Abstract
The liver has an extraordinary capacity to regenerate through activation of key molecular pathways. However, central regulators controlling liver regeneration remain insufficiently studied. Here, we show that B cell–deficient animals failed to induce sufficient liver regeneration after partial hepatectomy (PHx). Consistently, adoptive transfer of B cells could rescue defective liver regeneration. B cell–mediated lymphotoxin beta production promoted recovery from PHx. Absence of B cells coincided with loss of splenic cluster of differentiation 169–positive (CD169+) macrophages. Moreover, depletion of CD169+ cells resulted in defective liver regeneration and decreased survival, which was associated with reduced hepatocyte proliferation. Mechanistically, CD169+ cells contributed to liver regeneration by inducing hepatic interleukin‐6 (IL‐6) production and signal transducer and activator of transcription 3 activation. Accordingly, treatment of CD169+ cell–depleted animals with IL‐6/IL‐6 receptor rescued liver regeneration and severe pathology following PHx. Conclusion: We identified CD169+ cells to be a central trigger for liver regeneration, by inducing key signaling pathways important for liver regeneration.
Liver disease is a global health problem with millions of patients worldwide suffering from infections, toxic liver damage, and hepatocellular carcinoma. Liver tissue has an extraordinary potential to regenerate, an effect already described in Greek mythology. Since then, several key molecular pathways have been discovered to play important roles during liver regeneration, including nuclear factor kappa B, signal transducer and activator of transcription 3 (STAT3), and extracellular signal–regulated kinase (Erk).1 Following 70% reduction of liver mass through partial hepatectomy (PHx), tumor necrosis factor (TNF) is rapidly produced, and TNF receptor 1 (TNFR1) signaling is required to induce liver regeneration.2 Furthermore, the TNF superfamily members lymphotoxin (Lt) alpha and beta play a critical role during liver regeneration.3, 4 Consistently, mice deficient for both TNFRp55 and Ltβ receptor (LtβR) show delayed hepatocyte proliferation and impaired survival following PHx.5 Furthermore, a marked increase in interleukin‐6 (IL‐6) concentrations in the serum can be detected following loss of liver mass, and IL‐6‐deficient mice show delayed liver regeneration following PHx.6, 7, 8 Consistently, treatment with combined IL‐6 and soluble IL‐6 receptor (IL‐6R) can improve liver regeneration and induce rapid hepatocyte proliferation.6, 9 Moreover, epidermal growth factor receptor (EGFR) ligands including transforming growth factor alpha (TGF‐α) and amphiregulin are able to induce hepatocyte proliferation in vitro.1, 10 However, TGF‐α‐deficient animals exhibit normal recovery following PHx.11 In turn, amphiregulin‐deficient animals show delayed proliferation following loss of liver mass.12 Consistently, specific deletion of EGFR in hepatocytes resulted in decreased liver regeneration following PHx.13 Notably, TNF levels were strikingly increased in EGFRΔHEP animals following PHx, suggesting that factors important for liver regeneration can compensate for each other.13 The central, key players during liver regeneration, however, remain insufficiently studied.
The spleen is tightly connected to the liver with important blood circuits. Receiving its blood from the splenic artery, it feeds through the splenic vein after joining of the arteria mesenteria inferior and superior into the portal vein. Hence, cytokines and chemokines produced in the spleen can act directly on the liver. The spleen itself is organized into red and white pulp, with the separating marginal zone including the marginal sinus in between.14, 15 B cells account for about 50% of all cells in the spleen and are located in the white pulp and the marginal zone.14 B cells are critical for organization of the lymphoid tissue as B cell–deficient mice exhibit reduced presence of metallophilic cluster of differentiation 169–positive (CD169+) macrophages.16 CD169+ cells are located along the marginal sinus and ideally situated to capture pathogens.14, 17 Interestingly, maintenance of CD169+ cells depends on Ltα and Ltβ.18, 19, 20 Specifically, B cell–specific and T cell–specific, Ltβ‐deficient mice exhibit reduction of CD169+ cells in the spleen.18, 19 Moreover, as spleen‐resident macrophages, CD169+ cells can contribute to cytokine production during inflammation and infection.21, 22, 23 However, the contributions of B cells and CD169+ cells during liver regeneration remain insufficiently characterized.
In this study, we identified B cells and CD169+ cells as important players in liver regeneration following PHx. Specifically, genetic B‐cell deficiency resulted in reduced signaling cascades required for hepatocyte proliferation and limited survival following PHx. B cell–deficient mice exhibited reduced presence of CD169+ cells, which were critical for liver regeneration. Depletion of CD169+ cells resulted in reduced IL‐6 expression and reduced regeneration following PHx. Consistently, treatment of CD169+ cell–depleted animals with IL‐6/IL‐6R could rescue the severe pathology we observed in our setting.
Materials and Methods
Animals
This study was carried out in accordance with the German Animal Welfare Act and the guidelines of Shoochow University. The protocol was approved by the local authorities. B cell–activating factor receptor–negative (Baffr–/–), joining heavy chain–negative (Jh–/–), CD169‐DTR (diphtheria toxin [DT] receptor) mice have been described and were kept on a C57Bl/6 background.18, 24, 25 Laparotomy was performed predominantly on male mice at 10‐14 weeks of age using isoflurane inhalation narcosis, as described.26 For PHx the left lateral and the left and right median liver lobes together with the gallbladder were excised subsequent to a one‐step ligature using a 5‐0 suture tie (Ethicon, Somerville, NJ).5 Sham operations were performed in an identical manner without ligating and removing liver lobes. For splenectomy, the splenic artery and vein were ligated with a single‐knot 5‐0 suture at the same time as PHx or otherwise indicated in the figure legends. Next, connective tissue and spleen were removed. After irrigating the abdomen with 0.9% NaCl, both abdominal layers were closed with a running 5‐0 suture (Ethicon).26 Directly after surgery and 24 and 48 hours post‐PHx mice received 5 mg/kg carprofen (Rimadyl; Pfizer, Würselen, Germany). As expected, splenectomized animals did not show any sign of pathology (Fig. 1A). Mice exhibiting severe disease symptoms were sacrificed and considered as dead. CD169+ cells in the CD169‐DTR animals were depleted by injecting two doses of 100 ng DT (Sigma) before the PHx. Wild‐type (WT; C57Bl/6) mice were used as controls. Mice were 10‐14 weeks old. For blood and tissue collection mice were anesthetized (100 mg/kg ketamine, 10 mg/kg xylazine; Vétoquinol GmbH, Ravensburg, Germany), weighed, and bled through the vena cava inferior; and serum was collected. The liver and spleen were removed, rinsed in phosphate‐buffered saline (PBS), and weighed to calculate the liver weight to body weight ratio and the spleen weight. Liver and spleen samples were stored at –80 °C for histology and RNA and protein extraction.
Figure 1.
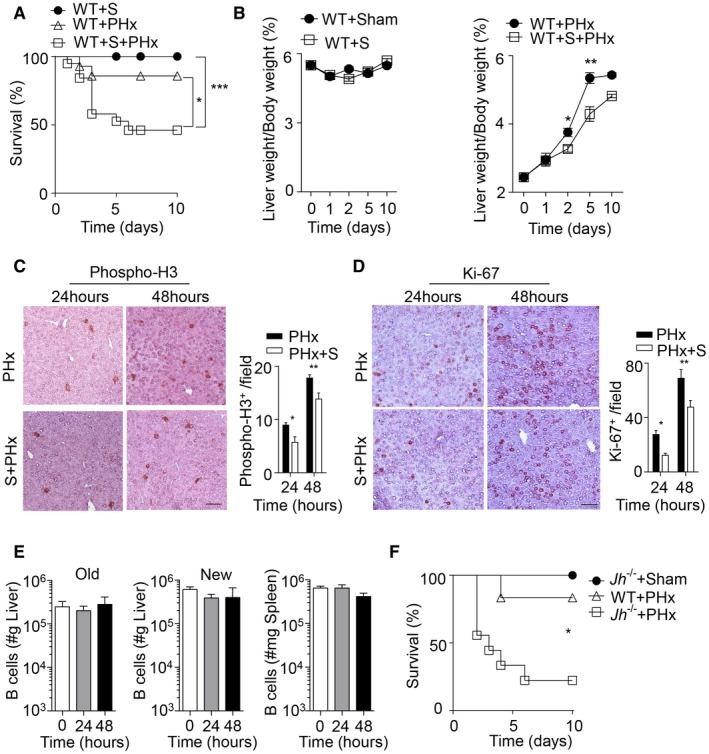
Decreased liver regeneration in splenectomized and B cell–deficient mice following PHx. (A) Survival of splenectomized, 70% PHx, and splenectomized mice followed by PHx (PHx+S) was monitored (n = 14‐19). (B) The liver weight/body weight ratio was determined at the indicated time points in WT sham‐operated mice and splenectomized mice (left panel) and in PHx WT mice and splenectomized mice (PHx+S) (right panel) (n = 3‐5). (C,D) Sections of snap‐frozen liver tissue from 70% PHx and splenectomized mice followed by PHx (PHx+S) at the indicated time points were stained with (C) anti‐phospho‐H3 and (D) anti‐Ki‐67 antibodies. Representative sections for each time point are shown (n = 4; scale bar, 100 μm). Right panels indicate quantification. (E) B‐cell numbers were determined by flow cytometry in the newly regenerated (“New,” n = 7‐8) and remaining (“Old,” n = 3‐4) liver lobes and spleen tissue (n = 7‐8) at indicated time points after 70% PHx. Results were calculated according to the liver (grams) and spleen (milligrams) weights. (F) Survival of Jh–/– mice (n = 9) after 70% PHx compared to sham‐operated Jh–/– mice (n = 3) and WT mice (n = 6). Error bars in all experiments represent SEM; *P < 0.05, **P < 0.01, ***P < 0.001. Abbreviation: S, splenectomy.
LtβR Antibody Treatment and IL‐6/IL‐6R Injection
In order to induce LtβR signaling, mice were intraperitoneally injected with two doses of 200 μg LtβR antibody agonist (clone 4H8) 24 hours before and 24 hours after PHx.27 DT‐treated CD169‐DTR mice were injected with two doses of 20 μg IL‐6/IL‐6R protein 24 hours before and immediately after PHx.
Purification of B Cells
For B‐cell purification, single‐cell suspensions of splenocytes were enriched following the manufacturer's instructions with a CD45R (B220) MicroBeads mouse kit (Miltenyi).
Histology
Histological analysis on snap‐frozen tissue (liver, spleen) was performed with hematoxylin and eosin (H&E) stain (Sigma‐Aldrich, St. Louis, MO) and terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick‐end labeling (TUNEL; Roche). Snap‐frozen tissue sections were stained with antibodies: CD169 (clone MOMA‐1; ABD Serotec, Dusseldorf, Germany), B220 (eBioscience, San Diego, CA), Ki‐67 (Abcam), phospho‐histone H3 (phospho‐H3; Millipore).
Flow‐Cytometric Analysis
Different immune populations were identified in single‐cell solutions from naive liver and spleen samples and spleen and liver samples newly regenerated (“new”) and remaining lobes (“old”) 24 and 48 hours after PHx using anti‐B220, anti‐CD21, anti‐CD23, anti–progammed death ligand 1, anti‐NK1.1, anti‐CD3, anti‐CD19, anti‐CD11b, anti‐Siglec‐H, anti‐CD8a, and anti‐CD11c antibodies; anti–major histocompatibility complex class II; and anti‐CD40, anti‐CD80, anti‐CD86, anti‐F4/80, anti‐Ly6C, anti‐CD138, anti–immunoglobulin M, anti‐CD38, anti‐CD62L, anti‐CD5, anti‐IgD, anti‐CD1d antibodies, and 7‐amino‐actinomycin D. All antibodies were obtained from eBioscience, except anti‐CD169 (3D6.112), which was obtained from AbD Serotec. For quantification of cell populations, calibration beads were added to assess cell numbers (BD, San Diego, CA).
Serum Biochemistry
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured using the automated biochemical analyzer Spotchem EZ SP‐4430 (Arkray, Amstelveen, The Netherlands) and the Spotchem EZ Reagent Strips Liver‐1.
Quantitative RT‐PCR
RNA purification and RT‐PCR analyses of liver and spleen were performed according to the manufacturer’s instructions (TRIzol reagent and iTaq Universal probes or SYBR Green One‐Step Kit; BioRad). Expression of Ltα, Ltβ, IL‐6, and TNFα was determined with fluorescein amidite probes (Applied Biosystems). Expression levels of other genes were tested using the following primer sequences: Egf_F, AGAAGGCTACGAAGGAGACG; Egf_R, AGAGTCAGGGCAACTCAGTC; Hbegf_F, GCAAATGCCTCCCTGGTTAC; Hbegf_R, GGACGACAGTACTACAGCCA; Areg_F, GCGAGGATGACAAGGACCTA; Areg_R, TCGTTTCCAAAGGTGCACTG; Tgfa_F, GCTCTGGAGAACAGCACATC; Tgfa_R, ACATGCTGGCTTCTCTTCCT; Tgfb1_F, TTGCTTCAGCTCCACAGAGA; Tgfb1_R, CAGAAGTTGGCATGGTAGCC; Hgf_F, CCAGAGGTACGCTACGAAGT; Hgf_R, CTGTGTGATCCATGGGACCT; Fgf1_F, CTCGCAGACACCAAATGAGG; Fgf1_R, CTTCTTGAGGCCCACAAACC; Vegfa_F, TTGAGACCCTGGTGGACATC; Vegfa_R, GGGCTTCATCGTTACAGCAG; Vegfb_F, GCCACCAGAAGAAAGTGGTG; Vegfb_R, ATTGCCCATGAGTTCCATGC; Vegfc_F, AGGCAGCTAACAAGACATGTCCAAC; Vegfc_R, GGGTCCACAGACATCATGGAATC; Fgf2_F, GGACGGCTGCTGGCTTCTAA; Fgf2_R, CCAGTTCGTTTCAGTGCCACATAC; Pdgfb_F, ATGAAATGCTGAGCGACCAC; Pdgfb_R, TCCCTCGAGATGAGCTTTCC. For analysis, the expression levels of all target genes were normalized to the β‐actin expression or glyceraldehyde 3‐phosphate dehydrogenase (Delta cycle threshold [ΔCt]). Gene expression values were calculated with the ΔΔCt method, with untreated WT mice as controls to which all other samples were normalized. Relative quantities (RQ) were determined with the equation RQ = 2–ΔΔCt.
Immunoblotting
Liver tissue was lysed in PBS containing 1% Triton X‐100, protease inhibitors (Sigma), and PhosSTOP (1 tablet/10 mL). Immunoblots were probed with primary antibody: phospho‐Stat3, Stat3, phospho‐Erk1/2, Erk1/2, inhibitor of kappa B alpha (IκBα), IL‐6, proliferating cell nuclear antigen (PCNA), and β‐actin (Cell Signalling Technology), followed by secondary antibody and enhanced chemiluminescence detection or fluorescence secondary antibody and detected by a LI‐COR imager.
Statistical Analyses
Data are expressed as mean ± SEM. Statistically significant differences between two different groups were analyzed using the Student t test. Statistically significant differences between groups in experiments involving more than one time point were determined with two‐way analysis of variance (repeated measurements). All quantifications were analyzed by ImageJ.
Results
B Cells are Important for Liver Regeneration
Based on previous data describing a beneficial role of the spleen during liver regeneration following PHx in mice,28 we found that a proportion of splenectomized C57Bl/6 mice developed severe pathology (Fig. 1A; Supporting Fig. S1A). Consistently, the liver weight/body weight ratio following PHx was reduced in the absence of spleen tissue when compared to PHx controls (Fig. 1B).28 Moreover, the activity of ALT and AST, indicators of liver damage, was markedly increased in the blood of splenectomized mice after PHx compared to PHx only or splenectomy controls (Supporting Fig. S1B). Although splenectomized mice exhibited histological changes in liver tissue, we did not observe a significant increase in TUNEL staining (Supporting Fig. S1C,D). However, staining for proliferation indicators such as Ki‐67 and phospho‐H3 was reduced in liver tissue of splenectomized animals (Fig. 1C,D; Supporting Fig. S1E). Next, we investigated, which splenic factors may be important for liver regeneration. B cells account for about 50% of all cells in the spleen and are located in the white pulp and the marginal zone.14 However, we did not observe any difference in the presence of B‐cell subsets following PHx in the spleen and liver (Fig. 1E; Supporting Fig. S2A‐C), while T‐cell numbers increased in the regenerating liver tissue following PHx, which is consistent with the literature (Supporting Fig. S2D).4 Nevertheless, when we subjected B cell–deficient Jh –/– mice to PHx, we found reduced survival following PHx when compared to WT controls (Fig. 1F). This effect was not due to surgical complications or infection as sham‐operated Jh –/– mice showed no signs of disease and survived after surgery (Fig. 1F). In contrast to splenectomized mice, B cell–deficient animals did not exhibit increased activity of liver transaminases when compared to WT controls (Fig. 2A). Consistently, we could not detect histological differences or increased TUNEL staining following PHx in Jh–/– mice compared to WT mice (Fig. 2B; Supporting Fig. S3A). Deficiency of B‐cell survival promoting the B cell–activating factor of the TNF family (BAFF) results in B‐cell lymphopenia in mice and humans.25, 29 Accordingly, Baffr –/– mice displayed severe disease symptoms following PHx compared to WT and sham‐operated mice (Fig. 2C). In parallel to Jh–/– mice, Baffr–/– animals exhibited only slight increases in liver transaminases and, compared to control liver tissue, comparable histological appearance in H&E tissue sections (Fig. 2D; Supporting Fig. S3B). However, expression of phospho‐H3 was reduced in B cell–deficient and BAFFR‐deficient mice compared to WT controls following PHx (Fig. 2E; Supporting Fig. S3C,D). Furthermore, expression of Ki‐67 was delayed in the absence of B cells or BAFFR (Fig. 2F; Supporting Fig. S3E,F). These data indicate that B cells provide important factors for hepatocyte proliferation and liver regeneration.
Figure 2.
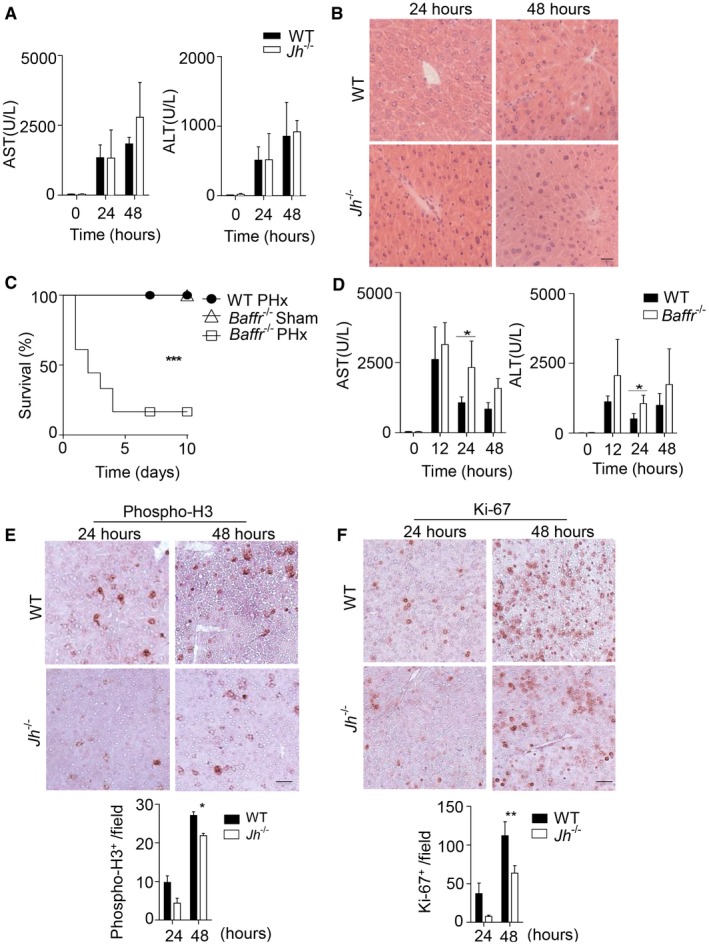
B cells play a crucial role in liver regeneration after PHx. (A) The activity of AST and ALT was measured in serum of WT and Jh–/– mice following PHx at the indicated time points (n = 4‐5). (B) Sections of snap‐frozen liver tissue from WT and Jh–/– mice following PHx were stained with H&E. One representative set of n = 3 is shown (scale bar, 200 μm). (C) Survival of Baffr–/– mice (n = 18) after 70% PHx compared to sham‐operated Baffr–/– mice (n = 4) and WT mice after 70% PHx (n = 7) was monitored. (D) The activity of AST and ALT was measured in serum of WT and Baffr–/– mice following PHx at the indicated time points (n = 4‐5). (E,F) Sections of snap‐frozen liver tissue from WT and Jh–/– mice following PHx were stained with (E) anti‐phospho‐H3 and (F) anti‐Ki‐67 antibodies. Representative sections for each time point are shown (n = 3; scale bar, 100 μm). Lower panels indicate quantification. Error bars in all experiments represent SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we wondered which B cell–derived factors are important for liver regeneration. B cells express Ltα and Ltβ, which are critical for lymphoid tissue organization. We found decreased expression levels of Ltβ in Jh –/– mice compared to WT controls in both the spleen and liver after PHx and in naive mice (Fig. 3A,B), while the difference in Ltα expression was not significant after PHx in the liver in our setting (Supporting Fig. S4). This led us to speculate that Ltβ plays a major role in regulating B cell–mediated liver regeneration in our setting. To further validate that Ltβ is produced by B cells, we adoptively transferred B cells into Jh–/– mice (Fig. 3C), which rescued Ltβ expression levels in the liver (Fig. 3D,E). Consistently, when we adoptively transferred B cells into Jh–/– mice, we could rescue severe disease development following PHx (Fig. 3F). Furthermore, B‐cell transfer into PHx Baffr –/– mice prevented severe disease (Fig. 3G). Because lymphotoxins are known to be critical for liver regeneration,30 we hypothesized that B cell–derived Ltβ expression may contribute to liver regeneration. Consistently, when we applied an agonistic anti‐LtβR antibody prior to PHx, we observed protection of Jh –/– mice (Fig. 3H). Taken together, these data indicate that B cells are critical contributors to liver regeneration.
Figure 3.
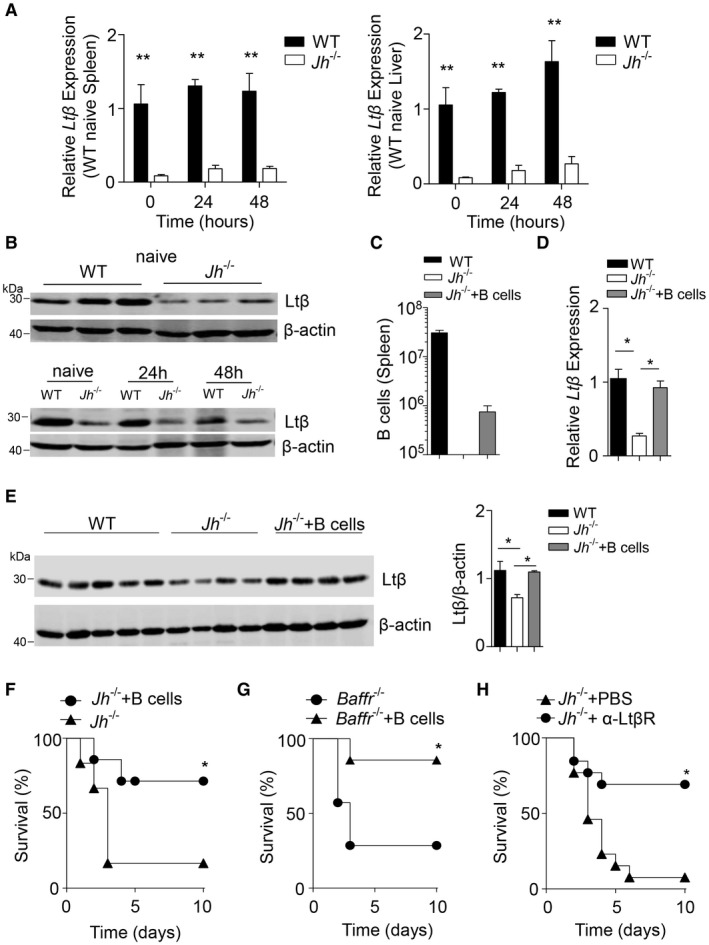
B cell–derived Ltβ contributes to liver regeneration after PHx. (A) RNA expression of Ltβ was measured in spleen and liver tissue from WT and Jh–/– mice at the indicated time points post–70% PHx (n = 3‐4). (B) Protein level of Ltβ was measured in liver tissue from naive WT and Jh–/– and from WT and Jh–/– mice at the indicated time points post–70% PHx. (C‐E) Purified B cells (2 × 106) from WT mice were intravenously injected into Jh–/– mice. After 48 hours, (C) B‐cell numbers were determined in the spleen by flow cytometry (n = 4‐6). (D) RNA levels of Ltβ were measured in liver tissue (n = 3‐5). (E) The protein level of Ltβ was measured in liver tissue. (F) Survival of Jh–/– mice without (n = 6) or after (n = 7) B‐cell transfer was determined following 70% PHx. (G) Survival of Baffr–/– mice without (n = 7) or after (n = 7) B‐cell transfer was determined following 70% PHx. (H) Survival of untreated (n = 13) and agonist LtβR antibody–treated (n = 13) Jh–/– mice was monitored after 70% PHx. Error bars in all experiments represent SEM; *P < 0.05, **P < 0.01.
CD169+ Cells are Critical for Liver Regeneration Following PHx
Ltα and Ltβ are important cytokines for the maintenance of CD169+ macrophages in the spleen.18, 19, 20 B cells are critical for organization of the lymphoid tissue as B cell–deficient mice exhibit reduced presence of metallophilic CD169+ macrophages (Fig. 4A,B).15, 23 Following adoptive transfer of B cells into Jh–/– mice, CD169+ cells were restored (Fig. 4C). Therefore, we hypothesized that B cell–mediated maintenance of CD169+ cells contributes to liver regeneration. Interestingly, we observed higher numbers of CD169+ cells in the regenerating but also the remaining liver lobe using flow cytometry (Fig. 4D). Notably, following splenectomy the increase of CD169+ cells was abolished (Supporting Fig. S5A). Furthermore, we observed a slight increase of CD169+ cells in the spleen compared to unoperated mice (Fig. 4E). CD169+ cells can be depleted by injection of DT into CD169‐DTR mice.24 To exclude effects of DT on other cell types, we administered DT to WT mice before and after PHx and observed no effects on liver regeneration and survival (Supporting Fig. S5B,C). Absence of CD169+ cells resulted in slightly increased AST activity in the serum after PHx when compared to WT controls (Fig. 4F). Furthermore, DT‐treated CD169‐DTR mice succumbed after PHx compared to control mice (Fig. 4G). Taken together, we identified CD169+ cells to be important for liver regeneration following PHx.
Figure 4.
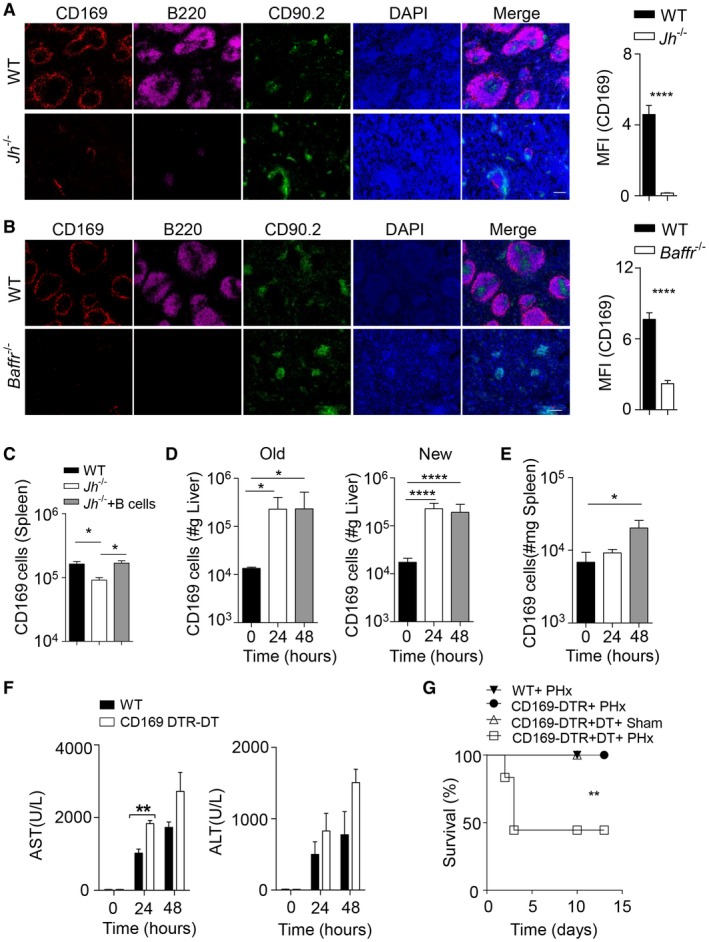
CD169+ cells contribute to liver regeneration. (A) Sections of snap‐frozen spleen tissue from WT and Jh–/– mice were stained with anti‐CD169, anti‐B220, and anti‐CD90.2 antibodies. Representative sections are shown (n = 4‐5; scale bar, 100 μm). Right panel indicates average and SEM of mean fluorescence intensities of CD169 staining. (B) Sections from snap‐frozen spleen tissue from WT and Baffr–/– mice were stained with anti‐CD169 anti‐B220 and anti‐CD90.2 antibodies. Representative sections are shown (n = 4‐5; scale bar, 100 μm). Right panel indicates average and SEM of mean fluorescence intensities of CD169 staining. (C) Purified B cells (2 × 106) from WT mice were adoptively transferred into Jh–/– mice. After 48 hours, CD169‐cell numbers were determined in the spleen by flow cytometry (n = 4‐6). (D,E) CD169+ cells were measured by flow cytometry in the newly regenerated (n = 7‐8) and remaining (“Old”) (n = 3‐4) liver lobes (D) and spleen tissue (n = 7‐8) (E) at the indicated time points after 70% PHx. Results were calculated according to liver (grams) and spleen (milligrams) weights. (F) The activity of AST and ALT was measured in serum of WT and DT‐treated CD169‐DTR mice following PHx at the indicated time points (n = 3). (G) Survival of DT‐treated CD169‐DTR mice after PHx (n = 12) compared to WT mice (n = 8) after PHx, CD169‐DTR mice (n = 5) after PHx, and sham‐operated, DT‐treated CD169‐DTR mice (n = 4) was monitored. Error bars in all experiments represent SEM; *P < 0.05, **P < 0.01, ****P < 0.0001. Abbreviations: DAPI, 4′,6‐diamidino‐2‐phenylindole; MFI, mean fluorescence intensity.
Consistent with the findings in B cell–deficient mice, we observed no histological differences in the absence or presence of CD169+ cells in H&E tissue sections (Supporting Fig. S5D). Moreover, we could not detect increased TUNEL staining following PHx in DT‐treated CD169‐DTR mice compared to untreated controls (Supporting Fig. S5E). However, we observed delayed liver regeneration following PHx in DT‐treated CD169‐DTR mice in comparison to control animals as evident by a decreased liver/body weight ratio (Fig. 5A). Furthermore, we found reduced presence of phospho‐H3 and reduced expression of Ki‐67 cells in the liver tissue in DT‐treated CD169‐DTR mice in comparison to control animals (Fig. 5B,C; Supporting Fig. S5F). Consistently, expression of PCNA, which is involved in DNA repair and synthesis, was reduced in the liver as a result of CD169+ cell depletion compared to WT mice following PHx (Fig. 5D). We next analyzed the liver morphology in DT‐treated CD169‐DTR mice at later time points such as 13 days after PHx. We observed a slight but significant reduction in liver weight in DT‐treated mice compared to untreated controls (Fig. 5E). Together these data suggest that CD169+ cells promote proliferation following PHx and therefore contribute to liver regeneration.
Figure 5.
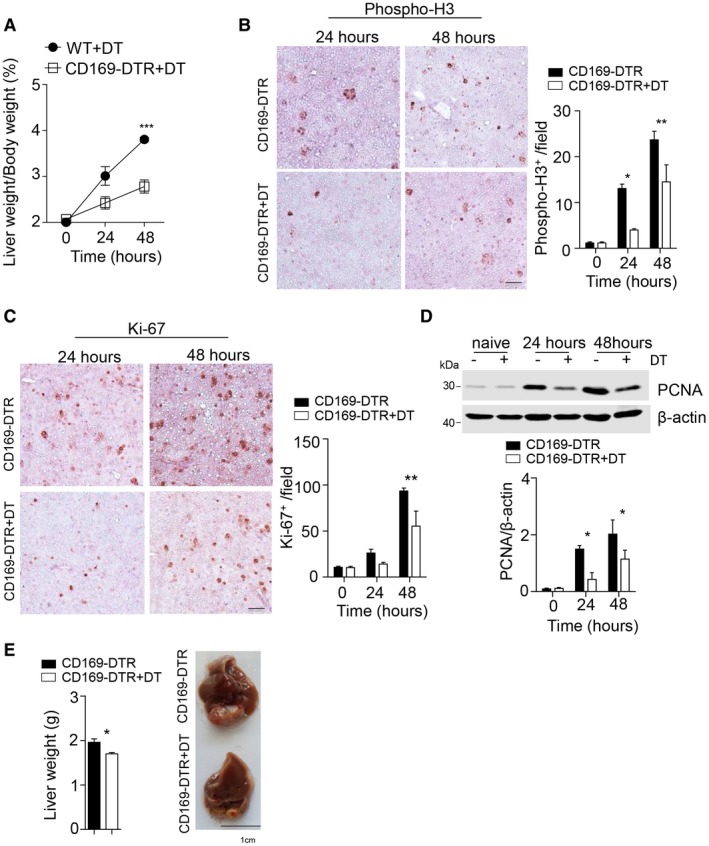
CD169+ cells promote the presence of hepatic proliferation markers after PHx. (A) The liver weight/body weight ratio was determined after 70% PHx in DT‐treated WT mice and DT‐treated CD169‐DTR mice followed by PHx (n = 3). (B,C) Sections of snap‐frozen liver tissue from CD169‐DTR and DT‐treated CD169‐DTR mice at the indicated time points after 70% PHx were stained with anti‐phospho‐H3 (B) and anti‐Ki‐67 (C) antibodies. Representative sections for each time point are shown (n = 3; scale bar, 100 μm). Right panels indicate quantification. (D) Protein level of PCNA was measured at the indicated time points after 70% PHx in DT‐treated CD169‐DTR and CD169‐DTR mice (n = 4). Lower panel indicates quantification. (E) Liver weight (left panel) and liver morphology (right panel) were determined at day 13 after 70% PHx of DT‐treated CD169‐DTR and CD169‐DTR mice (n = 3‐5). Error bars in all experiments represent SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
CD169+ Cells Promote IL‐6 Production During Liver Regeneration
To define how CD169+ cells contribute to liver regeneration, we analyzed RNA expression levels of genes encoding for cytokines important for liver regeneration (Fig. 6A). We found decreased IL‐6 expression in B cell–deficient mice in the liver when compared to control animals in our setting (Fig. 6A,B). Transfer of B cells into Jh–/– mice could restore IL‐6 expression levels in the liver (Fig. 6C). Because IL‐6‐deficient mice show delayed liver regeneration,6, 7, 8 we speculated that CD169+ cells might trigger liver regeneration by inducing expression of IL‐6. In line with that, DT‐treated CD169‐DTR mice showed reduced RNA expression levels of Il‐6 in liver tissue compared to control animals 6 and 48 hours after PHx (Fig. 6D,E). Moreover, IL‐6 expression was reduced on a protein level 48 hours following PHx in the absence of CD169+ cells (Fig. 6F). Notably, we also observed an early increase in Il‐6 expression levels in the absence of CD169+ cells and, accordingly, STAT3 phosphorylation 12 hours after PHx (Fig. 6D,G). However, consistent with the reduced expression of IL‐6, we observed lower expression of phospho‐STAT3 in liver tissue harvested from CD169+ cell–deficient mice compared to CD169+ cell–competent mice 24 and 48 hours after PHx (Fig. 6G; Supporting Fig. S6). Notably, we observed similar induction of phospho‐Erk and a similar decrease of IκBα, indicating that other signaling pathways participating in liver regeneration remain intact in the absence of CD169+ cells in our setting (Fig. 6G; Supporting Fig. S6).
Figure 6.
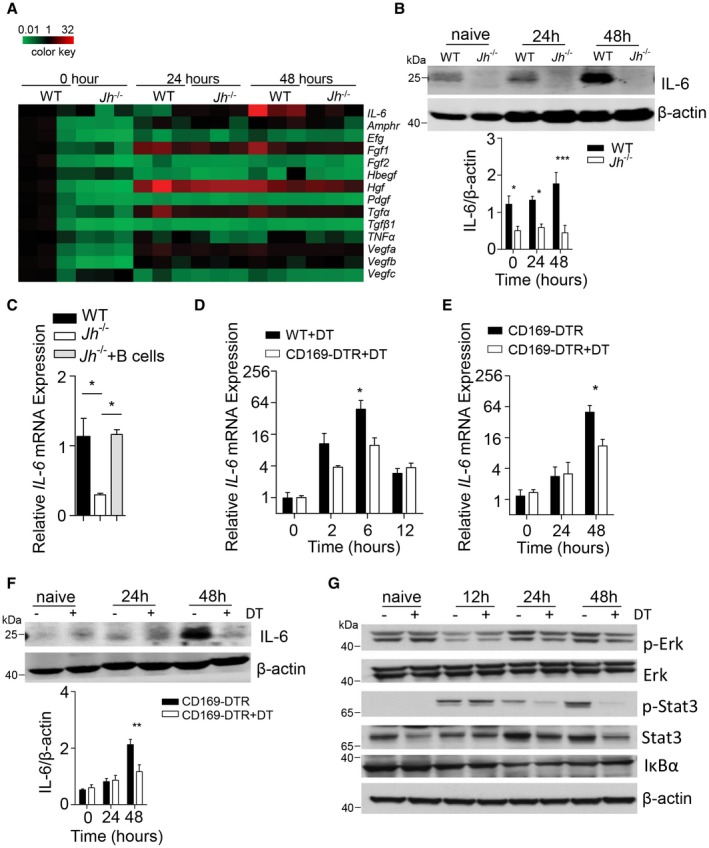
Defective activation of IL‐6 signaling in the absence of CD169+ cells. (A) RNA expression level of cytokines important for liver regeneration was determined in the liver tissue from WT and Jh –/– mice at the indicated time points post–70% PHx (n = 3). (B) Protein expression of IL‐6 was determined in liver tissue from WT and Jh –/– mice at the indicated time points after 70% PHx (n = 3). Lower panel indicates quantification. (C) RNA expression levels of IL‐6 was measured in the liver tissue of WT and Jh –/– mice without or with purified B cells (n = 3‐5). (D,E) RNA expression levels of Il‐6 were determined in liver tissue from DT‐treated WT, CD169‐DTR, and DT‐treated CD169‐DTR mice as labeled at the indicated time points after 70% PHx (n = 3‐5). (F) Protein expression of IL‐6 was determined in liver tissue from CD169‐DTR and DT‐treated CD169‐DTR mice at the indicated time points after 70% PHx (n = 3). Lower panel indicates quantification. (G) Protein lysates of liver tissue from CD169‐DTR mice and DT‐treated CD169‐DTR mice at the indicated time points after PHx were blotted and stained with anti‐phospho‐Erk, anti‐Erk, anti‐phospho‐STAT3, anti‐STAT3, anti‐IκBα, and anti‐β‐actin antibodies (one representative of n = 3 blots is shown). Error bars in all experiments represent SEM; *P < 0.05, **P < 0.01, ***P < 0.001. Abbreviations: Amphr, amphiregulin; Hbegf, heparin‐binding EGF‐like growth factor; Hgf, hepatocyte growth factor; Fgf, fibroblast growth factor; Pdgf, platelet‐derived growth factor; Vegf, vascular endothelial growth factor.
To explore whether IL‐6R signaling can compensate for CD169+ cells, we administered IL‐6/IL‐6R protein before and after PHx in DT‐treated CD169‐DTR mice.6 As expected, we found increased STAT3 phosphorylation following injection with IL‐6/IL‐6R in both CD169+ cell–competent and CD169+ cell–depleted animals (Fig. 7A). Notably, the liver weight/body weight ratio in CD169‐DTR DT‐treated animals after PHx following treatment with IL‐6/IL‐6R was comparable to that in untreated CD169‐DTR mice following PHx (Fig. 7B), indicating that injection of IL‐6/IL‐6R could restore liver regeneration in CD169+ cell–depleted mice. Consistently, we found increased expression of phospho‐H3 and Ki‐67 following treatment with IL‐6/IL‐6R of CD169‐DTR mice when compared to CD169‐DTR controls (Fig. 7C). Moreover, PCNA expression was increased after IL‐6/IL‐6R treatment in the absence of CD169+ cells after PHx (Fig. 7D). Furthermore, we found that IL‐6/IL‐6R protein significantly rescued the pathology following PHx in CD169‐DTR animals (Fig. 7E). In conclusion, we identified that B cell–mediated maintenance of CD169+ cells contributes to IL‐6 production and liver regeneration.
Figure 7.
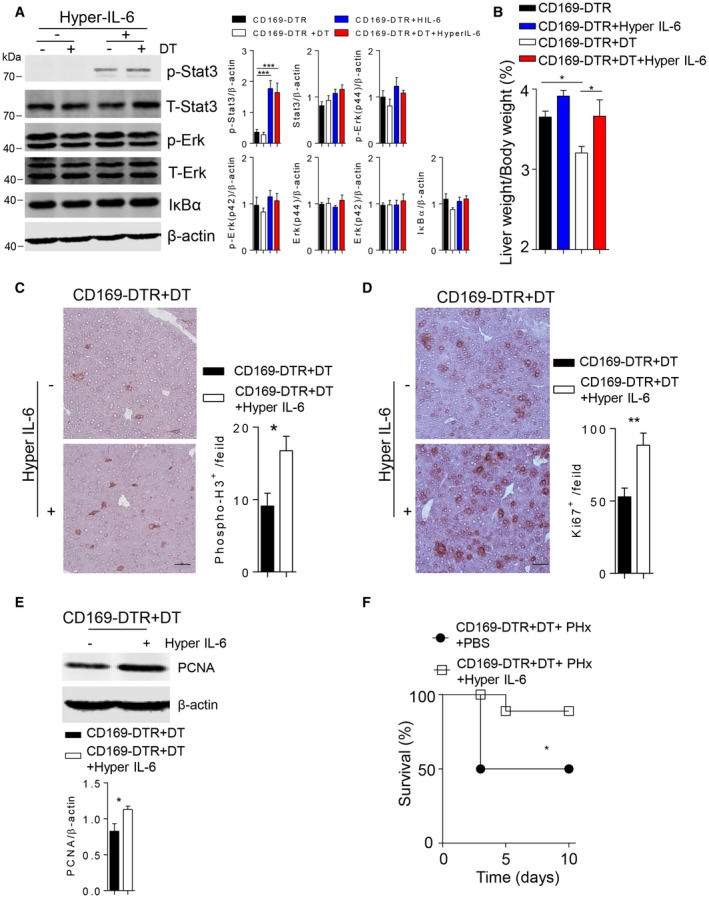
IL‐6/IL‐6R treatment can rescue liver regeneration in the absence of CD169+ cells. (A) Protein lysates of liver tissue from CD169‐DTR mice or DT‐treated CD169‐DTR mice following IL‐6/IL‐6R treatment were blotted and stained with anti‐phospho‐Erk, anti‐Erk, anti‐phospho‐STAT3, anti‐STAT3, anti‐IκBα, and anti‐β‐actin antibodies. One representative of n = 6 blots is shown. Right panels indicate quantification. (B) The liver weight/body weight ratio was determined at 48 hours after 70% PHx in CD169‐DTR mice and DT‐treated CD169‐DTR mice and following IL‐6/IL‐6R treatment (n = 3‐5). (C,D) Sections of snap‐frozen liver tissue from DT‐treated CD169‐DTR mice without or after IL‐6/IL‐6R treatment at 48 hours after 70% PHx were stained with (C) anti‐phospho‐H3 and (D) anti‐Ki‐67 antibodies. Representative sections for each time point are shown (n = 3‐5; scale bars, [C] 50 μm, [D], 100 μm). Right panels indicate quantification. (E) Protein level of PCNA was measured in DT‐treated CD169‐DTR mice in the absence and presence of IL‐6/IL‐6R treatment at 48 hours after 70% PHx (n = 5). Lower panel indicates quantification. (F) Survival of DT‐treated CD169‐DTR mice without or after IL‐6/IL‐6R treatment was monitored (n = 8‐9). Error bars in all experiments represent SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In this study, we identify that B cells promoted maintenance of CD169+ cells, which are critical for liver regeneration. Consistently, depletion of CD169+ cells resulted in reduced IL‐6 expression following PHx and consequently reduced activation of STAT3 signaling pathways. Application of IL‐6/IL‐6R could rescue defective liver regeneration in the absence of CD169+ cells.
Liver regeneration is triggered by key signaling pathways, which are regulated through several cytokines. Specifically, the TNF superfamily members TNF, Ltα, and Ltβ have been shown to be important for liver regeneration. Ltα and Ltβ production by T cells play an important role during liver regeneration. Notably, B cell–specific and T cell–specific Ltα‐deficient and Ltβ‐deficient mice display also reduced presence of CD169+ cells.19 Hence, Lt could exhibit effects not only on hepatocytes but also on CD169+ cells and thereby affect IL‐6‐mediated signaling. Specifically, we observed reduced IL‐6 expression in liver tissue from DT‐treated CD169‐DTR mice compared to control animals. Consistently, defective IL‐6 signaling results in impaired liver regeneration, a transient effect which can result in altering the severity of the phenotype.6, 7 Similarly, we observed severe pathology in a proportion of animals following splenectomy and PHx, although other studies, which also reported a supporting role of the spleen during liver regeneration in mice, did not indicate a severe pathology.28 Because at later time points we observed only slight but significant decreases in liver mass in the absence of CD169+ cells, the effects described here might be also transient. We speculated that underlying mechanisms following defective liver regeneration in B cell–deficient and CD169+ cell–deficient animals likely preceded the development of disease symptoms and therefore analyzed early time points following PHx. Consistently, we found reduced presence of proliferation markers and reduced liver weight/body weight ratios during these time points. However, treatment with IL‐6/IL‐6R improved expression of hepatic proliferation markers, liver weight/body weight ratio, and observed pathology in the absence of CD169+ cells.6
The spleen is ideally situated to trigger liver regeneration. The blood from spleen tissue directly feeds into the portal vein circulation. Accordingly, cytokines and chemokines produced in the spleen are able to trigger their effects in the liver. As discussed above, several immune factors contribute to or regulate liver regeneration. Splenectomy has been shown to be beneficial in clinical settings of liver cirrhosis and portal hypertension or hypersplenism.31, 32 These beneficial effects have been attributed to reduction of the portal circulation.33 Furthermore, experiments using rats have shown that splenectomy can result in reduced concentrations of TGFβ and consequently reduced inhibitory effects on liver regeneration.34, 35 Interestingly, these effects increase with the amount of liver mass removed.35 In mice, splenectomy results in delayed liver regeneration following PHx.28 The role of B cells in the regulation of liver functions during acute and chronic liver disease remains controversial.36, 37 In our settings, splenectomy, B‐cell deficiency, and deletion of CD169+ cells resulted in impaired liver regeneration following PHx. One explanation could be that in rats CD169+ cells from other lymphoid tissue compensate for loss of splenic CD169+ cells. Furthermore, increased IL‐6/IL‐6R signaling might compensate for the splenic loss of CD169+ cells. In our setting, treatment with IL‐6/IL‐6R could prevent severe pathology in the absence of CD169+ cells. Future studies could compare the activation of B cells and CD169+ cells during chronic liver disease and liver pathology.
In conclusion, we identified in our model systems that B cell–mediated maintenance of CD169+ cells contributes to liver regeneration.
Potential conflict of interest
Nothing to report.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BAFFR
B cell–activating factor receptor
- CD169
cluster of differentiation 169
- ΔCt
Delta cycle threshold
- DT
diphtheria toxin
- DTR
DT receptor
- EGFR
epidermal growth factor receptor
- Erk
extracellular signal–regulated kinase
- H&E
hematoxylin and eosin
- IκBα
inhibitor of kappa B alpha
- IL‐6
interleukin 6
- IL‐6R
IL‐6 receptor
- Jh
joining heavy chain
- Lt
lymphotoxin
- LtβR
Ltβ receptor
- PCNA
proliferating cell nuclear antigen
- PHx
partial hepatectomy
- phospho‐H3
phospho‐histone H3
- Stat3
signal transducer and activator of transcription 3
- TGFβ
transforming growth factor beta
- TNFα
tumor necrosis factor alpha
- TUNEL
terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick‐end labeling
- WT
wild type
Supporting information
Supported by the German Research Council (SFB974, KFO217, LA‐2558/5‐1), by the Jürgen Manchot Graduate School MOI III and by US NIH grant R01AI067890.
References
Author names in bold designate shared co‐first authorship.
- 1. Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010;176:2‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA 1997;94:1441‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anders RA, Subudhi SK, Wang J, Pfeffer K, Fu YX. Contribution of the lymphotoxin beta receptor to liver regeneration. J Immunol 2005;175:1295‐1300. [DOI] [PubMed] [Google Scholar]
- 4. Tumanov AV, Koroleva EP, Christiansen PA, Khan MA, Ruddy MJ, Burnette B, et al. T cell‐derived lymphotoxin regulates liver regeneration. Gastroenterology 2009;136:694‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sorg UR, Behnke K, Degrandi D, Reich M, Keitel V, Herebian D, et al. Cooperative role of lymphotoxin beta receptor and tumor necrosis factor receptor p55 in murine liver regeneration. J Hepatol 2016;64:1108‐1117. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt‐Arras D, Rose‐John S. IL‐6 pathway in the liver: from physiopathology to therapy. J Hepatol 2016;64:1403‐1415. [DOI] [PubMed] [Google Scholar]
- 7. Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin‐6‐deficient mice. Science 1996;274:1379‐1383. [DOI] [PubMed] [Google Scholar]
- 8. Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V, et al. Mitosis and apoptosis in the liver of interleukin‐6‐deficient mice after partial hepatectomy. Hepatology 1999;29:403‐411. [DOI] [PubMed] [Google Scholar]
- 9. Peters M, Blinn G, Jostock T, Schirmacher P, Zum Buschenfelde KHM, Galle PR, et al. Combined interleukin 6 and soluble interleukin 6 receptor accelerates murine liver regeneration. Gastroenterology 2000;119:1663‐1671. [DOI] [PubMed] [Google Scholar]
- 10. Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, et al. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol 1996;132:1133‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell WE, Kaufmann WK, Sitaric S, Luetteke NC, Lee DC. Liver regeneration and hepatocarcinogenesis in transforming growth factor‐alpha‐targeted mice. Mol Carcinog 1996;15:183‐189. [DOI] [PubMed] [Google Scholar]
- 12. Berasain C, Garcia‐Trevijano ER, Castillo J, Erroba E, Lee DC, Prieto J, et al. Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology 2005;128:424‐432. [DOI] [PubMed] [Google Scholar]
- 13. Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci USA 2007;104:17081‐17086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol 2008;8:764‐775. [DOI] [PubMed] [Google Scholar]
- 15. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue‐resident macrophages. Nat Immunol 2013;14:986‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nolte MA, Arens R, Kraus M, van Oers MH, Kraal G, van Lier RA, et al. B cells are crucial for both development and maintenance of the splenic marginal zone. J Immunol 2004;172:3620‐3627. [DOI] [PubMed] [Google Scholar]
- 17. Honke N, Shaabani N, Cadeddu G, Sorg UR, Zhang DE, Trilling M, et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol 2012;13:51‐57. [DOI] [PubMed] [Google Scholar]
- 18. Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity 2002;17:239‐250. [DOI] [PubMed] [Google Scholar]
- 19. Tumanov AV, Grivennikov SI, Shakhov AN, Rybtsov SA, Koroleva EP, Takeda J, et al. Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting. Immunol Rev 2003;195:106‐116. [DOI] [PubMed] [Google Scholar]
- 20. Futterer A, Mink K, Luz A, Kosco‐Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 1998;9:59‐70. [DOI] [PubMed] [Google Scholar]
- 21. Asano K, Takahashi N, Ushiki M, Monya M, Aihara F, Kuboki E, et al. Intestinal CD169+ macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat Commun 2015;6:7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moseman EA, Iannacone M, Bosurgi L, Tonti E, Chevrier N, Tumanov A, et al. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity 2012;36:415‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu HC, Huang J, Khairnar V, Duhan V, Pandyra AA, Grusdat M, et al. BAFFR deficiency results in limited CD169+ macrophage function during viral infection. J Virol 2015;89:4748‐4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, et al. CD169‐positive macrophages dominate antitumor immunity by crosspresenting dead cell‐associated antigens. Immunity 2011;34:85‐95. [DOI] [PubMed] [Google Scholar]
- 25. Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt‐Supprian M. TNF family member B cell–activating factor (BAFF) receptor–dependent and –independent roles for BAFF in B cell physiology. J Immunol 2004;173:2245‐2252. [DOI] [PubMed] [Google Scholar]
- 26. Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg 2003;16:99‐102. [PubMed] [Google Scholar]
- 27. Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, et al. The lymphotoxin‐beta receptor induces different patterns of gene expression via two NF‐kappaB pathways. Immunity 2002;17:525‐535. [DOI] [PubMed] [Google Scholar]
- 28. Furuya S, Kono H, Hara M, Hirayama K, Tsuchiya M, Fujii H. Interleukin‐17A plays a pivotal role after partial hepatectomy in mice. J Surg Res 2013;184:838‐846. [DOI] [PubMed] [Google Scholar]
- 29. Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, Bohm J, et al. B‐cell activating factor receptor deficiency is associated with an adult‐onset antibody deficiency syndrome in humans. Proc Natl Acad Sci USA 2009;106:13945‐13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tumanov AV, Koroleva EP, Christiansen PA, Khan MA, Ruddy MJ, Burnette B, et al. T cell–derived lymphotoxin regulates liver regeneration. Gastroenterology 2009;136:694‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawanaka H, Akahoshi T, Kinjo N, Harimoto N, Itoh S, Tsutsumi N, et al. Laparoscopic splenectomy with technical standardization and selection criteria for standard or hand‐assisted approach in 390 patients with liver cirrhosis and portal hypertension. J Am Coll Surg 2015;221:354‐366. [DOI] [PubMed] [Google Scholar]
- 32. Tomikawa M, Akahoshi T, Sugimachi K, Ikeda Y, Yoshida K, Tanabe Y, et al. Laparoscopic splenectomy may be a superior supportive intervention for cirrhotic patients with hypersplenism. J Gastroenterol Hepatol 2010;25:397‐402. [DOI] [PubMed] [Google Scholar]
- 33. Eipel C, Abshagen K, Ritter J, Cantre D, Menger MD, Vollmar B. Splenectomy improves survival by increasing arterial blood supply in a rat model of reduced‐size liver. Transpl Int 2010;23:998‐1007. [DOI] [PubMed] [Google Scholar]
- 34. Lee SC, Jeong HJ, Choi BJ, Kim SJ. Role of the spleen in liver regeneration in relation to transforming growth factor‐beta 1 and hepatocyte growth factor. J Surg Res 2015;196:270‐277. [DOI] [PubMed] [Google Scholar]
- 35. Kim J, Kim CJ, Ko IG, Joo SH, Ahn HJ. Splenectomy affects the balance between hepatic growth factor and transforming growth factor‐beta and its effect on liver regeneration is dependent on the amount of liver resection in rats. J Korean Surg Soc 2012;82:238‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest 2005;115:3072‐3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richards JA, Bucsaiova M, Hesketh EE, Ventre C, Henderson NC, Simpson K, et al. Acute liver injury is independent of B cells or immunoglobulin M. PLoS One 2015;10:e0138688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


