Abstract
Objectives
The pulsed‐dye laser has long been a gold standard in the treatment of poikiloderma of Civatte. Recent advances in pulsed dye laser technology enable output energies 50% higher, enabling beam diameters of up to 15 mm with clinically relevant fluences. In this study, we investigate this new laser for treatment of this condition.
Materials and Methods
Twenty subjects were enrolled in the study. A total of four treatments were administered at monthly intervals. Blinded assessment of digital, cross‐polarized photographs taken at baseline and two months following the last treatment was performed by blinded physician raters using an 11‐point clearance scale. Subject reported pain scores immediately following treatment and side effects at all visits were recorded by the investigator.
Results
Seventeen subjects completed the study. Blinded reviewers correctly identified the baseline photo in 48 of 51 cases (94%). All three reviewers mis‐identified the same subjects. The blinded reviewers scored 14 out of the 17 subjects with an improvement greater than 40% and 10 out of the 17 subjects greater than 50%. Average improvement was 49% for all 17 subjects. Side effects were limited to mild edema, and mild to moderate erythema and purpura. Pain scores averaged 3.5 on using an 11‐point scale.
Conclusion
This study demonstrates the safety and effectiveness of a new pulsed‐dye laser with a 15 mm spot and 50% higher fluences for the treatment of poikiloderma of Civatte. Lasers Surg. Med. 51:54–58, 2019. © 2018 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: poikiloderma of civatte, laser, treatment, pulsed dye laser
INTRODUCTION
Poikiloderma of Civatte (POC) is a chronic vascular and pigmentary disorder typically involving the lateral and inferior central neck region. POC is characterized by the presence of reticulate erythema, and brown pigmentation often on a background of chronic photodamage including sagging skin and wrinkles. POC has been described as a variant of rosacea involving the neck instead of the face. The similarities to rosacea include sun‐exposure as the obvious main cause of the observed changes that clearly spare portions of the neck shielded from sunlight, as well as the main hallmark of telangiectasias. The differences between rosacea and POC, often referred to as “rosacea of the neck” by the lead author when speaking with patients, is the more reticulate pattern of blood vessels in POC as well often prominent hyperpigmentation that accompanies the erythema of POC. This difference is most likely due to the differences in skin between the face and neck, and the types of changes that occur following chronic sun‐exposure in each location. The vessels in POC may leak more red cells and/or induce more inflammation than facial vessels, resulting in both hemosiderin and melanin being deposited in the dermis, along with the ectatic vessels in POC, as compared to classic cases of rosacea. In addition to being exposed to the sun, neck skin is often exposed to fragrances from various topical products, and authors have speculated that phototoxic or photosensitivity reactions may also contribute to the development of this condition.1, 2, 3
Numerous lasers and light sources including: pulsed‐dye lasers (PDLs),4, 5, 6, 7 intense pulsed light sources (IPLs),8, 9 long pulse‐duration neodymium‐doped yttrium‐aluminum garnet (Nd:YAG) lasers incorporating a potassium‐titanyl‐phosphate (KTP) frequency‐doubling crystal,10 and fractionated non‐ablative11 and ablative12 lasers have all been used to remove the extra blood vessels and pigment comprising POC. The intense vascularity of POC makes this an ideal condition to treat with vascular specific lasers such as the PDL and KTP lasers. Here we present a study investigating the use of a new generation PDL, capable of delivering 50% more energy than previous generation PDLs, thus enabling the use of a larger 15 mm diameter treatment beam.
MATERIALS AND METHODS
The purpose of this study is to evaluate the safety and effectiveness of a high‐energy, long pulse‐duration, extended sub‐pulse, larger spot‐size, 595 nm PDL for the treatment of chronic photodamage, telangiectasias and pigmentation, or POC, on the neck.
Subject Population
The study protocol was approved by an independent institutional review board (IRB) and was open to subjects ages 21–70 years of age with Fitzpatrick skin types I–IV. All subjects provided signed, informed consent prior to enrollment. Subjects who had previous laser treatments to the neck, a history of isotretinoin use in the six months prior to the study, dermatitis at the treatment site, a history of psoriasis or vitiligo, or were pregnant, were excluded from the study. Twenty subjects were enrolled, 13 females and 7 males, ages 47–61 and averaging 55 years of age, with Fitzpatrick skin types I–IV, and exhibiting POC on the neck as determined by the treating physician.
Pulsed Dye Laser
A newly designed, prototype PDL (VBeam Prima, Syneron‐Candela, Wayland, MA) was used in this study. The new PDL incorporates a redesigned and optimized laser cavity delivering 12 J maximum energy, and a zoom handpiece enabling beam diameters from 3 to 15 mm selectable in 0.5 mm increments. The 12 J total energy represents a 50% increase over the previous generation PDL, and the 15 mm diameter spot size is a 56% increase in area compared to the previous 12 mm maximum beam diameter. The available pulse durations range from 0.45 to 40 ms. The VBeam Prima is also equipped with a dynamic cooling device utilizing a cryogen spray, as was the previous generation PDL, to provide epidermal protection and reduce discomfort during treatments.
Laser Treatment
All subjects received four treatments to the entire neck area spaced one month apart, and a follow‐up visit for photographs eight weeks following the final treatment. All treatments were performed using a 15 mm spot size at a pulse repetition rate of 1.5 Hz. A pulse duration of 1.5 ms was used for all treatments. The dynamic cooling device was used for all treatments using a cryogen spray duration of 40 ms administered 20 ms prior to delivery of each laser pulse.
Treatment fluences were determined by the treating physician based on prior experience and by observing immediate clinical responses of erythema, transient purpura, or purpura. The average fluences delivered were 5.0 ± 0.3, 5.3 ± 0.3, 5.6 ± 0.3, and 5.7 ± 0.6 J/cm2 (Mean ± SD) for treatments 1, 2, 3, and 4, respectively. Treatment fluences ranged from 4.25 to 5.25 J/cm2 for treatment 1, 4.50–5.55 J/cm2 for treatment 2, 4.75–6.00 J/cm2 for treatment 3, and 4.00–6.25 J/cm2 for treatment 4 (Table 1).
Table 1.
Laser Treatment Parameters
| Spot size | Tx1 | Tx2 | Tx3 | Tx4 | |
|---|---|---|---|---|---|
| 15 | Average fluence (J/cm2) | 5.0 | 5.3 | 5.6 | 5.7 |
| Fluence Std Dev | 0.3 | 0.3 | 0.3 | 0.6 | |
| Range | 4.25–5.25 | 4.5–5.55 | 4.75–6.0 | 4.0–6.25 | |
| Mean # of pulse | 195 | 160 | 182 | 197 | |
| Range | 137–313 | 96–261 | 129–285 | 116–264 |
Blinded Evaluation of Digital Images
Three photographs of the neck were taken using a Canon Eos Rebel T6i camera with a 60 mm macro lens (Cannon U.S.A., Inc. Melville, NY) and a cross‐polarized ring flash (Canfield Scientific, Inc., Fairfield, N.J.). Left and right lateral and head‐on views of the subjects were photographed just prior to starting treatments and eight weeks after the final treatment. All photographs were taken with the same camera settings by a single photographer at a fixed focal length. Three blinded physician reviewers were asked to identify baseline and post‐treatment images from randomized, paired images for all subjects. The reviewers were first asked to identify the baseline and post‐treatment image and then rate the improvement in 10% increments using an 11‐point scale (0% no improvement to 100% improvement, or complete clearance). If the baseline image was incorrectly identified the evaluation was given a negative score, for example, a score of 20% would be recorded as −20%.
Side effects
Immediately following each treatment session, purpura, erythema, and edema were evaluated by the treating physician using a 4‐point severity scale (0 = absent, 1 = mild, 2 = moderate, and 3 = severe). The subjects were also asked to evaluate their pain on a 11‐point scale (0 = No Pain to 10 = Most Painful). At the final follow‐up visit, the investigator also recorded any edema, hypopigmentation, hyperpigmentation, or scarring using the above scale.
RESULTS
Thirteen female and seven male subjects were enrolled in the study, with three female subjects dropping out before completing the study. Of the subjects who completed the study, two subjects had skin Fitzpatrick skin type I, three subjects had skin type II, eleven subjects had skin Type III, and one subject had skin type IV. Subject ages ranged from 47 to 61 years of age with a mean of 55 ± 4 years (Mean ± SD) (Table 2).
Table 2.
Subject Demographics
| 20 Subject enrolled in the study | |
|---|---|
| 7 Male and 10 Female subjects CR (3 Female subjects dropped out) | |
| Mean Age (range) | 55 (47 to 61) |
| Skin type | |
| I | 2 |
| II | 3 |
| III | 11 |
| IV | 1 |
Blinded Evaluation of Digital Images
Blinded reviewers correctly identified the baseline images in 16 of the 17 subjects who completed the study (94% of images correctly identified). For the remaining subject, all reviewers misidentified the baseline image. The blinded reviewers were requested to rate the improvement on a 11‐point scale from 0% to 100% in 10% increments. The mean improvement for all three reviewers for all subjects was a mean improvement score of 4.9 (range = −6–9, 95%CI 4.0–5.8). This corresponds to a 49.2% ± 16.5 (mean ± sem) improvement, and this improvement was statistically significant (X2 = 103.9, two‐tail P < 0.0001). The mean improvement score for the 16 subjects whose baseline image was correctly identified by all three blinded reviewers was 55.6%. For the 16 subjects whose baseline was correctly identified, the mean improvement scores ranged from 37 to 80%. The one subject with a misidentified baseline image was given a negative improvement score (score = −53%), indicating that the baseline image looked on average 53% improved, compared to the post‐treatment image. Ten of the 17 subjects (58.8%) had improvement of greater than 50% (Fig. 1).
Figure 1.
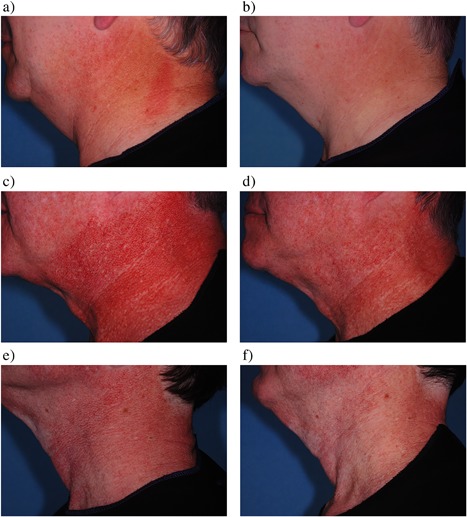
Baseline (a, c, e) and post‐treatment (b, d, f) cross‐polarized images. Mean subject improvement assessed 2‐months post four treatments from blinded review was 63% (a,b), 53% (c,d), and 77% (e,f), respectively.
Side effects
Pain reported by subjects after each treatment using a 0–10‐point scale averaged scores of 2.9 ± 1.9, 3.6 ± 2.1, 3.8 ± 1.9, and 3.8 ± 2.1 (Mean ± SD), for treatments 1, 2, 3, and 4, respectively. Pain scores increased with higher fluences. Side effects immediately following treatment ranged from no to mild purpura, mild to moderate erythema, and no to mild edema. There were no reported cases of hyperpigmentation or hypopigmentation. The investigator noted no purpura, erythema, edema, hyperpigmentation, hypopigmentation, or scarring at the final follow‐up visit.
DISCUSSION
In this study we demonstrate that the newly designed PDL is safe and effective for the treatment of POC. Improvement as rated by blinded observers evaluating cross‐polarized baseline and eight‐week post‐treatment images averaged an improvement of approximately 50%. Side effects, known to be quite low with the PDL, were limited to purpura, erythema, and edema. No scarring or pigmentary changes were seen, although the neck is a less forgiving area for laser treatment than the face in terms of scarring from laser treatments. For example, scarring is more likely following ablative laser treatment to the neck as compared to the face, but is an extremely rare occurrence following PDL treatment due to the safety profile of the device. As with other laser treatments, the neck is more likely to be sensitive to laser treatment than the face, and often requires lower fluences to achieve clearance of POC as compared to facial redness. A single subject looked significantly better on his pre‐treatment images as opposed to post‐treatment in the current study, and this spurious result is not surprising given the sensitivity of neck skin to irritation from numerous common stimuli. Acute erythema and clinical and sub‐clinical inflammation are common in people with rosacea, as well as those with POC. Virtually any stimulus including topicals, face washes, temperature fluctuations, exercise, or even emotions can elicit a flushing response in these sites.1, 2, 3 When evaluating patient responses to laser treatment for both rosacea and POC, an overall level of improvement as evidenced by numerous photographs at different time points, as well as reporting of objective symptoms or treatments being used such as the need to wear make‐up or cover‐up, incidences of flushing over a week or month, or the frequency of use or number of topical products used to treat rosacea, provide a better picture of the overall response than the appearance on a single time point. The single subject in which the baseline image was mis‐identified likely had flushing of the areas of involvement by POC making the post‐treatment image look worse, since developing vessels de novo would likely take longer than the study period. Still, using a single time point, the results of this study strongly demonstrate the safety and effectiveness of the newly designed PDL for treating POC.
Because the reticulate pattern of erythema is quite dense in areas of POC, leaving redness behind in the spaces between a given treatment, called “foot printing” because it leaves a “foot print” of the laser beam as an area of clearing in a background of remaining redness, is likely to be significantly reduced using a large 15 mm beam diameter, compared to smaller beam diameters. This is due to the size and likelihood of missed areas decreasing when juxtaposing fewer spots due to the larger area treated by the 15 mm beam diameter, which is 125% larger than the most commonly used 10 mm diameter spot. Foot printing resolves with each subsequent laser treatment since subsequent treatments are likely to cover missed areas. In the current study a pulse‐duration of 1.5 ms was used, rather than the more commonly used 6–10 ms pulse durations that are much less likely than shorter pulse durations to result in purpura or foot printing. However, the treating physician in this study has found that shorter pulse durations are more effective for treating POC than longer ones, and that patients are able to hide their necks post‐treatment easier than facial skin, and are therefore more likely to allow purpuric treatments. The development of purpura depends upon both fluence and pulse duration, with higher fluences and shorter pulse durations increasing the risk of bruising. The selected fluences used in the current study resulted in no or mild purpura.
Both vascular‐targeting lasers1, 2, 3, 4, 5, 6, 7, 10 and intense pulsed light sources (IPLs)8, 9 have been used to treat POC. The PDL was shown to treat POC almost three decades ago.4 Subsequent studies confirmed the ability of these early PDLs to treat POC.5, 6 These early generation PDLs delivered 585 nm laser light using a 0.45 ms pulse‐duration. The shorter pulse‐duration and wavelength, as compared to the device used in the current study, had a higher risk of foot printing and side effects due to the higher peak powers and greater melanin and hemoglobin absorption compared with current devices. Severe depigmentation was described in a series of patients following higher fluence treatment of POC with an early generation PDL, as compared with lower fluences.7 Modern PDLs can deliver pulse‐durations form 0.45 to 40 ms and emit at 595 nm, making them much safer, and more effective, than these very early‐generation PDLs. Two very large studies demonstrated improvement of POC after IPL treatment. IPLs deliver broad spectrum light, so much of the administered light is very poorly absorbed by hemoglobin, thus being deposited as heat, and contributing to potential side effects while not doing the work of removing unwanted vessels. This non‐specific absorption of light decreases the therapeutic window of IPLs compared with vascular lasers such as the PDL and KTP lasers, especially in more darkly pigmented or tan patients. Still, IPLs have been used quite successfully to treat POC as evidenced by two large studies performed by experienced users.8, 9 KTP lasers are vascular‐specific like the PDL and can remove POC quite successfully.10 The shorter 532 nm wavelength, as compared to the 595 nm PDL wavelength, is more strongly absorbed by melanin, making the KTP potentially riskier in more darkly pigmented or tan patients, but may also target the dermal melanin more strongly than 595 nm. Epidermal cooling is a component of PDL and KTP lasers and provides protection to the epidermis and comfort during treatment, reducing the risk of side effects. Even non‐ablative and ablative fractionated lasers, which are not vascular specific, have been used to treat POC.11, 12 These lasers were first used to treat POC because there are dermal changes associated with the photodamage that results in POC, and some vascular effects are seen on histopathologic examination of skin treated with carbon dioxide (CO2) lasers and improvement of vascularity has been observed following non‐ablative fractionated laser treatments. Because vascularity is the hallmark of POC, non‐ablative fractionated lasers should probably be used as an adjunct therapy for POC. In addition, caution should be exercised when using CO2 lasers on neck skin, as scarring is more likely than on facial skin.
In conclusion, the newly designed, high fluence PDL with a large 15 mm beam diameter is highly effective for treating POC with a very favorable side‐effect profile. Future studies of treating POC with varying pulse durations utilizing non‐purpuric settings, and combination treatments with other devices and topical products could elucidate the benefits of combination treatment approaches more commonly used in clinical practice.
ACKNOWLEDGMENTS
Dr. Bernstein received equipment loan and funding for this study, and is a consultant and equity holder in Syneron‐Candela. Dr. Schomacker, Dr. Paranjape, and Mr. Jones were employees of Syneron‐Candela at the time this work was done.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
REFERENCES
- 1. Civatte A. Poikilodermie reticulee pigmentaire du visage et du col. Ann Derm Syph 1923;6:605–620. [Google Scholar]
- 2. Geronemus R. Poikiloderma of civatte. Arch Dermatol 1990;126:547–548. [PubMed] [Google Scholar]
- 3. Katoulis AC, Stavianeas NG, Panayiotides JG, Bozi E, Vamavasakis E, Kalogeromitros D, Georgaia S. Poikiloderma of Civatte: a histopathological and ultrastructural study. Dermatology 2007;214:177–182. [DOI] [PubMed] [Google Scholar]
- 4. Wheeland R, Applebaum J. Flashlamp‐pumped pulsed dye laser therapy for Poikiloderma of Civatte. J Dermatol Surg Oncol 1990;16:12–16. [DOI] [PubMed] [Google Scholar]
- 5. Clark RE, Jimenez‐Acosta F. Poikiloderma of Civatte. Resolution after treatment with the pulsed dye laser. N C Med J 1994;55:234–235. [PubMed] [Google Scholar]
- 6. Haywood RM, Monk BE. Treatment of poikiloderma of Civatte with the pulsed dye laser: a series seven cases. J Cutan Laser Ther 1999;1:45–48. [DOI] [PubMed] [Google Scholar]
- 7. Meijs MM, Blok FA, de Rie MA. Treatment of poikiloderma of Civatte with the pulsed dye laser: a series of patients with severe depigmentation. J Eur Acad Dermatol Venereol 2006;20(10):1248–1251. [DOI] [PubMed] [Google Scholar]
- 8. Rusciani A, Motta A, Fino P, Menichini G. Treatment of poikiloderma of Civatte using intense pulsed light source: 7 years of experience. Dermatol Surg 2008;34(3):314–319. [DOI] [PubMed] [Google Scholar]
- 9. Weiss RA, Goldman MP, Weiss MA. Treatment of poikiloderma of Civatte with an intense pulsed light source. Dermatol Surg 2000;26(9):823–827. [DOI] [PubMed] [Google Scholar]
- 10. Batta K, Hinson C, Cotterill JA, Foulds IS. Treatment of poikiloderma of Civatte with potassium titanyl phosphate (KTP) laser. Br J Dermatol 1999;140:1191–1192. [PubMed] [Google Scholar]
- 11. Behroozan DS, Goldberg LH, Glaich AS, Dai T, Friedman PM. Fractional photothermolysis for treatment of poikiloderma of Civatte. Dermatol Surg 2006;32(2):298–301. [DOI] [PubMed] [Google Scholar]
- 12. Tierney EP, Hanke CW. Treatment of Poikiloderma of Civatte with ablative fractional laser resurfacing: prospective study and review of the literature. J Drugs Dermatol 2009;8(6):527–534. [PubMed] [Google Scholar]


