Summary
Ticagrelor is an antiplatelet agent for adults with coronary artery disease. The inhibition of platelet activation may decrease the frequency of vaso‐occlusion crisis (VOC) in sickle cell disease (SCD). The HESTIA2 study (NCT02482298) randomised 87 adults with SCD (aged 18–30 years) 1:1:1 to twice‐daily ticagrelor 10, 45 mg or placebo for 12 weeks. Numerical decreases from baseline in mean proportion of days with patient‐reported pain (primary endpoint) were seen in all three groups, as well as in pain intensity and analgesic use, with no significant differences between placebo and ticagrelor treatment groups. Plasma ticagrelor concentrations and platelet inhibition increased with dose. Adverse events were distributed evenly across groups and two non‐major bleeding events occurred per group. Ticagrelor was well tolerated with a low bleeding risk, but no effect on diary‐reported pain was detected. Potential effects on frequency of VOCs will need to be evaluated in a larger and longer study.
Keywords: pain, safety, sickle cell disease, ticagrelor, vaso‐occlusion crisis
Sickle cell disease (SCD) is an autosomal recessive inherited disorder resulting in an altered haemoglobin β‐chain (Ware et al, 2017). Sickled erythrocytes adhere to the vascular endothelium and the subsequent obstruction leads to painful ischaemia, i.e. vaso‐occlusive crisis (VOC) (Novelli & Gladwin, 2016). VOCs are associated with hospitalisation (Raphael et al, 2012), reduced quality of life (Dampier et al, 2011) and increased mortality (Darbari et al, 2013).
Platelets are activated during the non‐crisis ‘steady state’ and are further activated during painful SCD episodes (Lee et al, 2006). Evidence suggests an association between soluble CD40 ligand (a marker of platelet activation) and frequency of pain episodes in adults with SCD (Ataga et al, 2012). Thus, inhibition of platelet activation may be a potential SCD therapeutic option. A study in SCD patients demonstrated that the anti‐platelet drug ticlopidine resulted in 66% fewer pain crises versus placebo (Cabannes et al, 1984). The DOVE phase III trial in 341 children and adolescents with SCD reported a numerical reduction (not statistically significant) in VOCs with the antiplatelet agent prasugrel (328 events, 2·30 events/person‐year) versus placebo (408 events, 2·77 events/person‐year) with a trend towards improvement in adolescents (Heeney et al, 2016). Although there is some support for platelet inhibition having potential to reduce SCD‐associated pain crisis, the effect of platelet inhibition on daily, self‐reported, SCD‐related pain is less clear. A smaller study with prasugrel for 30 days showed a trend towards decreased self‐reported pain in adults with SCD (Wun et al, 2013).
Ticagrelor is an orally administered, direct‐acting, reversibly binding P2Y12 receptor antagonist that inhibits adenosine diphosphate‐induced platelet aggregation (van Giezen et al, 2009), and inhibits cellular uptake of adenosine by inhibiting the equilibrative nucleoside transporter 1 (Armstrong et al, 2014). Based on two phase III trials (PLATO: Wallentin et al, 2009; PEGASUS‐TIMI 54: Bonaca et al, 2015), ticagrelor is currently indicated for the prevention of atherothrombotic events in adult patients with acute coronary syndromes (ACS) or a history of myocardial infarction and a high risk of developing an atherothrombotic event (https://www.medicines.org.uk/emc/medicine/23935).
The phase IIb study, HESTIA2 (NCT02482298) was conducted along with the ticagrelor paediatric programme in SCD and was the first study of ticagrelor in adult patients with SCD. An extensive programme in healthy volunteers and adults with cardiovascular disease established the clinical pharmacology profile of ticagrelor (Teng, 2015). Therefore, HESTIA2 dose selection was based on pharmacokinetic/pharmacodynamic modelling and simulation, and ticagrelor doses of 10 and 45 mg twice daily (bid) were predicted to provide mean reductions in P2Y12 reaction units (PRU, a measure of platelet aggregation) at 2 h postdosing of 40–50% and 80–90%, respectively. These doses provide a wide range of peak inhibition of the P2Y12 receptor.
The primary objective of HESTIA2 was to evaluate the efficacy of ticagrelor versus placebo in reducing the number of days with self‐reported pain due to SCD in young adults. Secondary efficacy objectives included assessing the reduction of self‐reported pain intensity due to SCD and the reduction in analgesic use in patients with SCD. The safety and tolerability of ticagrelor were also evaluated.
Methods
Patients
Key inclusion criteria were males and females (negative serum/urine pregnancy tests at enrolment/randomisation and using contraceptives if of child‐bearing potential), aged 18–30 years, confirmed medical history/diagnosis of homozygous sickle cell (HbSS) or sickle beta‐zero thalassemia (HbSβ0, by high‐performance liquid chromatography), and ≥4 days of pain during the 4‐week single‐blind placebo baseline period (prior to randomisation). For patients on hydroxycarbamide, a stable dose for 3 months prior to enrolment was required. For patients on erythropoietin, the drug must have been prescribed for the preceding 6 months and at a stable dose for ≥3 months prior to randomisation.
Patients were excluded if they had a history of transient ischaemic attack or clinically overt cerebrovascular accident, severe head trauma, intracranial haemorrhage, intracranial neoplasm, arteriovenous malformation or aneurysm. Patients were not to be receiving chronic red blood cell transfusion therapy or chronic treatment with anticoagulants or antiplatelet drugs. Other exclusion criteria included current bleeding, an increased risk of bleeding complications, moderate or severe hepatic impairment, haemoglobin <40 g/l or platelet count <100 × 109/l.
Study design and treatments
HESTIA2 was a phase IIb, randomised, double‐blind, double‐dummy, parallel‐group, multicentre study (Fig 1). Participants were randomised 1:1:1 to receive ticagrelor 10, 45 mg or placebo bid, respectively. Randomisation was performed using an interactive voice response system/interactive web response system.
Figure 1.
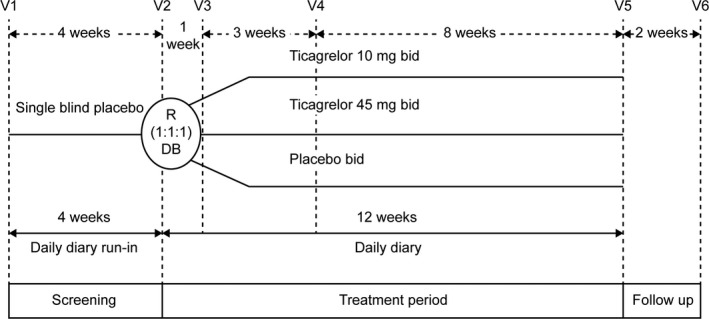
Study design. bid, twice daily; DB, double blind; R, randomisation; V, visit.
The study consisted of three periods over 18 weeks (Fig 1): a 4‐week, single‐blind placebo run‐in period for baseline assessments of pain‐related variables; then a 12‐week, double‐blind treatment period; and a 2‐week follow‐up period after treatment completion.
Randomised patients received one of three regimens, receiving one tablet bid (orally) from each bottle to preserve blinding (double‐dummy): ticagrelor 10 mg plus matching placebo for ticagrelor 45 mg; ticagrelor 45 mg plus matching placebo for ticagrelor 10 mg; matching placebo for ticagrelor 10 and 45 mg.
Study visits occurred at screening/enrolment, at randomisation and thereafter at 1 week, 4 and 12 weeks (end of treatment). A follow‐up phone call was scheduled 2 weeks after the last intake of study drug to collect adverse events (AEs) and any changes in concomitant mediations.
All patients provided written, informed consent before any study‐specific procedures. At each site, the final study protocol, amendments, and informed consent documentation were approved by an Ethics Committee and/or Institutional Review Board, or national regulatory authorities. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization/Good Clinical Practice Guidelines, and followed applicable regulatory requirements and AstraZeneca's policy on bioethics.
Assessments
The primary efficacy variable was the proportion of days with diary‐reported SCD pain during the 12‐week treatment period. Secondary efficacy variables included SCD pain intensity, and the proportion of days with analgesic use. Pain was recorded daily in an electronic diary using a handheld device from the start of the baseline period until the end of the randomised treatment period. Every evening, patients were to rate the intensity of the worst pain in the previous 24 h using an 11‐point scale; where 0 represented ‘no pain’ and 10 represented ‘pain as bad as you can imagine’. Patients recorded analgesic use (yes/no); type of pain medication was not recorded. Concomitant medications (including pain medication) were recorded by the investigator. Each time study drug was returned, compliance was assessed based on patient interview and tablet count. Days absent from school/work were recorded in the electronic diary (exploratory assessment).
Blood samples for pharmacokinetic analyses were collected at randomisation, 2 h after the first dose of study drug and on day 7 (±3 days) at 0 h (pre‐dose) and 2 h post‐dose. Ticagrelor and AR‐C124910XX (active metabolite) plasma concentrations were determined using a fully validated, liquid chromatography tandem mass spectrometry assay; lower limits of quantification were 5·0 and 2·5 ng/ml, respectively, from 100 μl of human plasma (Sillén et al, 2010).
PRU (VerifyNow®, Accumetrics, Inc., San Diego, CA, USA) blood samples were obtained pre‐dose at randomisation and then at the same time points as for the pharmacokinetic analysis.
Safety
Adverse events were recorded throughout the study and classified using the Medical Dictionary for Regulatory Activities (MedDRA) version 19.0 (https://www.meddra.org/sites/default/files/guidance/file/intguide_19_0_english.pdf).
Bleeding events were categorised as major, clinically relevant non‐major, or minor by the investigators (Mitchell et al, 2011). Major bleeding was any fatal bleeding, clinically overt bleeding (with a haemoglobin decrease ≥20 g/l), bleeding that was retroperitoneal, pulmonary, intracranial or otherwise involved the central nervous system, or bleeding requiring surgical intervention (operating suite). Clinically relevant, non‐major bleeding was overt bleeding for which a blood product was administered and not directly attributable to the underlying medical condition, or bleeding that required medical or surgical intervention (not an operating suite). Minor bleeding was any overt or macroscopic evidence of bleeding not fulfilling criteria for either major bleeding or clinically relevant, non‐major bleeding.
Patients underwent a physical examination at enrolment and at the end of treatment. Vital signs were assessed at every visit (except follow‐up). Clinical laboratory safety assessments (standard haematology, clinical chemistry, urinalysis and pregnancy testing) were conducted at enrolment, during week 4 and at the end of treatment. A pregnancy test was also conducted at randomisation before start of ticagrelor or matching placebo dosing.
Sample size and data analyses
Assuming the proportion of days with pain with placebo was 0·56 and the common standard deviation (SD) was 0·4, based on published data (Smith et al, 2008; Wun et al, 2013), 30 patients/group were expected to detect a difference in the proportion of days with pain of 0·17 (17 percentage points) by use of a 90% confidence interval (CI).
The proportion of days with pain due to SCD was calculated as the number of days in the treatment period with any reported SCD pain (i.e. worst pain score >0) divided by the total number of days reported in the electronic diary during the treatment period.
SCD pain intensity was based on the average of the daily worst pain values (electronic diary) during the treatment period, including days with a pain intensity of 0. A post‐hoc analysis investigated pain intensity during days with pain (i.e. worst pain score >0).
The proportion of days with analgesic use was calculated as the number of days with analgesic use in the treatment period divided by the number of days reported in the electronic diary during the treatment period.
Baseline values for efficacy variables were calculated as the average reported during the 4 weeks of single‐blind placebo baseline period before randomisation.
A post‐hoc visual exploration of the baseline proportion of days with pain versus haemoglobin and leucocytes baseline levels investigated the potential relationship between daily pain and levels of these laboratory parameters.
Statistical analyses were conducted using SAS® version 9.4 (SAS Institute, Inc., Cary, NC, USA). Continuous variables were presented as descriptive statistics (n, mean and SD), and categorical variables were summarised as number of patients and percentage by group. The proportion of days with SCD pain from baseline to week 12 was analysed using a mixed model analysis of covariance (MMANCOVA) adjusting for treatment, country, baseline proportion of days of pain and baseline hydroxycarbamide use. Treatment, hydroxycarbamide and baseline proportion of pain were fixed effects, and country was a random effect. A sensitivity analysis was performed using the same MMANCOVA model excluding country and baseline hydroxycarbamide use. Two additional sensitivity analyses were conducted to assess possible implications of missing electronic diary data for days of pain, using the primary MMANCOVA model and including only those patients with <10% or <20% of daily electronic diary pain data missing during treatment. For each analysis, least squares (LS) mean differences and 90% CIs were calculated between ticagrelor 45 mg and placebo, and ticagrelor 10 mg and placebo. The MMANCOVA model was applied to the proportion of days with analgesic use, based on the same methodology as for the primary variable and including a sensitivity analysis which excluded country and baseline hydroxycarbamide use. Handling of multiplicity was not pre‐specified. Hence, no formal hypothesis test were performed for the secondary variables.
Results
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy, available at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Patients and treatment
This study was conducted at 26 centres in eight countries. The countries (number of centres) were Lebanon (two), Egypt, France, Italy, Kenya, Turkey (three in each), UK (four) and the USA (five). The first patient was enrolled on 09 July 2015, and the study was completed on 16 November 2016.
Of 194 enrolled patients, 87 were randomised (Fig 2). Key reasons for non‐randomisation were failure to meet inclusion/exclusion criteria, with 29 patients having <4 days of pain during the 4‐week, single‐blind, placebo run‐in period and 71 patients failing other criteria. Most patients (n = 78; 90%) completed randomised treatment, and three patients/group discontinued treatment (Fig 2). Reasons for discontinuation were: withdrew consent (n = 5, in one patient an exertional dyspnoea AE at the end of the 12‐week period contributed to the decision); due to an AE (n = 1, hepatic ischaemia); met study‐specific withdrawal criteria (n = 1; emerging indication for surgery); lost to follow‐up (n = 1); and other reason (n = 1; incorrectly randomised despite not fulfilling pain inclusion criterion).
Figure 2.
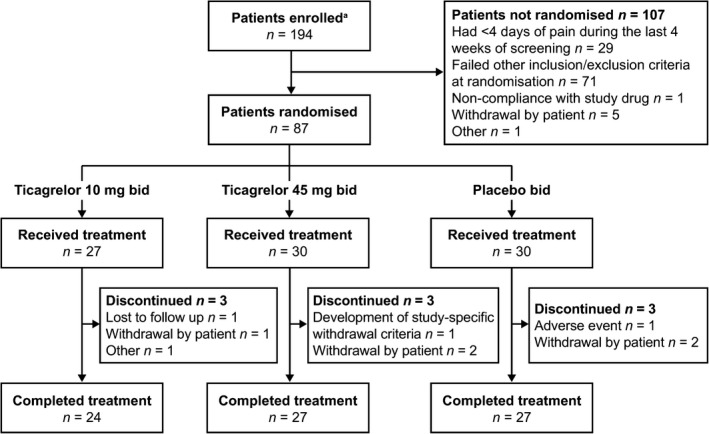
Patient disposition. bid, twice daily. aInformed consent received.
Baseline characteristics were generally comparable between groups (Table 1). Overall mean age was 22·2 years, and more patients had HbSS than HbSβ0 thalassaemia, reflecting the general SCD population. Most patients across all groups used concomitant medications during the treatment period, with no notable differences in the types of concomitant medications used among groups. The proportion of patients using paracetamol in the ticagrelor 10 mg, ticagrelor 45 mg and placebo groups was 67%, 53% and 57%, respectively. Hydroxycarbamide was used by 56%, 60% and 53% of patients in the ticagrelor 10 mg, ticagrelor 45 mg and placebo groups, respectively.
Table 1.
Baseline demographic characteristics of the randomised population
| Characteristic | Ticagrelor 10 mg bid (n = 27) | Ticagrelor 45 mg bid (n = 30) | Placebo bid (n = 30) |
|---|---|---|---|
| Age, mean (SD), years | 21·9 (2·7) | 23·2 (3·7) | 21·6 (3·4) |
| Sex, n (%) | |||
| Male | 12 (44·4) | 14 (46·7) | 14 (46·7) |
| Female | 15 (55·6) | 16 (53·3) | 16 (53·3) |
| BMI, mean (SD), kg/m2 | 21·1 (3·7) | 21·0 (3·7) | 21·8 (6·2) |
| Race, n (%) | |||
| White | 12 (44·4) | 13 (43·3) | 15 (50·0) |
| Black/African American | 14 (51·9) | 17 (56·7) | 15 (50·0) |
| Mixed | 1 (3·7) | 0 | 0 |
| SCD genotype, n (%) | |||
| HbSβ0 | 3 (11·1) | 6 (20·0) | 7 (23·3) |
| HbSS | 24 (88·9) | 24 (80·0) | 23 (76·7) |
| Proportion of days with pain due to SCD during screening period, median (min., max.) | 0·30 (0·00, 1·00) | 0·34 (0·14, 1·00) | 0·42 (0·08, 1·00) |
bid, twice daily; BMI, body mass index; HbSS, homozygous sickle cell; HbSβ0, sickle beta‐zero thalassemia; max, maximum; min, minimum; SCD, sickle cell disease; SD, standard deviation.
The mean (SD) duration of study drug exposure was 76·9 (25·7) days for ticagrelor 10 mg, 80·8 (16·6) days for ticagrelor 45 mg and 81·2 (17·3) days for placebo. Treatment compliance was high and similar across groups. The mean (SD) compliance with treatment was 94·0% (22·5), 92·6% (10·3) and 90·3% (15·3) for the ticagrelor 10 mg, ticagrelor 45 mg and placebo groups, respectively.
Primary variable: proportion of days with patient‐reported SCD pain
During the baseline period, the mean (SD) proportion of days with patient‐reported SCD pain was ticagrelor 10 mg: 0·43 (0·33), ticagrelor 45 mg: 0·52 (0·34), and placebo; 0·50 (0·32), respectively. The mean proportion of days with pain decreased numerically from baseline to week 12 for all groups. No significant differences were seen between ticagrelor and placebo in the change from baseline in the proportion of days with pain, adjusted for other variables, such as hydroxycarbamide use and baseline proportion of days with pain (Fig 3). The reduction in the adjusted change from baseline was numerically greater with placebo versus ticagrelor 10 mg and 45 mg. The LS mean difference for ticagrelor 10 mg versus placebo was 0·045 (90% CI: −0·061, 0·151; i.e. in favour of placebo), and for ticagrelor 45 mg versus placebo was 0·080 (90% CI: −0·023, 0·183; i.e. in favour of placebo). Comparable results were reported with the sensitivity analyses excluding country and baseline hydroxycarbamide use, and for a separate sensitivity analysis including only subsets of patients with <10% or <20% of daily pain data missing during treatment.
Figure 3.
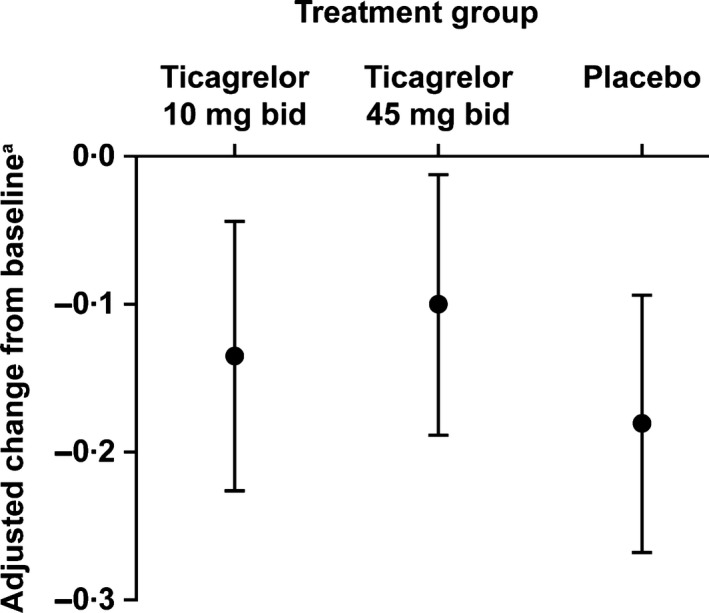
Change in the proportion of days with pain due to sickle cell disease from baseline to week 12. bid, twice daily. aLeast squares mean (black spot) and 90% confidence interval (vertical line) for the proportion of days with pain from baseline to week 12 were obtained using mixed model analysis of covariance with terms for treatment group, baseline proportion of days with pain, country and baseline hydroxycarbamide use. The baseline proportion of days with pain, treatment and hydroxycarbamide were entered into the model as fixed effects, with country as a random effect. Baseline was defined as day −28 to day 0 prior to study drug administration. The baseline proportion of days with pain was calculated as the proportion of days with any pain during the last 4 weeks of the screening period.
Secondary variables
During the baseline period, the mean (SD) patient‐reported daily worst pain intensity (scale 0–10) was generally low: ticagrelor 10 mg: 1·38 (1·49), ticagrelor 45 mg: 2·17 (2·40), placebo: 1·78 (1·45). In all groups, mean (SD) patient‐reported daily worst pain intensity values were numerically lower during treatment versus baseline: ticagrelor 10 mg: 1·15 (1·55), ticagrelor 45 mg: 1·74 (2·28), placebo: 1·02 (1·11).
The mean proportion of days of analgesic use was numerically reduced from baseline to week 12 in all groups. The reduction in the adjusted change from baseline for the proportion of days of analgesic use was numerically greater with placebo versus ticagrelor 10 mg and 45 mg. The LS mean difference for ticagrelor 10 mg versus placebo was 0·119 (90% CI: 0·035, 0·204 i.e. in favour of placebo) and for ticagrelor 45 mg versus placebo was 0·098 (90% CI: 0·016, 0·180 i.e. in favour of placebo).
Exploratory endpoints
No notable differences between groups were seen in the proportion of days absent from school/work. Baseline period mean (SD) proportion of days absent from school/work was 0·03 (0·05) for ticagrelor 10 mg, 0·09 (0·17) for ticagrelor 45 mg and 0·06 (0·11) for placebo. Treatment period mean (SD) proportion of days absent from school/work values was 0·04 (0·07) for ticagrelor 10 mg, 0·05 (0·14) for ticagrelor 45 mg and 0·07 (0·14) for placebo.
A post‐hoc analysis of pain intensity, only including days with pain, showed no differences between groups. Baseline mean (SD) pain intensity was 2·95 (1·56) for ticagrelor 10 mg, 3·45 (2·10) for ticagrelor 45 mg and 3·39 (1·60) for placebo. Treatment period mean (SD) pain intensity was 3·18 (1·58) for ticagrelor 10 mg, 3·27 (1·97) for ticagrelor 45 mg and 3·47 (1·89) for placebo:.
A visual post‐hoc assessment of baseline proportion of days with pain versus levels of haemoglobin and leucocytes at screening showed no obvious association (data not shown).
Pharmacokinetics and pharmacodynamics
Plasma ticagrelor and AR‐C12910XX exposure increased approximately dose proportionally, and concentrations were higher with the 45 mg versus the 10 mg dose. After the first dose of ticagrelor, mean (SD) ticagrelor plasma concentrations at 2 h post‐dosing were 36·4 (20·7) and 202 (130) ng/ml for the 10 and 45 mg groups, respectively. At steady‐state, mean (SD) ticagrelor plasma concentrations at 2 h post‐dosing were 49·3 (28·3) and 245 (130) ng/ml for the 10 and 45 mg bid groups, respectively. For AR‐C1290XX, mean (SD) plasma concentrations were 8·7 (6·9) and 54·6 (41·1) ng/ml 2 h after a single ticagrelor dose of 10 or 45 mg, respectively.
A pharmacodynamic effect (PRU reduction) was confirmed with ticagrelor, and a clear dose–response relationship was seen for PRU. Mean (SD) baseline PRU values prior to dosing were 264 (62·0), 255 (48·4) and 240 (38·8) for ticagrelor 10 and 45 mg and placebo, respectively. At 2 h post‐dose following 1 week of treatment, mean (SD) PRU absolute values were 191 (67·4), 55·3 (62·2) and 242 (37·9) for ticagrelor 10 and 45 mg and placebo, respectively, and the mean (SD) changes from baseline PRU were −79 (46·7; ticagrelor 10 mg), −200 (57·6; ticagrelor 45 mg) and 2·6 (32·4; placebo). The steady‐state absolute PRU values and relative change from baseline at 2 h post‐dose are shown in Fig 4.
Figure 4.
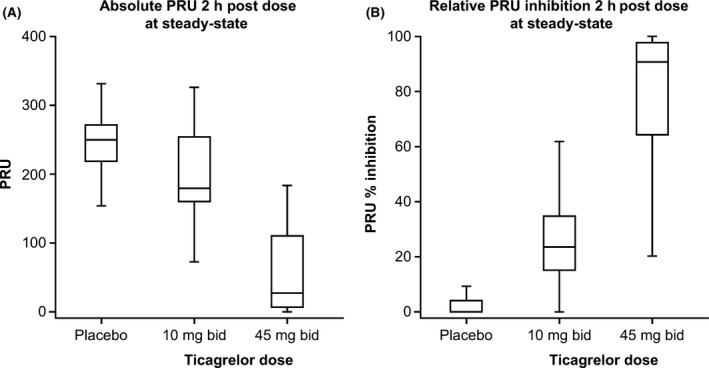
Effects of ticagrelor on platelet activity (PRU). bid, twice daily; PRU, P2Y12 reaction units. Whiskers: 1·5× interquartile range; Boxes: upper and lower quartiles; Mid‐line: median; outliers are not shown. Percent inhibition was calculated versus baseline values before start of study drug according to (1 − [observed PRU/baseline PRU]) × 100. Values are imputed to be between 0% and 100% relative inhibition. Patients with missing baseline values were imputed with the median baseline PRU value to calculate relative change.
Safety
The proportion of patients experiencing AEs during treatment was similar across the groups, with no relation to ticagrelor dose (Table 2). One patient (placebo) experienced a serious AE of hepatic ischaemia and discontinued treatment. One patient (ticagrelor 45 mg) experienced an AE of exertional dyspnoea of severe intensity on day 86 at the end of the 12‐week treatment period, leading to treatment discontinuation. Neither of these events were judged by the investigator to be study drug‐related. No deaths occurred during the study.
Table 2.
Summary of adverse events in safety population (patients taking study drug)
| Categorya n (%) | Ticagrelor 10 mg bid (n = 26)b | Ticagrelor 45 mg bid (n = 30) | Placebo bid (n = 30) |
|---|---|---|---|
| Any AE | 19 (73·1) | 21 (70·0) | 20 (66·7) |
| Any AE leading to treatment discontinuation | 0 | 1 (3·3) | 1 (3·3) |
| Any AE leading to dose interruption | 2 (7·7) | 4 (13·3) | 2 (6·7) |
| Any AE leading to death | 0 | 0 | 0 |
| Any SAE | 6 (23·1) | 5 (16·7) | 6 (20·0) |
| Most common AEsc | |||
| Arthralgia | 6 (23·1) | 9 (30·0) | 6 (20·0) |
| Pain in extremity | 4 (15·4) | 9 (30·0) | 5 (16·7) |
| Headache | 11 (42·3) | 8 (26·7) | 8 (26·7) |
| Sickle cell anaemia with crisis | 5 (19·2) | 5 (16·7) | 3 (10·0) |
| Pneumonia | 2 (7·7) | 4 (13·3) | 2 (6·7) |
| Back pain | 4 (15·4) | 4 (13·3) | 8 (26·7) |
| Non‐cardiac chest pain | 3 (11·5) | 4 (13·3) | 3 (10·0) |
| Abdominal pain | 5 (19·2) | 3 (10·0) | 3 (10·0) |
| Nausea | 1 (3·8) | 3 (10·0) | 1 (3·3) |
| Musculoskeletal pain | 3 (11·5) | 3 (10·0) | 2 (6·7) |
| Urinary tract infection | 2 (7·7) | 2 (6·7) | 4 (13·3) |
| Epistaxis | 0 | 2 (6·7) | 1 (3·3) |
| Vomiting | 2 (7·7) | 2 (6·7) | 1 (3·3) |
| Musculoskeletal chest pain | 1 (3·8) | 2 (6·7) | 1 (3·3) |
| Dysmenorrhoea | 1 (3·8) | 2 (6·7) | 0 |
| Fatigue | 1 (3·8) | 2 (6·7) | 2 (6·7) |
| Pain | 0 | 2 (6·7) | 1 (3·3) |
| Upper respiratory infection | 1 (3·8) | 1 (3·3) | 4 (13·3) |
| Gastroenteritis | 0 | 1 (3·3) | 3 (10·0) |
| Oropharyngeal pain | 2 (7·7) | 1 (3·3) | 2 (6·7) |
| Toothache | 2 (7·7) | 0 | 1 (3·3) |
| Cough | 2 (7·7) | 0 | 0 |
| Most common SAEsc | |||
| Sickle cell anaemia with crisis | 5 (19·2) | 3 (10·0) | 3 (10·0) |
| Gastroenteritis | 0 | 1 (3·3) | 2 (6·7) |
AE, adverse event; bid, twice daily; MedDRA, Medical Dictionary for Regulatory Activities version 19.0; SAE, serious adverse event.
Patients with multiple events in the same category are counted only once in that category. Patients with events in >1 category are counted once in each of those categories. Includes AEs with an onset date on or after the first dose of study medication during the treatment period and through the date of the last dose of study medication.
One randomised patient was discontinued early due to being incorrectly randomised, and no post‐dose data was collected.
At least two patients in any treatment group based on MedDRA Preferred Terms – in order of decreasing proportion in the ticagrelor 45 mg group.
Most AEs were mild or moderate, with the most common AEs being headache, arthralgia and pain in extremities. Severe AEs were experienced by 7 (26·9%), 4 (13·3%) and 3 patients (10·0%) in the ticagrelor 10 mg, ticagrelor 45 mg and placebo groups, respectively. The proportion of patients experiencing a serious AE during treatment was similar across the groups (Table 2). No serious AEs were considered to be treatment‐related as judged by the investigator. The most common serious AE was sickle cell anaemia with crisis.
Overall, six patients (2/group) experienced a total of six bleeding events, none of which were major. Five events were classified as clinically relevant, non‐major bleeding events: nose bleed (2 patients on ticagrelor 45 mg and 1 on placebo); blood in urine (one patient on ticagrelor 10 mg); and vaginal bleeding (one placebo patient). One event of haematuria was classified as minor bleeding (one patient on ticagrelor 10 mg). Two bleeding events were considered by the investigator to be treatment‐related: one case of intermittent vaginal bleeding (placebo) and one of bilateral epistaxis (ticagrelor 45 mg).
There were no clinically meaningful differences across the groups in changes from baseline in haematology, clinical laboratory results or vital signs.
Discussion
HESTIA2 is the first study of the efficacy and safety of ticagrelor in young adults with SCD. Numerical reductions in the proportion of days with pain were observed in all treatment groups, including placebo, with no difference between treatments. There were no benefits observed with ticagrelor regarding secondary pain‐related variables (intensity of SCD pain, proportion of days with analgesic use), or exploratory variables (absence from study/work, pain intensity for days with pain). Ticagrelor was well tolerated with no increased bleeding risk versus placebo. Although there was no effect of ticagrelor on the primary endpoint, the trial only lasted 12 weeks (mean exposure: placebo = 81·2 days; ticagrelor 10 mg = 76·9 days; ticagrelor 45 mg = 80·8 days), and a longer drug exposure might be required to better evaluate an investigational drug considering the numerical pain improvement with placebo.
The lack of effect of ticagrelor, an antiplatelet agent, on reducing daily, self‐reported SCD‐related pain in young adults with SCD might be explained by the heterogeneous and complex nature of pain in SCD. Daily questions about pain recorded in electronic diaries may have also captured pain unrelated to ischaemia, including chronic and neuropathic pain. Therefore, any benefits from platelet inhibition alone might not be sufficient to alleviate all types of SCD‐related pain as reported by patients in a diary. For example, SUSTAIN, a 12‐month, randomised, controlled phase II study in teenage and adult patients with SCD evaluated the effects of crizanlizumab, an antibody to P‐selectin (Ataga et al, 2017). P‐selectin is released from activated platelets and promotes formation of cell aggregates during sickling (Matsui et al, 2001). In SUSTAIN, P‐selectin inhibition resulted in a significant reduction of the VOC event rate, with no significant effects in patient‐reported variables using the Brief Pain Inventory questionnaire (Ataga et al, 2017). A randomised, double‐blind, 30‐day study with prasugrel in adults with SCD (aged 18–55 years) demonstrated no significant effect on patient‐reported pain versus placebo, although the mean pain intensity was lower with prasugrel (1·8) compared with placebo (2·4) (Wun et al, 2013). The subsequent phase III DOVE trial in children and adolescents aged 2–17 years with SCD continued to evaluate the effects of prasugrel over a longer time, and there was a numerical difference between prasugrel and placebo in VOC event rate, and the VOC event rate Anderson–Gill model curves clearly diverged over time, although this trend was not reflected in the diary data on pain‐related symptoms (Heeney et al, 2016). However, the reduction in platelet activity with prasugrel was only modest in DOVE (Jakubowski et al, 2017) and was increased in the current study.
An important observation in HESTIA2 is that the rate of self‐reported pain decreased in all groups versus baseline, including placebo, potentially due to frequent healthcare encounters that may improve disease management, which is important to study in the future. This finding further highlights the complexity and difficulties with pain assessment in SCD. It is well recognised that pain in SCD is complex and consists of disease‐related, non‐disease‐related and psychosocial factors (Mathur et al, 2016).
There are some important strengths with the HESTIA2 study. A high number of patients (almost 90%) completed the randomised ticagrelor treatment period, and tablet compliance was also high. Following administration of ticagrelor at 45 mg to SCD patients, ticagrelor and AR‐C124910XX plasma concentrations at 2 h post‐dose at steady‐state were similar to steady‐state plasma levels in healthy adult volunteers following multiple doses of 50 mg ticagrelor (Butler & Teng, 2010). Ticagrelor and AR‐C124910XX plasma concentrations in HESTIA2 increased approximately proportionally with ticagrelor dose, as expected. In addition, ticagrelor‐related platelet inhibition in HESTIA2 patients with SCD, as shown by an inhibition of PRU, also increased with ticagrelor dose, and was in keeping with the PRU reduction observed in patients with stable coronary artery disease treated with ticagrelor (Gurbel et al, 2009). Collectively, these findings suggest that the lack of efficacy of ticagrelor at the highest dose of 45 mg bid in reducing self‐reported pain in patients with SCD could not be attributed to low ticagrelor exposure or effect on platelet inhibition in adults with SCD.
The current study demonstrated that both ticagrelor doses (10 and 45 mg bid) were well tolerated in young adults with SCD without drug‐related serious AEs or serious bleeding events. The current AE profile of ticagrelor in young adults receiving 10 or 45 mg bid for 12 weeks was in line with what are common medical issues in patients with SCD (Yawn et al, 2014; Novelli & Gladwin, 2016). As the platelet inhibition achieved in HESTIA2 was higher than in the DOVE trial (Jakubowski et al, 2017), it is crucial to note the relative safety of this intervention for future trials combining ticagrelor with other SCD treatments.
There are also some important limitations of the HESTIA2 study. As eligible patients had to experience (and record) pain for a certain number of days during the run‐in period, a regression to the mean may explain the numerical reduction in all three groups during the treatment period and a longer study duration may be needed to fully evaluate any effects beyond placebo. It is crucial to note that ultimate endpoints to assess efficacy of treatments in SCD have yet to be defined, and it is recognised that self‐reported pain is a challenging endpoint. In addition, this study was not designed to evaluate the impact of ticagrelor on the rate of VOCs as the treatment duration in HESTIA2 was only 12 weeks, which may not have been long enough to see an effect on VOCs. In the DOVE trial with prasugrel, differences between treatment arms in VOC rate started to emerge after the first 3 months of treatment (Heeney et al, 2016). Another potential limitation of HESTIA2 is the relatively small number of patients per group.
In conclusion, in this first study of ticagrelor in young adult patients with SCD, ticagrelor was well tolerated and no increased bleeding risk was observed. No effect could be detected on self‐reported SCD‐related pain as captured in a daily diary. Whether platelet inhibition has a potential to impact VOC events is still to be evaluated in a larger and longer study.
Authorship contributions
J.K., and C.A. contributed to the conception and design (protocol development and/or design advice) of HESTIA2. J.K., M.R.A., B.K. and V.N, contributed to data acquisition. M.R.A., C.A., C.G., M.R. and M.L‐Z contributed to data analysis. J.K., B.K., C.A., C.G., M.R. and M.L‐Z contributed to data interpretation. All authors also contributed to the critical review of the important intellectual content of the article and final approval of the version submitted.
Disclosures of conflicts of interest
J.K. chaired the HESTIA2 Steering Committee and is serving on the HESTIA3 Steering Committee and received funding for consultancy for both activities from AstraZeneca; her Institute received funding to support the HESTIA2 research from AstraZeneca. M.R.A. has received advisory board and steering committee fees from AstraZeneca. B.K. received advisory board fees from AstraZeneca. V.N. has no conflicts of interest. C.A., C.G., M.R., and M.L‐Z are employees of AstraZeneca.
Acknowledgements
The authors would like to thank all participating patients, investigators and trial site staff for making this study possible. The authors also thank Jackie Phillipson from Zoetic Science (UK), who provided medical writing support funded by AstraZeneca. This study was funded by AstraZeneca.
References
- Armstrong, D. , Summers, C. , Ewart, L. , Nylander, S. , Sidaway, J.E. & van Giezen, J.J. (2014) Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. Journal of Cardiovascular Pharmacology and Therapeutics, 19, 209–219. [DOI] [PubMed] [Google Scholar]
- Ataga, K.I. , Brittain, J.E. , Desai, P. , May, R. , Jones, S. , Delaney, J. , Strayhorn, D. , Hinderliter, A. & Key, N.S. (2012) Association of coagulation activation with clinical complications in sickle cell disease. PLoS ONE, 7, e29786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga, K.I. , Kutlar, A. , Kanter, J. , Liles, D. , Cancado, R. , Friedrisch, J. , Guthrie, T.H. , Knight‐Madden, J. , Alvarez, O.A. , Gordeuk, V.R. , Gualandro, S. , Colella, M.P. , Smith, W.R. , Rollins, S.A. , Stocker, J.W. & Rother, R.P. (2017) Crizanlizumab for the prevention of pain crises in sickle cell disease. New England Journal of Medicine, 376, 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaca, M.P. , Bhatt, D.L. , Cohen, M. , Steg, P.G. , Storey, R.F. , Jensen, E.C. , Magnani, G. , Bansilal, S. , Fish, M.P. , Im, K. , Bengtsson, O. , Oude Ophuis, T. , Budaj, A. , Theroux, P. , Ruda, M. , Hamm, C. , Goto, S. , Spinar, J. , Nicolau, J.C. , Kiss, R.G. , Murphy, S.A. , Wiviott, S.D. , Held, P. , Braunwald, E. & Sabatine, M.S. ; PEGASUS‐TIMI 54 Steering Committee and Investigators . (2015) Long‐term use of ticagrelor in patients with prior myocardial infarction. New England Journal of Medicine, 372, 1791–1800. [DOI] [PubMed] [Google Scholar]
- Butler, K. & Teng, R. (2010) Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. British Journal of Clinical Pharmacology, 70, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabannes, R. , Lonsdorfer, J. , Castaigne, J.P. , Ondo, A. , Plassard, A. & Zohoun, I. (1984) Clinical and biological double‐blind‐study of ticlopidine in preventive treatment of sickle‐cell disease crises. Agents and Actions. Supplements, 15, 199–212. [PubMed] [Google Scholar]
- Dampier, C. , LeBeau, P. , Rhee, S. , Lieff, S. , Kesler, K. , Ballas, S. , Rogers, Z. & Wang, W. ; Comprehensive Sickle Cell Centers (CSCC) Clinical Trial Consortium (CTC) Site Investigators . (2011) Health‐related quality of life in adults with sickle cell disease (SCD): a report from the comprehensive sickle cell centers clinical trial consortium. American Journal of Hematology, 86, 203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbari, D.S. , Wang, Z. , Kwak, M. , Hildesheim, M. , Nichols, J. , Allen, D. , Seamon, C. , Peters‐Lawrence, M. , Conrey, A. , Hall, M.K. , Kato, G.J. & Taylor, J.G. (2013) Severe painful vaso‐occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLoS ONE, 8, e79923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Giezen, J.J. , Nilsson, L. , Berntsson, P. , Wissing, B.M. , Giordanetto, F. , Tomlinson, W. & Greasley, P.J. (2009) Ticagrelor binds to human P2Y(12) independently from ADP but antagonizes ADP‐induced receptor signaling and platelet aggregation. Journal of Thrombosis and Haemostasis, 7, 1556–1565. [DOI] [PubMed] [Google Scholar]
- Gurbel, P.A. , Bliden, K.P. , Butler, K. , Tantry, U.S. , Gesheff, T. , Wei, C. , Teng, R. , Antonino, M.J. , Patil, S.B. , Karunakaran, A. , Kereiakes, D.J. , Parris, C. , Purdy, D. , Wilson, V. , Ledley, G.S. & Storey, R.F. (2009) Randomized double‐blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation, 120, 2577–2585. [DOI] [PubMed] [Google Scholar]
- Heeney, M.M. , Hoppe, C.C. , Abboud, M.R. , Inusa, B. , Kanter, J. , Ogutu, B. , Brown, P.B. , Heath, L.E. , Jakubowski, J.A. , Zhou, C. , Zamoryakhin, D. , Agbenyega, T. , Colombatti, R. , Hassab, H.M. , Nduba, V.N. , Oyieko, J.N. , Robitaille, N. , Segbefia, C.I. , Rees, D.C. & DOVE Investigators . (2016) A multinational trial of prasugrel for sickle cell vaso‐occlusive events. New England Journal of Medicine, 374, 625–635. [DOI] [PubMed] [Google Scholar]
- Jakubowski, J.A. , Hoppe, C.C. , Zhou, C. , Smith, B.E. , Brown, P.B. , Heath, L.E. , Inusa, B. , Rees, D.C. , Small, D.S. , Gupta, N. , Yao, S. , Heeney, M. & Kanter, J. (2017) Real‐time dose adjustment using point‐of‐care platelet reactivity testing in a double‐blind study of prasugrel in children with sickle cell anaemia. Thrombosis and Haemostasis, 117, 580–588. [DOI] [PubMed] [Google Scholar]
- Lee, S.P. , Ataga, K.I. , Orringer, E.P. , Phillips, D.R. & Parise, L.V. (2006) Biologically active CD40 ligand is elevated in sickle cell disease: potential role for platelet mediated inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 1626–1631. [DOI] [PubMed] [Google Scholar]
- Mathur, V.A. , Kiley, K.B. , Carroll, C.P. , Edwards, R.R. , Lanzkron, S. , Haythornthwaite, J.A. & Campbell, C.M. (2016) Disease‐related, nondisease‐related, and situational catastrophizing in sickle cell disease and its relationship with pain. The Journal of Pain, 17, 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, N.M. , Borsig, L. , Rosen, S.D. , Yaghmai, M. , Varki, A. & Embury, S.H. (2001) P‐selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood, 98, 1955–1962. [DOI] [PubMed] [Google Scholar]
- Mitchell, L.G. , Goldenberg, N.A. , Male, C. , Kenet, G. , Monagle, P. & Nowak‐Göttl, U. ; Perinatal and Paediatric Haemostasis Subcommittee of the SSC of the ISTH . (2011) Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. Journal of Thrombosis and Haemostasis, 9, 1856–1858. [DOI] [PubMed] [Google Scholar]
- Novelli, E.M. & Gladwin, M.T. (2016) Crises in sickle cell disease. Chest, 149, 1082–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael, J.L. , Mei, M. , Mueller, B.U. & Giordano, T. (2012) High resource hospitalizations among children with vaso‐occlusive crises in sickle cell disease. Pediatric Blood Cancer, 58, 584–590. [DOI] [PubMed] [Google Scholar]
- Sillén, H. , Cook, M. & Davis, P. (2010) Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 878, 2299–2306. [DOI] [PubMed] [Google Scholar]
- Smith, W.R. , Penberthy, L.T. , Bovbjerg, V.E. , McClish, D.K. , Roberts, J.D. , Dahman, B. , Aisiku, I.P. , Levenson, J.L. & Roseff, S.D. (2008) Daily assessment of pain in adults with sickle cell disease. Annals of Internal Medicine, 148, 94–101. [DOI] [PubMed] [Google Scholar]
- Teng, R. (2015) Ticagrelor: pharmacokinetic, pharmacodynamic, and pharmacogenetic profile – an update. Clinical Pharmacokinetics, 54, 1125–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallentin, L. , Becker, R.C. , Budaj, A. , Cannon, C.P. , Emanuelsson, H. , Held, C. , Horrow, J. , Husted, S. , James, S. , Katus, H. , Mahaffey, K.W. , Scirica, B.M. , Skene, A. , Steg, P.G. , Storey, R.F. , Harrington, R.A. , & PLATO Investigators . Freij, A. & Thorsén, M. (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine, 361, 1045–1057. [DOI] [PubMed] [Google Scholar]
- Ware, R.E. , de Montalembert, M. , Tshilolo, L. & Abboud, M.R. (2017) Sickle cell disease. The Lancet, 390, 311–323. [DOI] [PubMed] [Google Scholar]
- Wun, T. , Soulieres, D. , Frelinger, A.L. , Krishnamurti, L. , Novelli, E.M. , Kutlar, A. , Ataga, K.I. , Knupp, C.L. , McMahon, L.E. , Strouse, J.J. , Zhou, C. , Heath, L.E. , Nwachuku, C.E. , Jakubowski, J.A. , Riesmeyer, J.S. & Winters, K.J. (2013) A double‐blind, randomized, multicenter phase 2 study of prasugrel versus placebo in adult patients with sickle cell disease. Journal of Hematology & Oncology, 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn, B.P. , Buchanan, G.R. , Afenyi‐Annan, A.N. , Ballas, S.K. , Hassell, K.L. , James, A.H. , Jordan, L. , Lanzkron, S.M. , Lottenberg, R. , Savage, W.J. , Tanabe, P.J. , Ware, R.E. , Murad, M.H. , Goldsmith, J.C. , Ortiz, E. , Fulwood, R. , Horton, A. & John‐Sowah, J. (2014) Management of sickle cell disease: summary of the 2014 evidence‐based report by expert panel members. Journal of the American Medical Association, 312, 1033–1048. [DOI] [PubMed] [Google Scholar]


