP agents and occurrence of MH during treatment with ibrutinib.
Table SIX. AC and AP agents used concomitantly with ibrutinib in patients with and without an MH event.
Table SX. HAS‐BLED risk scores and occurrence of MH during treatment with ibrutinib or comparators.
Fig S1. Incidence of MH over 24 months by 3‐month intervals.
Acknowledgments
This study was sponsored by Pharmacyclics LLC, an AbbVie Company. Editorial support was provided by Allison Cherry, PhD and funded by Pharmacyclics LLC, an AbbVie Company.
Presented in part at the 59th annual meeting of the American Society of Hematology
References
- Alberelli, M.A. , Innocenti, I. , Sica, S. , Laurenti, L. & De Candia, E. (2016) PO‐54 ‐ Clinical and laboratory characterization of platelet dysfunction caused by ibrutinib treatment in patients with chronic lymphocytic leukemia. Thrombosis Research, 140(Suppl. 1), S196. [DOI] [PubMed] [Google Scholar]
- Atkinson, B.T. , Ellmeier, W. & Watson, S.P. (2003) Tec regulates platelet activation by GPVI in the absence of Btk. Blood, 102, 3592–3599. [DOI] [PubMed] [Google Scholar]
- Brown, J.R. (2017) How I treat CLL patients with ibrutinib. Blood, 131, 379–386. [DOI] [PubMed] [Google Scholar]
- Brown, J.R. , Moslehi, J. , O'Brien, S. , Ghia, P. , Hillmen, P. , Cymbalista, F. , Shanafelt, T.D. , Fraser, G. , Rule, S. , Kipps, T.J. , Coutre, S. , Dilhuydy, M.S. , Cramer, P. , Tedeschi, A. , Jaeger, U. , Dreyling, M. , Byrd, J.C. , Howes, A. , Todd, M. , Vermeulen, J. , James, D.F. , Clow, F. , Styles, L. , Valentino, R. , Wildgust, M. , Mahler, M. & Burger, J.A. (2017) Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica, 102, 1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, J.A. , Tedeschi, A. , Barr, P.M. , Robak, T. , Owen, C. , Ghia, P. , Bairey, O. , Hillmen, P. , Bartlett, N.L. , Li, J. , Simpson, D. , Grosicki, S. , Devereux, S. , McCarthy, H. , Coutre, S. , Quach, H. , Gaidano, G. , Maslyak, Z. , Stevens, D.A. , Janssens, A. , Offner, F. , Mayer, J. , O'Dwyer, M. , Hellmann, A. , Schuh, A. , Siddiqi, T. , Polliack, A. , Tam, C.S. , Suri, D. , Cheng, M. , Clow, F. , Styles, L. , James, D.F. & Kipps, T.J. (2015) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. New England Journal of Medicine, 373, 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye, A.P. , Unsworth, A.J. , Vaiyapuri, S. , Stainer, A.R. , Fry, M.J. & Gibbins, J.M. (2015) Ibrutinib inhibits platelet integrin alphaIIbbeta3 outside‐in signaling and thrombus stability but not adhesion to collagen. Arteriosclerosis, Thrombosis, and Vascular Biology, 35, 2326–2335. [DOI] [PubMed] [Google Scholar]
- Byrd, J.C. , Furman, R.R. , Coutre, S.E. , Flinn, I.W. , Burger, J.A. , Blum, K.A. , Grant, B. , Sharman, J.P. , Coleman, M. , Wierda, W.G. , Jones, J.A. , Zhao, W. , Heerema, N.A. , Johnson, A.J. , Sukbuntherng, J. , Chang, B.Y. , Clow, F. , Hedrick, E. , Buggy, J.J. , James, D.F. & O'Brien, S. (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. New England Journal of Medicine, 369, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, J.C. , Brown, J.R. , O'Brien, S. , Barrientos, J.C. , Kay, N.E. , Reddy, N.M. , Coutre, S. , Tam, C.S. , Mulligan, S.P. , Jaeger, U. , Devereux, S. , Barr, P.M. , Furman, R.R. , Kipps, T.J. , Cymbalista, F. , Pocock, C. , Thornton, P. , Caligaris‐Cappio, F. , Robak, T. , Delgado, J. , Schuster, S.J. , Montillo, M. , Schuh, A. , de Vos, S. , Gill, D. , Bloor, A. , Dearden, C. , Moreno, C. , Jones, J.J. , Chu, A.D. , Fardis, M. , McGreivy, J. , Clow, F. , James, D.F. & Hillmen, P. (2014) Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. New England Journal of Medicine, 371, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, J.C. , Furman, R.R. , Coutre, S.E. , Burger, J.A. , Blum, K.A. , Coleman, M. , Wierda, W.G. , Jones, J.A. , Zhao, W. , Heerema, N.A. , Johnson, A.J. , Shaw, Y. , Bilotti, E. , Zhou, C. , James, D.F. & O'Brien, S. (2015) Three‐year follow‐up of treatment‐naive and previously treated patients with CLL and SLL receiving single‐agent ibrutinib. Blood, 125, 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, J.C. , Harrington, B. , O'Brien, S. , Jones, J.A. , Schuh, A. , Devereux, S. , Chaves, J. , Wierda, W.G. , Awan, F.T. , Brown, J.R. , Hillmen, P. , Stephens, D.M. , Ghia, P. , Barrientos, J.C. , Pagel, J.M. , Woyach, J. , Johnson, D. , Huang, J. , Wang, X. , Kaptein, A. , Lannutti, B.J. , Covey, T. , Fardis, M. , McGreivy, J. , Hamdy, A. , Rothbaum, W. , Izumi, R. , Diacovo, T.G. , Johnson, A.J. & Furman, R.R. (2016) Acalabrutinib (ACP‐196) in relapsed chronic lymphocytic leukemia. New England Journal of Medicine, 374, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, J.C. , Hillmen, P. , O'Brien, S.M. , Barrientos, J.C. , Reddy, N.M. , Coutre, S.E. , Tam, C.S. , Mulligan, S.P. , Jäger, U. , Barr, P.M. , Furman, R.R. , Kipps, T.J. , Thornton, P. , Pagel, J.M. , Burger, J.A. , Jones, J.A. , Dai, S. , Vezan, R.N. , James, D.F. & Brown, J.R. (2017a) Long‐term efficacy and safety with ibrutinib (ibr) in previously treated chronic lymphocytic leukemia (CLL): up to four years follow‐up of the RESONATE study. Journal of Clinical Oncology, 35, 7510–7510. [Google Scholar]
- Byrd, J.C. , Owen, R. , O'Brien, S.M. , Brown, J.R. , Hillmen, P. , Bitman, B. , Chernyukhin, N. , Hamdy, A. , Izumi, R. , Patel, P. , Schwartz‐Sagi, L. , Tucker, E. , Fowler, N.H. , Streetly, M.J. , Wiestner, A. , Rule, S. & Wang, M. (2017b) Pooled analysis of safety data from clinical trials evaluating acalabrutinib monotherapy in hematologic malignancies. Blood, 130, 4326–4326. [DOI] [PubMed] [Google Scholar]
- Caron, F. , Leong, D.P. , Hillis, C. , Fraser, G. & Siegal, D. (2017) Current understanding of bleeding with ibrutinib use: a systematic review and meta‐analysis. Blood Advances, 1, 772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanan‐Khan, A. , Cramer, P. , Demirkan, F. , Fraser, G. , Silva, R.S. , Grosicki, S. , Pristupa, A. , Janssens, A. , Mayer, J. , Bartlett, N.L. , Dilhuydy, M.S. , Pylypenko, H. , Loscertales, J. , Avigdor, A. , Rule, S. , Villa, D. , Samoilova, O. , Panagiotidis, P. , Goy, A. , Mato, A. , Pavlovsky, M.A. , Karlsson, C. , Mahler, M. , Salman, M. , Sun, S. , Phelps, C. , Balasubramanian, S. , Howes, A. & Hallek, M. ; HELIOS Investigators . (2016) Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double‐blind, phase 3 study. The Lancet Oncology, 17, 200–211. [DOI] [PubMed] [Google Scholar]
- Coutre, S.E. , Furman, R.R. , Flinn, I.W. , Burger, J.A. , Blum, K. , Sharman, J. , Jones, J. , Wierda, W. , Zhao, W. , Heerema, N.A. , Johnson, A.J. , Tran, A. , Zhou, C. , Bilotti, E. , James, D.F. , Byrd, J.C. & O'Brien, S. (2017) Extended treatment with single‐agent ibrutinib at the 420 mg dose leads to durable responses in chronic lymphocytic leukemia/small lymphocytic lymphoma. Clinical Cancer Research, 23, 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyling, M. , Jurczak, W. , Jerkeman, M. , Silva, R.S. , Rusconi, C. , Trneny, M. , Offner, F. , Caballero, D. , Joao, C. , Witzens‐Harig, M. , Hess, G. , Bence‐Bruckler, I. , Cho, S.G. , Bothos, J. , Goldberg, J.D. , Enny, C. , Traina, S. , Balasubramanian, S. , Bandyopadhyay, N. , Sun, S. , Vermeulen, J. , Rizo, A. & Rule, S. (2016) Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle‐cell lymphoma: an international, randomised, open‐label, phase 3 study. Lancet, 387, 770–778. [DOI] [PubMed] [Google Scholar]
- Gifkins, D.M. , Matcho, A. , Yang, H. , Xu, Y. , Gooden, M.A. & Wildgust, M. (2015) Incidence of major hemorrhage among CLL and MCL patients compared to the general elderly population: an analysis of the US SEER‐medicare linked database. Blood, 126, 3268. [Google Scholar]
- Jones, J.A. , Hillmen, P. , Coutre, S. , Tam, C. , Furman, R.R. , Barr, P.M. , Schuster, S.J. , Kipps, T.J. , Flinn, I.W. , Jaeger, U. , Burger, J.A. , Cheng, M. , Ninomoto, J. , James, D.F. , Byrd, J.C. & O'Brien, S.M. (2017) Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single‐agent ibrutinib. British Journal of Haematology, 178, 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel, S. , Horton, L. , Ysebaert, L. , Levade, M. , Burbury, K. , Tan, S. , Cole‐Sinclair, M. , Reynolds, J. , Filshie, R. , Schischka, S. , Khot, A. , Sandhu, S. , Keating, M.J. , Nandurkar, H. & Tam, C.S. (2015) Ibrutinib inhibits collagen‐mediated but not ADP‐mediated platelet aggregation. Leukemia, 29, 783–787. [DOI] [PubMed] [Google Scholar]
- Kazianka, L. , Drucker, C. , Skrabs, C. , Thomas, W. , Melchardt, T. , Struve, S. , Bergmann, M. , Staber, P.B. , Porpaczy, E. , Einberger, C. , Heinz, M. , Hauswirth, A. , Raderer, M. , Pabinger, I. , Thalhammer, R. , Egle, A. , Wendtner, C.M. , Follows, G. , Hoermann, G. , Quehenberger, P. , Jilma, B. & Jaeger, U. (2017) Ristocetin‐induced platelet aggregation for monitoring of bleeding tendency in CLL treated with ibrutinib. Leukemia, 31, 1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunk, P.R. , Mock, J. , Devitt, M.E. , Palkimas, S. , Sen, J. , Portell, C.A. & Williams, M.E. (2016) Major bleeding with ibrutinib: more than expected. Blood, 128, 3229–3229. [Google Scholar]
- Levade, M. , David, E. , Garcia, C. , Laurent, P.A. , Cadot, S. , Michallet, A.S. , Bordet, J.C. , Tam, C. , Sie, P. , Ysebaert, L. & Payrastre, B. (2014) Ibrutinib treatment affects collagen and von Willebrand factor‐dependent platelet functions. Blood, 124, 3991–3995. [DOI] [PubMed] [Google Scholar]
- Lipsky, A.H. , Farooqui, M.Z. , Tian, X. , Martyr, S. , Cullinane, A.M. , Nghiem, K. , Sun, C. , Valdez, J. , Niemann, C.U. , Herman, S.E. , Saba, N. , Soto, S. , Marti, G. , Uzel, G. , Holland, S.M. , Lozier, J.N. & Wiestner, A. (2015) Incidence and risk factors of bleeding‐related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica, 100, 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlik, A. , Barr, H. , Dotson, E. , Byrd, J.C. , Blum, K.A. , Awan, F.T. , Woyach, J.A. , Maddocks, K.J. , Christian, B.A. & Jones, J. (2016) Major bleeding complications among patients treated with ibrutinib and concomitant antiplatelet, anticoagulant, or supplemental therapy. Blood, 128, 4387–4387. [Google Scholar]
- Pisters, R. , Lane, D.A. , Nieuwlaat, R. , de Vos, C.B. , Crijns, H.J. & Lip, G.Y. (2010) A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest, 138, 1093–1100. [DOI] [PubMed] [Google Scholar]
- Schulman, S. & Kearon, C. ; for the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. Journal of Thrombosis and Haemostasis, 3, 692–694. [DOI] [PubMed] [Google Scholar]
- Seymour, J.F. , Opat, S. , Cull, G. , Trotman, J. , Gottlieb, D. , Simpson, D. , Marlton, P. , Anderson, M. , Ku, M. , Ritchie, D.S. , Ratnasingam, S. , Augustson, B. , Kim, W. , Wang, L. , Xue, L. , Hilger, J. , Huang, J. , Hedrick, E. , Roberts, A.W. & Tam, C.S. (2017) High overall response rate with the BTK inhibitor BGB‐3111 in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma: an update on safety and activity. Hematological Oncology, 35, 234–235. [Google Scholar]
- Shatzel, J.J. , Olson, S.R. , Tao, D.L. , McCarty, O.J.T. , Danilov, A.V. & DeLoughery, T.G. (2017) Ibrutinib‐associated bleeding: pathogenesis, management and risk reduction strategies. Journal of Thrombosis and Haemostasis, 15, 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, C.S. , Simpson, D. , Opat, S. , Kim, W.S. , Wang, M. , Cull, G. , Johnston, P.B. , Munoz, J. , Lee, W.‐S. , Marlton, P. , Gottlieb, D. , Wang, L. , Huang, J. , Hilger, J. , Xue, L. , Ro, S. & Trotman, J. (2017) Safety and activity of the highly specific BTK inhibitor BGB‐3111 in patients with indolent and aggressive non Hodgkin's lymphoma. Blood, 130, 152–152. [Google Scholar]
- Treon, S.P. , Tripsas, C.K. , Meid, K. , Warren, D. , Varma, G. , Green, R. , Argyropoulos, K.V. , Yang, G. , Cao, Y. , Xu, L. , Patterson, C.J. , Rodig, S. , Zehnder, J.L. , Aster, J.C. , Harris, N.L. , Kanan, S. , Ghobrial, I. , Castillo, J.J. , Laubach, J.P. , Hunter, Z.R. , Salman, Z. , Li, J. , Cheng, M. , Clow, F. , Graef, T. , Palomba, M.L. & Advani, R.H. (2015) Ibrutinib in previously treated Waldenstrom's macroglobulinemia. New England Journal of Medicine, 372, 1430–1440. [DOI] [PubMed] [Google Scholar]
- Walter, H.S. , Rule, S.A. , Dyer, M.J. , Karlin, L. , Jones, C. , Cazin, B. , Quittet, P. , Shah, N. , Hutchinson, C.V. , Honda, H. , Duffy, K. , Birkett, J. , Jamieson, V. , Courtenay‐Luck, N. , Yoshizawa, T. , Sharpe, J. , Ohno, T. , Abe, S. , Nishimura, A. , Cartron, G. , Morschhauser, F. , Fegan, C. & Salles, G. (2016) A phase 1 clinical trial of the selective BTK inhibitor ONO/GS‐4059 in relapsed and refractory mature B‐cell malignancies. Blood, 127, 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M.L. , Rule, S. , Martin, P. , Goy, A. , Auer, R. , Kahl, B.S. , Jurczak, W. , Advani, R.H. , Romaguera, J.E. , Williams, M.E. , Barrientos, J.C. , Chmielowska, E. , Radford, J. , Stilgenbauer, S. , Dreyling, M. , Jedrzejczak, W.W. , Johnson, P. , Spurgeon, S.E. , Li, L. , Zhang, L. , Newberry, K. , Ou, Z. , Cheng, N. , Fang, B. , McGreivy, J. , Clow, F. , Buggy, J.J. , Chang, B.Y. , Beaupre, D.M. , Kunkel, L.A. & Blum, K.A. (2013) Targeting BTK with ibrutinib in relapsed or refractory mantle‐cell lymphoma. New England Journal of Medicine, 369, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M.L. , Blum, K.A. , Martin, P. , Goy, A. , Auer, R. , Kahl, B.S. , Jurczak, W. , Advani, R.H. , Romaguera, J.E. , Williams, M.E. , Barrientos, J.C. , Chmielowska, E. , Radford, J. , Stilgenbauer, S. , Dreyling, M. , Jedrzejczak, W.W. , Johnson, P. , Spurgeon, S.E. , Zhang, L. , Baher, L. , Cheng, M. , Lee, D. , Beaupre, D.M. & Rule, S. (2015) Long‐term follow‐up of MCL patients treated with single‐agent ibrutinib: updated safety and efficacy results. Blood, 126, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiczer, T.E. , Levine, L.B. , Brumbaugh, J. , Coggins, J. , Zhao, Q. , Ruppert, A.S. , Rogers, K. , McCoy, A. , Mousa, L. , Guha, A. , Heerema, N.A. , Maddocks, K. , Christian, B. , Andritsos, L.A. , Jaglowski, S. , Devine, S. , Baiocchi, R. , Woyach, J. , Jones, J. , Grever, M. , Blum, K.A. , Byrd, J.C. & Awan, F.T. (2017) Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Advances, 1, 1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ysebaert, L. , Levade, M. , Cedric, G. , Michallet, A.‐S. , Tam, C. , Pierre, S. & Payrastre, B. (2014) Elucidation of mild bleeding disorders reported under ibrutinib (Imbruvica(R)) therapy: implications for optimal clinical management. Blood, 124, 3296. [Google Scholar]
- Yun, S. , Vincelette, N.D. , Acharya, U. & Abraham, I. (2017) Risk of atrial fibrillation and bleeding diathesis associated with ibrutinib treatment: a systematic review and pooled analysis of four randomized controlled trials. Clinical Lymphoma Myeloma and Leukemia, 17, e13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Exploratory analysis by HAS‐BLED risk score.
Table SI. Clinical studies included in the integrated analysis.
Table SII. Incidence of all bleeding events by severity.
Table SIII. Types of MH in the total ibrutinib pool by preferred term.
Table SIV. Brief narratives of patients treated with ibrutinib with MH events leading to death from non‐RCT studies.
Table SV. Summary of ibrutinib treatment modifications and outcomes due to MH in the total ibrutinib pool.
Table SVI. AC and AP agents used concomitantly with ibrutinib.
Table SVII. Use of AC agents and occurrence of MH during treatment with ibrutinib.
Table SVIII. Use of AP agents and occurrence of MH during treatment with ibrutinib.
Table SIX. AC and AP agents used concomitantly with ibrutinib in patients with and without an MH event.
Table SX. HAS‐BLED risk scores and occurrence of MH during treatment with ibrutinib or comparators.
Fig S1. Incidence of MH over 24 months by 3‐month intervals.


