Summary
Elevated blood pressure (BP) is a major determinant of morbidity and mortality burden related to cardio‐metabolic risk. Current guidelines indicate that controlling and lowering BP promotes cardiovascular (CV) risk reduction. Among antihypertensive agents, angiotensin receptor blockers (ARBs) are characterized by an efficacy profile equivalent to other antihypertensive agents and are provided with excellent tolerability and low discontinuation rates during chronic treatments. Moreover, CV outcomes are reduced by ARBs. Olmesartan is a long‐lasting ARB which proved to achieve a comparable or more effective action in lowering BP when compared to other ARBs. Olmesartan, in fact, displayed a larger and more sustained antihypertensive effect over the 24 hours, with a buffering effect on short‐term BP variability. These are important features which differentiate olmesartan from the other principles of the same class and that may help to control the increased CV risk in the presence of high BP variability. Olmesartan shows similar benefits as other ARBs in terms of all‐cause and CV mortality, and a favorable tolerability profile. Combination of olmesartan with long‐lasting calcium‐channel blockers and thiazide diuretics represents a rational and effective therapy. Thus, ARBs, including olmesartan, represent one of the most effective and safe treatments for patients with arterial hypertension.
Keywords: ambulatory blood pressure, angiotensin receptor blockers, arterial hypertension, blood pressure, blood pressure variability, olmesartan
1. HYPERTENSION IS THE STRONGEST RISK FACTOR FOR CARDIOVASCULAR DISEASE
In the last three decades, ischemic heart disease, stroke, and diabetes became leading causes of mortality and years of life lost (YLL), although with some divergences among countries in the ratio of observed and expected YLLs which is based on sociodemographic indexes.1 Worldwide, the morbidity and mortality burden associated to cardio‐metabolic risk appears to be largely influenced by a rise in blood pressure (BP) levels, body mass index, glucose, and cholesterol. These risk factors are responsible of more than 60% of global death from cardiovascular disease (CVD), chronic kidney disease (CKD), and diabetes, with the high BP having the largest effect.2 In 2013, high systolic blood pressure (SBP) accounted for 10.4 million deaths and 208.1 million Disability‐Adjusted Life Years.3 People with hypertension (BP ≥140/90 mm Hg or those receiving BP‐lowering drugs) have a higher lifetime risk of overall CVD at 30 years of age (63.3% vs 46.1% of those with normal BP) and tend to develop CVD 5.0 years earlier.4 Even among individuals without known vascular disease at baseline, a rise in BP levels is associated strongly with the age‐specific mortality rates from CV events.5
A 20 mm Hg difference in usual SBP is approximately equivalent in its hazards to a 10 mm Hg difference in usual diastolic blood pressure (DBP). In particular, an elevation of 20 mm Hg accounts for many increases in strokes, coronary syndromes, and mortality.6 These relationships between BP levels and vascular mortality show a remarkable, continuous association starting from SBP of 115 mm Hg and DBP of 75 mm Hg for each decade of age.5
2. THE IMPORTANCE OF REDUCING BLOOD PRESSURE TO PREVENT CARDIOVASCULAR OUTCOMES
The benefits of hypertension treatments aimed to lower BP for prevention of CVD are well established. A 10 mm Hg decrease in SBP reduces the risk of major CVD events by 20%, coronary heart disease by 17%, stroke by 27%, heart failure by 28%, and all‐cause mortality by 13%,7 while a decrease in DBP has been linearly related to a lower risk of recurrent stroke (P = 0.026) and all‐cause mortality (P = 0.009).8 Reduction of CV risk is proportional to the decrease of SBP, DBP, and pulse pressure (PP), but the logarithmic nature of this relationship implies that risk reduction increases to a progressively smaller extent the larger the BP reduction.9
The effects of BP lowering are broadly similar even in the presence of concomitant comorbidities or previous CVD, coronary heart disease, or cerebrovascular disease.7 Indeed, the extent of both SBP and DBP decrease is directly associated with the magnitude of risk reduction in recurrent cerebrovascular and CV events. These findings highlight the importance of a strict and aggressive BP control, which is increasingly being considered as the most essential therapeutic strategy for an effective CVD prevention, particularly in case of pre‐existing CVD. For instance, achieving a SBP <130 mm Hg seems to be invaluable for secondary stroke prevention in patients with cerebrovascular events.8
Controlling and lowering BP is per se functional to reduce CV risk, independently of the antihypertensive approaches used (Figure 1) and produces a significant improvement in outcome in all hypertension grades.10 For instance, in the presence of grade 1 hypertension at low‐to‐moderate risk, lowering BP induces a reduction in stroke (risk ratio, RR = 0.33), coronary events (RR = 0.68), and death (RR = 0.53); the SBP cut‐off values are across 150 and 140 mm Hg; below 130 mm Hg only stroke and all‐cause death are significantly reduced.9 Furthermore, after stratifying patients per increasing 10‐year incidence of CV death, the relative reduction in all outcomes by BP lowering does not differ among strata and the absolute risk decreases in very high‐risk patients.11 In patients at high risk for the presence of diabetes mellitus, BP reductions in 5‐10 mm Hg significantly contribute to reduce the relative risk of coronary heart disease, heart failure, and all‐cause death; these results are amplified in hypertensive patients with diabetes who show a significantly greater relative risk reduction of coronary heart disease and all‐cause death, obtained by a more intensive BP control.12 In general, when an intensive BP lowering is achieved, the risk ratio of stroke (RR = 0.71), coronary events (RR = 0.80), major CV events (RR = 0.75), and CV mortality (RR = 0.79) further improve; heart failure and all‐cause death do not change.13 Even in patients in the high normal range with an estimated risk higher than 10%, an intensive antihypertensive strategy aimed at reaching SBP targets of 120 mm Hg reduced the composite outcome compared to the standard strategy.14
Figure 1.
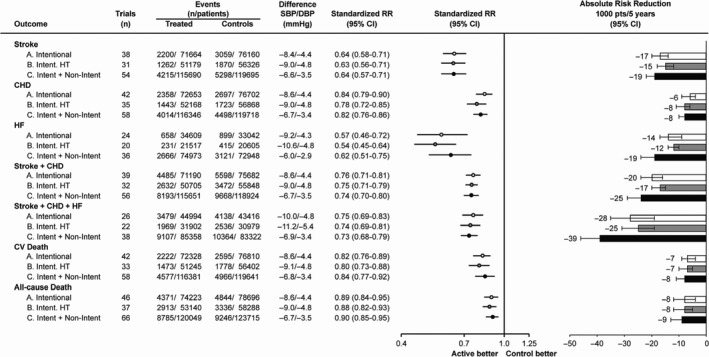
Relative and absolute risk reduction of various outcomes in trials of blood pressure (BP) lowering. A, Intentional BP‐lowering trials. B, Intentional BP‐lowering trials exclusively in hypertensive (HT) patients. C, Intentional and nonintentional BP‐lowering trials together. Standardized Mantel–Haenszel risk ratio (RR) is to a systolic BP (SBP)/diastolic BP (DBP) difference of 10/5 mm Hg. The column absolute risk reduction reports the number and 95% confidence interval (CI) of events prevented for every 1000 patients treated for 5 years with a standardized RR. CHD indicates coronary heart disease [Redrawn from 10 with permission]
Treatments aimed to lower BP may be important to reduce CVD risk even in individuals with normal or high normal blood pressure, but risk reduction is limited to stroke.15
3. THE PLACE OF ANGIOTENSIN RECEPTOR BLOCKERS IN THE TREATMENT OF HYPERTENSION AND ASSOCIATED CARDIOVASCULAR CONDITIONS
Systematic reviews have shown that BP lowering by all classes of antihypertensive drugs is matched with significant reductions in stroke and major CV events. Although some minor differences among classes have been observed for prevention of specific outcomes (eg, overall mortality), there is a general homogeneity in the observed benefits (Figure 2).10 This supports the concept that reduction in these events is highly related to the BP‐lowering properties beside potential‐specific drug properties.
Figure 2.
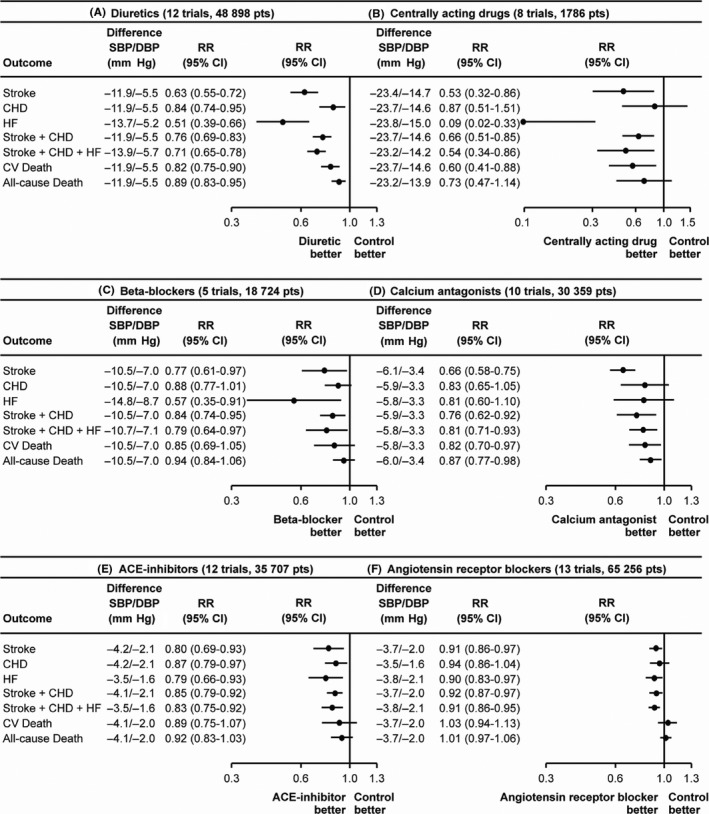
Relative risk reduction of various outcomes in trials of blood pressure (BP) lowering by different classes of drugs. A, Diuretics. B, Centrally acting drugs. C, Beta‐blockers. D, Calcium antagonists. E, Angiotensin‐converting enzyme (ACE) inhibitors. F, Angiotensin receptor blockers. CI indicates confidence intervals; DBP, diastolic blood pressure; pts, patients; RR, Mantel–Haenszel risk ratios; and SBP, systolic blood pressure [Redrawn from 10 with permission]
Current hypertension guidelines recommend angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) as suitable first choice for initiation and maintenance of antihypertensive treatment, either as monotherapy or in combination.16, 17 Combining drug regimens with complementary activity, where a second antihypertensive agent is used to block the compensatory responses to the first agent, can result in additional BP lowering, thus contributing to reach the BP goal, while combinations of drugs with similar mechanism of action should be avoided.16 For instance, data from high‐quality randomized clinical trials demonstrate that simultaneous administration of renin‐angiotensin system (RAS) blockers (ie, ACE‐inhibitor with ARB; ACE‐inhibitor or ARB with renin inhibitor aliskiren) increases CV and renal risk.16
Current guidelines are based on results of randomized controlled studies, mostly placebo‐controlled, and mostly carried out between 1965 and 1995. These trials provided solid evidence in favor of antihypertensive therapy in patients with SBP >160 mm Hg or DBP >100 mm Hg, who would now be classified as grade 2 and 3 hypertensives. Evidence for drug treatment in individuals with low‐to‐moderate risk (grade 1 hypertension) is conversely limited, since no trial has specifically addressed this condition. A pharmacological treatment is supported even in these patients who have the potential to become at high risk, since treatments can be personalized to enhance efficacy and tolerability and have a good cost‐benefit ratio.17
Accounting for equivalent antihypertensive properties, the effects of drug classes on overall CV risk are similar, but some difference can be noticed in reducing specific risks: diuretics result as superior in preventing heart failure; beta‐blockers are less effective in protecting from stroke; calcium antagonists are superior in preventing stroke and all‐cause death, but inferior in preventing coronary heart disease; ARBs are more effective in preventing heart failure.18
Indeed, ARBs are associated with a significant relative reduction in heart failure (−10%), stroke (−9%), and major CV events—composite of stroke, coronary heart disease, heart failure—(−9%), although they produce a rather small difference in SBP/DBP compared to placebo (about −3.7/−2.0 mm Hg), which may be due to the fact that in comparative studies patients in the placebo groups were often treated with active CV drugs. ARBs have additional properties which make them particularly effective in reducing proteinuria and improving outcomes in chronic heart failure. These properties are especially important to treat patients with nephropathy, since the relationship between BP and progression of CKD and incident end‐stage renal disease is direct and progressive. Several randomized trials have clearly demonstrated that RAS blockade is more effective in reducing albuminuria than either placebo or other antihypertensive agents in diabetic nephropathy, nondiabetic nephropathy, and in patients with CVD and is also effective in preventing incident microalbuminuria.19, 20 Hypertension is a leading cause for heart failure, and BP lowering has been described as an invaluable strategy to prevent this complication in all patients, including in very elderly patients.23 Correcting cardiac overstimulation by sympathetic system and the RAS, rather than exclusively lowering BP is the mechanism by which these agents contribute to reduce the risk of heart failure. Angiotensin blockade is a major therapeutic strategy in patients with heart failure, both by providing a balanced reduction in preload and after‐load when a reduced systolic function occurs and by antagonizing vasoconstriction and salt and water retention which perpetuate the disease and promote progression toward the congestive or the end‐stage states. Finally, RAS blockade has also a beneficial influence on cardiac remodeling.24
In most randomized controlled large trials performed with ARBs over the last 20 years, in different clinical conditions, these compounds have revealed systematically noninferior to ACE‐inhibitors and often superior to other drug comparators. Thus, in the LIFE (Losartan Intervention For Endpoint reduction) and MOSES (MOrbidity and mortality after Stroke, Eprosartan compared with nitrendipine for Secondary prevention) studies in hypertension ARBs were superior to beta‐blockers and calcium antagonists, respectively.25, 26 In the RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan), IDNT (Irbesartan Diabetic Nephropathy Trial) and IRMA (IRbesartan in patients with type 2 diabetes and MicroalbuminuriA) studies in CKD ARBs revealed superior to conventional optimal therapy, as they did for onset of microalbuminuria in the ROADMAP (Randomized Olmesartan and Diabetes MicroAlbuminuria Prevention).22, 27, 28, 29 In the ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) study in high‐risk patients with a large proportion of hypertensives, ARBs performed equally to ACE‐inhibitors, as they systematically did in heart failure (ELITE and ELITE II—Evaluation of Losartan in the Elderly, Val‐HefT—Valsartan Heart Failure Trial and CHARM—Candesartan in Heart failure Assessment of moRtality and Morbidity), in postmyocardial state (OPTIMAAL—OPtimal Trial In Myocardial infarction with Angiotensin II Antagonist Losartan, VALIANT—VALsartan In Acute myocardial iNfarcTion).30, 31, 32, 33, 34, 35, 36 Also in prevention of new onset of diabetes, a meta‐analysis has shown similar benefits with ARBs or ACE‐inhibitors.37 Finally, in large registries and meta‐analyses ARBs have displayed better tolerability and safety, as well as persistence on treatment.38, 39 The impressive size of this evidence‐based medicine literature with ARBs, obtained with the different compounds in the class, indeed strongly suggests a beneficial impact on CV outcomes as a class effect of ARBs.
4. ARE ANGIOTENSIN RECEPTOR BLOCKERS ALL EQUALS? THE ROLE OF OLMESARTAN
Angiotensin receptor blockers have a well‐recognized antihypertensive activity, and although they share many common features, some pharmacokinetic and pharmacodynamic differences have been found among the components of this group (Table 1).
Table 1.
ARBs currently indicated for the treatment of hypertension
| Drug | Usual starting dose (mg/d) | Usual dose, range (mg/d) | Daily frequency | Other indications approved beside hypertension |
|---|---|---|---|---|
| Azilsartan | 40 or 70 | 40‐80 | 1 | None |
| Candesartan | 16 | 8‐32 | 1 | Treatment of heart failure (NYHA Classes II‐IV) |
| Eprosartan | 600 | 600‐800 | 1 or 2 | None |
| Irbesartan | 150 | 150‐300 | 1 | Diabetic nephropathy when serum creatinine is increased and proteinuria present in patients with hypertension and type 2 diabetes |
| Losartan | 50 | 50‐100 | 1 or 2 |
Diabetic nephropathy when serum creatinine is increased and proteinuria present in patients with hypertension and type 2 diabetes Stroke reduction in patients with hypertension and left ventricular hypertrophy (nonblack only) |
| Olmesartan | 20 | 20‐40 | 1 | None |
| Telmisartan | 40 | 20‐80 | 1 | Cardiovascular risk reduction in patients unable to take ACE‐inhibitors |
| Valsartan | 80 or 160 | 80‐320 | 1 |
Treatment of heart failure (NYHA Classes II‐IV) Reduction of CV mortality in clinically stable patients with left ventricular failure or dysfunction following myocardial infarction. |
ACE, Angiotensin‐Converting Enzyme; ARB, Angiotensin II Receptor Blocker; CV, Cardiovascular; NYHA, New York Heart Association.
The head‐to head comparison among ARBs is quite controversial, since each published study shows some confounding factors and study designs are often heterogeneous. However, two recent meta‐analyses try to summarize the results of randomized trials and provide an overview on the effect of ARBs in BP lowering, highlighting the differences among the components of this class. In this review, we focus our attention on results concerning olmesartan.
In a meta‐analysis on 22 studies (4892 subjects), Wang et al40 indicated that olmesartan provided greater reduction in both DBP and SBP than losartan and in SBP compared to valsartan, while the BP response rates and the incidence of adverse events were similar among the class. Tsoi et al performed a network meta‐analysis to compare both direct and indirect treatments at 24 weeks: Although none significant difference was achieved, olmesartan resulted as more effective in reducing BP than losartan (−3.99 mm Hg) (Figure 3). Olmesartan showed similar odd ratio for all‐cause mortality as other ARBs, and it did not show any difference in terms of CV mortality (Figure 3).41
Figure 3.
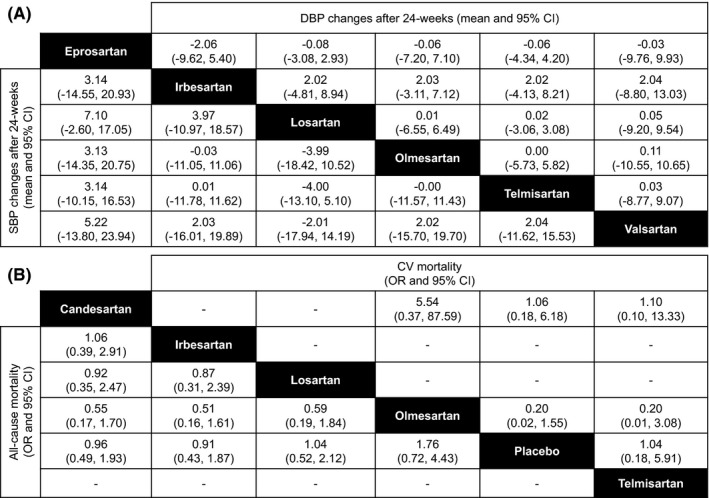
A, Relative treatment effects of Angiotensin Receptor Blockers at 24 weeks in terms of the absolute change (weighted mean difference and 95% credible interval or CI) in systolic blood pressure (SBP) and diastolic blood pressure (DBP). To interpret: weighted mean difference <0 favors the column‐defining treatment (eg, eprosartan, compared to irbesartan, is associated with increase in blood pressure by +3.14/+2.06 mm Hg). B, Relative treatment effects (odds ratio, OR, and 95% credible interval or CI) of Angiotensin Receptor Blockers on all‐cause and cardiovascular (CV)‐related mortality. To interpret: odds ratio >1 favors the column‐defining treatment [Redrawn from 41 with permission]
As mentioned above, BP lowering is important in the elderly to prevent serious CV events. ARBs, including olmesartan, are among the first treatment choice for treatment of hypertension in older people. In a recent meta‐analysis performed by Redon et al42 including 25 studies in patients aged 60‐79 years, of which 10 based on head‐to‐head comparisons of olmesartan (n = 770) vs other ARBs (n = 691), including losartan, irbesartan, valsartan and candesartan, SBP and DBP reductions after 8‐52 weeks of olmesartan monotherapy were significantly larger than those obtained with other ARBs [SBP: 1.38 (3.56, +0.79) and DBP: 1.02 (2.28, +0.24 mm Hg)].
Interestingly, olmesartan seems to possess some anthiatherogenic and vasoprotective effects which add to its hypotensive activity. Studies in different animal models have demonstrated that the full blockade of angiotensin II receptors, obtained with olmesartan, can interfere with the atherogenic process independently of blood pressure reduction, likely due to the significant reduction in inflammatory markers induced by treatment with the drug.43, 44 In large randomized placebo or active drug controlled studies performed in patients with hypertension, stable angina or type 2 diabetes, long‐term treatment with olmesartan showed reduction in the levels of markers of vascular inflammation (high sensitive C‐reactive protein, alpha tumor necrosis factor, interleukine‐6, and macrophage chemotactic protein‐1), regression of atheromatous plaque volume at the carotid and coronary artery, regression of vascular hypertrophy, and microalbuminuria prevention.46
5. OLMESARTAN AND 24‐HOUR BLOOD PRESSURE CONTROL
Considering the slight differences in their pharmacokinetic and pharmacodynamic, the choice of specific ARBs may affect the entity and the timing of BP reduction and the dose may be particularly important. Although specific drug or dose usually does not affect the reduction in office and awake SBP, they may influence the effects on SBP over the 24 hours, at night, and during the last 4 hours. Furthermore, variation in dosing may enhance the possibility of prolonging BP reduction during the nocturnal period. Concerning DBP, the type of ARB is determinant to modify BP reduction in the office, during the 24 hours, in the awake and sleep periods; the dosage is even important for differential nocturnal DBP reduction.47 Compared to other ARBs, olmesartan shows efficacy in reducing 24‐hour SBP (P = 0.03) and DBP (P = 0.002), awake BP [with a significant difference only for DBP (P = 0.04)], sleep SBP (P = 0.008) and DBP (P = 0.002), and SBP (P = 0.02) and DBP (P = 0.04) during the last 4 hours of the dosing interval (Figure 4).47 Therefore, olmesartan provides additional advantages in controlling BP as compared to other ARBs, since it allows a larger BP reduction over the 24 hours.48
Figure 4.
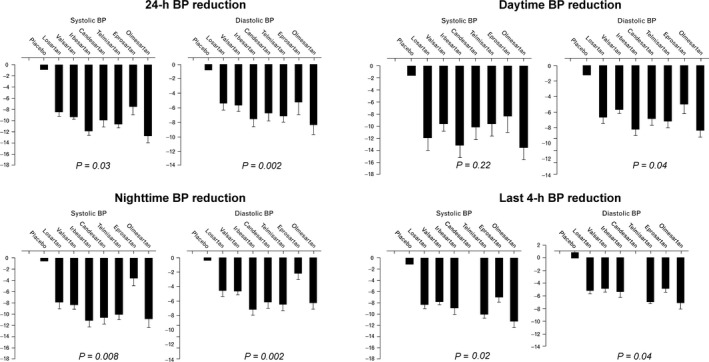
Reduction in the values of systolic and diastolic blood pressure (BP) over the 24 hours, the daytime, the nighttime and the last 4 hours of the dosing interval for different angiotensin receptor blockers and placebo. Values are adjusted by the baseline BP values, dose, age, and number of participants included in every cohort and are expressed as average and standard error. P value for the difference across treatments [Redrawn from 47 with permission]
Additional features of olmesartan, when its efficacy is evaluated over the 24 hours, are represented by a more homogeneous control and a stabilizing effect on BP variability (BPV). Olmesartan maintains SBP and DBP at lower levels over a 24‐hour period than ARB comparators: irbesartan achieves a larger SBP and DBP drops in the first few hours, while olmesartan largely reduces BP from 5 to 15 hours and in the last 5 hours of the dosing period. At 24‐hour time‐point, the mean SBP and DBP are approximately 3‐5 mm Hg lower with olmesartan than with losartan, valsartan, or irbesartan.49 Olmesartan significantly reduces mean 24‐hour BP and night‐time BP, compared to losartan and after 8 weeks, 20.6% of patients treated with olmesartan achieve the goal of 24‐hour ambulatory BP <130/80 mm Hg, compared to 9.0%, 9.2% and 14.2% with losartan, valsartan, and irbesartan.49 Therefore, olmesartan provides a favorable action in lowering and, especially, controlling BP and this aspect is particularly important for reducing CV risk. Indeed, although average BP values are usually considered as the main determinant of CV events related to hypertension, short‐term fluctuations in BP levels, and variations in prolonged periods of time should be attentively monitored. Evidence from longitudinal and observational studies has indicated that short‐term BPV within the 24 hours may have a nonmarginal contribution to CV risk. An initial increase in BPV within 24 hours is an independent predictor of progression of subclinical organ damage, CV events, and CV mortality.50, 51 Similarly, long‐term day‐by‐day BPV is associated with a higher prevalence and severity of cardiac, vascular, and renal organ damage and with an increased risk of fatal and nonfatal CV events.51 The impact on 24‐hour BP control, BPV, and 24‐hour distribution of BP reduction has been recently determined for olmesartan alone or in combination with one or two other antihypertensive drugs in a large pooled individual data analysis of ten randomized controlled studies.52 Active treatment with olmesartan or comparators, but not placebo, reduced SBP and DBP during the whole 24 hours, and the reduction was well maintained during the day and during the night, with larger effects during the waking hours (Figure 5). Interestingly the mean BP reduction was significantly larger after combination treatment than with monotherapies and increased with the intensity of the combination. Placebo had no effect on BPV, small effects were observed under monotherapies, whereas the greatest effect was reported in the combination groups, when olmesartan was combined with dihydropyridine calcium‐channel blockers or thiazide diuretic or was present in a triple combination therapy.52, 53 Treatment with olmesartan monotherapy resulted in smoothness indexes significantly larger than with active control, and dual and triple combinations achieved smoothness indexes significantly larger than under corresponding monotherapies; the treatment on variability index (TOVI) showed the same trend of smoothness index (Figure 6). Therefore, olmesartan administered in combination with one or two other antihypertensive drugs, allowed a superior 24‐hour BP control than placebo or monotherapies (even including olmesartan).52 The achievement of a more homogeneous and sustained BP control, with reduced BPV, may represent a desirable feature of a given antihypertensive drug treatment, because it may help in preventing the CV consequence associated with uncontrolled arterial hypertension.52, 54, 55
Figure 5.
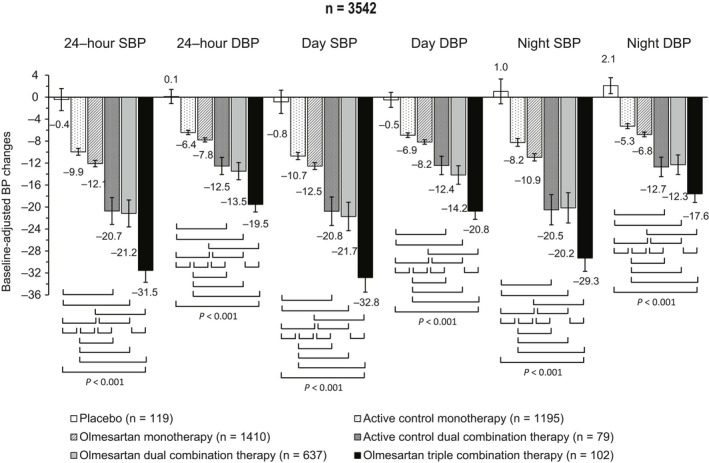
Adjusted 24 hour, day and night systolic (SBP) and diastolic blood pressure (DBP) mean changes (95% confidence interval) from baseline after double blind treatment with placebo (n = 119), active control monotherapy (n = 1195), olmesartan monotherapy (n = 1410), active control dual combination therapy (n = 79), olmesartan dual combination therapy (n = 637), and olmesartan triple combination therapy (n = 102). The statistical significance of differences between individual pairs of treatments is indicated by the P‐value. Changes are adjusted for baseline value, age, sex, body mass index, and region [Redrawn from 52 with permission]
Figure 6.
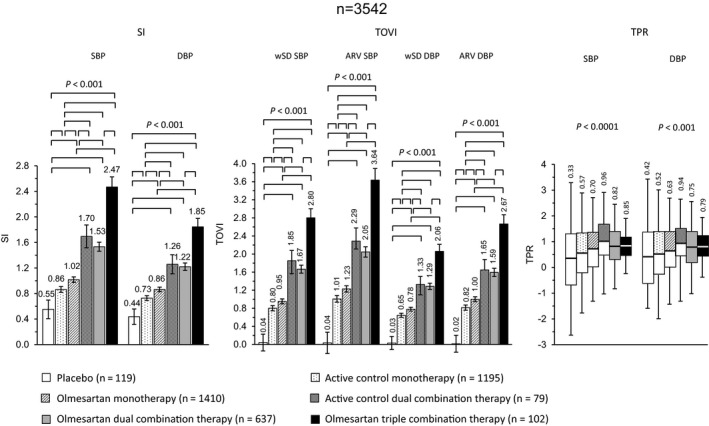
Adjusted average (95% confidence interval) smoothness index (SI) and treatment on variability index (TOVI), and boxplots of trough‐to‐peak ratio (TPR) after double blind treatment with placebo (n = 119), active control monotherapy (n = 1195), olmesartan monotherapy (n = 1410), active control dual combination therapy (n = 79), olmesartan dual combination therapy (n = 637), and olmesartan triple combination therapy (n = 102). Data are shown for systolic (SBP) and diastolic blood pressure (DBP). The statistical significance of differences between individual pairs of treatments is indicated by the P‐value. SI and TOVI data are adjusted for age, sex, mass index, and region [Redrawn from 52 with permission]
6. SAFETY OF ANGIOTENSIN RECEPTOR BLOCKERS
All classes of antihypertensive drugs, although able to significantly reduce the risk of major CV events, significantly increase the risk of adverse events leading to permanent treatment discontinuations, with the distinct exception of ARBs which differ from all other classes for the lowest risk of discontinuation. As shown in Figure 7 , the risk of discontinuation for an adverse event was reduced by 29% with ARB treatment, by 10% with diuretics, and by 2% with calcium‐channel blockers, whereas it was increased by 18% with ACE‐inhibitors and by 13% with beta‐blockers.56
Figure 7.
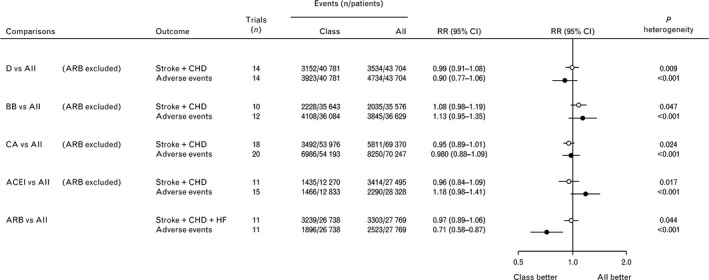
Comparisons of the effects of blood pressure‐lowering treatments based on diuretics, beta‐blockers, calcium antagonists, and angiotensin‐converting enzyme inhibitors vs all other classes of drugs excluding angiotensin receptor blockers. The type of cardiovascular events considered was the composite of stroke and coronary heart disease or the composite of stroke, coronary heart disease, and heart failure, as indicated. ACEI, angiotensin‐converting enzyme inhibitors; All, all other classes together; ARB, angiotensin receptor blockers; BB, beta‐blockers; CA, calcium antagonists; CHD, coronary heart disease; CI, confidence interval; D, diuretics; HF, heart failure; RR, risk ratio [Redrawn from 56 with permission]
Since ARBs act on renin‐angiotensin system to produce the angiotensin II that is not only an effective hypertensive agent, but it also regulates cell growth, their potential for an increased risk of cancer has been widely investigated. Except a meta‐analysis that in 2010 described a relationship between cancer and ARBs, no evidence supports that the use of ARBs raises the risk of tumor development.57 In this regard, a critical commentary took into consideration the mechanisms underlying the potential link between ARBs and cancer and concluded that, if anything, ARBs are likely to be involved with a prevention of cancer, rather than a promotion.58 This is supported by Yang et al,59 which analyzed data from almost 4 million of patients involved in six cohort studies and four case–control studies and found that ARB‐based therapy was not significantly associated with an increased risk of cancer (RR = 0.87, 95% CI: 0.75, 1.01). Some data evidence that ARBs could decrease the lung cancer risk (OR = 0.81, 95% CI: 0.69‐0.54), but subgroup analysis by race indicated that Asians treated with ARBs showed a decreased risk of lung cancer (OR = 0.60; 95% CI: 0.54‐0.67; P < 0.00001), while Caucasians did not show any significant reduction (OR = 0.90; 95% CI: 0.79‐1.02; P = 0.11).60, 61 Therefore, since the preventive role of ARBs in lung cancer is still controversial, it is possible to exclude that ARBs increase the overall risk of cancer occurrence.
ARBs have demonstrated excellent safety profiles alone and in combination with other antihypertensive therapies during the past 20 years. The tolerability profiles of ARBs are similar to placebo and superior to ACE inhibitors. For example, ACE inhibitors increase the risk of cough two‐ to threefold over placebo and may cause up to 0.1%‐0.2% rates of angioedema, while with ARBs, the risk of cough was comparable to placebo (RR = 1.01, 95% CI: 0.74‐1.39).56 However, compared to placebo, ARBs were associated with higher risk of renal dysfunction, hypotension, and hyperkalemia; despite these findings, discontinuation events in patients treated with ARBs, diuretics, or placebo were similar.62
Olmesartan shows similar tolerability in terms of incidence of adverse events, as other ARBs (losartan, valsartan, candesartan, and irbesartan), with similar relative risk of dizziness, headache, and diarrhea as losartan or valsartan and it is well tolerated even in combination with diuretics or amlodipine.41 In 2012, a case series of sprue‐like syndrome at the Mayo Clinic which was linked to the use of olmesartan was reported. This syndrome is characterized by severe diarrhea and sprue‐like histopathologic findings in the intestine, often with increased subepithelial collagen.59 Some clinicopathological findings were described in patients taking ARBs other than olmesartan; therefore, the sprue‐like enteropathy, which has indeed a low incidence, seems to be a class effect, rather than an olmesartan‐related adverse event.63, 64, 65, 66
7. CONCLUSIONS
Lowering and controlling BP are important strategies to decrease the risk of CV events and CV mortality in both hypertensive and normotensive individuals. Current guidelines recommend the use of ACE‐inhibitors or ARBs to initiate an antihypertensive therapy; while BP lowering is per se useful to reduce CV risk, the use of ARBs may be convenient for many reasons: (a) ARBs have a protective effect against heart failure and stroke; (b) thanks to their ancillary properties, ARBs are important to reduce the risk of proteinuria and chronic heart failure; (c) ARBs show a favorable tolerability profile, with a low discontinuation rate compared to other antihypertensive drug classes. Among ARBs, olmesartan achieves a more homogeneous and sustained BP control over the 24 hours, with a consistent buffering effect on BPV. This property is particularly important to further reduce CV risk and to treat patients who present increased short‐term BP fluctuations.
CONFLICTS OF INTEREST
SO and MV received a grant for the preparation of the manuscript.
AUTHOR CONTRIBUTIONS
Authors meet the criteria for authorship for this manuscript and gave final approval to the version to be published. SO and MV conceived the idea behind the review. They both contributed to finalize the manuscript.
ETHICS
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Omboni S, Volpe M. Management of arterial hypertension with angiotensin receptor blockers: Current evidence and the role of olmesartan. Cardiovasc Ther. 2018;36:e12471 10.1111/1755-5922.12471
Funding information
Article was supported by Menarini International Operations Luxembourg SA (MIOL).
REFERENCES
- 1. GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration . Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2(8):634‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2013 Risk Factors Collaborators , Forouzanfar MH, Alexander L, Anderson HR, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1·25 million people. Lancet. 2014;383(9932):1899‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903‐1913. [DOI] [PubMed] [Google Scholar]
- 6. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335(8693):827‐838. [DOI] [PubMed] [Google Scholar]
- 7. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387(10022):957‐967. [DOI] [PubMed] [Google Scholar]
- 8. Katsanos AH, Filippatou A, Manios E, et al. Blood pressure reduction and secondary stroke prevention: a systematic review and metaregression analysis of randomized clinical trials. Hypertension. 2017;69(1):171‐179. [DOI] [PubMed] [Google Scholar]
- 9. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta‐analyses, and meta‐regression analyses of randomized trials. J Hypertens. 2014;32(12):2285‐2295. [DOI] [PubMed] [Google Scholar]
- 10. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: a critical reappraisal. Circ Res. 2015;116(6):1058‐1073. [DOI] [PubMed] [Google Scholar]
- 11. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk‐overview and meta‐analyses of randomized trials. J Hypertens. 2014;32(12):2305‐2314. [DOI] [PubMed] [Google Scholar]
- 12. Thomopoulos C, Parati G, Zanchetti A. Effects of blood‐pressure‐lowering treatment on outcome incidence in hypertension: 10 ‐ Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta‐analyses of randomized trials. J Hypertens. 2017;35(5):922‐944. [DOI] [PubMed] [Google Scholar]
- 13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels ‐ updated overview and meta‐analyses of randomized trials. J Hypertens. 2016;34(4):613‐622. [DOI] [PubMed] [Google Scholar]
- 14. The SPRINT Research Group . A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomopoulos C, Parati G, Zanchetti A. Effects of blood‐pressure‐lowering treatment on outcome incidence. 12. Effects in individuals with high‐normal and normal blood pressure: overview and meta‐analyses of randomized trials. J Hypertens. 2017;35(11):2150‐2160. [DOI] [PubMed] [Google Scholar]
- 16. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13‐e115. [DOI] [PubMed] [Google Scholar]
- 17. Williams B, Mancia G, Spiering W, et al.; Authors/Task Force Members . 2018 ESC/ESH Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953‐2041. [DOI] [PubMed] [Google Scholar]
- 18. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure‐lowering on outcome incidence in hypertension: 5. Head‐to‐head comparisons of various classes of antihypertensive drugs ‐ overview and meta‐analyses. J Hypertens. 2015;33(7):1321‐1341. [DOI] [PubMed] [Google Scholar]
- 19. Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin‐angiotensin system and cardiovascular risk. Lancet. 2007;369:1208‐1219. [DOI] [PubMed] [Google Scholar]
- 20. Kunz R, Friedrich C, Wolbers M, Mann JF. Meta‐analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30‐48. [DOI] [PubMed] [Google Scholar]
- 21. Ruggenenti P, Fassi A, Ilieva AP, et al. Effects of verapamil added‐on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT‐B randomized trial. J Hypertens. 2011;29:207‐216. [DOI] [PubMed] [Google Scholar]
- 22. Haller H, Ito S, Izzo JL Jr, et al. ROADMAP trial investigators. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907‐917. [DOI] [PubMed] [Google Scholar]
- 23. Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐ designed overviews of randomised trials. Lancet. 2003;362:1527‐1535. [DOI] [PubMed] [Google Scholar]
- 24. Ferrario CM. Cardiac remodelling and RAS inhibition. Ther Adv Cardiovasc Dis. 2016;10(3):162‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dahlöf B, Devereux RB, Kjeldsen SE, et al.; LIFE Study Group . Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995‐1003. [DOI] [PubMed] [Google Scholar]
- 26. Schrader J, Lüders S, Kulschewski A, et al.; MOSES Study Group . Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES). Stroke 2005;36(6):1218‐1226. [DOI] [PubMed] [Google Scholar]
- 27. Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163(13):1555‐1565. [DOI] [PubMed] [Google Scholar]
- 28. Berl T, Hunsicker LG, Lewis JB, et al.; Irbesartan Diabetic Nephropathy Trial . Collaborative Study Group. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med. 2003;138(7):542‐549. [DOI] [PubMed] [Google Scholar]
- 29. Parving HH, Lehnert H, Bröchner‐Mortensen J, Gomis R, Andersen S, Arner P. Irbesartan in patients with type 2 diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870‐878. [DOI] [PubMed] [Google Scholar]
- 30. The ONTARGET Investigators . Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547‐1559. [DOI] [PubMed] [Google Scholar]
- 31. Pitt B, Segal R, Martinez FA, et al. on behalf of the ELITE Study Investigators. Randomised trial of losartan versus captopril in patients over 65 with heart failure. Lancet. 1997;349(9054):747‐752. [DOI] [PubMed] [Google Scholar]
- 32. Pitt B, Poole‐Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial‐the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355(9215):1582‐1587. [DOI] [PubMed] [Google Scholar]
- 33. Cohn JN, Tognoni G for the Valsartan Heart Failure Trial Investigators . A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667‐1675. [DOI] [PubMed] [Google Scholar]
- 34. Young JB, Dunlap ME, Pfeffer MA, et al.; Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees . Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low‐left ventricular ejection fraction trials. Circulation. 2004;110(17):2618‐2626. [DOI] [PubMed] [Google Scholar]
- 35. Gayet JL; Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan . The OPTIMAAL trial: losartan or captopril after acute myocardial infarction. Lancet. 2002;360(9348):1884‐1885. [DOI] [PubMed] [Google Scholar]
- 36. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893‐1906. [DOI] [PubMed] [Google Scholar]
- 37. Tocci G, Paneni F, Palano F, et al. Angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers and diabetes: a meta‐analysis of placebo‐controlled clinical trials. Am J Hypertens. 2011;24(5):582‐590. [DOI] [PubMed] [Google Scholar]
- 38. Corrao G, Zambon A, Parodi A, et al. Discontinuation of and changes in drug therapy for hypertension among newly‐treated patients: a population‐based study in Italy. J Hypertens. 2008;26(4):819‐824. [DOI] [PubMed] [Google Scholar]
- 39. Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin‐converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol. 2018;71(13):1474‐1482. [DOI] [PubMed] [Google Scholar]
- 40. Wang L, Zhao JW, Liu B, Shi D, Zou Z, Shi XY. Antihypertensive effects of olmesartan compared with other angiotensin receptor blockers: a meta‐analysis. Am J Cardiovasc Drugs. 2012;12(5):335‐344. [DOI] [PubMed] [Google Scholar]
- 41. Tsoi B, Akioyamen LE, Bonner A. et al. Comparative efficacy of angiotensin II antagonists in essential hypertension: systematic review and network meta‐analysis of randomised controlled trials. Heart Lung Circ. 2018;27(6):666‐682. [DOI] [PubMed] [Google Scholar]
- 42. Redon J, Weber MA, Reimitz PE, Wang JG. Comparative effectiveness of an angiotensin receptor blocker, olmesartan medoxomil, in older hypertensive patients. J Clin Hypertens (Greenwich). 2018;20(2):356‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miyazaki M, Takai S. Anti‐atherosclerotic efficacy of olmesartan. J Hum Hypertens. 2002;16(Suppl 2):S7‐S12. [DOI] [PubMed] [Google Scholar]
- 44. Sukumaran V, Watanabe K, Veeraveedu PT, et al. Olmesartan, an AT1 antagonist, attenuates oxidative stress, endoplasmic reticulum stress and cardiacinflammatory mediators in rats with heart failure induced by experimental autoimmune myocarditis. Int J Biol Sci. 2011;7(2):154‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gounder VK, Arumugam S, Arozal W, et al. Olmesartan protects against oxidative stress possibly through the Nrf2 signaling pathway and inhibits inflammation in daunorubicin‐induced nephrotoxicity in rats. Int Immunopharmacol. 2014;18(2):282‐289. [DOI] [PubMed] [Google Scholar]
- 46. la Sierra A, Volpe M. Olmesartan‐based therapies: an effective way to improve blood pressure control and cardiovascular protection. J Hypertens. 2013;31(Suppl 1):S13‐7. [DOI] [PubMed] [Google Scholar]
- 47. Fabia MJ, Abdilla N, Oltra R, Fernandez C, Redon J. Antihypertensive activity of angiotensin II AT1 receptor antagonists: a systematic review of studies with 24 h ambulatory blood pressure monitoring. J Hypertens. 2007;25(7):1327‐1336. [DOI] [PubMed] [Google Scholar]
- 48. Zhang X, Zhang H, Ma Y, Che W, Hamblin MR. Management of Hypertension Using Olmesartan Alone or in Combination. Cardiol Ther. 2017;6(1):13‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith D, Dubiel R, Jones M. Use of 24‐hours ambulatory blood pressure monitoring to assess antihypertensive efficacy. Am J Cardiovasc Drugs. 2005;5(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 50. Parati G, Ochoa JE, Salvi P, Lombardi C, Bilo G. Prognostic value of blood pressure variability and average blood pressure levels in patients with hypertension and diabetes. Diabetes Care. 2013;36(Suppl. 2):S312‐S324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Omboni S, Kario K, Bakris G, Parati G. Effect of antihypertensive treatment on 24‐h blood pressure variability: pooled individual data analysis of ambulatory blood pressure monitoring studies based on olmesartan mono or combination treatment. J Hypertens. 2018;36(4):720‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Volpe M, Santolamazza C, Mastromarino V, Coluccia R, Battistoni A, Tocci G. Monotherapy and dual combination therapies based on olmesartan: a comprehensive strategy to improve blood pressure control. High Blood Press Cardiovasc Prev. 2017;24(3):243‐253. [DOI] [PubMed] [Google Scholar]
- 54. Volpe M, Santolamazza C, Mastromarino V, Coluccia R, Battistoni A, Tocci G. Triple combination therapies based on olmesartan: a personalized therapeutic approach to improve blood pressure control. High Blood Press Cardiovasc Prev. 2017;24(3):255‐263. [DOI] [PubMed] [Google Scholar]
- 55. Volpe M, Tocci G, de la Sierra A, et al. Personalised single‐pill combination therapy in hypertensive patients: an update of a practical treatment platform. High Blood Press Cardiovasc Prev. 2017;24(4):463‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thomopoulos C, Parati G, Zanchetti A. Effects of blood‐pressure‐lowering treatment in hypertension: 9. Discontinuations for adverse events attributed to different classes of antihypertensive drugs: meta‐analyses of randomized trials. J Hypertens. 2016;34(10):1921‐1932. [DOI] [PubMed] [Google Scholar]
- 57. Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin‐receptor blockade and risk of cancer: meta‐analysis of randomised controlled trials. Lancet Oncol. 2010;11(7):627‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deszi CA. A review of clinical studies on angiotensin II receptor blockers and risk of cancer. Int J Cardiol. 2014;177(3):748‐753. [DOI] [PubMed] [Google Scholar]
- 59. Yang Y, Zhang F, Skrip L, et al. Lack of an association between angiotensin receptor blocker based therapy and increased risk of cancer: evidence from large observational studies. PLoS ONE. 2015;10(3):e0119775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang J, Liu J, Chen J, et al. Angiotensin receptor blockers (ARBs) reduce the risk of lung cancer: a systematic review and meta‐analysis. Int J Clin Exp Med. 2015;8(8):12656‐12660. [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang W, Liang Z, Li J, Cai S. Angiotensin receptor blockers use and the risk of lung cancer: A meta‐analysis. J Renin Angiotensin Aldosterone Syst. 2015;16(4):768‐773. [DOI] [PubMed] [Google Scholar]
- 62. Abraham HM, White CM, White WB. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015;38(1):33‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Burbure N, Lebwohl B, Arguelles‐Grande C, Green PH, Bhagat G, Lagana S. Olmesartan‐associated sprue‐like enteropathy: a systematic review with emphasis on histopathology. Hum Pathol. 2016;50:127‐134. [DOI] [PubMed] [Google Scholar]
- 64. Zanelli M, Negro A, Santi R, et al. Letter: sprue‐like enteropathy associated with angiotensin II receptor blockers other than olmesartan. Aliment Pharmacol Ther. 2017;46(4):471‐473. [DOI] [PubMed] [Google Scholar]
- 65. Choi EY, McKenna BJ. Olmesartan‐associated enteropathy: a review of clinical and histologic findings. Arch Pathol Lab Med. 2015;139(10):1242‐1247. [DOI] [PubMed] [Google Scholar]
- 66. Malfertheiner P, Ripellino C, Cataldo N. Severe intestinal malabsorption associated with ACE inhibitor or angiotensin receptor blocker treatment. An observational cohort study in Germany and Italy. Pharmacoepidemiol Drug Saf. 2018;27(6):581‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]


