Abstract
Background
Patients with moderate‐to‐severe psoriasis require long‐term treatment, yet few trials compare outcomes beyond a short‐term induction period. Quantitative comparisons of long‐term outcomes in patients with psoriasis are limited. To our knowledge, no network meta‐analysis (NMA) of such data has been performed.
Objective
To compare novel systemic therapies, both biologic and non‐biologic, approved for moderate‐to‐severe psoriasis by conducting a systematic review (SR) and NMA of Psoriasis Area and Severity Index (PASI) outcomes measured at or around 1 year.
Methods
An SR was conducted to identify studies reporting PASI 75, PASI 90 and PASI 100 responses. Feasibility of an NMA on maintenance phase endpoints was assessed and sources of heterogeneity considered. Data appropriate for analysis were modelled using a Bayesian multinomial likelihood model with probit link. Wherever possible, data corresponding to an intention‐to‐treat approach with non‐responder imputation were used.
Results
Twenty‐four studies reporting outcomes at 40–64 weeks were identified, but heterogeneity in study design allowed synthesis of only 17. Four 52‐week randomized controlled trials (RCTs) comprised the primary analysis, which found brodalumab was significantly more efficacious than secukinumab, ustekinumab and etanercept. Secukinumab was also more efficacious than ustekinumab and both outperformed etanercept. In a secondary analysis, evidence from 13 additional studies and 4 further therapies (adalimumab, apremilast, infliximab and ixekizumab) was included by comparing long‐term outcomes from active interventions to placebo outcomes extrapolated from induction. Results were consistent with the primary analysis: brodalumab was most effective, followed by ixekizumab and secukinumab, then ustekinumab, infliximab and adalimumab. Etanercept and apremilast had the lowest expected long‐term efficacy. Results were similar when studies with low prior exposure to biological therapies were excluded.
Conclusion
Results suggest that brodalumab is associated with a higher likelihood of sustained PASI response, including complete clearance, at week 52 than comparators. Further long‐term active‐comparator RCT data are required to better assess relative efficacy across therapies.
Introduction
Psoriasis is a common inflammatory skin condition, estimated to affect 2–3% of the worldwide population.1 Moderate‐to‐severe chronic plaque psoriasis symptoms have a significant negative impact on patient quality of life2 and are associated with a considerable economic burden.3 Approximately 90% of cases require long‐term therapy4; therefore, therapies with favourable efficacy and safety as demonstrated in longer‐term trials stand to make a meaningful difference to the lives of patients.5
Treatments such as the anti‐tumour necrosis factor (TNF) therapies, adalimumab, etanercept and infliximab, and the interleukin (IL)‐12/23 inhibitor, ustekinumab, transformed the treatment of psoriasis when they were approved. More recently, three therapies focusing on the IL‐17 pathway have been approved: secukinumab and ixekizumab, both IL‐17A inhibitors, and brodalumab, a human monoclonal antibody which targets the IL‐17 receptor A (IL‐17RA) on keratinocytes and immune cells. These biological therapies, along with the phosphodiesterase 4 (PD4) inhibitor apremilast, have proven to be effective options for many patients, though they are typically available only to patients with moderate‐to‐severe disease who have failed or are ineligible for conventional systemic therapy.
Despite their importance, comparisons of long‐term outcomes in patients with psoriasis are limited due to complicated trial designs and inconsistencies in analysis and data handling methods used.6 Many long‐term trials have multiple phases, are not clear or consistent in how they deal with imputations of missing observations or even in which population outcomes are being analysed. For these reasons, most systematic literature reviews (SLRs) and meta‐analyses in psoriasis have focused on induction phase outcomes. One 2015 review and meta‐analysis compared 24‐week outcomes of standard systemic and biological therapies,7 though the authors also noted limitations of the long‐term data available. Since then, several 52‐week randomized controlled trials (RCTs) have been published demonstrating the longer‐term efficacy of some licensed therapies. To our knowledge, no formal synthesis of these outcomes has been attempted.
With so many therapies licensed for moderate‐to‐severe psoriasis and only a few compared directly in a head‐to‐head fashion, traditional pairwise meta‐analysis alone is insufficient to guide practical clinical decision making. Network meta‐analysis (NMA) offers a set of methods to visualize and interpret a broad evidence base and to determine the comparative efficacy of multiple interventions.8 The technique borrows strength from indirect evidence to enable the simultaneous evaluation of relative effects that have not been investigated directly in RCTs9 and has been used extensively to evaluate short‐term effects of psoriasis treatments.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24
The objective of this study was to compare novel systemic therapies, both biologic and non‐biologic, approved for the treatment of moderate‐to‐severe psoriasis by conducting a SLR and NMA of Psoriasis Area and Severity Index (PASI) outcomes measured at or around 1 year.
Materials and methods
Systematic literature review
An SLR was performed to identify RCT evidence that assessed the efficacy of biologic therapies and apremilast in adult patients with moderate‐to‐severe chronic plaque psoriasis. PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analyses and network meta‐analyses) reporting guidelines were followed throughout.25, 26
MEDLINE, Embase and Cochrane Library databases were searched for articles published in English from 2000 to 31 August 2016 (Table S1). Search strings combined terms related to psoriasis, to specific therapies and to RCTs. Screening of potentially relevant publications was performed double‐blind, with a third reviewer resolving any differences as to eligibility. Supplementary searching included a bibliography review, congress abstract searching and hand searching. Bibliographies of included studies were cross‐referenced with the search results to identify additional studies. Abstracts of relevant disease‐specific and health economics and outcomes research congresses from 2013 to 31 August 2016 were searched. Finally, a hand search was performed in February 2017 to identify additional full‐text publications reporting on trials that had been included as abstracts in the original SLR.
Only RCTs comparing an intervention of interest – adalimumab, apremilast, brodalumab, etanercept, infliximab, ixekizumab, secukinumab and ustekinumab – with any comparator, including placebo and unlicensed doses of biological and non‐biological systemic therapies, were included in the systematic review. The NMA included only doses of biological therapies and apremilast licensed by the European Medicines Agency (EMA). The main outcome of interest was the proportion of patients achieving 75%, 90% and 100% improvements in PASI score at between 40‐ and 64‐week follow‐up (PASI 75, PASI 90 and PASI 100).
For each study meeting the inclusion criteria, study design details, patient demographics, therapy details, efficacy endpoints and statistical analyses were extracted, with particular attention paid to patient follow‐up and the handling of missing data. The methodological quality of included studies was assessed using the Cochrane Risk of Bias Tool.27 Potential risk of bias was determined by assessing heterogeneity of treatment and outcome characteristics as well as study and patient characteristics.
Analysis
To determine the feasibility and appropriateness of analysis, included studies were compared to assess heterogeneity in terms of treatment and outcome characteristics as well as study and patient characteristics. The planning and execution of all analyses adhered to internationally recommended methods.28, 29 Relevant study results were combined by means of a hierarchical Bayesian NMA of PASI responses using an ordered probit model to estimate probabilities of achieving different levels of response (e.g. PASI 75, PASI 90 and PASI 100). This is the preferred model when synthesizing ordered categorical data as it makes efficient use of all available trial data, even where different trials use different thresholds or report different numbers of thresholds, by assuming that the treatment effect is the same regardless of response level.29, 30 Prior exposure to biological therapies varies across trials of psoriasis and is thought to be an potential effect modifier; therefore, a sensitivity analysis was run excluding studies which do not report or in which <5% of patients report prior experience with biologics.
Results were generated using both fixed‐ and random‐effects models, and compared for goodness of fit to the data, calculated as the overall mean residual deviance. The model with the lowest deviance information criterion (DIC) was considered to have the ‘best’ fit to the data.29 Inconsistency between direct and indirect estimates of effect was assessed for any loops in the evidence network using the two‐stage Bucher method.30, 31
All analyses were performed using WinBUGS version 1.4 statistical software with non‐informative priors. An initial burn‐in of at least 20 000 simulations was used, and convergence was confirmed through visual inspection the Brook–Gelman–Rubin diagnostic and history plots. This was followed by 50 000 simulations on three chains to estimate the sampled parameters. Results are calculated as risk ratios (RRs) for each treatment compared to one another. Point estimates of the median value are presented, along with 95% credible intervals (95% CrI) reflecting the range of true effects with 95% probability. A numerical summary of each treatment's rank distribution, called the surface under the cumulative ranking (SUCRA) curve, is also presented.
Results
Literature search results
Electronic searches identified 3441 publications, to which supplementary searching added a total of 31 additional references. After deduplication, 2997 titles and abstracts were screened, and full‐text versions of 225 publications were assessed. In total, 98 publications, covering 67 RCTs, were included (Fig. 1).
Figure 1.
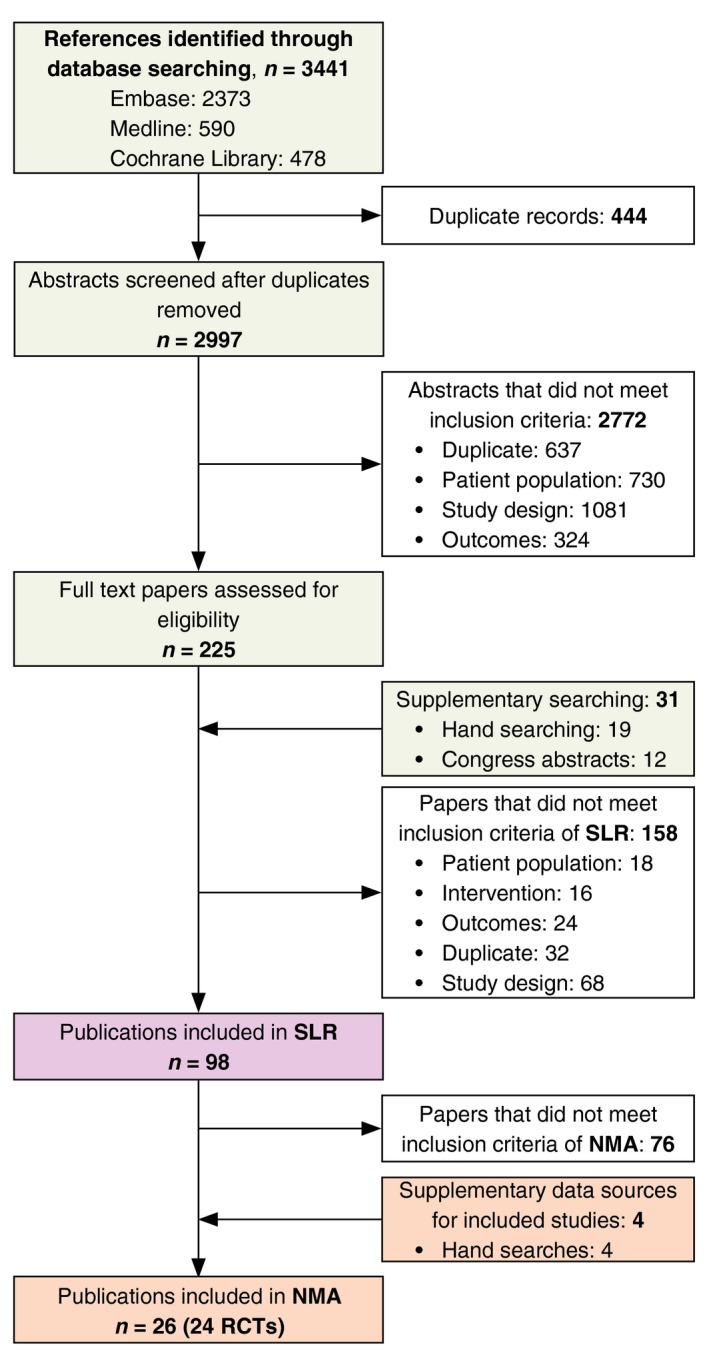
PRISMA flow diagram of SLR.
Evidence network
Of the 98 publications identified in the SLR, 62 publications describing 54 RCTs reported PASI outcomes for a licensed dose of biologic therapy or apremilast at the end of a 10‐ to 16‐week induction period. Twenty‐four of these RCTs also reported on PASI outcomes measured at 40–64 weeks of follow‐up, but placebo‐ and active‐controlled data were limited. Most control arms did not continue beyond the short‐term induction period, yet controlled data are necessary to calculate comparative effect estimates.
Five studies were long‐term RCTs for which maintenance phase data were available for at least two trial arms. Fourteen studies were RCTs with a short‐term induction phase followed by an observational maintenance phase in which patients originally randomized to placebo crossed over to active therapy. Five studies were short‐term RCTs with a crossover maintenance phase followed by a rerandomized withdrawal‐controlled phase among treatment responders. From this heterogeneous data set of longer‐term outcomes, several analyses were planned and implemented (Fig. 2).
Figure 2.
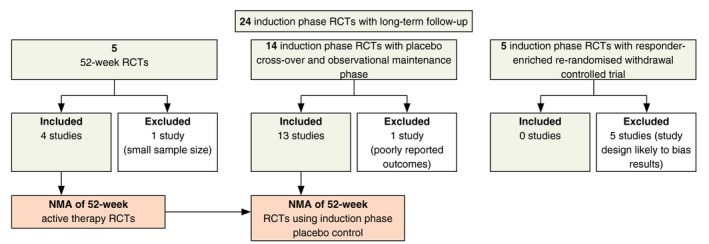
Study selection for network meta‐analysis (NMA).
Analysis 1: NMA of 52‐week active therapy RCTs
The primary analysis utilized data from four of the active‐controlled 52‐week RCTs32, 33, 34 evaluating brodalumab, ustekinumab (weight‐based dosing: 45 mg if <100 kg; 90 mg if >100 kg), secukinumab and etanercept (50 mg BIW). The fifth study, PIECE,35 was excluded due to its small sample size (n = 19) and the risk for Type II error.
Analysis 2: NMA of 52‐week RCTs using induction phase placebo control
Building on the primary network, a secondary analysis was undertaken using evidence from 13 of the 14 crossover maintenance phase trials. The PHOENIX 2 trial was excluded due to outcomes being insufficiently reported for use in the analysis.36, 37 By design, these maintenance phases are like single‐arm studies, and thus data are non‐comparative. To use these data in the NMA, maintenance phase responses from licensed active therapy arms were compared to induction phase outcomes from placebo arms. This assumes that if patients randomized to placebo at the start of the trial had continued receiving placebo, there would have been no change in their likelihood or level of response. Making this assumption allowed for the inclusion of data for adalimumab, apremilast, infliximab and ixekizumab, as well as additional data for etanercept, secukinumab and ustekinumab (45 and 90 mg). Only data for patients starting on the licensed induction dose followed by the licensed maintenance dose were used. Maintenance phase data relating to patients who crossed over from unlicensed induction doses were excluded.
Data from the five studies with a withdrawal‐controlled phase were not included in the secondary analysis.38, 39, 40, 41 The responder‐enrichment design of these studies, that is the restriction of rerandomization only to patients who reached a predefined level of response, may bias results in favour of the active intervention.
A description of the 22 RCTs included in the primary and secondary analyses is provided in Table 1, and an evidence network for both analyses is provided in Fig. 3. A total of 2244 patients were included in the four 52‐week RCTs. A total of 6113 patients were included in the 17 trials forming the augmented network for PASI response. In the sensitivity analysis around prior biologic exposure, six studies42, 43, 44, 45, 46, 47 were excluded from the augmented network. Study‐level outcomes are reported in Table S2. Direct and indirect treatment effects were compared in the loops of evidence observed in the augmented network (Analysis 2), but no significant inconsistency was identified.
Table 1.
Baseline characteristics of studies included in primary and secondary analyses
| Study | Treatment | Sample size | Age (years) | Male | Weight (kg) | PsA | Disease duration (years) | Previous therapies | Percentage psoriasis‐affected BSA | PASI score | DLQI score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ind. | Maint. | Phototherapy (UVB or PUVA) | Systemic non‐biologic | Biologic | ||||||||||||||||
| n | n | Mean | SD | % | Mean | SD | % | Mean | SD | % | % | % | Mean | SD | Mean | SD | Mean | SD | ||
| AMAGINE‐2a , 34 | Brodalumab 210 mg | 612 | 334 | 45 | 13 | 69 | 91.0 | 23.0 | 19 | 19.0 | 12.0 | 52 | 77 | 29 | 26 | 16 | 20.3 | 8.3 | 14.7 | 7.1 |
| Ustekinumab (WBD) | 300 | 289 | 45 | 13 | 68 | 91.0 | 24.0 | 17 | 19.0 | 13.0 | 50 | 75 | 28 | 27 | 19 | 20.0 | 8.4 | 15.1 | 7.2 | |
| AMAGINE‐3a , 34 | Brodalumab 210 mg | 624 | 342 | 45 | 13 | 69 | 90.0 | 23.0 | 20 | 18.0 | 12.0 | 40 | 68 | 25 | 28 | 18 | 20.4 | 8.3 | 14.5 | 7.2 |
| Ustekinumab (WBD) | 313 | 301 | 45 | 13 | 68 | 90.0 | 22.0 | 20 | 18.0 | 12.0 | 44 | 70 | 24 | 28 | 20.1 | 8.4 | 14.6 | 7.4 | ||
| CLEARa , 32, 63 | Secukinumab 300 mg | 337 | 337 | 45.2 | 14.0 | 68 | 87.4 | 20.0 | 21 | 19.6 | 12.9 | 65 | 14 | 33 | 18 | 21.7 | 8.5 | |||
| Ustekinumab (WBD) | 339 | 339 | 44.6 | 13.7 | 74 | 87.2 | 22.1 | 16 | 16.1 | 11.2 | 66 | 13 | 32 | 17 | 21.5 | 8.1 | ||||
| FIXTUREa , 33 | Secukinumab 300 mg | 327 | 312 | 44.5 | 13.2 | 68 | 83.0 | 21.6 | 15 | 15.8 | 12.3 | 60 | 12 | 34 | 19 | 23.9 | 9.9 | 13.3 | ||
| Etanercept 50 mg BIW | 326 | 305 | 43.8 | 13.0 | 71 | 84.6 | 20.5 | 14 | 16.4 | 12.0 | 63 | 14 | 34 | 18 | 23.2 | 9.8 | 13.4 | |||
| PHOENIX 164 | Placebo | 255 | 44.8 | 11.3 | 72 | 94.2 | 23.5 | 35 | 20.4 | 11.7 | 59 | 56 | 50 | 28 | 17 | 20.4 | 8.6 | 11.8 | 7.4 | |
| Ustekinumab 45 mg | 255 | 251 | 44.8 | 12.5 | 69 | 93.7 | 23.8 | 29 | 19.7 | 11.7 | 68 | 55 | 53 | 27 | 18 | 20.5 | 8.6 | 11.1 | 7.1 | |
| Ustekinumab 90 mg | 255 | 246 | 46.2 | 11.3 | 68 | 93.8 | 23.9 | 37 | 19.6 | 11.1 | 67 | 55 | 51 | 25 | 15 | 19.7 | 7.6 | 11.6 | 6.9 | |
| Igarashi 201243 | Placebo | 32 | 49.0 | 84 | 71.2 | 10.9 | 3 | 16.0 | 11.2 | 63 | 66 | 0 | 50 | 23 | 30.3 | 11.8 | 10.5 | 6.2 | ||
| Ustekinumab 45 mg | 64 | 64 | 45.0 | 83 | 73.2 | 15.4 | 9 | 15.8 | 8.2 | 56 | 73 | 2 | 47 | 24 | 30.1 | 12.9 | 11.4 | 6.5 | ||
| Ustekinumab 90 mg | 62 | 58 | 44.0 | 76 | 71.1 | 14.0 | 11 | 17.3 | 10.7 | 82 | 84 | 0 | 47 | 20 | 28.7 | 11.2 | 10.7 | 6.4 | ||
| JUNCTURE65, 66 | Placebo | 61 | 43.7 | 12.7 | 62 | 90.2 | 21.2 | 20 | 19.9 | 12.2 | 48 | 21 | 26 | 15 | 19.4 | 6.7 | ||||
| Secukinumab 300 mg | 60 | 60 | 46.6 | 14.2 | 77 | 91.0 | 23.1 | 23 | 21.0 | 13.5 | 50 | 25 | 26 | 13 | 18.9 | 6.4 | ||||
| FEATURE67, 68 | Placebo | 59 | 46.5 | 14.1 | 66 | 88.4 | 21.6 | 20.2 | 14.2 | 49 | 44 | 21.1 | 8.5 | |||||||
| Secukinumab 300 mg | 59 | 59 | 45.1 | 12.6 | 64 | 92.6 | 25.9 | 18.0 | 11.9 | 34 | 39 | 33 | 18 | 20.7 | 8.0 | |||||
| ERASURE33 | Placebo | 248 | 45.4 | 12.6 | 69 | 89.7 | 25.0 | 27 | 17.3 | 12.4 | 44 | 29 | 30 | 16 | 21.4 | 9.1 | 12.0 | |||
| Secukinumab 300 mg | 245 | 238 | 44.9 | 13.5 | 69 | 88.8 | 24.0 | 23 | 17.4 | 11.1 | 52 | 29 | 33 | 19 | 22.5 | 9.2 | 13.9 | |||
| UNCOVER‐338, 69 | Placebo | 193 | 46.0 | 12.0 | 71 | 91.0 | 21.0 | 18.0 | 13.0 | 31 | 43 | 17 | 29 | 17 | 21.0 | 8.0 | 13.0 | 7.0 | ||
| Ixekizumab 80 mg Q2W→Q4W | 385 | 362 | 46.0 | 13.0 | 66 | 90.0 | 23.0 | 18.0 | 12.0 | 39 | 44 | 15 | 28 | 17 | 21.0 | 8.0 | 12.0 | 7.0 | ||
| X‐PLORE70 | Placebo | 42 | 46.5 | 67 | 93.6 | 22.6 | 29 | 18.0 | 13.3 | 50 | 50 | 36 | 28 | 19 | 21.8 | 10.0 | ||||
| Adalimumab 40 mg Q2W | 43 | 38 | 50.0 | 70 | 91.6 | 19.9 | 26 | 19.3 | 12.8 | 56 | 40 | 60 | 27 | 17 | 20.2 | 7.6 | ||||
| Gordon 200642 | Placebo | 52 | 43.0 | 20–70 | 65 | 94.0 | 50–147 | 31 | 19.0 | 1–40 | 0 | 28 | 7–75 | 16.0 | 5–40 | 12.2 | 8.1 | |||
| Adalimumab 40 mg Q2W | 46 | 42 | 46.0 | 20–71 | 71 | 93.0 | 63–159 | 33 | 21.0 | 1–58 | 0 | 29 | 6–58 | 16.7 | 5–39 | 13.3 | 8.8 | |||
| LIBERATE44 | Placebo | 84 | 43.4 | 14.9 | 70 | 89.5 | 23.1 | 16.6 | 12.1 | 83 | 0 | 27 | 16 | 19.4 | 6.8 | 11.4 | 6.3 | |||
| Apremilast 30 mg BID | 83 | 74 | 46.0 | 13.6 | 59 | 88.5 | 19.8 | 19.7 | 12.7 | 80 | 0 | 27 | 16 | 19.3 | 7.0 | 13.6 | 6.7 | |||
| Tyring 200647 | Placebo | 306 | 45.6 | 12.1 | 70 | 91.0 | 33 | 19.7 | 11.4 | 0 | 27 | 17 | 18.1 | 7.4 | 12.5 | 6.7 | ||||
| Etanercept 50 mg BIW | 311 | 304 | 45.8 | 12.8 | 65 | 92.6 | 35 | 20.1 | 12.3 | 0 | 27 | 18 | 18.3 | 7.6 | 12.1 | 6.7 | ||||
| Torii 201046 | Placebo | 19 | 43.3 | 12.3 | 74 | 69.7 | 8.9 | 37 | 11.1 | 6.4 | 74 | 95 | 50 | 27 | 33.1 | 15.6 | 10.5 | 6.8 | ||
| Infliximab 5 mg/kg | 35 | 32 | 46.9 | 13.0 | 63 | 68.5 | 13.4 | 29 | 14.2 | 8.9 | 63 | 94 | 46 | 21 | 31.9 | 12.8 | 12.7 | 6.8 | ||
| EXPRESS45, 71 | Placebo | 77 | 43.8 | 12.6 | 79 | 89.3 | 18.7 | 29 | 17.3 | 11.1 | 71 | 46 | 0 | 34 | 18 | 22.8 | 8.7 | 11.8 | 7.5 | |
| Infliximab 5 mg/kg | 301 | 281 | 42.6 | 11.7 | 69 | 85.9 | 20.1 | 31 | 19.1 | 11.0 | 65 | 42 | 0 | 34 | 19 | 22.9 | 9.3 | 12.7 | 7.0 | |
| EXPRESS II48 | Placebo | 208 | 44.4 | 12.5 | 69 | 91.1 | 22.6 | 26 | 50 | 13 | 28 | 18 | 19.8 | 7.7 | 13.4 | 7.3 | ||||
| Infliximab 5 mg/kg | 314 | 150 | 44.5 | 13.0 | 65 | 92.2 | 23.2 | 28 | 55 | 14 | 29 | 16 | 20.4 | 7.5 | 13.1 | 7.0 | ||||
The analyses do not include placebo arms from AMAGINE‐2, AMAGINE‐3, CLEAR and FIXTURE trials.
BID, twice daily; BIW, twice weekly; BSA, body surface area; DLQI, dermatology life quality index; Ind, induction; Maint, maintenance; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PUVA, psoralen and ultraviolet A; Q2W, every 2 weeks; Q4W, every 4 weeks; SD, standard deviation; UVB, ultraviolet B; WBD, weight‐based dose.
Figure 3.
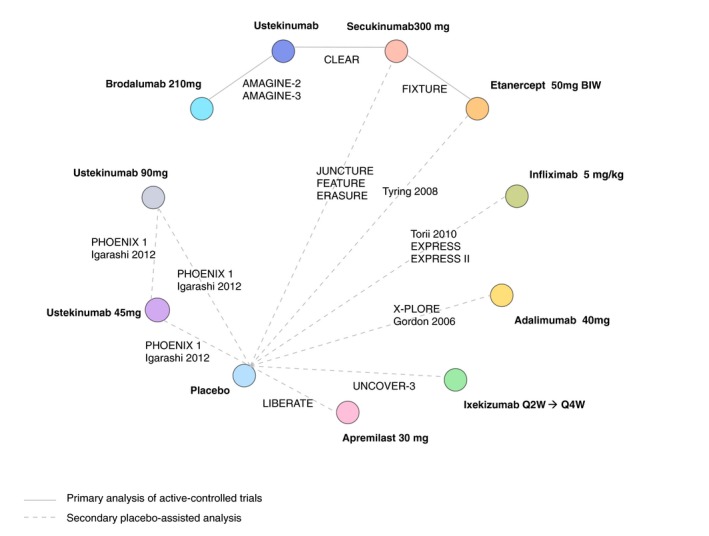
Network diagram of Psoriasis Area and Severity Index (PASI) responses – primary and secondary analyses.
Risk of bias
The risk of bias among the included studies was somewhat heterogeneous, but most were rated as being low risk of bias (Figure S1). Of the 17 included RCTs, four (24%) reported an adequate randomization method and 14 (82%) supplied sufficient information to assess whether allocation concealment was properly ensured. In four studies, the blinding of participants and personnel was insufficient as the long‐term extension was open label.42, 47, 48 In all studies, the risk of attrition bias was low, as incomplete outcome data were sufficiently addressed. The risk of reporting bias was low in most of the studies. The risk of bias for each study is presented in Figure S2.
Efficacy
Analysis 1: NMA of 52‐week active therapy RCTs
The relative treatment effects for comparisons between brodalumab, ustekinumab (weight‐based dosing), secukinumab and etanercept (50 mg twice weekly) are presented in Table 2. Results indicate that brodalumab is associated with significantly higher proportions of PASI 75, PASI 90 and PASI 100 responders compared to secukinumab, ustekinumab and etanercept. Secukinumab was also found to be more efficacious than ustekinumab, and both were found to outperform etanercept.
Table 2.
NMA of 52‐week active therapy RCTs (Analysis 1): results on PASI responses
| Intervention vs. | Comparator | Median risk ratio (95% credible interval) | ||
|---|---|---|---|---|
| PASI 75 | PASI 90 | PASI 100 | ||
| BRO | SEC | 1.10 (1.01–1.41) | 1.17 (1.03–1.61) | 1.32 (1.06–2.02) |
| UST | 1.27 (1.05–1.94) | 1.46 (1.1–2.41) | 1.90 (1.26–3.46) | |
| ETN | 1.65 (1.11–3.65) | 2.11 (1.23–5.34) | 3.31 (1.58–10.00) | |
| SEC | UST | 1.15 (1.03–1.48) | 1.23 (1.05–1.67) | 1.4 (1.12–2.07) |
| ETN | 1.49 (1.10–2.66) | 1.79 (1.19–3.49) | 2.48 (1.44–5.39) | |
| UST | ETN | 1.28 (1.05–2.00) | 1.43 (1.09–2.42) | 1.73 (1.18–3.29) |
Results from fixed‐effect multinomial likelihood model with probit link and presented as risk ratios, with 95% credible intervals in parentheses below.
BRO, brodalumab 210 mg; ETN, etanercept 50 mg twice weekly; NMA, network meta‐analysis; PASI, Psoriasis Area and Severity Index; RCTs, randomized controlled trials; SEC, secukinumab 300 mg; UST, ustekinumab (45 mg if <100 kg; 90 mg if >100 kg).
Analysis 2: NMA of 52‐week RCTs using induction phase placebo control
Results of the analysis in which maintenance phase outcomes for active therapies were compared to induction phase outcomes for placebo showed all treatments to be significantly more efficacious than placebo. In terms of PASI responses, the most effective therapies in the network were brodalumab followed by ixekizumab and secukinumab, whereas apremilast showed the poorest efficacy of achieving any level of PASI response (Table 3 and Figure S3).
Table 3.
NMA of 52‐week RCTs using induction phase placebo control (Analysis 2): results on PASI responses
| Intervention vs. | Comparator | Median risk ratio (95% Credible Interval) | ||
|---|---|---|---|---|
| PASI 75 | PASI 90 | PASI 100 | ||
| BRO | SEC | 1.1 (1.01, 1.41) | 1.18 (1.03, 1.63) | 1.32 (1.06, 2.02) |
| IXE | 1.06 (0.96, 1.46) | 1.11 (0.93, 1.73) | 1.21 (0.89, 2.23) | |
| UST | 1.27 (1.05, 1.94) | 1.49 (1.11, 2.48) | 1.91 (1.27, 3.48) | |
| UST 45 mg | 1.2 (1.02, 2.04) | 1.37 (1.04, 2.72) | 1.68 (1.09, 4.06) | |
| UST 90 mg | 1.13 (1, 1.75) | 1.24 (1, 2.21) | 1.43 (1, 3.1) | |
| APR | 3.22 (1.36, 16.4) | 5.47 (1.75, 36.35) | 11.61 (2.72, 103.4) | |
| ADA | 1.31 (1.02, 2.89) | 1.57 (1.04, 4.32) | 2.07 (1.08, 7.5) | |
| ETN | 1.63 (1.11, 3.54) | 2.15 (1.25, 5.43) | 3.26 (1.57, 9.64) | |
| INF | 1.25 (1.02, 2.26) | 1.45 (1.06, 3.11) | 1.84 (1.12, 4.84) | |
| PBO | 19.86 (3.5, 231) | 53.14 (6.7, 775.6) | 201.7 (18.01, 3683) | |
| SEC | IXE | 0.98 (0.82, 1.16) | 0.96 (0.74, 1.24) | 0.93 (0.64, 1.37) |
| UST | 1.15 (1.03, 1.48) | 1.25 (1.06, 1.71) | 1.42 (1.13, 2.09) | |
| UST 45 mg | 1.09 (0.96, 1.57) | 1.15 (0.93, 1.87) | 1.26 (0.89, 2.39) | |
| UST 90 mg | 1.03 (0.89, 1.35) | 1.05 (0.84, 1.54) | 1.09 (0.76, 1.84) | |
| APR | 2.91 (1.33, 12.4) | 4.58 (1.67, 24.39) | 8.66 (2.42, 58.94) | |
| ADA | 1.19 (0.95, 2.26) | 1.32 (0.91, 3.05) | 1.56 (0.86, 4.58) | |
| ETN | 1.48 (1.1, 2.59) | 1.82 (1.2, 3.49) | 2.45 (1.44, 5.18) | |
| INF | 1.13 (0.97, 1.73) | 1.23 (0.96, 2.13) | 1.39 (0.93, 2.84) | |
| PBO | 18.03 (3.44, 170) | 44.82 (6.46, 504.3) | 151.3 (16.51, 2003) | |
| IXE | UST | 1.17 (1.01, 1.68) | 1.29 (1.01, 2.08) | 1.5 (1.02, 2.8) |
| UST 45 mg | 1.12 (0.96, 1.66) | 1.2 (0.94, 2.04) | 1.35 (0.91, 2.72) | |
| UST 90 mg | 1.05 (0.9, 1.43) | 1.09 (0.84, 1.68) | 1.16 (0.77, 2.11) | |
| APR | 2.98 (1.34, 13) | 4.78 (1.7, 26.17) | 9.29 (2.52, 65.72) | |
| ADA | 1.22 (0.96, 2.38) | 1.38 (0.94, 3.28) | 1.67 (0.9, 5.1) | |
| ETN | 1.5 (1.1, 2.85) | 1.87 (1.21, 4.05) | 2.59 (1.44, 6.51) | |
| INF | 1.16 (0.99, 1.84) | 1.28 (0.98, 2.33) | 1.48 (0.96, 3.25) | |
| PBO | 18.51 (3.46, 177.2) | 46.92 (6.56, 534.5) | 162.7 (17.11, 2210) | |
| UST | UST 45 mg | 0.97 (0.74, 1.25) | 0.94 (0.65, 1.37) | 0.91 (0.54, 1.56) |
| UST 90 mg | 0.91 (0.67, 1.11) | 0.86 (0.57, 1.16) | 0.78 (0.45, 1.25) | |
| APR | 2.5 (1.27, 9.27) | 3.62 (1.5, 16.57) | 6 (1.94, 35.29) | |
| ADA | 1.04 (0.75, 1.77) | 1.07 (0.66, 2.19) | 1.11 (0.54, 2.91) | |
| ETN | 1.27 (1.05, 1.93) | 1.43 (1.09, 2.37) | 1.69 (1.17, 3.12) | |
| INF | 1 (0.77, 1.36) | 0.99 (0.69, 1.54) | 0.99 (0.58, 1.82) | |
| PBO | 15.54 (3.32, 122.8) | 35.42 (5.96, 326.1) | 104.7 (13.98, 1133) | |
| UST 45 mg | UST 90 mg | 0.95 (0.79, 1.02) | 0.91 (0.72, 1.03) | 0.87 (0.63, 1.04) |
| APR | 2.61 (1.3, 9.76) | 3.86 (1.56, 17.79) | 6.63 (2.1, 39.03) | |
| ADA | 1.08 (0.79, 1.86) | 1.13 (0.7, 2.35) | 1.22 (0.59, 3.23) | |
| ETN | 1.31 (1.05, 2.16) | 1.51 (1.09, 2.8) | 1.85 (1.16, 3.97) | |
| INF | 1.03 (0.8, 1.44) | 1.06 (0.72, 1.67) | 1.09 (0.62, 2.05) | |
| PBO | 16.25 (3.38, 127) | 37.97 (6.18, 344) | 116.4 (15.09, 1220) | |
| UST 90 mg | APR | 2.78 (1.32, 11.16) | 4.28 (1.63, 21.28) | 7.78 (2.3, 49.65) |
| ADA | 1.14 (0.88, 2.08) | 1.24 (0.81, 2.74) | 1.41 (0.73, 3.98) | |
| ETN | 1.4 (1.08, 2.46) | 1.67 (1.15, 3.33) | 2.17 (1.3, 5.02) | |
| INF | 1.09 (0.89, 1.61) | 1.15 (0.84, 1.94) | 1.26 (0.77, 2.53) | |
| PBO | 17.33 (3.43, 149.5) | 42.07 (6.37, 426.3) | 136.7 (16.04, 1619) | |
| APR | ADA | 0.43 (0.12, 0.83) | 0.31 (0.07, 0.73) | 0.19 (0.03, 0.62) |
| ETN | 0.52 (0.18, 0.88) | 0.4 (0.12, 0.82) | 0.29 (0.07, 0.73) | |
| INF | 0.4 (0.11, 0.79) | 0.28 (0.06, 0.66) | 0.17 (0.03, 0.51) | |
| PBO | 5.83 (2.29, 22.01) | 9.09 (3.11, 39.46) | 16.05 (4.62, 82.94) | |
| ADA | ETN | 1.18 (0.82, 2.04) | 1.3 (0.76, 2.63) | 1.48 (0.69, 3.76) |
| INF | 0.96 (0.57, 1.39) | 0.93 (0.46, 1.6) | 0.9 (0.34, 1.98) | |
| PBO | 14.51 (3.29, 107.6) | 31.98 (5.81, 283.5) | 90.38 (13.29, 976.8) | |
| ETN | INF | 0.8 (0.49, 0.99) | 0.71 (0.39, 0.98) | 0.6 (0.28, 0.96) |
| PBO | 12.14 (3.13, 68.57) | 24.49 (5.3, 154.4) | 61.07 (11.2, 428.2) | |
| INF | PBO | 15.57 (3.35, 117.1) | 35.53 (6.05, 309.4) | 105.3 (14.43, 1053) |
Results from fixed‐effect multinomial likelihood model with probit link.
ADA, adalimumab 40 mg Q2W; APR, apremilast 30 mg BID; BID, twice daily; BIW, twice weekly; BRO, brodalumab 210 mg; ETN, etanercept 50 mg BIW; INF, infliximab 5 mg/kg; IXE, ixekizumab 80 mg Q2W→Q4W; NMA, network meta‐analysis; PASI, Psoriasis Area and Severity Index; PBO, placebo; Q2W, every 2 weeks; Q4W, every 4 weeks; RCTs, randomized controlled trials; SEC, secukinumab 300 mg; UST, ustekinumab (45 mg if <100 kg; 90 mg if >100 kg).
Figure 4 presents the cumulative ranking curves for each treatment along with an estimate of the SUCRA line. A SUCRA would be 100% when a treatment is certain to be the best and 0% when it is certain to be the worst. Brodalumab was ranked the most efficacious therapy in 79% of Bayesian iterations, giving it the highest SUCRA value of 97%. Ixekizumab and secukinumab had the next highest SUCRA values, at 83% and 77%, respectively. Of active therapies, etanercept and apremilast had the lowest SUCRA values, at just 22% and 10%, respectively.
Figure 4.
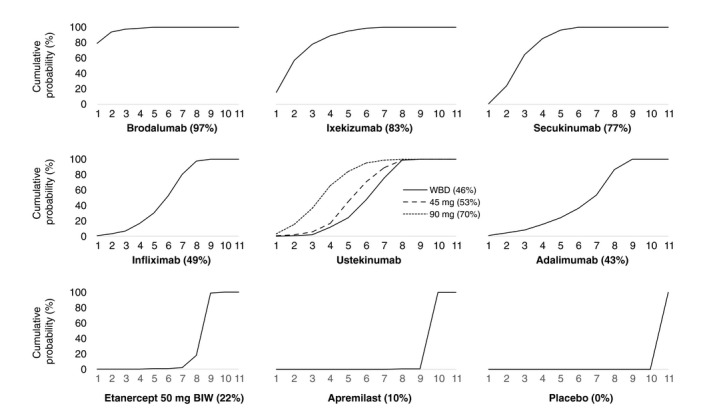
Cumulative ranking probability plots and surface under the cumulative ranking (SUCRA) for each treatment included in network meta‐analysis (NMA) of 52‐week randomized controlled trials (RCTs) using induction phase placebo control (Analysis 2). On the horizontal axis is the possible rank of each treatment according to the magnitude of its treatment effect across all measures of Psoriasis Area and Severity Index (PASI) response (from the best rank [1] to worst [11]). On the vertical axis is the cumulative probability for each treatment to be the best option, among the best two options, among the best three options and so on. If a treatment always ranks first, then the SUCRA = 100%; if a treatment always ranks last, then the SUCRA = 0%. WBD, weight‐based dose.
Results from the sensitivity analysis including only studies with at least 5% of patients reporting prior biologic exposure were consistent with the analysis including all studies regardless of prior biologic exposure (Table S3). With the exception of apremilast, for which there were no data in this sensitivity analysis, the relative rank and statistical significance of treatments effects between treatments were unchanged.
Discussion
We performed a comprehensive systematic review and NMA summarizing the long‐term RCT evidence available for the efficacy of systemic biologic and non‐biologic drugs in the treatment of patients with moderate‐to‐severe psoriasis. This is the first NMA to synthesize maintenance phase outcomes reported at or around 1 year of follow‐up.
The synthesis of four RCTs reporting PASI outcomes at week 52 for brodalumab, ustekinumab, secukinumab and etanercept showed brodalumab to be associated with the highest likelihood of response. Based on these results, patients treated with brodalumab are 30% more likely to experience a complete clearance of psoriasis (PASI 100) at 1 year than patients treated with secukinumab, almost twice as likely as those treated with a weight‐based dose of ustekinumab and more than three times as likely as patients treated with a 50 mg twice weekly dose of etanercept.
We supplemented this ‘purer’ network with the inclusion of placebo‐controlled RCTs reporting maintenance phase outcomes for other licensed therapies, including adalimumab, apremilast, infliximab and ixekizumab. The newest generation biologic therapies – brodalumab, ixekizumab and secukinumab – were the best performing treatments, followed by ustekinumab, infliximab and adalimumab. Etanercept and apremilast had the lowest expected long‐term efficacy.
These findings are generally consistent with published NMAs on the induction phase efficacy of biologics for psoriasis and with a published meta‐analysis of 24‐week outcomes. Nast et al.7 ranked drugs based on PASI 75 responses at 24 weeks, with the best results reported for infliximab, secukinumab and ustekinumab followed by adalimumab, etanercept and apremilast.
Patient registries, such as BADBIR in the United Kingdom and DERMBIO in Denmark, are another valuable source of long‐term data on the efficacy of biologic therapies for psoriasis. Several studies reporting analyses of drug survival from registry or other observational data sets have been published, and they showed ustekinumab to have similar49 or longer drug survival compared to anti‐TNF agents.50, 51, 52, 53, 54, 55, 56, 57, 58, 59 It will be some time before published data are available for the newest biologics – brodalumab, ixekizumab and secukinumab – yet the RCT evidence and this analysis suggest that they will outperform older biologics in the long‐term.
Limitations
A number of challenges arise when attempting to quantitatively summarize the results of long‐term studies in psoriasis. They vary in study design, in their method of analysis and in their handling of missing data. Wherever possible, we used data from the intention‐to‐treat analysis population and the more conservative non‐responder imputation method. We included outcomes for patients receiving the licensed regimen of the intervention throughout each study, thus excluding patients who had crossed over following induction.
In the majority of long‐term RCTs, the placebo groups were discontinued after induction. As comparative data are fundamental to meta‐analysis (both pairwise and network), we imputed these missing long‐term outcomes for placebo by carrying forward responses measured at the end of induction (10–16 weeks). We are aware that this approach is associated with uncertainties; however, the stability of placebo responses recorded between the end of induction up to week 24 in four RCTs45, 60, 61, 62 lends some support to our assumption. In their meta‐analysis of 24‐week outcomes, Nast et al.7 dealt with this problem by calculating a mean placebo response from three studies that reported placebo data up to week 24 and used it as a model for trials without a long‐term placebo control, a method of adjustment that breaks randomization. More long‐term direct evidence is required to increase the quantity and validity of possible comparisons, thus active‐controlled RCTs make the most sense ethically and methodologically.
The number of therapies coming to market for the treatment of moderate‐to‐severe psoriasis is evolving rapidly, and already licensed therapies are still being evaluated in active‐comparator trials. Since the search was performed for this systematic review, three treatments have received marketing authorization for psoriasis in either the United States, Europe or both: guselkumab, certolizumab pegol and tildrakizumab. Due to their very nature, systematic reviews are challenged by the rate of publications in this disease area, and further updates will inevitably be required as and when long‐term RCT data mature for these newer therapies.
Conclusions
This NMA of long‐term RCT evidence demonstrates that high levels of sustained efficacy can be expected from IL‐17A inhibitor secukinumab, and IL‐17RA receptor blocker brodalumab, with brodalumab achieving the highest rates of PASI response up to and including PASI 100. Our secondary analysis indirectly compared the IL‐17 blocking agents with older biologics and apremilast, and although there was more uncertainty, it supported the conclusion that IL‐17A inhibitors and the IL‐17RA receptor blocker are the most efficacious medicines in the currently available treatment arsenal to treat moderate‐to‐severe psoriasis. Long‐term active‐comparator trials are needed to validate these conclusions.
Supporting information
Figure S1. Risk of bias graph: review author's judgements about each risk of bias item presented as percentages across all included studies.
Figure S2. Risk of bias summary for individual studies.
Figure S3. Network meta‐analysis (NMA) of 52‐week randomized controlled trials (RCTs) using induction phase placebo control (Analysis 2): results as probabilities of achieving each level of Psoriasis Area and Severity Index (PASI) response.
Table S1. Embase search terms.
Table S2. Study‐level response data.
Table S3. Sensitivity analysis of network meta‐analysis (NMA) of 52‐week randomized controlled trials (RCTs) using induction phase placebo control (Analysis 2) excluding studies with <5% of patients reporting prior biologic exposure: results on Psoriasis Area and Severity Index (PASI) responses.
Acknowledgement
The authors thank Sarah Bermingham of Symmetron Ltd for providing editorial assistance.
Conflicts of interest
Laura Sawyer and Laura Cornic are Symmetron employees and consultants to LEO Pharma for this study; Carl Gibbons and Anders Holmen Møller are LEO Pharma employees. Lars Åke Levin has received consulting fees from LEO Pharma; Gregor B Jemec has received honoraria from AbbVie, Coloplast, Galderma, InflaRx, LEO Pharma, MSD, Pierre‐Fabre and UCB, and his department received grants from AbbVie, Actelion, Janssen‐Cilag, LEO Pharma, Novartis, Regeneron, Serono and UCB for his participation as an investigator.
Funding source
This study was funded by LEO Pharma A/S.
References
- 1. Goff KL, Karimkhani C, Boyers LN et al The global burden of psoriatic skin disease. Br J Dermatol 2015; 172: 1665–1668. [DOI] [PubMed] [Google Scholar]
- 2. de Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc 2004; 9: 140–147. [DOI] [PubMed] [Google Scholar]
- 3. Feldman SR, Burudpakdee C, Gala S, Nanavaty M, Mallya UG. The economic burden of psoriasis: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res 2014; 14: 685–705. [DOI] [PubMed] [Google Scholar]
- 4. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263–271. [DOI] [PubMed] [Google Scholar]
- 5. van de Kerkhof PC. The relevance of biologics for the treatment of patients with psoriasis. Br J Dermatol 2009; 161: 1213–1214. [DOI] [PubMed] [Google Scholar]
- 6. Langley RG, Reich K. The interpretation of long‐term trials of biologic treatments for psoriasis: trial designs and the choices of statistical analyses affect ability to compare outcomes across trials. Br J Dermatol 2013; 169: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 7. Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and safety of systemic long‐term treatments for moderate‐to‐severe psoriasis: a systematic review and meta‐analysis. J Invest Dermatol 2015; 135: 2641–2648. [DOI] [PubMed] [Google Scholar]
- 8. Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta‐analysis. BMJ 2013; 346: f2914. [DOI] [PubMed] [Google Scholar]
- 9. Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005; 331: 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bansback N, Sizto S, Sun H, Feldman S, Willian MK, Anis A. Efficacy of systemic treatments for moderate to severe plaque psoriasis: systematic review and meta‐analysis. Dermatology 2009; 219: 209–218. [DOI] [PubMed] [Google Scholar]
- 11. Collins A, Hawe E, Vickers A et al Secukinumab 300 mg demonstrates higher probability of efficacy than other biologics in psoriasis: indirect comparison. Poster presented at the 48th Australasian College of Dermatologists Annual Scientific Meeting. 2015.
- 12. Fan T, Bennett H, Smith N, Marin M, Shuvayu S. Mixed treatment comparison of infliximab with ustekinumab in patients with moderate to severe psoriasis. Br J Dermatol 2011; 165: e38–e39. [Google Scholar]
- 13. Galvan‐Banqueri M, Gil RM, Ramos BS, Bautista Paloma FJ. Biological treatments for moderate‐to‐severe psoriasis: indirect comparison. J Clin Pharm Ther 2013; 38: 121–130. [DOI] [PubMed] [Google Scholar]
- 14. Gupta AK, Daigle D, Lyons DCA. Network meta‐analysis of treatments for chronic plaque psoriasis in Canada. J Cutan Med Surg 2014; 18: 371–378. [DOI] [PubMed] [Google Scholar]
- 15. Hartz S, Walzer S, Dutronc Y, Kiri HS, Schacht A, Dakin H. Network meta‐analysis to evaluate the efficacy of ixekizumab in the treatment of moderate to severe psoriasis. Value Health 2016; 19: A576. [Google Scholar]
- 16. Jabbar‐Lopez ZK, Yiu ZZN, Ward V et al Quantitative evaluation of biologic therapy options for psoriasis: a systematic review and network meta‐analysis. J Invest Dermatol 2017; 137: 1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamel SA, Myer KA, Younes N, Zhou JA, Maibach H, Maibach HI. Placebo response in relation to clinical trial design: a systematic review and meta‐analysis of randomized controlled trials for determining biologic efficacy in psoriasis treatment. Arch Dermatol Res 2012; 304: 707–717. [DOI] [PubMed] [Google Scholar]
- 18. Lin VW, Ringold S, Devine EB. Comparison of ustekinumab with other biological agents for the treatment of moderate to severe plaque psoriasis. Arch Dermatol 2012; 148: 1403–1410. [DOI] [PubMed] [Google Scholar]
- 19. Odom D, Brogan A, Talbird S, Schenkel B. A meta‐analysis of randomized, controlled trials of ustekinumab and adalimumab for moderate‐to‐severe psoriasis. Value Health 2013; 16: A112. [Google Scholar]
- 20. Reich K, Burden AD, Eaton JN, Hawkins NS. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta‐analysis of randomized controlled trials. Br J Dermatol 2012; 166: 179–188. [DOI] [PubMed] [Google Scholar]
- 21. Signorovitch JE, Betts KA, Yan YS et al Comparative efficacy of biological treatments for moderate‐to‐severe psoriasis: a network meta‐analysis adjusting for cross‐trial differences in reference arm response. Br J Dermatol 2015; 172: 504–512. [DOI] [PubMed] [Google Scholar]
- 22. Strober B, Checchio T, Gupta P et al A dose‐response model‐based meta‐analysis to compare tofacitinib to other psoriasis treatments. J Eur Acad Dermatol Venereol 2016; 30(Suppl. 6): 94. [Google Scholar]
- 23. Wilson JL, Standfield L, Paech D, Mulani P. Comparative effectiveness of Adalimumab and Etanercept in patients with chronic plaque psoriasis. Australas J Dermatol 2012; 53: 54. [Google Scholar]
- 24. Woolacott N, Hawkins N, Mason A et al Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technol Assess 2006; 10: 1–258. [DOI] [PubMed] [Google Scholar]
- 25. Liberati A, Altman DG, Tetzlaff J et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hutton B, Salanti G, Caldwell DM et al The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JP, Altman DG, Gotzsche PC et al The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoaglin DC, Hawkins N, Jansen JP et al Conducting indirect‐treatment‐comparison and network‐meta‐analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health 2011; 14: 429–437. [DOI] [PubMed] [Google Scholar]
- 29. Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta‐analysis of randomized controlled trials. Med Decis Making 2013; 33: 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013; 33: 641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. J Clin Epidemiol 1997; 50: 683–691. [DOI] [PubMed] [Google Scholar]
- 32. Blauvelt A, Reich K, Tsai TF et al Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate‐to‐severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol 2017; 76: 60–69.e9. [DOI] [PubMed] [Google Scholar]
- 33. Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014; 371: 326–338. [DOI] [PubMed] [Google Scholar]
- 34. Lebwohl M, Strober B, Menter A et al Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med 2015; 373: 1318–1328. [DOI] [PubMed] [Google Scholar]
- 35. de Vries AC, Thio HB, de Kort WJ et al A prospective randomised controlled trial comparing infliximab and etanercept in patients with moderate to severe chronic plaque type psoriasis Psoriasis Infliximab versus Etanercept Comparison Evaluation, the PIECE study. Br J Dermatol 2016; 176: 624–633. [DOI] [PubMed] [Google Scholar]
- 36. Langley RG, Lebwohl M, Krueger GG et al Long‐term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate‐to‐severe psoriasis: results from the PHOENIX 2 study through 5 years of follow‐up. Br J Dermatol 2015; 172: 1371–1383. [DOI] [PubMed] [Google Scholar]
- 37. Papp KA, Langley RG, Lebwohl M et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 52‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 2). Lancet 2008; 371: 1675–1684. [DOI] [PubMed] [Google Scholar]
- 38. Gordon KB, Blauvelt A, Papp KA et al Phase 3 trials of ixekizumab in moderate‐to‐severe plaque psoriasis. N Engl J Med 2016; 375: 345–356. [DOI] [PubMed] [Google Scholar]
- 39. Papp K, Reich K, Leonardi CL et al Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol 2015; 73: 37–49. [DOI] [PubMed] [Google Scholar]
- 40. Papp KA, Reich K, Paul C et al A prospective phase III, randomized, double‐blind, placebo‐controlled study of brodalumab in patients with moderate‐to‐severe plaque psoriasis. Br J Dermatol 2016; 175: 273–286. [DOI] [PubMed] [Google Scholar]
- 41. Paul C, Cather J, Gooderham M et al Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol 2015; 173: 1387–1399. [DOI] [PubMed] [Google Scholar]
- 42. Gordon KB, Langley RG, Leonardi C et al Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double‐blind, randomized controlled trial and open‐label extension study. J Am Acad Dermatol 2006; 55: 598–606. [DOI] [PubMed] [Google Scholar]
- 43. Igarashi A, Kato T, Kato M, Song M, Nakagawa H, Japanese Ustekinumab Study Group . Efficacy and safety of ustekinumab in Japanese patients with moderate‐to‐severe plaque‐type psoriasis: long‐term results from a phase 2/3 clinical trial. J Dermatol 2012; 39: 242–252. [DOI] [PubMed] [Google Scholar]
- 44. Reich K, Gooderham M, Green L et al The efficacy and safety of apremilast, etanercept and placebo in patients with moderate‐to‐severe plaque psoriasis: 52‐week results from a phase IIIb, randomized, placebo‐controlled trial (LIBERATE). J Eur Acad Dermatol Venereol 2017; 31: 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reich K, Nestle FO, Papp K et al Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 2005; 366: 1367–1374. [DOI] [PubMed] [Google Scholar]
- 46. Torii H, Nakagawa H, Japanese Infliximab Study investigators . Infliximab monotherapy in Japanese patients with moderate‐to‐severe plaque psoriasis and psoriatic arthritis. A randomized, double‐blind, placebo‐controlled multicenter trial. J Dermatol Sci 2010; 59: 40–49. [DOI] [PubMed] [Google Scholar]
- 47. Tyring S, Gottlieb A, Papp K et al Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double‐blind placebo‐controlled randomised phase III trial. Lancet 2006; 367: 29–35. [DOI] [PubMed] [Google Scholar]
- 48. Menter A, Feldman SR, Weinstein GD et al A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate‐to‐severe plaque psoriasis. J Am Acad Dermatol 2007; 56: 31.e1‐15. [DOI] [PubMed] [Google Scholar]
- 49. Menting SP, Sitaram AS, Bonnerjee‐van der Stok HM, de Rie MA, Hooft L, Spuls PI. Drug survival is not significantly different between biologics in patients with psoriasis vulgaris: a single‐centre database analysis. Br J Dermatol 2014; 171: 875–883. [DOI] [PubMed] [Google Scholar]
- 50. Dávila‐Seijo P, Dauden E, Carretero G et al Survival of classic and biological systemic drugs in psoriasis: results of the BIOBADADERM registry and critical analysis. J Eur Acad Dermatol Venereol 2016; 30: 1942–1950. [DOI] [PubMed] [Google Scholar]
- 51. Gniadecki R, Bang B, Bryld LE, Iversen L, Lasthein S, Skov L. Comparison of long‐term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol 2015; 172: 244–252. [DOI] [PubMed] [Google Scholar]
- 52. Inzinger M, Wippel‐Slupetzky K, Weger W et al Survival and effectiveness of tumour necrosis factor‐alpha inhibitors in the treatment of plaque psoriasis under daily life conditions: report from the psoriasis registry Austria. Acta Derm Venereol 2016; 96: 207–212. [DOI] [PubMed] [Google Scholar]
- 53. Menter A, Papp KA, Gooderham M et al Drug survival of biologic therapy in a large, disease‐based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol 2016; 30: 1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shalom G, Cohen AD, Ziv M et al Biologic drug survival in Israeli psoriasis patients. J Am Acad Dermatol 2017; 76: 662–9.e1. [DOI] [PubMed] [Google Scholar]
- 55. Umezawa Y, Nobeyama Y, Hayashi M et al Drug survival rates in patients with psoriasis after treatment with biologics. J Dermatol 2013; 40: 1008–1013. [DOI] [PubMed] [Google Scholar]
- 56. van den Reek JM, Zweegers J, Kievit W et al ‘Happy’ drug survival of adalimumab, etanercept and ustekinumab in psoriasis in daily practice care: results from the BioCAPTURE network. Br J Dermatol 2014; 171: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 57. Vilarrasa E, Notario J, Bordas X, Lopez‐Ferrer A, Gich IJ, Puig L. ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis): a retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatol 2016; 74: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 58. Warren RB, Smith CH, Yiu ZZ et al Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of dermatologists biologic interventions register (BADBIR). J Invest Dermatol 2015; 135: 2632–2640. [DOI] [PubMed] [Google Scholar]
- 59. Zweegers J, van den Reek JMPA, van de Kerkhof PC et al Body mass index predicts discontinuation due to ineffectiveness and female sex predicts discontinuation due to side‐effects in patients with psoriasis treated with adalimumab, etanercept or ustekinumab in daily practice: a prospective, comparative, long‐term drug‐survival study from the BioCAPTURE registry*. Br J Dermatol 2016; 175: 340–347. [DOI] [PubMed] [Google Scholar]
- 60. Asahina A, Nakagawa H, Etoh T, Ohtsuki M, Adalimumab MSG. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol 2010; 37: 299–310. [DOI] [PubMed] [Google Scholar]
- 61. Gottlieb AB, Matheson RT, Lowe N et al A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol 2003; 139: 1627–1632. [DOI] [PubMed] [Google Scholar]
- 62. Krueger GG, Langley RG, Leonardi C et al A human interleukin‐12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 2007; 356: 580–592. [DOI] [PubMed] [Google Scholar]
- 63. Thaci D, Blauvelt A, Reich K et al Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015; 73: 400–409. [DOI] [PubMed] [Google Scholar]
- 64. Leonardi CL, Kimball AB, Papp KA et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet 2008; 371: 1665–1674. [DOI] [PubMed] [Google Scholar]
- 65. Lacour JP, Paul C, Jazayeri S et al Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. J Eur Acad Dermatol Venereol 2017; 31: 847–856. [DOI] [PubMed] [Google Scholar]
- 66. Paul C, Lacour JP, Tedremets L et al Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol 2015; 29: 1082–1090. [DOI] [PubMed] [Google Scholar]
- 67. Blauvelt A, Prinz JC, Gottlieb AB et al Secukinumab administration by pre‐filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol 2015; 172: 484–493. [DOI] [PubMed] [Google Scholar]
- 68. Gottlieb AB, Blauvelt A, Prinz JC et al Secukinumab self‐administration by prefilled syringe maintains reduction of plaque psoriasis severity over 52 weeks: results of the FEATURE trial. J Drugs Dermatol 2016; 15: 1226–1234. [PubMed] [Google Scholar]
- 69. Griffiths CE, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551. [DOI] [PubMed] [Google Scholar]
- 70. Gordon KB, Duffin KC, Bissonnette R et al A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med 2015; 373: 136–144. [DOI] [PubMed] [Google Scholar]
- 71. Reich K, Nestle FO, Papp K et al Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate‐to‐severe psoriasis: a randomized controlled trial. Br J Dermatol 2006; 154: 1161–1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Risk of bias graph: review author's judgements about each risk of bias item presented as percentages across all included studies.
Figure S2. Risk of bias summary for individual studies.
Figure S3. Network meta‐analysis (NMA) of 52‐week randomized controlled trials (RCTs) using induction phase placebo control (Analysis 2): results as probabilities of achieving each level of Psoriasis Area and Severity Index (PASI) response.
Table S1. Embase search terms.
Table S2. Study‐level response data.
Table S3. Sensitivity analysis of network meta‐analysis (NMA) of 52‐week randomized controlled trials (RCTs) using induction phase placebo control (Analysis 2) excluding studies with <5% of patients reporting prior biologic exposure: results on Psoriasis Area and Severity Index (PASI) responses.


