Abstract
CSL112 (apolipoprotein A‐I [human]) is a novel intravenous formulation of plasma‐derived apolipoprotein A‐I (apoA‐I) that enhances cholesterol efflux capacity. Renal impairment is a common comorbidity in acute myocardial infarction patients and is associated with impaired lipid metabolism. The aim of this phase 1 study was to assess the impact of moderate renal impairment on the pharmacokinetic and pharmacodynamic profile of CSL112. Sixteen subjects with moderate renal impairment and 16 age‐, sex‐, and weight‐matched subjects with normal renal function participated in the study. Within each renal function cohort, subjects were randomized 3:1 to receive a single intravenous infusion of CSL112 2 g (n = 6) or placebo (n = 2) or CSL112 6 g (n = 6) or placebo (n = 2). At baseline, subjects with moderate renal impairment versus normal renal function had higher total cholesterol efflux, ABCA1‐dependent cholesterol efflux capacity, and pre‐β1‐high‐density lipoprotein (HDL) levels. Infusing CSL112 resulted in similar, immediate, robust, dose‐dependent elevations in apoA‐I and cholesterol efflux capacity in both renal function cohorts and significantly greater elevations in pre‐β1‐HDL (P < .05) in moderate renal impairment. Lecithin‐cholesterol acyltransferase activity, demonstrated by a time‐dependent change in the ratio of unesterified to esterified cholesterol, did not differ by renal function. No meaningful changes in proatherogenic lipid levels were observed. Moderate renal impairment did not impact the ability of CSL112 to enhance cholesterol efflux capacity. CSL112 may represent a novel therapy to reduce the risk of early recurrent cardiovascular events following acute myocardial infarction in patients with or without moderate renal impairment.
Keywords: pharmacodynamics, atherosclerosis, chronic kidney disease, lipids, clinical trials
Following acute myocardial infarction, patients experience a high risk of cardiovascular events, especially recurrent acute myocardial infarction and cardiovascular death. This risk is greatest in the first weeks and months following acute myocardial infarction, the subacute period, with half the events in the following year occurring in the first month after acute myocardial infarction.1 Approximately 50% of these recurrent events can be attributed to lipid‐laden plaques in the coronary arteries distant from the culprit lesion.2 Pharmacological therapies that reduce the burden of atherosclerosis by decreasing low‐density lipoprotein‐cholesterol levels are the mainstay of chronic treatment for secondary prevention. However, the clinical benefit for cardiovascular outcomes with high‐intensity statin therapy and proprotein convertase subtilisin/kexin type 9 inhibitors is not evident in the subacute period and is first observed several months after treatment initiation.3, 4, 5 Therefore, new strategies are needed to reduce the risk of early recurrent cardiovascular events in the subacute period.
CSL112 (apolipoprotein A‐I [human]) is a novel intravenous formulation of plasma‐derived apolipoprotein A‐I (apoA‐I), the primary functional component of high‐density lipoprotein (HDL). It is currently in development to reduce the risk of early recurrent cardiovascular events following acute myocardial infarction. CSL112 is designed to enhance cholesterol efflux capacity, the ability of HDL to remove cholesterol from lipid‐laden macrophages. Cholesterol efflux capacity is the first step in the process of reverse cholesterol transport, the major atheroprotective function of HDL.6 A higher capacity for cholesterol efflux is associated with a reduced risk of developing cardiovascular disease.7, 8 Importantly, cholesterol efflux capacity is an independent risk factor whose contribution is not significantly decreased on correction for other cardiovascular risk factors including HDL‐cholesterol (HDL‐C) levels.7, 8 Low levels of cholesterol efflux capacity in adults with established coronary artery disease have also been associated with a dramatically increased risk of cardiovascular‐related death.9, 10 Rapidly enhancing cholesterol efflux capacity immediately following an acute myocardial infarction, a period in which cholesterol efflux capacity is known to be impaired,11, 12 is hypothesized to lead to a reduction in cardiovascular events by reducing plaque burden and stabilizing nonculprit plaque lesions in the coronary arteries that are vulnerable to rupture.
Infusion of CSL112 has now been studied in healthy subjects,13 patients with stable cardiovascular disease,14 and in patients in the first 7 days after a myocardial infarction.15 In each case, infusion of CSL112 caused a robust increase in cholesterol efflux, and interestingly, the ABCA1‐dependent portion of cholesterol efflux capacity was enhanced more strongly than ABCA1‐independent efflux. Because ABCA1‐dependent efflux predominates in plaque macrophages, the basis for this finding was further explored.16 In vitro studies showed that on introduction into plasma, CSL112 interacts with and remodels endogenous HDL to yield large amounts of lipid‐poor apoA‐I (pre‐β1‐HDL), the preferred acceptor of cholesterol from ABCA1. Additional studies showed that CSL112 enhances not only cholesterol efflux capacity but also esterification of the cholesterol by lecithin‐cholesterol acyltransferase (LCAT).13, 17 Cholesterol esterification is the second step of reverse cholesterol transport and acts to prevent backflow of cholesterol into cells.18
Renal impairment is a prevalent comorbid condition in acute myocardial infarction patients, with approximately 30% of subjects having stage 3 chronic kidney disease. Moreover, chronic kidney disease is associated with a progressively higher mortality rate in acute myocardial infarction patients as chronic kidney disease severity increases.19 Unique facets of renal impairment, however, might affect the response of patients to therapy with CSL112.
First, the kidney is a major site of apoA‐I clearance,20, 21 and pharmacokinetics may be affected. More importantly, HDL is known to become dysfunctional in chronic kidney disease, with reduced cholesterol efflux capacity and vasoprotective, antioxidative, and anti‐inflammatory properties.22, 23 The disease processes that affect endogenous apoA‐I in renal impairment patients might also affect infused apoA‐I.
Finally, it is noteworthy that although published work has observed a dramatic reduction in cholesterol efflux capacity in patients with end‐stage renal disease on dialysis, the impact of moderate renal impairment or stage 3 chronic kidney disease (estimated glomerular filtration rate [eGFR] ≥30 and <60 mL/min/1.73 m2) on cholesterol efflux capacity is less clear or well studied (for a review see Kon et al, 2015).21 For these reasons, we sought to understand the impact of renal impairment on the pharmacokinetic (PK)/pharmacodynamic (PD) profile of CSL112. Here we report the findings of a phase 1 study in subjects with moderate renal impairment compared with normal renal function. To our knowledge this is the first study assessing cholesterol efflux capacity in patients with moderate renal impairment and matched controls.
Methods
Ethical Approval and Consent
The study protocol was approved by the institutional review board at each of the participating centers, and subjects provided informed consent. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. This study (NCT02427035) was conducted at 4 centers in Europe: Quintiles Drug Research Unit at Guy's Hospital, London, United Kingdom; CMFT, Manchester, United Kingdom; APEX, Munich, Germany; and Charite Research, Berlin, Germany.
Study Design
This was a phase 1 double‐blind, randomized, placebo‐controlled single‐ascending‐dose study conducted to assess PK, safety, and PD of CSL112 in adults with moderate renal impairment compared with healthy adults with normal renal function. Full details of the study design are described in Tortorici et al.24
Briefly, adults with moderate renal impairment and sex‐, age‐, and weight‐matched subjects with normal renal function were enrolled. In total, 32 subjects (n = 16 with moderate renal impairment and n = 16 with normal renal function) were randomized 3:1 (CSL112:placebo) by renal function into 4 cohorts, each with 8 subjects (n = 6 CSL112 [2 or 6 g] and n = 2 placebo). CSL112 was administered during a 16‐day active treatment period that included a mandatory in‐house stay (in the study unit), and patients were followed up at several outpatient visits The maximum study duration was approximately 120 days.
Study Population
Briefly, subjects were male or female, aged 18 to 85 years, with a body weight ≥50 kg. Moderate renal impairment patients had stable chronic renal impairment, defined as an eGFR ≥30 and <60 mL/min/1.73 m2 within 6 months of screening. Subjects with normal renal function had an eGFR ≥90 mL/min/1.73 m2 and were determined to be healthy based on detailed medical history and physical examination. The main exclusion criteria are described in Tortorici et al.24
Study Product, Dose, and Administration
CSL112 is a novel intravenous formulation of plasma‐derived apoA‐I, the primary functional component of HDL, reconstituted into disk‐shaped lipoproteins with phosphatidylcholine. Lyophilized CSL112 was reconstituted with sterile water for injection and was dosed based on total protein content. Both CSL112 (2 or 6 g) and placebo (0.9% sodium chloride solution) were administered as a single 2‐hour intravenous infusion.
Pharmacokinetic and Pharmacodynamics Assessments
Plasma apoA‐I was measured before dosing and at 2, 4, 6, 8, 12, 24, 48, 72, 96, and 144 hours using an immunoturbidimetric method.
Plasma and serum cholesterol efflux capacity biomarkers of reverse cholesterol transport: total, ABCA1‐dependent and ABCA1‐independent cholesterol efflux capacity, and pre‐β1‐HDL were measured before dosing and at 2, 4, 8, 24, 48, and 72 hours.
Cholesterol efflux capacity, total and ABCA1‐independent, was measured after incubation of apolipoprotein B‐depleted serum in vitro with macrophages preloaded with radiolabeled cholesterol with and without cyclic adenosine monophosphate induction.25 ABCA1‐dependent cholesterol efflux capacity was calculated as the difference between total cholesterol efflux capacity and ABCA1‐independent cholesterol efflux capacity. Pre‐β1‐HDL was measured using a sandwich enzyme‐linked immunosorbent assay (Sekisui, Japan) employing a conformational‐specific antibody to apoA‐I within pre‐β1‐HDL.26 The area under the effect curve from time point zero (baseline) to 24 hours following infusion (AUEC0‐24) was calculated for total, ABCA1‐independent, and ABCA1‐dependent cholesterol efflux capacity and pre‐β1‐HDL.
HDL‐C, HDL‐esterified cholesterol (HDL‐EC), HDL‐unesterified cholesterol (HDL‐UC), total cholesterol, and non‐HDL‐C were assessed before dosing and at 2, 4, 8, 24, 48, 72, 96, and 144 hours using standard enzymatic methods.
Apolipoprotein B, predose and at 144 hours, and high‐sensitivity C‐reactive protein (hsCRP), predose and at 24 and 48 hours, were measured by an immunoturbidimetric method. Triglycerides were determined following elimination of free glycerol, subsequent hydrolysis of triglycerides with lipoprotein lipase, and detection of the glycerol generated in a colorimetric reaction using glycerol phosphate‐oxidase predose and at 144 hours.
Statistical Analysis
The planned sample size for this study was n = 16 moderate renal impairment and n = 16 normal renal function subjects. This empirical sample size was considered to be sufficient to assess PK. Subjects receiving placebo were pooled within renal function cohorts (n = 4) in the analysis.
A 2‐sample t test was used to compare baseline cholesterol efflux capacity and lipoprotein parameters between patients with moderate renal impairment and those with normal renal function.
When measured postinfusion, the following parameters were corrected for baseline levels: apoA‐I; total, ABCA1‐independent, and ABCA1‐dependent cholesterol efflux; total cholesterol and HDL‐C, to ascertain the effect of CSL112 irrespective of baseline levels. AUEC0‐24 was calculated using WinNonLin v6.4 from concentration/activity‐time data following infusion of CSL112 for baseline‐corrected data. The relationship between biomarker exposures (AUEC0‐24 for total, ABCA1‐independent, and ABCA1‐dependent cholesterol efflux capacity and pre‐β1‐HDL) and CSL112 dose was compared between those with renal impairment and those with normal renal function. A linear model with terms for renal function group, dose (as a continuous variable), and treatment by renal function group interaction was used to assess whether the slopes were parallel for the 2 renal function groups. P < .05 was considered statistically significant. The data and statistical analysis complied with the recommendations on the experimental design and analysis as published by the British Journal of Clinical Pharmacology.27
Results
Baseline Characteristics
In this phase 1 multicenter, double‐blind, placebo‐controlled ascending‐dose study, 32 subjects were randomized to receive a single intravenous infusion of CSL112 or placebo, n = 16 with moderate renal impairment and n = 16 with normal renal function (age‐, sex‐, and weight‐matched). The baseline characteristics of subjects by renal function group are shown in Table 1.
Table 1.
Subject Demographics and Baseline Characteristics by Renal Function Group
| Normal Renal Function (n = 16) | Moderate Renal Impairment (n = 16) | P for Difference | |
|---|---|---|---|
| Age, years | 55 (7) | 69 (9) | N/A |
| Sex (male), n (%) | 11 (68.8) | 11 (68.8) | N/A |
| White, n (%) | 16 (100) | 16 (100) | N/A |
| Weight, kg | 78 (10.8) | 80.5 (16.6) | N/A |
| BMI, kg/m2 | 26.23 (2.89) | 27.88 (4.64) | N/A |
| eGFR, mL/min/1.73 m2 | 100.5 (6.0) | 49.1 (7.7) | N/A |
| Apolipoprotein A‐I, mg/dL | 141 (19.0) | 143 (21.3) | 0.828 |
| Cholesterol efflux capacity, % efflux/4 hours | |||
| Total | 9.03 (1.75) | 11.50 (2.49) | 0.003 |
| ABCA1‐independent | 7.02 (1.29) | 7.85 (1.56) | 0.110 |
| ABCA1‐dependent | 2.01 (1.22) | 3.65 (1.68) | 0.004 |
| Pre‐β1‐HDL, g/L | 0.016 (0.003) | 0.023 (0.01) | 0.014 |
| Total cholesterol, mg/dL | 191 (36) | 188 (34) | 0.834 |
| HDL‐cholesterol, mg/dL | 52 (8) | 53 (12) | 0.731 |
| HDL‐unesterified cholesterol, mg/dL | 15 (3) | 15 (3) | 1.000 |
| HDL‐esterified cholesterol, mg/dL | 37 (6) | 38 (9) | 0.692 |
| Non‐HDL‐cholesterol, mg/dL | 140 (37) | 136 (31) | 0.743 |
| Apolipoprotein B, mg/dL | 91 (24) | 89 (18) | 0.780 |
| Triglycerides, mg/dL | 132 (57) | 141 (63) | 0.687 |
| C‐reactive protein, mg/L | 1.7 (3.5) | 2.3 (3.9) | 0.645 |
| Comorbidities, n (%) | |||
| Type 2 diabetes mellitus | 0 | 4 (25.0) | n.d. |
| Hyperlipidemia | 0 | 1 (6.3) | n.d. |
| Hypertension | 0 | 13 (81.3) | n.d. |
| Concomitant medications, n (%) | |||
| Statins | 0 | 5 (31.3) | n.d. |
| ACE inhibitors | 0 | 10 (62.5) | n.d. |
| Thiazides | 0 | 5 (31.3) | n.d. |
ACE, angiotensin‐converting enzyme; BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; N/A, not applicable; n.d., not determined.
Values are shown as mean (standard deviation) unless otherwise stated.
At baseline, total and ABCA1‐dependent cholesterol efflux capacity were significantly higher in moderate renal impairment subjects than in subjects with normal renal function (1.3‐fold and 1.8‐fold, respectively). There was no significant difference in ABCA1‐independent cholesterol efflux capacity. Baseline pre‐β1‐HDL was significantly higher (1.4‐fold) in moderate renal impairment versus normal renal function. All other lipid and lipoprotein levels and high‐sensitivity hsCRP were similar in the renal function groups at baseline (Table 1).
At baseline, there was a significant inverse correlation between eGFR and total, ABCA1‐independent, and ABCA1‐dependent cholesterol efflux capacity and pre‐β1‐HDL levels. The lower eGFR in subjects with moderate renal impairment was significantly associated with higher cholesterol efflux capacity and pre‐β1‐HDL levels (Figure 1).
Figure 1.
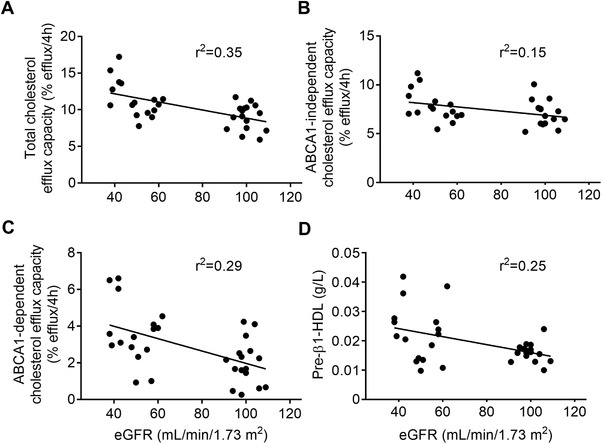
Correlation of baseline estimated glomerular filtration rate, cholesterol efflux capacity, and pre‐β1‐HDL. Shown are individual data points (n = 32) and regression lines for cholesterol efflux capacity; total (A), ABCA1‐independent (B), ABCA1‐dependent (C), and pre‐β1‐high density lipoprotein (D) by baseline estimated glomerular filtration rate (eGFR). Linear regression analysis showed that eGFR was significantly correlated with all parameters shown at baseline (p values not shown). Goodness of fit shown as r2.
Pharmacokinetics of apoA‐I
The infusion of 2 or 6 g of CSL112 resulted in a rapid dose‐dependent increase in plasma apoA‐I concentrations, which peaked at the end of the 2‐hour infusion and remained above baseline 72 hours postdose. Infusion of the highest dose, 6 g CSL112, resulted in an approximately 2‐fold increase in apoA‐I concentrations from baseline in both renal function groups. The mean apoA‐I concentration‐time profiles were similar in the renal function groups (Figure 2). Full details of the PK analysis are reported elsewhere.24
Figure 2.
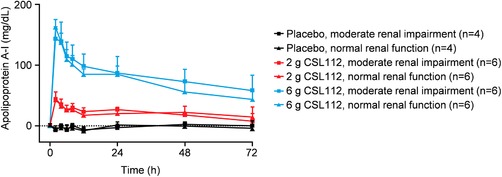
Apolipoprotein A‐I pharmacokinetic profile following infusion of CSL112. Shown are baseline‐corrected mean ± standard deviation apoA‐I plasma concentration by dose group and renal function from predose to 72 hours postdose.
CSL112 Strongly Elevates Cholesterol Efflux Capacity and Pre‐β1‐HDL Levels in a Dose‐Dependent Manner
Strong elevations in total, ABCA1‐independent and ABCA1‐dependent cholesterol efflux capacity were observed following CSL112 infusion, with similar effects observed in both renal function groups (Figure 3A‐C). Following infusion of 6 g CSL112, total cholesterol efflux capacity increased by approximately 2.5‐fold, ABCA1‐independent cholesterol efflux capacity by approximately 2‐fold, and ABCA1‐dependent by approximately 4‐fold. CSL112 also dramatically increased pre‐β1‐HDL levels (Figure 3D) in both renal function groups by up to approximately 20‐fold with 6 g CSL112.
Figure 3.
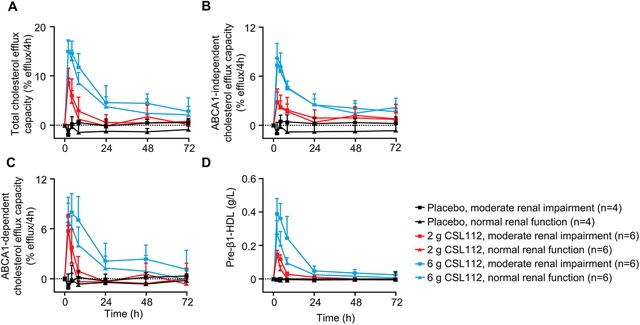
Cholesterol efflux capacity and pre‐β1‐HDL following infusion of CSL112. Shown are baseline‐corrected mean ± standard deviation cholesterol efflux capacity; total (A), ABCA1‐independent (B), ABCA1‐dependent (C), and pre‐β1‐high density lipoprotein (D) by dose group and renal function group from predose to 72 hours postdose.
Increases in total cholesterol efflux, ABCA1‐independent cholesterol efflux, ABCA1‐dependent cholesterol efflux capacity, and pre‐β1‐HDL following CSL112 infusion were dose dependent (Figure 4A‐D). For cholesterol efflux capacity parameters, there was no significant difference between renal function groups in the relationship between AUEC0‐24 and CSL112 dose (Figure 4A‐C); however, for pre‐β1‐HDL, the dose‐dependent increase was greater for subjects with moderate renal impairment than for subjects with normal renal function (Figure 4D). There was a trend toward greater elevation in ABCA1‐dependent cholesterol efflux capacity in moderate renal impairment compared with normal renal function (Figure 4C), although this difference did not reach statistical significance.
Figure 4.
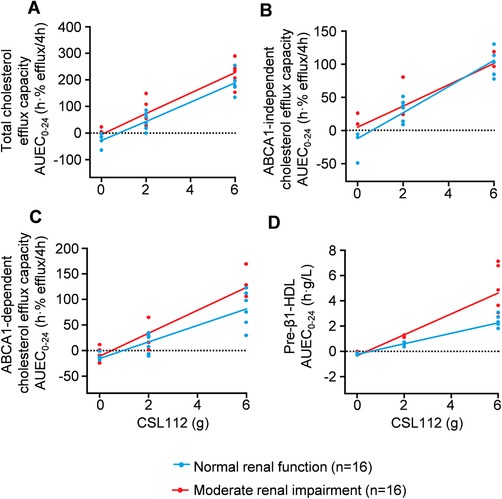
Dose‐dependent increases in cholesterol efflux capacity and pre‐β1‐HDL following infusion of CSL112. Shown are individual data points for the area under the effect curve from 0 to 24 hours (AUEC0‐24) for cholesterol efflux capacity; total (A), ABCA1‐independent (B), ABCA1‐dependent (C), and pre‐β1‐high density lipoprotein (D). Regression lines for area under the effect curve over 24 hours (AUEC0‐24) by CSL112 dose for renal function groups were compared for parallelism, as described in the Methods section. Slopes were not significantly different for total, ABCA1‐independent, or ABCA1‐dependent cholesterol efflux capacity. The dose‐dependent increase in pre‐β1‐HDL was significantly greater for subjects with moderate renal impairment than for those with normal renal function.
CSL112 Infusion Leads to Mobilization and Esterification of Cholesterol
Total cholesterol and HDL‐C increased dose dependently following infusion of CSL112 (Figure 5). Because CSL112 does not contain cholesterol, the increase in total plasma cholesterol indicates recruitment of cholesterol from tissues into plasma. Total cholesterol and HDL‐C increased to a similar degree in both renal function groups.
Figure 5.
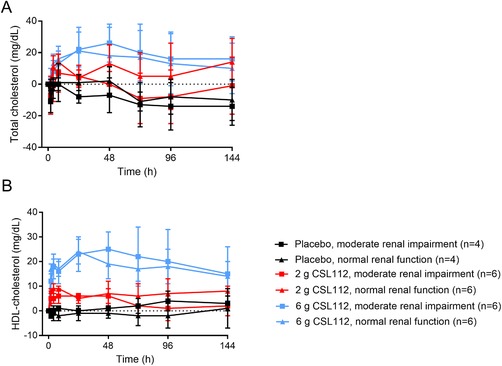
Baseline‐corrected total and high density lipoprotein‐cholesterol (HDL‐C) following infusion of CSL112. Shown are baseline‐corrected mean ± standard deviation total cholesterol (A) and HDL‐C (B) from predose to 144 hours postdose in subjects with moderate renal impairment and normal renal function.
Following infusion of CSL112, there was a transient dose‐dependent increase in HDL‐UC, which peaked at the end of the 2‐hour infusion and then declined. This was followed by an increase in HDL‐EC, peaking 24 hours postdose and exceeding the level of HDL‐UC. For the 6‐g CSL112 group, both HDL‐UC and HDL‐EC levels were sustained above baseline levels 144 hours postdose. Within dose groups, CSL112 had a similar impact on levels of HDL‐UC and HDL‐EC in both renal function groups (Figure 6). These findings demonstrate a precursor‐product kinetic relationship between HDL‐UC and HDL‐EC following CSL112 infusion that is suggestive of rapid esterification by LCAT.
Figure 6.
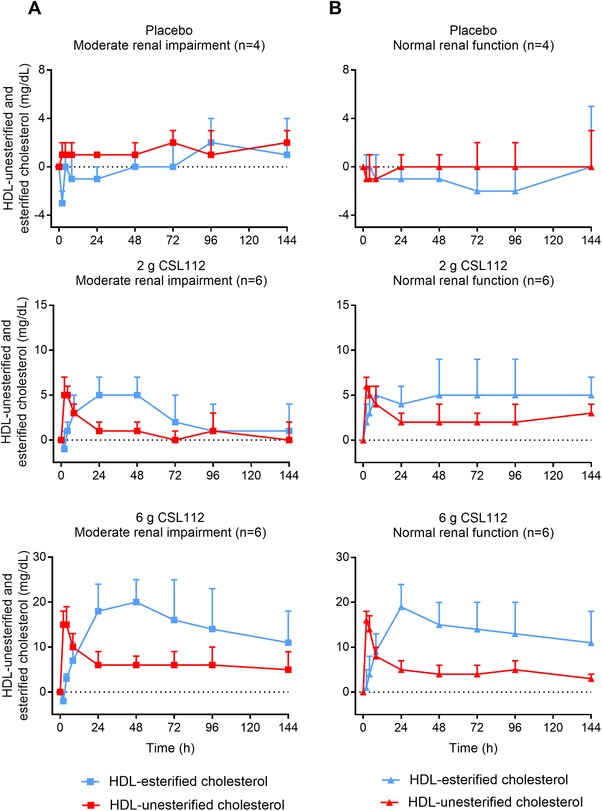
Conversion of unesterified to esterified cholesterol following infusion of CSL112. Shown are baseline‐corrected mean ± standard deviation high‐density lipoprotein (HDL)‐esterified cholesterol and HDL‐unesterified cholesterol from predose to 144 hours postdose for placebo and by CSL112 dose in subjects with moderate renal impairment (A) and normal renal function (B).
Infusion of CSL112 did not significantly alter plasma levels of the proatherogenic lipids apolipoprotein B, non‐HDL‐C, or triglycerides compared with baseline levels, in either renal function group (Supplementary Figure S1A‐C). CSL112 infusion also did not alter levels of plasma hsCRP, a biomarker of inflammation, from baseline to 24 or 48 hours postdose (Supplementary Figure S1D).
Safety
CSL112 was well tolerated during the study. There were no clinically significant changes in renal function and no serious related adverse events. The full safety data are reported elsewhere.24
Discussion
Patients with chronic renal insufficiency have an elevated risk of death, cardiovascular events, and hospitalization.28 Importantly, acute myocardial infarction patients with moderate to severe renal impairment (eGFR < 40 mL/min/1.73 m2) have an approximately 1.5‐fold increased risk of experiencing a recurrent cardiovascular event compared with acute myocardial infarction patients with normal renal function to mild renal impairment (eGFR ≥ 75 mL/min/1.73 m2) and an estimated 3‐fold higher rate of reinfarction.29
In this phase 1 study, a single infusion of CSL112 caused similar elevation of apoA‐I and cholesterol efflux capacity in subjects with normal renal function and in patients with moderate renal impairment. Moreover, both groups demonstrated similar dose dependence for elevation of cholesterol efflux capacity. These findings are consistent with our recent studies on patients with mild renal impairment in which no differences in cholesterol efflux capacity between normal renal function and mild renal impairment subjects could be detected after infusion of CSL112 (Gille et al, unpublished data, 2018).
Infusion of CSL112 was followed by first a rise in HDL‐UC cholesterol and then a rise in HDL‐EC, with a characteristic precursor/product time course indicative of LCAT activity. As with the elevation of cholesterol efflux capacity activity, a similar pattern of cholesterol esterification was observed in moderate renal impairment and normal renal function subjects. These data are consistent with previous work that showed strong elevation of LCAT activity ex vivo30 and rapid esterification of cholesterol on infusion of CSL112 in healthy adults.13 Taken together, this suggests no diminution of effect of CSL112 in either cholesterol efflux capacity or LCAT activation in patients with moderate renal impairment.
Baseline measures in subjects with moderate renal impairment showed elevated total cholesterol efflux capacity and ABCA1‐dependent cholesterol efflux capacity compared with subjects with normal renal function. Further comparison showed a clear relationship of these parameters with eGFR. Nevertheless, no differences were observed in levels of apoA‐I or HDL‐C. This finding suggests that moderate renal impairment is associated with enhanced cholesterol efflux capacity on a per‐HDL basis.
The literature offers little precedent for the finding of elevated cholesterol efflux capacity in moderate renal impairment because most prior studies in subjects with low eGFR have focused mainly on patients with severe renal disease (eGFR < 30 mL/min/1.73 m2), quite different from those studied here. The severely impaired patients were generally observed to have both reduced cholesterol efflux capacity and reduced levels of HDL‐C.31, 32, 33, 34, 35 Only 1 previous study measured efflux in patients with moderate renal impairment (eGFR, ∼50 mL/min/1.73 m2) similar to the ones studied here. These authors observed that cholesterol efflux capacity was no different from that of healthy control subjects.36 However, the moderate renal impairment patients in this study had apoA‐I and HDL‐C levels lower than in control subjects. Thus, if cholesterol efflux capacity is normalized to either apoA‐I level or HDL‐C, elevated cholesterol efflux capacity on a per‐HDL basis is seen in both the current study and that of Baragetti et al.36
The mechanisms underlying enhanced cholesterol efflux capacity per HDL in moderate renal impairment are not fully evident at this time. We have observed that baseline ABCA1‐dependent cholesterol efflux capacity is more strongly elevated than ABCA1‐independent cholesterol efflux capacity. Consistent with this finding, we have also observed elevated pre‐β1‐HDL in baseline samples of patients with moderate renal impairment. Because the pre‐β1 species of HDL are the preferred acceptors of cholesterol from ABCA1, elevated pre‐β1‐HDL levels are likely a direct cause of elevated ABCA1‐dependent cholesterol efflux capacity. But the cause of elevated pre‐β1‐HDL is, in turn, uncertain. One possibility is that renal impairment reduces the activity of LCAT, an enzyme that drives conversion of pre‐β1‐HDL to HDL3. A reduction in LCAT‐mediated conversion of pre‐β1‐HDL to HDL3 was previously reported in hemodialysis patients,37 and LCAT activity progressively decreased and HDL‐UC levels increased over a 9‐year period in a longitudinal study of patients on long‐term dialysis.38 In chronic kidney disease patients not requiring hemodialysis, chronic kidney disease was associated with lower circulating levels of LCAT, decreased LCAT activity, and increased pre‐β1‐HDL levels.39 Any changes in LCAT in the present study are likely to be modest given our observation of strong cholesterol esterification on infusion of CSL112.
A second possible explanation for elevated pre‐β1‐HDL and cholesterol efflux capacity in renal impairment may involve downregulation of ABCA1 expression on peripheral mononuclear cells in renal impairment, as recently reported by Ganda et al.40 This may lead to an increase in pre‐β1‐HDL because of reduced ability to metabolize pre‐β1‐HDL to HDL3. Further, it is possible that reduced LCAT and reduced ABCA1 expression act in combination to slow the metabolism of pre‐β1‐HDL to HDL3 and thereby affect ABCA1‐dependent cholesterol efflux capacity in renal impairment patients. Regardless of which effect predominates, the time‐course observations reported here are consistent with the hypothesis that metabolism of pre‐β1‐HDL to HDL3 is slowed in renal impairment patients. After infusion of CSL112, the rate of clearance of both pre‐β1‐HDL and ABCA1‐dependent cholesterol efflux capacity is slower in subjects with moderate renal impairment. These findings suggest dysregulation of HDL metabolism in patients with moderate renal impairment, and this dysregulation may underlie their increased cardiovascular risk.
Conclusions
CSL112 (apolipoprotein A‐I [human]) enhances biomarkers of reverse cholesterol transport similarly in subjects with moderate renal impairment and in those with normal renal function. Increased cholesterol efflux capacity may help to stabilize plaques in the coronary arteries that are vulnerable to rupture.41 Therefore, CSL112 may provide a novel therapy to rapidly lower the burden of atherosclerosis and reduce the risk of early recurrent cardiovascular events in acute myocardial infarction patients, with or without renal impairment.
Declaration of Conflicting Interests
All authors are employees of CSL Limited or CSL Behring.
Supporting information
Supplementary Figure S1. Proatherogenic lipid and inflammatory biomarker levels following infusion of CSL112.
Acknowledgments
The authors thank Kate Holliday of Meridian HealthComms, Plumley, UK, for providing medical writing assistance, which was funded by CSL Behring, King of Prussia, Pennsylvania, in accordance with Good Publication practice (GPP3).
Funding
This work was funded by CSL Behring.
Contributions
All authors contributed to the design of the study and/or assisted with the data analysis/interpretation of the data. All authors assisted in the preparation of the manuscript, reviewed the manuscript, and provided their approval for submission. All authors agree to be accountable for all aspects of the work presented.
Data‐Sharing Statement
Individual participant data will not be shared.
References
- 1. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post‐myocardial infarction patients: nationwide real world data demonstrate the importance of a long‐term perspective. Eur Heart J. 2015;36(19):1163–1170. [DOI] [PubMed] [Google Scholar]
- 2. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural‐history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–235. [DOI] [PubMed] [Google Scholar]
- 3. Briel M, Vale N, Schwartz GG, et al. Updated evidence on early statin therapy for acute coronary syndromes: meta‐analysis of 18 randomized trials involving over 14,000 patients. Int J Cardiol. 2012;158(1):93–100. [DOI] [PubMed] [Google Scholar]
- 4. Vale N, Nordmann AJ, Schwartz GG, et al. Statins for acute coronary syndrome. Cochrane Database Syst Rev. 2014;9:CD006870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. [DOI] [PubMed] [Google Scholar]
- 6. Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(suppl):S189–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case‐control study. Lancet Diabetes Endocrinol. 2015;3(7):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang J, Xu J, Wang J, et al. Prognostic usefulness of serum cholesterol efflux capacity in patients with coronary artery disease. Am J Cardiol. 2016;117(4):508–514. [DOI] [PubMed] [Google Scholar]
- 10. Liu C, Zhang Y, Ding D, et al. Cholesterol efflux capacity is an independent predictor of all‐cause and cardiovascular mortality in patients with coronary artery disease: a prospective cohort study. Atherosclerosis. 2016;249:116–124. [DOI] [PubMed] [Google Scholar]
- 11. Annema W, Willemsen HM, de Boer JF, et al. HDL function is impaired in acute myocardial infarction independent of plasma HDL cholesterol levels. J Clin Lipidol. 2016;10(6):1318–1328. [DOI] [PubMed] [Google Scholar]
- 12. Hafiane A, Jabor B, Ruel I, Ling J, Genest J. High‐density lipoprotein mediated cellular cholesterol efflux in acute coronary syndromes. Am J Cardiol. 2014;113(2):249–255. [DOI] [PubMed] [Google Scholar]
- 13. Gille A, Easton R, D'Andrea D, Wright SD, Shear CL. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34(9):2106–2114. [DOI] [PubMed] [Google Scholar]
- 14. Tricoci P, D'Andrea DM, Gurbel PA, et al. Infusion of reconstituted high‐density lipoprotein, CSL112, in patients with atherosclerosis: Safety and pharmacokinetic results from a Phase 2a randomized clinical trial. J Am Heart Assoc. 2015;4(8):e002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibson CM, Korjian S, Tricoci P, et al. Safety and tolerability of CSL112, a reconstituted infusible, plasma‐derived apolipoprotein A‐I, after acute myocardial infarction. Circulation. 2016;134:1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Didichenko SA, Navdaev AV, Cukier AM, et al. Enhanced HDL functionality in small HDL species produced upon remodeling of HDL by reconstituted HDL, CSL112: effects on cholesterol efflux, anti‐Inflammatory and antioxidative activity. Circ Res. 2016;119(6):751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gille A, D'Andrea D, Easton R, et al. CSL112, a novel formulation of human apolipoprotein A‐I, dramatically increases cholesterol efflux capacity in patients with stable atherothrombotic disease: a multicenter, randomized, double‐blind, placebo‐controlled, ascending‐dose study. Circulation. 2013;128:A15780. [Google Scholar]
- 18. Rosenson RS, Brewer HB, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125(15):1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox CS, Muntner P, Chen AY, et al. Use of evidence‐based therapies in short‐term outcomes of ST‐segment elevation myocardial infarction and non‐ST‐segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121(3):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glass CK, Pittman RC, Keller GA, Steinberg D. Tissue sites of degradation of apoprotein A‐I in the rat. J Biol Chem. 1983;258(11):7161–7167. [PubMed] [Google Scholar]
- 21. Kon V, Yang H, Fazio S. Residual cardiovascular risk in chronic kidney disease: role of high‐density lipoprotein. Arch Med Res. 2015;46(5):379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacek R, Anna G, Danilo F, Timo S, Andrzej W. Chronic kidney disease ‐ different role for HDL? Curr Med Chem. 2014;21(25):2910–2916. [DOI] [PubMed] [Google Scholar]
- 23. Vaziri ND. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol. 2016;12(1):37–47. [DOI] [PubMed] [Google Scholar]
- 24. Tortorici MA, Duffy D, Evans R, et al. Pharmacokinetics and safety of CSL112 (apolipoprotein A‐I [human]) in adults with moderate renal impairment and normal renal function. Clin Pharmacol Drug Dev. 2018. 10.1002/cpdd.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de la Llera‐Moya M, Drazul‐Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high‐density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30(4):796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miida T, Miyazaki O, Nakamura Y, et al. Analytical performance of a sandwich enzyme immunoassay for pre beta 1‐HDL in stabilized plasma. J Lipid Res. 2003;44(3):645–650. [DOI] [PubMed] [Google Scholar]
- 27. Curtis MJ, Bond RA, Spina D, et al. Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol. 2015;172(14):3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. [DOI] [PubMed] [Google Scholar]
- 29. Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–1295. [DOI] [PubMed] [Google Scholar]
- 30. Diditchenko S, Gille A, Pragst I, et al. Novel formulation of a reconstituted high‐density lipoprotein (CSL112) dramatically enhances ABCA1‐dependent cholesterol efflux. Arterioscler Thromb Vasc Biol. 2013;33(9):2202–2211. [DOI] [PubMed] [Google Scholar]
- 31. Meier SM, Wultsch A, Hollaus M, et al. Effect of chronic kidney disease on macrophage cholesterol efflux. Life Sci. 2015;136:1–6. [DOI] [PubMed] [Google Scholar]
- 32. Holzer M, Birner‐Gruenberger R, Stojakovic T, et al. Uremia alters HDL composition and function. J Am Soc Nephrol. 2011;22(9):1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holzer M, Schilcher G, Curcic S, et al. Dialysis modalities and HDL composition and function. J Am Soc Nephrol. 2015;26(9):2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamamoto S, Yancey PG, Ikizler TA, et al. Dysfunctional high‐density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012;60(23):2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shroff R, Speer T, Colin S, et al. HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J Am Soc Nephrol. 2014;25(11):2658–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baragetti A, Norata GD, Sarcina C, et al. High density lipoprotein cholesterol levels are an independent predictor of the progression of chronic kidney disease. J Intern Med. 2013;274(3):252–262. [DOI] [PubMed] [Google Scholar]
- 37. Miida T, Miyazaki O, Hanyu O, et al. LCAT‐dependent conversion of prebeta1‐HDL into alpha‐migrating HDL is severely delayed in hemodialysis patients. J Am Soc Nephrol. 2003;14(3):732–738. [DOI] [PubMed] [Google Scholar]
- 38. Mekki K, Bouchenak M, Remaoun M, Belleville JL. Effect of long‐term hemodialysis on plasma lecithin: cholesterol acyltransferase activity and the amounts and compositions of HDL2 and HDL3 in hemodialysis‐treated patients with chronic renal failure: a 9‐year longitudinal study. Med Sci Monit. 2004;10(8):CR439–446. [PubMed] [Google Scholar]
- 39. Calabresi L, Simonelli S, Conca P, et al. Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J Intern Med. 2015;277(5):552–561. [DOI] [PubMed] [Google Scholar]
- 40. Ganda A, Yvan‐Charvet L, Zhang Y, et al. Plasma metabolite profiles, cellular cholesterol efflux, and non‐traditional cardiovascular risk in patients with CKD. J Mol Cell Cardiol. 2017;112:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kingwell BA, Chapman MJ, Kontush A, Miller NE. HDL‐targeted therapies: progress, failures and future. Nat Rev Drug Discov. 2014;13(6):445–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Proatherogenic lipid and inflammatory biomarker levels following infusion of CSL112.


