Abstract
Background
Bioabsorbable steroid‐releasing implants (mometasone furoate, 370 μg) are effective for improving postsurgical outcomes in the frontal sinus ostia (FSO). In this study we evaluated the effect of these implants on frontal outcomes in various patient subgroups with chronic rhinosinusitis (CRS) using pooled data from 2 randomized, controlled trials (RCTs).
Methods
A total of 160 subjects were enrolled in 2 RCTs. After surgery, subjects were randomized to receive an implant in 1 FSO with the contralateral side as control. Data through day 90 from the 2 studies were pooled and subgroup analyses were performed.
Results
At day 30, relative to controls, steroid‐releasing implants significantly reduced the need for postoperative interventions by 46.8% (95% confidence interval [CI], −60.7 to −27.9), for surgical interventions by 51.2% (95% CI, −68.2 to −25.2), and for oral steroid interventions by 37.2% (95% CI, −54.6 to −13.1) in the pooled data set. At day 90, statistically significant reductions (p < 0.05) in the need for postoperative interventions (relative reduction [RR], 30.2%), restenosis/occlusion rate (RR, 31.7%), and inflammation score (absolute difference, −6.0), and increase in estimated FSO diameter (absolute difference, 1 mm), favoring the treated side, were observed. Subgroup analyses of the pooled data showed statistically significant improvements (p < 0.05) at day 90 in restenosis/occlusion rate, and estimated FSO diameter, favoring the treated side across subgroups, with no statistically significant subgroup‐by‐treatment interactions.
Conclusion
Bioabsorbable steroid‐releasing sinus implants improve outcomes of frontal sinus surgery through 90 days, irrespective of asthma status, previous endoscopic sinus surgery, extent of surgery, extent of polyps, or Lund‐Mackay computed tomography stage in the FSO.
Keywords: corticosteroid, mometasone furoate, endoscopic sinus surgery, frontal sinus, revision ESS, asthma
Treatment of the frontal sinus in patients with chronic rhinosinusitis (CRS) presents unique challenges for the otolaryngologist.1 Greater anatomic complexity, angled visualization, specialized instrumentation, and proximity to critical structures make surgical management of the frontal sinus far more demanding than that of other paranasal sinuses.2 Postoperative circumferential scarring and inflammation often results in restenosis and often occlusion of the frontal sinus ostium (FSO). This may result in recurrent acute or subacute frontal sinusitis, chronic frontal sinusitis, and/or delayed mucoceles.3 In addition, middle turbinate lateralization and the ubiquitous force of gravity often render the FSO inaccessible to topical therapies.4, 5, 6 Historically, endoscopic frontal sinus surgery has generally been less successful when compared with the other paranasal sinuses, secondary to preoperative conditions, extent of sinusotomy, and challenges of postoperative medical management of the frontal sinus.2, 7, 8
Innovations in surgical management and instrumentation have improved longer term frontal sinus outcomes. Advanced surgical techniques,9 mucosa‐sparing techniques, mucosal grafts,10 and advances in instrumentation, such as the angled microdebrider, specialized frontal punches, balloon sinuplasty, image guidance, and high speed drills, have all contributed to these improvements. As a result, longer term patency rates after frontal sinus surgery have steadily improved from the lower range of 25% reported historically.11
However, despite significant progress in surgical techniques and instrumentation, as well as strategies to address chronic inflammation, postoperative “medical” and “surgical” failures continue to damper frontal sinus surgery results. The frustrating propensity of the FSO to stenose and exhibit recurrent polypoid inflammation remains an “Achilles heel” for the sinus surgeon. Although a minority of authors reported excellent long‐term frontal recess patency with advanced surgical procedures, the vast majority of the literature has shown wide‐ranging (from 67.6% to 92%) frontal sinus patency after frontal sinusotomy.2, 8, 12, 13
Currently, there are 2 United States Food and Drug Administration‒approved bioabsorbable steroid‐releasing sinus implants for the FSO. These implants, each releasing 370 μg of mometasone furoate (MF) over 30 days, were studied in 2 double‐blind randomized, controlled trials (RCTs) in 160 CRS patients undergoing endoscopic sinus surgery (ESS). The reported significant reductions in the need for postoperative interventions at 30 days in both RCTs established the efficacy of these implants in the FSO.14, 15 The objective of the pooled data analysis was to further evaluate the longer term outcomes and the effects of baseline clinical characteristics on the efficacy of the implants to determine which subsets of patients may benefit most from use of the implants in the FSO after ESS.16 We addressed this question by assessing endoscopic outcomes at 90 days in the following CRS patient subgroups: asthmatics vs nonasthmatics; primary vs revision ESS; minimal vs significant frontal recess polyposis; extent of surgery (Draf IIA or Draf IIB); and extent of frontal disease, based on Lund‐Mackay (LM) frontal computed tomography (CT) stage.
Patients and methods
Trial design
The study designs and outcomes of the 2 RCTs have been published previously14, 15 and are summarized in what follows. Both studies were of identical design—prospective, randomized, and blinded—using an intrapatient control in 80 CRS patients. Institutional review board approval was obtained for each study, and written informed consent was obtained from all patients before screening and study entry. Endoscopic evaluations were performed by clinical investigators through 90 days after implant placement. Implants were removed at day 21 to allow blinded assessment by an independent sinus surgeon at day 30 based on a centralized review of video‐endoscopies, which were edited to remove all patient identifying information.
Study population
The study population comprised adult (≥18 years of age) patients diagnosed with CRS17 who were scheduled to undergo bilateral ESS (primary or revision) and had evidence of bilateral frontal sinus disease based on CT (LM score ≥1 on each side).14, 15 Frontal sinus surgery was required to be performed using a Draf IIa or IIb dissection method with traditional instrumentation, balloon, or a combination of both, provided the same instrumentation and technique was utilized on both sides. Concurrent septoplasty for access to the paranasal sinuses was permitted, as was bilateral surgical treatment of other paranasal sinuses. Patients were randomized in the study after a successful completion of the ESS procedure without any complications; creation of a minimum of a 5‐mm FSO bilaterally; and confirmation that the FSO were amenable to placement of the steroid‐releasing sinus implant.
Intervention
After successful bilateral frontal sinusotomy, each patient was randomized to receive 1 steroid‐releasing implant (PROPEL® Mini or PROPEL® Contour; Intersect ENT, Inc, Menlo Park, CA) in 1 FSO (treatment side) while the contralateral FSO served as the control. Patients were maintained on standardized medical therapy throughout the study periods. Oral and/or intranasal corticosteroid use was allowed preoperatively. Postoperatively, hemostatic packing material was allowed to be placed within the ethmoid sinuses as long as it did not reside within the study implants. Septal splints were allowed as well. A 10‐day course of antibiotics was required, starting within 1 day of surgery. Intranasal steroid sprays were allowed starting at 14 days post‐ESS, and oral steroids were prescribed, if warranted, by the investigator. If patients were on orally inhaled steroids for control of asthma, they were maintained on such treatment throughout the study. Patients were encouraged to use saline sprays or irrigation during the follow‐up period. All medications taken by the patient were documented throughout the studies.
Outcome measures
The primary efficacy endpoint for both RCTs was reduction in the need for postoperative interventions at 30 days based on a centralized, blinded video‐endoscopy review by the same independent sinus surgeon. Postoperative intervention was a composite end point defined as either surgical intervention required to debride obstructive adhesions or scar tissue formation in the frontal recess/FSO (defined as grades 2 or 3 on the adhesion/scarring scale), and/or oral steroid intervention warranted to resolve recurrent inflammation or polypoid edema in the frontal recess/FSO (Table 1). Endoscopic evaluation by clinical investigators at baseline, day 30, and day 90 was performed using the same grading scales in both studies,14, 15 and included grading of patency of the FSO, visually estimated FSO diameter (in millimeters), and inflammation score using a 100‐mm visual analog scale (VAS) (Table 1). Baseline evaluations were performed before patients underwent ESS, except for FSO diameter assessment, which was performed after ESS. Safety assessment was based on all adverse events reported throughout the 90‐day timepoint.
Table 1.
Endoscopic grading scales for the frontal recess/frontal sinus ostia
| Characteristic | Score definition |
|---|---|
| Inflammation |
|
| Polypoid edema |
|
| Patency |
|
| Adhesion/scarring |
|
| Need for oral steroid interventions | Clinician judgment whether oral steroid intervention is warranted to resolve recurrent inflammation or polypoid edema (yes/no) |
| Need for surgical interventions | Defined as grade 2 or 3 on the adhesion/scarring scale |
| Need for postoperative interventions |
|
FSO = frontal sinus ostia; VAS = visual analog scale.
Pooled data and subgroup analyses
Data from the 2 RCTs were pooled for the 30‐day and 90‐day analyses. It was reasonable to pool the data because both studies had identical study design, eligibility criteria, treatment methods, and efficacy end‐point assessments, and they also enrolled patients with similar baseline clinical characteristics (Table 2, Table 3). In addition, the same centralized reviewer graded the video‐endoscopies from both studies. Efficacy outcomes at day 90 were analyzed in patient subgroups based on their clinical characteristics at baseline. Patients’ subgroups included: those with vs without asthma, as diagnosed by a physician; those undergoing primary vs revision ESS; those undergoing Draf IIA/balloon dilation only vs Draf IIB procedure; those with vs without high polyp burden (defined as polypoid edema of grade 2 in one or both FSOs) at baseline; and those with LM CT stage 2 in either frontal sinus at baseline.
Table 2.
Patients’ characteristics and clinical comorbidities (N = 160)
| Patients’ characteristic | RCT 1 | RCT 2 | Pooled data |
|---|---|---|---|
| Age, in yearsa | 49.9 (13.91) | 49.5 (13.36) | 49.7 (13.59) |
| Male sexb | 46 (57.5) | 53 (66.3) | 99 (61.9%) |
| White raceb | 68 (85.0) | 62 (77.5) | 130 (81.3%) |
| Patients with previous ESSb | 41 (51.2) | 41 (51.2) | 82 (51.2%) |
| Aspirin intolerance or allergyb | 6 (7.5) | 7 (8.8) | 13 (8.1%) |
| Physician‐diagnosed asthmab | 30 (37.5) | 36 (45.0) | 66 (41.3%) |
| Samter triadb | 6 (7.5) | 5 (6.3) | 11 (6.9%) |
| Smokersb | 28 (35.1) | 25 (31.3) | 53 (33.1%) |
| Total LM scorea | 15.8 (4.82) | 14.8 (4.87) | 15.3 (4.86) |
aData expressed as mean (SD).
bData expressed as number (%).
ESS = endoscopic sinus surgery; LM = Lund‐Mackay; SD = standard deviation.
Table 3.
Baseline endoscopic and CT scores of the frontal sinus
| RCT 1 | RCT 2 | |||||
|---|---|---|---|---|---|---|
| n = 80 | n = 80 | Pooled, n = 160 | ||||
| Characteristic* | T | C | T | C | T | C |
| Adhesion/scarring—clinically significant amount,a n (%) | 13 (16.7) | 17 (21.8) | 10 (17.5) | 11 (19.3) | 23 (17.0) | 28 (20.7) |
| Polypoid edema ‐ expanded amount,b n (%) | 53 (68.8) | 56 (72.7) | 41 (58.6) | 37 (52.9) | 94 (63.9) | 93 (63.3) |
| Estimated FSO diameter (mm), mean (SD) | 8.0 (2.16) | 8.0 (2.02) | 7.5 (1.82) | 7.5 (1.90) | 7.8 (2.01) | 7.7 (1.97) |
| Inflammation score (100‐mm VAS), mean (SD) | 68.8 (24.9) | 69.2 (26.6) | 63.2 (30.3) | 59.1 (30.8) | 66.1 (27.8) | 64.2 (29.1) |
| Frontal LM score, mean (SD) | 1.4 (0.49) | 1.4 (0.50) | 1.4 (0.49) | 1.4 (0.48) | 1.4 (0.49) | 1.4 (0.49) |
*Percent of sinuses were computed based on the number of evaluable sinuses for each characteristic. All baseline endoscopic assessments were performed before the endoscopic sinus surgery, except assessment of estimated FSO diameter, which was performed immediately after sinus surgery.
aAdhesion/scarring grades 2 and 3.
bPolypoid edema grade 2.
CT = computed tomography; FSO = frontal sinus ostium; LM = Lund‐Mackay; SD = standard deviation; VAS = visual analog scale.
Patients and sinuses were included in the analyses if they were part of the intention‐to‐treat population, which consisted of all patients and frontal sinuses with successful ESS and amenable to receiving an implant.
The categorical efficacy end points were analyzed using the McNemar exact test to obtain two‐sided p values, where only discordant pairs observations contribute to evidence of a treatment effect. Continuous end points were analyzed using a paired t test to obtain a side‐to‐side difference in scores. Statistical summaries, confidence intervals (CIs), and p values were generated using SAS version 9.4 (SAS Institute, Inc, Cary, NC). All statistical tests were two‐sided and interpreted at a 5% significance level. Descriptive statistics were provided for all summarized data, displaying the mean and standard deviation (SD) and 95% CI for continuous variables, and count and percentage for categorical variables. Absolute difference represents the mean difference between treatment and control FSO. Percent relative difference was calculated as the difference between percent of treatment and control divided by percent of control FSO for an outcome measure. The influence of subgroup on the treatment effect was analyzed using repeated‐measures analysis of variance (ANOVA) or the logistic regression model with treatment side, subgroup, and interaction of treatment side by subgroup factor as fixed effects.
Results
Patients’ demographics and baseline clinical characteristics
Of the 160 patients randomized, 61.9% of patients were male, 81.3% were white, 51.2% had undergone previous ESS, and 41.3% had a history of asthma (Table 2). Similar proportions of sinuses had polypoid edema of grade 2 (control: 94 [63.9%] vs treatment: 93 [63.3%]) in the FSO at baseline. The mean total LM score at screening was 15.3 (standard deviation [SD], 4.86), and the mean frontal LM score was 1.4 (SD, 0.49) on both frontal sinus sides (Table 3). The implants were successfully delivered in all 160 treated FSO, resulting in a 100% implant delivery success.
One hundred fifty‐eight of the 160 patients (98.8%) completed the 90‐day follow‐up. The day 30 video‐endoscopies of 128 patients were evaluable on both sides by the centralized reviewer for assessment of the primary efficacy end point. In a subset of 32 patients, the centralized reviewer was unable to judge 1 or both sides due to either suboptimal endoscopic video quality or inadequate imaging of the relevant anatomy, lateralization of middle turbinate, or recurrent polyposis within the frontoethmoidal cavity. Owing to suboptimal video quality of video‐endoscopies, or due to inadequate imaging of the FSO in some cases, the number of sinuses evaluable varied by outcome parameter based on ability to visualize relevant anatomy. For example, presence of frontal recess polyposis or a middle turbinate adhesion can prevent visualization of the FSO and, therefore, may have prevented estimation of ostial diameter or patency.
Efficacy outcomes at day 30
Based on the centralized independent blinded reviewer assessment at day 30, the proportion of FSO needing postoperative interventions on the treatment sides was 25.8% compared with 48.4% on the control sides, representing a 46.8% relative difference (RR; 95% CI, −60.7 to −27.9; Table 4). This difference was driven by both a 51.2% significant RR (95% CI, −68.2 to −25.2) in the need for surgical intervention and a 37.2% significant RR (95% CI, −54.6 to −13.1) in need for oral steroid intervention (Table 4).
Table 4.
Need for postoperative interventions in frontal sinus ostia at day 30 as judged by independent reviewer
| RCT 1 | RCT 2 | Pooled data | ||||
|---|---|---|---|---|---|---|
| Outcome* | Treatment (n = 80) | Control (n = 80) | Treatment (n = 80) | Control (n = 80) | Treatment (n = 160) | Control (n = 160) |
| Need for postoperative interventions | ||||||
| Evaluable, n | 67 | 67 | 61 | 61 | 128 | 128 |
| n (%) | 26 (38.8%) | 42 (62.7%) | 7 (11.5%) | 20 (32.8%) | 33 (25.8%) | 62 (48.4%) |
| p value | 0.0070 | 0.0023 | <0.0001 | |||
| Absolute difference (T − C) | 23.9% | 21.3% | 22.7% | |||
| Relative differencea (95% CI) | −38.1% (−55.7 to −13.4) | −65.0% (−82.3 to −30.7) | −46.8% (−60.7 to −27.9) | |||
| Need for surgical interventions | ||||||
| Evaluable, n | 59 | 59 | 58 | 58 | 117 | 117 |
| n (%) | 16 (27.1%) | 26 (44.1%) | 4 (6.9%) | 15 (25.9%) | 20 (17.1%) | 41 (35.0%) |
| p value | 0.0639 | 0.0074 | 0.0011 | |||
| Absolute difference (T − C) | 17.0% | 19.0% | 17.9% | |||
| Relative differencea (95% CI) | −38.5% (−61.6 to −1.5) | −73.3% (−90.0 to −28.9) | −51.2% (−68.2 to −25.2) | |||
| Need for oral steroid interventions | ||||||
| Evaluable, n | 67 | 67 | 61 | 61 | 128 | 128 |
| n (%) | 21 (31.3%) | 33 (49.3%) | 6 (9.8%) | 10 (16.4%) | 27 (21.1%) | 43 (33.6%) |
| p value | 0.0227 | 0.2891 | 0.0070 | |||
| Absolute difference (T − C) | 18.0% | 6.6% | 12.5% | |||
| Relative differencea (95% CI) | −36.4% (−55.8 to −8.4) | −40.0% (−70.7 to 22.7) | −37.2% (−54.6 to −13.1) | |||
* p < 0.05 considered statistically significant.
aRelative difference is calculated as percentage of absolute difference between treatment sides divided by control side. A negative value reflects a decrease in the outcome on the treatment side compared to control, while a positive value reflects an increase in the outcomes on the treatment side compared to control.
C = control side; CI = confidence interval; T = treatment side.
A total of 10 patients received oral steroids for the frontal recess/FSO before day 30, which may have had a confounding effect on the efficacy outcomes in the study. Sensitivity analyses performed in the original studies concluded that there was no impact of oral steroid use on the primary end‐point results. Any effect of oral steroids use would have been present on both FSO sides, and therefore was not expected to confound the efficacy outcomes analyses. Hence, the 10 patients who received oral steroids before day 30 were retained in this pooled analysis.
Efficacy outcomes at day 90
Pooled analysis of the endoscopic assessments at day 90 showed that the need for postoperative interventions continued to be significantly reduced (RR, 30.2%; 95% CI, −42.0 to −15.9) in sinuses that received the steroid‐releasing implant when compared with the control sides, driven by a significantly reduced need for both surgical (RR, 57.1%; 95% CI, −74.9 to −26.9) and oral steroid (RR, 22.6%; 95% CI, −35.3 to −7.5) interventions, as assessed by clinical investigators (Table 5). Significant reductions at day 90 favoring the steroid‐releasing implants were observed for rate of restenosis/occlusion (RR, 31.7%; 95% CI, −44.5 to −16.1) and inflammation score (absolute difference, −6.0; 95% CI, −10.4 to −1.7). A significantly larger estimated FSO diameter (absolute difference, 1.0 mm; 95% CI, 0.5‐1.5) at day 90 was observed, based on assessment by the investigators (Fig. 1 and Table 5).
Table 5.
Endoscopic outcomes in the frontal sinus ostia at day 90 according to clinical investigators
| RCT 1 | RCT 2 | Pooled | ||||
|---|---|---|---|---|---|---|
| Outcome | T (n = 80) | C (n = 80) | T (n = 80) | C (n = 80) | T (n = 160) | C (n = 160) |
| Need for postoperative interventions | ||||||
| Evaluable, n | 77 | 77 | 77 | 77 | 154 | 154 |
| n (%) | 21 (27.3%) | 31 (40.3%) | 23 (29.9%) | 32 (41.6%) | 44 (28.6%) | 63 (40.9%) |
| p value | 0.0129 | 0.0117 | 0.0002 | |||
| Absolute difference (T − C) | 13.0% | 11.7% | 12.3% | |||
| Relative differencea, (95% CI) | −32.3% (−49.2 to −9.7) | −28.1% (−43.4 to −8.7) | −30.2% (−42.0 to −15.9) | |||
| Need for surgical interventions | ||||||
| Evaluable, n | 77 | 77 | 77 | 77 | 154 | 154 |
| n (%) | 3 (3.9%) | 8 (10.5%) | 6 (8.5%) | 13 (18.3%) | 9 (6.1%) | 21 (14.3%) |
| p‐value | 0.1250 | 0.0156 | 0.0018 | |||
| Absolute difference (T − C) | 6.6% | 9.9% | 8.2% | |||
| Relative differencea (95% CI) | −62.5% (−87.0 to 8.1) | −53.8% (−74.3 to −17.0) | −57.1% (−74.9 to −26.9) | |||
| Need for oral steroid interventions | ||||||
| Evaluable, n | 77 | 77 | 77 | 77 | 154 | 154 |
| n (%) | 20 (25.3%) | 27 (34.2%) | 21 (26.6%) | 26 (32.9%) | 41 (25.9%) | 53 (33.5%) |
| p value | 0.0654 | 0.1250 | 0.0075 | |||
| Absolute difference (T − C) | 8.9% | 6.3% | 7.6% | |||
| Relative differencea (95% CI) | −25.9% (−44.0 to −2.0) | −19.2% (−35.3 to 0.8) | −22.6% (−35.3 to −7.5) | |||
| Restenosis/occlusion rate | ||||||
| Evaluable, n | 76 | 76 | 69 | 69 | 145 | 145 |
| n (%) | 27 (35.5%) | 35 (46.1%) | 16 (23.2%) | 28 (40.6%) | 43 (29.7%) | 63 (43.4%) |
| p value | 0.0768 | 0.0018 | 0.0003 | |||
| Absolute difference (T − C) | 10.8% | 17.4% | 13.8% | |||
| Relative differencea (95% CI) | −22.9% (−40.2 to −0.4) | −42.9% (−59.6 to −19.2) | −31.7% (−44.5 to −16.1) | |||
| Inflammation score (100 mm VAS) | ||||||
| Evaluable, n | 77 | 77 | 76 | 77 | 153 | 154 |
| Mean (SD) | 32.4 (33.27) | 39.0 (33.67) | 26.0 (31.17) | 31.9 (32.08) | 29.2 (32.30) | 35.5 (32.97) |
| p value | 0.0057 | 0.0633 | 0.0070 | |||
| Absolute difference (T − C) | −6.6 (−13.2 to 0.1) | −5.5 (−11.3 to 0.3) | −6.0 (−10.4 to −1.7) | |||
| Estimated FSO diameter (mm) | ||||||
| Evaluable, n | 76 | 77 | 68 | 68 | 144 | 145 |
| n (%) | 4.8 (3.24) | 3.9 (2.84) | 5.7 (3.22) | 4.7 (3.44) | 5.2 (3.25) | 4.3 (3.15) |
| p value | <0.0001 | 0.0095 | <0.0001 | |||
| Absolute difference (T − C) | 1.0 (0.4 to 1.6) | 1.0 (0.2 to 1.7) | 1.0 (0.5 to 1.5) | |||
* p < 0.05 considered statistically significant.
aRelative difference is calculated as percentage of absolute difference between treatment sides divided by control side. A negative value reflects a decrease in the outcome on the treatment side compared to control, while a positive value reflects an increase in the outcomes on the treatment side compared to control.
C = control side; CI = confidence interval; FSO = frontal sinus ostium; SD = standard deviation; T = treatment side.
Figure 1.
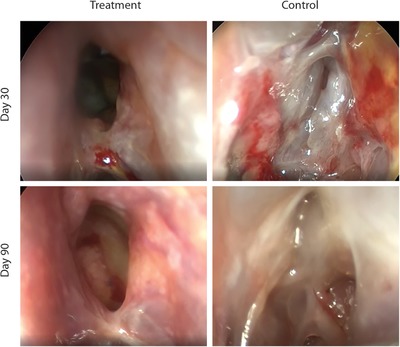
Endoscopic photographs from a study patient at 30‐day and 90‐day follow‐up. The sinus that received a steroid‐releasing sinus implant is normal at day 30 and continues to be normal at day 90, with no need for surgical and/or oral steroid intervention. Oral steroid intervention was determined to be needed on the control side.
Subgroup analyses of efficacy outcomes at day 90
Subgroup analyses of the effect of baseline clinical characteristics on the efficacy outcomes at day 90 are presented in Fig. 2. Inflammation score was significantly lower favoring treatment sides in patients with asthma, patients undergoing revision ESS, patients who underwent either a Draf IIA or Draf IIB procedure, and patients with high polyp burden. Statistically significantly larger estimated FSO diameter and decrease in restenosis/occlusion rate (p < 0.05) were attained at 90 days across all subgroups. Although the magnitude of the relative difference was similar, the restenosis/occlusion rate did not reach statistical significance between treatment sides in patients with lower LM CT stage and in patients undergoing a Draf IIB procedure.
Figure 2.
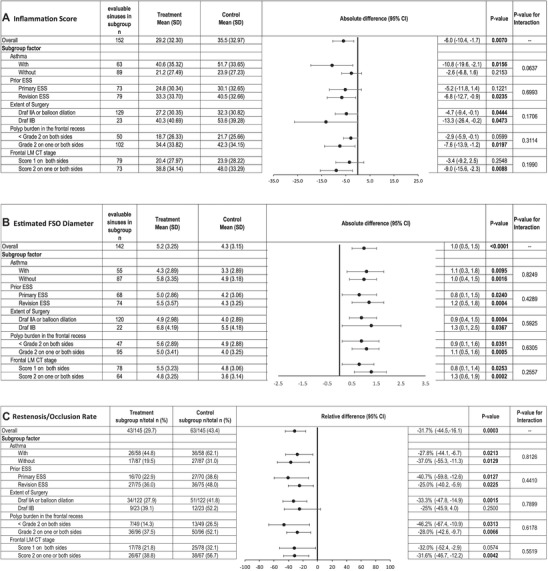
Forest plot of the treatment effect on day 90 end points by subgroups: (A) Absolute difference in inflammation scores. (B) Absolute difference in estimated FSO diameter. (C) Relative difference in restenosis/occlusion rate. The interaction p value is based on repeated measures analysis of variance model with treatment side and subgroup and interaction of treatment by subgroup as fixed effects. CI = confidence interval; ESS = endoscopic sinus surgery; FSO = frontal sinus ostia; LM = Lund‐Mackay; n = number of sinuses; SD = standard deviation.
Safety outcomes
There were no implant‐related adverse events in the study. A total of 5 serious adverse events (diverticulitis, pulmonary fungal infection, 2 events of deep vein thrombosis, and cerebrovascular accident) were reported in 4 patients but these were not related to the study implants.
Discussion
Outcomes of frontal sinus surgery are dependent on optimizing postoperative wound healing. Published studies have documented that the frontal sinuses remain the most challenging paranasal sinus to maintain patency post‐ESS given the anatomic configuration of the frontal sinus outflow tract.18 The challenging anatomy and the vulnerability of the frontal sinus to circumferential scarring, along with the underlying persistent or recurrent inflammation, often requires additional medical or surgical interventions to maintain a patent frontal sinus. The results of our pooled analysis demonstrate statistically significant reductions in favor of the steroid‐releasing implants in the need for postoperative interventions, including surgical and oral steroid intervention, as well as inflammation score and restenosis/occlusion rates. These outcomes were sustained through 90 days—well beyond the acute phase of wound healing of approximately 4 weeks.19 The results are consistent with outcomes from the previous meta‐analysis that included clinical studies conducted in the ethmoid sinus20 and support the role of steroid‐releasing implants in optimization of the postoperative wound‐healing process in the frontal recess.
Long‐term patency after endoscopic frontal sinus surgery has been reported to be as low as 25%.11 Novel instrumentation and surgical techniques over the past few decades have steadily improved frontal sinus ostial patency rates, ranging from 67.6% to 92%.2, 3, 7, 8, 21 However, given the technical demands of frontal sinus surgery, and the intrinsic propensity of the frontal sinus ostium to restenose, there remains a need for additional innovations to improve frontal sinus surgical outcomes. Results of this pooled analysis demonstrate a statistically significantly lower restenosis/occlusion rate at 90 days in favor of the steroid‐releasing implants compared with control sides in the post‐ESS setting. Furthermore, the estimated FSO diameter was significantly larger, by 1.0 mm, compared with the control sides, suggesting that the treated sides may be less likely to restenose.
Multiple studies have shown that the size of the frontal sinusotomy plays a vital role in predicting postoperative patency.3, 18, 19 Those studies concluded that an approximately 5‐mm postsurgical opening was necessary to meaningfully decrease the risk of FSO stenosis and maintain long‐term patency. A larger FSO has been a goal of sinus surgery to improve mucociliary drainage as well as allow for better penetration of topical therapies to address the chronic inflammation that characterizes CRS.18, 22 Results from this pooled analysis showed a statistically significantly larger FSO size (mean, 5.2 mm) and higher patency rates at 3 months, suggesting improved long‐term frontal sinus outcomes.
The prevalence of asthma, higher polyp burden, higher preoperative frontal LM CT stage, and history of previous ESS have been considered as potential risk factors for poor outcomes of endoscopic frontal sinus surgery.2, 3, 23, 24, 25 Chandra et al examined the success of frontal sinusotomy based on preoperative CT scans and diffuse polyposis at baseline and observed a significant correlation between advanced disease burden and the rate of surgical failure.2 In the current pooled analysis, over half the evaluable patients underwent revision ESS, just over 40% had asthma, nearly half (49%) of the patients had a preoperative frontal LM CT stage of 2 on 1 or both sides, and about two thirds of patients exhibited expanded polypoid edema (grade 2) in the frontal sinus ostia. Despite the potential relationship of poorer outcomes post‐ESS in patients with asthma, diffuse polypoid edema, and history of previous ESS,24 significant improvements in endoscopic outcomes favoring the steroid‐releasing implants were observed in the subgroup of patients with and without asthma as well as in patients undergoing either primary or revision ESS with either a Draf IIA or IIB procedure. Similarly, patients with preoperative complete opacification (LM score of 2, on 1 or both sides) of the frontal sinus showed significant improvements in endoscopic outcomes favoring the sinuses receiving implants; these patients are otherwise at higher risk of surgical failure.2 Although the magnitude of side‐to‐side differences observed in inflammation scores and restenosis/occlusion rates in the subgroup of patients with LM CT stage 1 also favored the treated sides, these results did not achieve statistical significance. The results from these subgroup analyses demonstrate the role of steroid‐releasing implants in optimizing outcomes of frontal sinus surgery in CRS patients with differing baseline clinical comorbidities.
Interpretation of the results from this pooled analysis entails some limitations. The intrapatient control design of the clinical trials included in the pooled analysis precluded assessment of patient‐reported outcomes between treatment groups. However, the study design addressed the potential confounding effect of interpatient variability, underlying comorbid conditions and concomitant medication use on efficacy outcomes. Another limitation is that, although the implants were removed at day 21, the clinical investigators were not blinded to treatment assignment when they performed the 90‐day endoscopic grading over 2 months later in both clinical studies. Also, although 20% (32 of 160) of video recordings were unable to be graded by the centralized reviewer, endoscopic outcomes graded by the reviewer at 30 days showed a significant treatment effect.
Conclusion
Bioabsorbable steroid‐releasing sinus implants that provide sustained release of corticosteroid can improve endoscopic outcomes of frontal sinus surgery, irrespective of asthma status, previous ESS, extent of surgery, or extent of polyps in the frontal recess. Through 90 days, the implants significantly reduced the restenosis/occlusion rate and the need for postoperative interventions when compared with surgery alone.
Acknowledgments
Statistical analyses were performed by independent biostatisticians, Saling Huang, PhD, and I‐Ling Hsiue. The pooled analysis results were presented at the Annual Meeting of American Rhinologic Society in Atlanta, GA on October 6, 2018.
How to Cite this Article: Singh A, Luong AU, Fong KJ, et al. Bioabsorbable steroid‐releasing implants in the frontal sinus ostia: a pooled analysis. Int Forum Allergy Rhinol. 2019;9:131–139.
Funding source for the study: Intersect ENT.
Potential conflict of interest: A.L.: 480 Biomedical, Aerin Medical, ENTvantage, Intersect ENT and Medtronic, consultant; J.K.H.: Intersect ENT, consulting fees during the study; A.S.: Intersect ENT, consultant; K.J.F.: Intersect ENT, consultant; J.P.S. Intersect ENT and Acclarent, consultant. J.S. and A.R.: employees of Intersect ENT. The remaining authors have no disclosures.
[Correction added on 12/6, after first online publication: A correction was made to Fig. 2C.]
References
- 1. Bury S, Singh A. Evaluation of a steroid releasing sinus implant for the treatment of patients undergoing frontal sinus surgery for chronic rhinosinusitis. Expert Rev Med Devices. 2017;14:93–101. [DOI] [PubMed] [Google Scholar]
- 2. Chandra RK, Palmer JN, Tangsujarittham T, Kennedy DW. Factors associated with failure of frontal sinusotomy in the early follow‐up period. Otolaryngol Head Neck Surg. 2004;131:514–518. [DOI] [PubMed] [Google Scholar]
- 3. Naidoo Y, Wen D, Bassiouni A, Keen M, Wormald PJ. Long‐term results after primary frontal sinus surgery. Int Forum Allergy Rhinol. 2012;2:185–190. [DOI] [PubMed] [Google Scholar]
- 4. Aggarwal R, Cardozo A, Homer JJ. The assessment of topical nasal drug distribution. Clin Otolaryngol Allied Sci. 2004;29:201–205. [DOI] [PubMed] [Google Scholar]
- 5. Hong SD, Jang JY, Kim JH, et al. The effect of anatomically directed topical steroid drops on frontal recess patency after endoscopic sinus surgery: a prospective randomized single blind study. Am J Rhinol Allergy. 2012;26:209–212. [DOI] [PubMed] [Google Scholar]
- 6. Kayarkar R, Clifton NJ, Woolford TJ. An evaluation of the best head position for instillation of steroid nose drops. Clin Otolaryngol Allied Sci. 2002;27:18–21. [DOI] [PubMed] [Google Scholar]
- 7. Chan Y, Melroy CT, Kuhn CA, et al. Long‐term frontal sinus patency after endoscopic frontal sinusotomy. Laryngoscope. 2009;119:1229–1232. [DOI] [PubMed] [Google Scholar]
- 8. Friedman M, Bliznikas D, Vidyasagar R, Joseph NJ, Landsberg R. Long‐term results after endoscopic sinus surgery involving frontal recess dissection. Laryngoscope. 2006;116:573–579. [DOI] [PubMed] [Google Scholar]
- 9. Draf W. Endonasal micro‐endoscopic frontal sinus surgery: the Fulda concept. Op Techn Otolaryngol. 1991;2:234–240. [Google Scholar]
- 10. Illing EA, Cho do Y, Riley KO, Woodworth BA. Draf III mucosal graft technique: long‐term results. Int Forum Allergy Rhinol. 2016;6:514–517. [DOI] [PubMed] [Google Scholar]
- 11. Ramadan HH. Surgical causes of failure in endoscopic sinus surgery. Laryngoscope. 1999;109:27–29. [DOI] [PubMed] [Google Scholar]
- 12. Kuhn FA, Church CA, Goldberg AN, et al. Balloon catheter sinusotomy: one‐year follow‐up‐outcomes and role in functional endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2008;139(suppl):S27–S37. [DOI] [PubMed] [Google Scholar]
- 13. Ting JY, Wu A, Metson R. Frontal sinus drillout (modified Lothrop procedure): long‐term results in 204 patients. Laryngoscope. 2014;124:1066–1070. [DOI] [PubMed] [Google Scholar]
- 14. Smith TL, Singh A, Luong A, et al. Randomized controlled trial of a bioabsorbable steroid‐releasing implant in the frontal sinus opening. Laryngoscope. 2016;126:2659–2664. [DOI] [PubMed] [Google Scholar]
- 15. Luong A, Ow RA, Singh A, et al. Safety and effectiveness of a bioabsorbable steroid‐releasing implant for the paranasal sinus ostia: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gray ST, Sedaghat AR. Frontal sinus drug‐eluting implants—effective, but for which patients and at what cost? JAMA Otolaryngol Head Neck Surg. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(suppl):S1–S39. [DOI] [PubMed] [Google Scholar]
- 18. DeConde AS, Smith TL. Outcomes after frontal sinus surgery: an evidence‐based review. Otolaryngol Clin North Am. 2016;49:1019–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosemann W, Kuhnel T, Held P, Wagner W, Felderhoff A. Endonasal frontal sinusotomy in surgical management of chronic sinusitis: a critical evaluation. Am J Rhinol. 1997;11:1–9. [DOI] [PubMed] [Google Scholar]
- 20. Han JK, Marple BF, Smith TL, et al. Effect of steroid‐releasing sinus implants on postoperative medical and surgical interventions: an efficacy meta‐analysis. Int Forum Allergy Rhinol. 2012;2:271–279. [DOI] [PubMed] [Google Scholar]
- 21. Askar MH, Gamea A, Tomoum MO, et al. Endoscopic Management of chronic frontal sinusitis: prospective quality of life analysis. Ann Otol Rhinol Laryngol. 2015;124:638–648. [DOI] [PubMed] [Google Scholar]
- 22. Grobler A, Weitzel EK, Buele A, et al. Pre‐ and postoperative sinus penetration of nasal irrigation. Laryngoscope. 2008;118:2078–2081. [DOI] [PubMed] [Google Scholar]
- 23. Matsuwaki Y, Ookushi T, Asaka D, et al. Chronic rhinosinusitis: risk factors for the recurrence of chronic rhinosinusitis based on 5‐year follow‐up after endoscopic sinus surgery. Int Arch Allergy Immunol. 2008;146(suppl 1):77–81. [DOI] [PubMed] [Google Scholar]
- 24. Smith TL, Mendolia‐Loffredo S, Loehrl TA, et al. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2005;115:2199–2205. [DOI] [PubMed] [Google Scholar]
- 25. Naidoo Y, Bassiouni A, Keen M, Wormald PJ. Risk factors and outcomes for primary, revision, and modified Lothrop (Draf III) frontal sinus surgery. Int Forum Allergy Rhinol. 2013;3:412–417. [DOI] [PubMed] [Google Scholar]


