Abstract
Background
In efforts to improve the implementation of survivorship care plans (SCPs), the authors assessed whether the impact of SCPs on patient‐reported outcomes differed between patients with an information‐seeking coping style (monitoring) versus those with an information‐avoiding coping style (blunting).
Methods
In the Registration System Oncological Gynecology (ROGY) Care Trial, 12 hospitals in the Netherlands were randomized to deliver SCP care or usual care. All patients with newly diagnosed endometrial and ovarian cancer in the SCP care arm received an SCP that was generated automatically by their oncology provider through the web‐based ROGY registration system. Outcomes (satisfaction with information provision and care, illness perceptions, and health care use) were measured directly after initial treatment and after 6, 12, and 24 months. Information coping style was measured at 12 months after initial treatment.
Results
Among patients who had a monitoring coping style (N = 123), those in the SCP care arm reported higher satisfaction with information provision (mean score: 73.9 vs 63.9, respectively; P = .04) and care (mean score: 74.5 vs 69.2, respectively; P = .03) compared with those in the usual care arm. Among patients who had a blunting coping style (N = 102), those in the SCP care arm reported a higher impact of the disease on life (mean score: 5.0 vs 4.5, respectively; P = .02) and a higher emotional impact of the disease (mean score: 5.4 vs 4.2, respectively; P = .01) compared with those in the usual care arm.
Conclusions
SCPs may be beneficial for patients who desire information about their disease, whereas SCPs may be less beneficial for patients who avoid medical information, suggesting a need for tailored SCP delivery to improve survivorship care.
Keywords: coping, gynecologic cancer, illness perception, information provision, patient satisfaction, survivorship care plan
Short abstract
Survivorship care plans may be beneficial for patients who desire detailed information about their cancer, whereas they may be less beneficial and perhaps even harmful for patients who prefer to avoid medical information. The current study emphasizes the need to individualize delivery of survivorship care plans according to patients’ information needs.
Introduction
For more than a decade, survivorship care plans (SCPs) have been broadly endorsed as a means to improve care coordination and to address unmet information needs in the growing population of cancer survivors.1 Since the first recommendation by the US Institute of Medicine in 2006,1 SCPs have been the focus of survivorship care research.2 Despite these efforts, the evidence base for the impact of SCPs among cancer survivors is still inconclusive, and directions for future implementation of SCPs remain under debate.3 Randomized controlled trials (RTCs) have failed to identify the benefits of SCP delivery on patient satisfaction, quality of life, and distress in various patient populations, including those with breast,4, 5 gynecologic,6, 7, 8 colorectal,9 and prostate10 cancers. However, SCPs may be beneficial for selected subgroups of survivors, such as underserved populations11 and patients who do not use other sources of medical information like the internet,12 indicating that we should focus on those individuals who benefit most from SCPs.
Ample research in health communication demonstrates that individuals respond differently to medical information because of different coping strategies.13, 14 Miller identified 2 main information coping styles for dealing with health threats like: monitoring and blunting.15 Monitors typically seek for information relevant for them with regard to their health threat, whereas blunters prefer to avoid medical information and distract from it. Monitors report more anxiety related to their cancer treatment,16 desire more voluminous and detailed information about their disease, and to be dissatisfied more often with the information they receive.17 Conversely, excessive information before a diagnostic procedure resulted in more self‐reported tension, depression, and physical discomfort among patients who had a blunting coping style.18 Hence, in a sample of gynecologic patients at risk of cancer, stress reduction appears to be most optimal for monitors when they receive detailed information, whereas blunters respond better when they receive minimal information.19 These findings suggest that SCPs may be more beneficial for cancer survivors who have a monitoring coping style compared with those who have a blunting coping style.
In efforts to improve the future implementation of SCPs, our objective was to determine whether information coping style moderates the impact of SCPs on patient‐reported outcomes among patients with gynecologic cancer in the pragmatic cluster‐randomized Registration System Oncological Gynecology (ROGY) Care Trial, including satisfaction with information provision and care, illness perceptions, and health care use. In the main analyses of that trial, the overall effects of SCPs were assessed among patients who had endometrial and ovarian cancer. We demonstrated that SCPs did not improve satisfaction with information provision and care but, rather, increased worry, emotional impact, and experienced symptoms among women with endometrial cancer7 and decreased trust in the treatment among those with ovarian cancer.8 However, we hypothesize that SCPs have a positive effect on satisfaction with information provision and care and on health care use among patients who have a monitoring coping style but not among those who have a blunting coping style, whereas SCPs may increase threatening illness perceptions in patients who have a blunting coping style but not those who have a monitoring coping style.
Materials and methods
Design
The ROGY Care Trial is a pragmatic, cluster RTC in which the objective is to assess the impact of automatically generated SCPs on patient‐reported outcomes among patients with endometrial and ovarian cancer during 2 years of follow‐up. Twelve hospitals in the Netherlands were randomized to deliver either SCP care or usual care. The trial was centrally approved by a Medical Research Ethics Committee and is registered as NCT01185626 on clinicaltrials.gov. Further details about the design are described in the trial protocol.20
Participants and Recruitment
After initial treatment, all patients who were newly diagnosed with endometrial cancer between April 2011 and October 2012 or with ovarian cancer between April 2011 and March 2014 were invited to participate by their own gynecologist with a letter and an informed‐consent form. After consent, questionnaires were sent to the patients after treatment and at 6, 12, and 24 months after treatment. Because of the pragmatic nature of the trial, exclusion criteria were limited. Only patients with borderline ovarian tumors, those who were receiving palliative care, and those who were unable to complete a Dutch questionnaire were excluded from participation.20 The current analysis includes only those patients who completed the questionnaire assessing information coping style at 12 months after initial treatment (Supporting Table 1). Primary effects of SCPs on patient‐reported outcomes in women with endometrial7 and ovarian8 cancer have been described elsewhere.
Randomization and Blinding
Randomization was performed, using a table of random numbers, by an independent researcher who was blinded to the identity of the hospitals. Patients, but not oncology providers or researchers who assessed the outcomes, were blinded to trial assignment.20
SCP Care Versus Usual Care
In the hospitals that provided usual care, standard care was delivered in accordance with Dutch oncology guidelines (www.oncoline.nl, accessed October 30, 2018). In the hospitals that provided SCP care, all oncology providers (gynecologists/gynecologic oncologists and oncology nurses) were instructed to provide an SCP to patients during the consultation at which the results of histopathology and the (adjuvant) treatment plan were discussed (ie, 7‐14 days after surgery). An updated version of the SCP optionally could be discussed in a follow‐up consultation. Providers received practical guidelines on the components of the SCP that minimally should be discussed with each patient during the consultation. The SCP was based on the Dutch translation of the National Academy of Medicine (NAM) SCP template,1 adjusted to the local situation.20 Texts of the SCP were based on pilot‐tested patient education material from the Dutch Cancer Society. In addition, the SCP was pilot‐tested on patients with low/intermediate educational levels to ensure that the SCP was understandable. The SCP contained a treatment summary and a follow‐up care plan, including detailed information on the short‐term and long‐term effects of the treatments received, the effects on social and sexual life, possible signs of recurrence and secondary tumors, and information on rehabilitation, psychosocial support, and supportive care services. Details about the intervention20 and implementation21 have been described elsewhere.
Measures
Age, socioeconomic status (SES), and clinical data were obtained from the Netherlands Cancer Registry.22 SES was based on the postal code of the patient’s area of area of residence23 and was categorized into low, medium, or high. Additional sociodemographic information was assessed in the first questionnaire. Marital status (married/living together vs divorced/widowed/never married) and employment status (having a paid job vs not having a paid job) were dichotomized. Comorbidity was assessed using the adapted Self‐Administered Comorbidity Questionnaire and was categorized into no comorbidities, 1 comorbidity, or >1 comorbidity.24 Disease‐related internet use and receipt of an SCP (“Did you receive a survivorship care plan?”) were treated as dichotomous measures.
Information coping style was assessed using the shortened version of the Threatening Medical Situations Inventory,25 which consists of 2 hypothetical descriptions of threatening medical situations. The internal consistency of the monitoring (Cronbach α = .79) and blunting (Cronbach α = .73) subscales were good, and test‐retest reliability has been established as sufficient for both scales (Pearson correlation, 0.64‐0.83).25 A sum score was calculated by subtracting the blunting subscale score from the monitoring subscale score, as previously described.15 Individuals with sum scores below or equal to the median were categorized as monitors, and individuals with scores above the median were categorized as blunters.
Outcome scales were assessed in each questionnaire. Information provision was measured with the 25‐item European Organization for Research and Treatment Quality‐of‐Life Group Information Questionnaire (EORTC‐QLQINFO25),26 which has 4 multi‐item subscales (information about the disease, medical tests, treatment, and other care services) and 4 single‐item scales (information about different places of care, things you can do to help yourself get well, satisfaction with the information, and helpfulness of the information). Internal consistency of the scales was good in our sample (Cronbach α, 0.75‐0.90). Previously, test‐retest reliability (intraclass correlation [ICC], 0.71‐0.91) was established as good.26 Satisfaction with care was assessed using the 32‐item EORTC‐QLQ Inpatient Satisfaction Questionnaire (EORTC‐QLQINPATSAT32), which was adjusted to make the questionnaire appropriate for use during survivorship care27 using 2 multi‐item scales (physician’s interpersonal skills and nurses’ interpersonal skills) and 2 singe‐item scales (exchange of information between caregivers and general satisfaction with care). Internal consistency of the scales was good in our sample (Cronbach α, 0.93‐0.94). Previously, test‐retest reliability (ICC, 0.66‐0.85) was established as good.27 Illness perception was assessed using the Brief Illness Perception Questionnaire,28 which includes 8 single‐item scales. Test‐retest reliability (Pearson correlation, 0.42‐0.75) was fair to good.28 Health care use was assessed according to the number of visits to a medical specialist or primary care physician in relation to cancer in the past 6 months.
Statistical Analysis
Statistical analyses were conducted using SAS version 9.4. (SAS Institute Inc, Cary, NC). Differences in characteristics between patients who were included in the analyses and those who were lost to follow‐up, both between trial arms and between patients with monitoring and blunting coping styles, were compared using t tests for normally distributed continuous variables, Mann‐Whitney U tests for non‐normally distributed variables, and chi‐square tests for categorical variables.
Linear, multilevel regression analysis was performed to assess the moderating effect of information coping style on the impact of SCPs on patient‐reported outcomes. A random intercept on the patient level was included in the model to adjust for intradependency between repeated measures.29 Random intercepts on the hospital level (ICC < 0.16) and random slopes on the patient level (ICC < 0.14) were not included, because they did not improve the models.29 To assess the moderating effect of information coping style, an interaction term of information coping style and trial arm, with all a priori‐selected covariates (ie, cancer type, age, time since diagnosis, marital status, SES, employment status, comorbidities, disease stage, and treatment) was added to the model. Dependent variables were the information provision and care, illness perceptions, and health care use scales. For outcome scales in which the interaction term of coping style and trial arm was significant, the analyses were stratified by information coping style. For all analyses, patients in the SCP care arm were compared with all patients in the usual care arm (intention‐to‐treat analysis). In addition, per‐protocol analyses were conducted to compare patients in the SCP care arm who reported having received an SCP versus all patients in the usual care arm, because no SCPs were provided by the hospitals to patients in the usual care arm. Interactions with cancer type were assessed by using a 3‐way interaction of cancer type, coping style, and trial arm. Prior analyses revealed a moderating effect of disease‐related internet use on the outcome scales.12 Therefore, we also assessed whether there was a 3‐way interaction between disease‐related internet use, information coping style, and trial arm on any of the outcome scales when adjusted for covariates.
Results
In total, 221 patients with endometrial cancer and 174 with ovarian cancer participated in the trial. Patients with endometrial cancer who did not participate in the trial were older and had higher stage cancers, as previously described.7, 8 Furthermore, patients who had endometrial cancer in the SCP care arm completed the questionnaires later after diagnosis compared with those who had endometrial cancer in the usual care arm.7 Among patients with ovarian cancer, women in the SCP care arm had more comorbidities compared with those in the usual care arm.8
The current analysis included 131 patients (59%) with endometrial cancer and 95 patients (55%) with ovarian cancer who completed the questionnaires at 12 months after treatment (Supporting Table 1). Compared with the patients who were lost to follow‐up, patients who were included in the current analysis were younger, and those with endometrial cancer more often had a partner, had higher SES, had lower cancer stage, and received surgery, and they less often received chemotherapy (Table 1). Patients who had a monitoring coping style more often had a partner and used the internet to look up medical information compared with those who had a blunting coping style (Table 2). Additional analyses stratified by cancer type demonstrated that patients with ovarian cancer who had higher stage cancers more often had a monitoring coping style (P < .01). The monitoring and blunting groups were not of equal size, because 19 patients had median scores on the Threatening Medical Situations Inventory.
Table 1.
Baseline Characteristics of Participants Lost to Follow‐Up Versus Those Included in the Analyses Stratified by Cancer Type: Univariate Analysesa
| Endometrial Cancer: No. of Participants (%) | Ovarian Cancer: No. of Participants (%) | |||||
|---|---|---|---|---|---|---|
| Characteristic | Included in Analyses, N = 131 | Lost to Follow‐Up, N = 90 | P | Included in Analyses, N = 94 | Lost to Follow‐Up, N = 81 | P |
| Age at first questionnaire: Mean ± SD, y | 65.9 ± 8.7 | 71.3 ± 8.4 | < .01a | 61.9 ± 9.3 | 65.9 ± 12.7 | < .01a |
| SESb | ||||||
| High | 55 (42) | 18 (20) | < .01a | 39 (41) | 29 (36) | .85 |
| Intermediate | 49 (37) | 42 (47) | 37 (39) | 37 (46) | ||
| Low | 19 (15) | 24 (27) | 17 (55) | 14 (17) | ||
| Institutionalized/unknown | 8 (6) | 6 (7) | 1 (1) | 1 (1) | ||
| Marital statusc | ||||||
| Partner | 106 (81) | 55 (61) | < .01a | 74 (79) | 57 (70) | .20 |
| No partner | 25 (19) | 35 (39) | 20 (21) | 24 (30) | ||
| Employed | ||||||
| Yes | 24 (18) | 13 (14) | .34 | 33 (35) | 18 (22) | .06 |
| No | 98 (75) | 66 (73) | 61 (65) | 63 (78) | ||
| Time from diagnosis: Median [IQR], mo | 3.0 [1.8] | 3.0 [1.6] | .62 | 2.4 [2.4] | 2.8 (2.5) | .20 |
| FIGO stage | ||||||
| I | 122 (93) | 69 (77) | < .01a | 31 (33) | 21 (26) | .57 |
| II | 3 (2) | 4 (4) | 10 (11) | 6 (8) | ||
| III | 5 (4) | 13 (14) | 39 (41) | 37 (46) | ||
| IV | 1 (1) | 4 (4) | 14 (15) | 16 (20) | ||
| Treatment | ||||||
| Surgery | 131 (100) | 86 (97) | .03a | 88 (96) | 71 (88) | .054 |
| Chemotherapy | 2 (2) | 13 (14) | < .01a | 71 (75) | 64 (79) | .58 |
| Radiotherapy | 42 (32) | 33 (37) | .48 | |||
| Comorbidity | ||||||
| None | 24 (18) | 14 (16) | .17 | 29 (31) | 27 (33) | .33 |
| 1 | 29 (22) | 26 (29) | 33 (35) | 20 (25) | ||
| ≥2 | 77 (59) | 46 (51) | 31 (33) | 34 (42) | ||
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; IQR, interquartile range; SD, standard deviation; SES, socioeconomic status.
This P value indicates a statistically significant difference.
SES was based on the postal code of the patient’s residence.
Marital status included partner (married/living together) and no partner (divorced/widowed/never married). Values may not always add up to 100%, because percentages have been rounded off to whole numbers.
Table 2.
Characteristics of Patients Included in the Analyses Stratified by Information Coping Style: Univariate Analyses
| No. of Patients (%)a | |||
|---|---|---|---|
| Characteristic | Monitoring Coping Style, N = 123 | Blunting Coping Style, N = 102 | P |
| Age at first questionnaire: Mean ± SD, y | 63.3 (9.1) | 65.4 (9.2) | .10 |
| SESb | |||
| High | 54 (44) | 41 (40) | .30 |
| Intermediate | 46 (37) | 40 (39) | |
| Low | 16 (13) | 20 (19) | |
| Institutionalized/unknown | 7 (6) | 2 (2) | |
| Marital statusc | |||
| Partner | 108 (88) | 72 (70) | < .01d |
| No partner | 15 (12) | 31 (30) | |
| Employed | |||
| Yes | 33 (28) | 24 (24) | .46 |
| No | 83 (72) | 76 (76) | |
| Cancer type | |||
| Endometrial | 74 (60) | 57 (55) | .46 |
| Ovarian | 49 (40) | 46 (45) | |
| Time from diagnosis: Median [IQR], mo | 3.0 [2.0] | 2.8 [2.2] | .77 |
| FIGO stage | |||
| I | 80 (65) | 74 (72) | .06 |
| II | 8 (7) | 5 (5) | |
| III | 22 (18) | 22 (21) | |
| IV | 13 (11) | 2 (1) | |
| Treatment | |||
| Surgery | 120 (98) | 100 (98) | .86 |
| Chemotherapy | 42 (34) | 31 (30) | .52 |
| Radiotherapy | 15 (17) | 27 (19) | .73 |
| Comorbidity | |||
| None | 31 (26) | 22 (21) | .86 |
| 1 | 32 (26) | 30 (29) | |
| ≥2 | 58 (48) | 50 (49) | |
| Disease‐related internet use | |||
| Yes | 67 (54) | 36 (35) | < .01d |
| No | 56 (46) | 66 (65) | |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; IQR, interquartile range; SD, standard deviation; SES, socioeconomic status.
Note that the monitoring and blunting groups are not of equal size, because 19 patients had a median score on the Threatening Medical Situations Inventory.
SES was based on the postal code of the patient’s residence.
Marital status included partner (married/living together) and no partner (divorced/widowed/never married). Values may not always add up to 100%, because percentages have been rounded off to whole numbers.
This P value indicates a statistically significant difference.
There was no significant interaction between cancer type and information coping style for any of the outcome scales. Among patients with endometrial cancer and those with ovarian cancer, information coping style significantly moderated the impact of SCPs on the outcomes scales, as illustrated in Table 3. No moderating effect of information coping style on the impact of SCPs was observed on satisfaction with information about medical tests and other services, the interpersonal skills of the physician, exchange of information between caregivers, perceptions about the timeline, personal control, treatment control, identity, emotions and understanding of the disease, and cancer‐related contact with the medical specialist and primary care physician. Crude means at all time points stratified by information coping style and trial arm are illustrated in Figure 1. Overall, patients who had a monitoring coping style were more satisfied with information and care and had less threatening illness perceptions compared with those who had a blunting coping style. Stratified, multilevel linear regression analyses subsequently revealed that, among patients who had a monitoring coping style, compared with patients in the usual care arm, those in the SCP care arm reported higher receipt of information about treatments (β = 8.9; 95% confidence interval [CI], 2.2‐15.5; P < .01), information about things to do to get well (β = 11.6; 95% CI, 3.3‐19.9; P < .01), satisfaction with information received (β = 7.7; 95% CI, 0.3‐15.1; P = .04), perceived helpfulness of the information received (β = 8.4; 95% CI, 1.4‐15.3; P = .02), and higher general satisfaction with care (β = 6.2; 95% CI, 0.7; 11.8; P = .03). Among patients who had a blunting coping style, compared with patients in the usual care arm, those in the SCP care arm reported a higher impact of the disease on life (β = 0.9; 95% CI, 0.2‐1.7; P = .02) and more concerns about the illness (β = 1.1; 95% CI, 0.3‐1.9; P = .01) (Table 3). In addition, a significant 3‐way interaction was observed between disease‐related internet use and information coping style on the helpfulness scale: patients in the SCP care arm with a monitoring coping style who did not use the internet for medical information reported higher helpfulness of the information received (β = 14.7; 95% CI, 3.4‐25.9; P = .01), whereas those with a monitoring coping style who did use the internet for medical information did not report higher helpfulness (β = 6.8; 95% CI, −2.6, 16.2; P = .15).
Table 3.
Effects of the Intervention on Patients With Endometrial and Ovarian Cancer Stratified by Information Coping Style: Intention‐to‐Treat Analyses of the Overall Effects at All Time Points Combined (0, 6, 12, and 24 Months)a
| Monitoring Coping Style (NPatients = 123, NObservations = 453)b | Blunting Coping Style(NPatients = 201, NObservations = 347)b | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| Measurec | SCP Care | Usual Care | β (95% CI) | SCP Care | Usual Care | β (95% CI) |
| Satisfaction with information provision | ||||||
| Disease | 64.4 ± 20 | 58.9 ± 22 | 3.5 (−2.7, 9.7) | 60.7 ± 19 | 63.5 ± 23 | −4.8 (−12.5, 2.8) |
| Treatment | 57.0 ± 24 | 47.3 ± 24 | 8.9 (2.2‐15.5)d , e | 50.2 ± 24 | 54.7 ± 27 | −3.5 (−12.5, 5.6) |
| Things to do | 44.5 ± 29 | 34.4 ± 30 | 11.6 (3.3‐19.9)d , e | 40.4 ± 32 | 42.0 ± 36 | −3.3 (−14.5, 8.0) |
| Satisfaction | 73.9 ± 23 | 63.9 ± 24 | 7.7 (0.3‐15.1)d , f | 74.5 ± 21 | 75.1 ± 25 | −1.4 (−9.5, 6.7) |
| Helpfulness | 76.5 ± 24 | 66.8 ± 22 | 8.4 (1.4‐15.3)d , f | 75.0 ± 21 | 75.4 ± 24 | −1.2 (−9.1, 6.8) |
| Satisfaction with care | ||||||
| Nurse interpersonal skills | 75.0 ± 19 | 72.5 ± 19 | 2.9 (−4.3, 10.2) | 72.9 ± 18 | 79.9 ± 20 | −6.5 (−13.2, 0.3) |
| General satisfaction with care | 74.5 ± 18 | 69.2 ± 19 | 6.2 (0.7‐11.8)d , f | 74.5 ± 17 | 76.1 ± 20 | −1.7 (−7.2, 2.9) |
| Illness perceptions | ||||||
| How much illness affects life | 5.4 ± 2.7 | 5.2 ± 2.8 | 0.0 (−0.7, 0.8) | 5.0 ± 2.6 | 4.5 ± 2.6 | 0.9 (0.2‐1.7)d , f |
| How concerned about illness | 5.5 ± 2.7 | 5.4 ± 2.9 | 0.2 (−0.6, 1.1) | 5.4 ± 2.4 | 4.2 ± 2.6 | 1.1 (0.3‐1.9)d , f |
Abbreviations: CI, confidence interval; SCP, survivorship care plan; SD, standard deviation.
Note that linear, multilevel regression analyses stratified by coping style were performed and were adjusted for covariates. Only the scales on which the interaction term was significant are included in the table. Analyses report the results of the main effect of the intervention after diagnosis and after 6, 12, and 24 months stratified by coping style.
Crude mean and SD values are reported for SCP care and usual care. Unstandardized β values and CIs are reported for SCP care (with usual care as the reference group).
Scores on the 25‐item European Organization for Research and Treatment Quality‐of‐Life Group (EORTC‐QLQ) Information Questionnaire (measuring satisfaction with information provision) and the 32‐item EORTC‐QOL In‐Patient Satisfaction Questionnaire (measuring satisfaction with care) scales range from 0 to 100, with higher scores reflecting better perceived information and care received. Scores on the Brief Illness Perception Questionnaire (measuring illness perceptions) range from 1 to 10, with higher scores indicating more endorsement of that item.
These values indicate that the main effect of the intervention was significant in stratified analysis.
P < .01.
P < .05.
Figure 1.
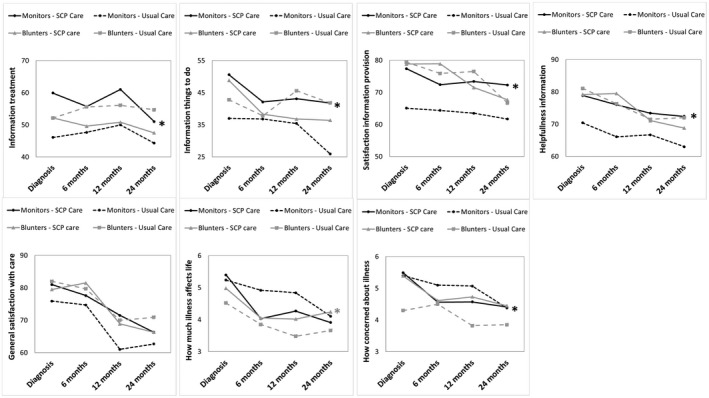
The mean values of trial outcomes stratified by trial arm and information coping style at 0, 6, 12, and 24 months are illustrated. Note that crude means are reported. Monitors are patients who have an information‐seeking coping style, and blunters are those who have an information‐avoiding coping style. Only outcomes that differed significantly between trial arms in either monitors or blunters are included. An asterisk indicates P < .05 in the overall intention‐to‐treat analysis. Detailed statistics are provided in Table 3. SCP indicates survivorship care plan.
In a per‐protocol analysis comparing patients in the SCP care arm who reported receipt of an SCP (endometrial cancer, N = 60 patients [87%]; ovarian cancer, N = 24 patients [75%]) with all patients in the usual care arm (endometrial cancer, N = 62 patients; ovarian cancer, N = 63 patients) produced similar results.
Discussion
The current study demonstrates that information coping style moderates the impact of SCPs on patient‐reported outcomes among patients with gynecologic cancers during 2 years of follow‐up. SCPs appear to improve satisfaction with information and care among patients who desire information about their disease (ie, monitors), whereas SCPs appears to increase worry and perceived consequences of the cancer among patients who avoid medical information (ie, blunters), suggesting a need for the tailored delivery of SCPs. No moderating effect of coping style was observed on the use of health care.
In line with the literature regarding information coping styles,17, 30 monitors generally were satisfied less with information and care compared with blunters, suggesting that there is room for improvement among patients with a monitoring coping style. Consistent with our hypothesis, SCPs appear to meet the high information needs among monitors by substantially increasing satisfaction in this subgroup (up to 12 points on a scale from 0 to 100), resulting in satisfaction levels similar to those observed among blunters. The other side of the coin is that blunters, who generally have lower baseline levels of distress,31 appear to experience increased worry and a higher impact of the disease on life when they receive an SCP. This is consistent with a trial among gynecologic patients demonstrating that stress reduction among blunters is most optimal when they receive minimal information, as opposed to excessive information.19 Excessive information may increase arousal among blunters, because they are confronted with detailed information about a health threat they initially did not worry about.18, 25 In a sample of gynecologic patients with a blunting coping style, this resulted in self‐reported tension, depression, and physical discomfort before and during a diagnostic procedure.19 In contrast, an information brochure for patients who were undergoing gastrointestinal coloscopy was beneficial in reducing anxiety among those who had a monitoring coping style.18 Hence, our results are in line with previous health information intervention studies.
In addition, we observed that monitors more often used the internet to look up information about their disease compared with blunters, whereas SCPs appeared most helpful for monitors who did not do so. These findings suggest that SCPs are most valuable for patients who desire information about their disease but do not have access to resources like the internet. This is in line with earlier findings from our trial, in which patients with endometrial cancer who did not use the internet benefited from SCPs.12 It is noteworthy that prior analyses of our trial demonstrated that more threatening illness perceptions because of the SCP (ie, more worry and experienced symptoms and lower trust in the treatment) resulted in worse long‐term health‐related quality of life and more anxiety.32 Therefore, we should be aware of the potential harmful effects of SCPs in patients with a blunting coping style. Yet appropriate counseling accompanied with the SCP may reduce the harmful effects on health‐related quality of life.33
It also is worth noting that, in a previous report on our trial, SCPs increased cancer‐related contact with the primary care physician among patients with endometrial cancer in the first year after treatment,7 which appeared to be related to anxiety.34 Therefore, we hypothesized that patients with an information coping style, who generally have higher levels of distress, would be encouraged more often to contact their care providers for additional questions and concerns with regard to the SCP. However, we did not observe a moderating effect of information coping style on health care use. Perhaps our findings were diluted by our overall analyses of all time points combined. Unfortunately, the numbers were too small to conduct analyses separately for each time point.
A limitation of this study includes the selective sample of patients who participated in our trial until at least 12 months after initial treatment and who completed the questionnaires included in the this analysis. Patients who were older and had higher stage cancers more often were lost to follow‐up, which may be because of death or ill‐health. Consequently, the number of patients in the current study was too small to conduct stratified analyses separately for those with endometrial cancer and ovarian cancer, because our trial was powered on an analyses of patients who were included from baseline (n = 75 per trial arm per cancer type). Although there were no significant interactions with cancer type, the magnitude of the moderating effect of coping style may differ between cancer types. Furthermore, although patients were told that they participated in an observational study, and response rates were high, patients with a blunting coping style may be under‐represented in our trial, because they do not like to be reminded of their cancer.31 This may have resulted in an underestimation of the potential harmful effects of SCPs among blunters. Furthermore, because of our pragmatic approach, not all patients in the intervention arm reported receipt of an SCP, resulting in an underestimation of the impact when all patients would receive an SCP. Also, unlike most trials, SCPs were provided after initial treatment while some patients were still receiving adjuvant treatment. This may have enlarged the detrimental impact on illness perceptions among blunters, because information may have been provided too early.
Another limitation is that information coping style was assessed at 12 months after initial treatment. Although it has been demonstrated that information coping style is fairly stable over a 1‐month period,25, 35 we observed that patients who had ovarian cancer with higher stage cancer more often had a monitoring coping style. Although information coping style has not previously been associated with disease‐related characteristics,36 our finding suggest that information coping style may be modified by the experience of a cancer diagnosis, and possibly even by the receipt of an SCP. However, the distribution of monitors and blunters did not differ between trial arms, indicating that this probably did not affect our results, although it does suggest that repeated assessments of information coping style may be needed to provide appropriate information when needs have been changed. Furthermore, there is no consensus on whether the outcomes in our analysis (eg, patient satisfaction) are the most relevant for evaluating SCPs. Future studies may need to focus on more proximal outcomes, such as the understanding of survivorship care issues, care provider roles, self‐management, and sense of control.3, 37
It is important to note that the SCPs provided in our trial were extensive documents (ie, up to 25 pages) containing detailed information about potential long‐term and late effects and explicit information about the chance of recurrence.20 The impact of such a voluminous SCP may be much greater compared with treatment summaries or brief SCPs comprising only a couple of pages of information.38 However, these extensive SCPs principally may meet the high information needs of monitors, whereas brief SCPs may be more beneficial for blunters. The heterogeneity of information needs among cancer survivors also may explain why neither brief nor extensive SCPs appear to be beneficial for patient populations as a whole in current SCP trials.3 Hence, either withholding information or providing information to all patients uniformly would not achieve patient satisfaction. Rather, we may need to develop distinct templates of SCPs that are tailored to patients’ information needs, which not only would improve survivors’ outcomes but also may contribute to the more efficient distribution of the limited resources in survivorship care. Hence, we believe that risk stratification according to information and care needs, similar to that applied in individualized follow‐up,39 is needed to accomplish the effective and efficient provision of survivorship information. Determining whether SCPs should be individualized according to information dose only (ie, extensive vs brief SCPs) or also on content (ie, focus on physical vs psychological aspects)40 will require further research. Possibly, a simple set of screening questions to determine information needs may be sufficient to use tailored SCPs in clinical practice, although repeated assessments may be required, because needs may change over time. This may be feasible particularly for a setting in which information is provided online. Furthermore, our findings may apply to other types of health information provision, suggesting that careful evaluation of the effects of various information provision interventions across coping styles is needed before implementation in clinical practice.
Conclusion
Although SCPs may not be helpful for all cancer survivors,7, 8 they appear to be valuable for some survivor subgroups. The current study demonstrates that SCPs may be beneficial for patients who desire detailed information about their cancer, whereas they may be less beneficial and perhaps even harmful for patients who prefer to avoid medical information. Our results emphasize the need to individualize the delivery of SCPs according to patients’ information needs.
Funding Support
The Registration System Oncological Gynecology (ROGY) Care Trial is supported by grant UVT 2010‐4743 from the Dutch Cancer Society. Nicole P. M. Ezendam was supported by a Fellowship grant from the Dutch Cancer Society (UVT‐2014‐6632).
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Belle H. de Rooij: Conceptualization, methodology, formal analysis, investigation, data curation, writing–original draft, and writing–review and editing. Nicole P.M. Ezendam: Conceptualization, methodology, investigation, data curation, writing–review and editing, and supervision. M. Caroline Vos: Investigation and writing–review and editing. Johanna M. A. Pijnenborg: Investigation and writing–review and editing. Dorry Boll: Investigation and writing–review and editing. Roy F. P. M. Kruitwagen: Investigation and writing–review and editing. Lonneke V. van de Poll‐Franse: Conceptualization, methodology, investigation, data curation, writing–review and editing, and supervision.
Supporting information
References
- 1. Hewitt M, Greenfield S, Stovall E, eds. From Cancer Patient to Cancer Survivor: Lost in Transition. Committee on Cancer Survivorship: Improving Quality Care and Quality of Life, National Cancer Policy Board Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 2. Nekhlyudov L, Ganz PA, Arora NK, Rowland JH. Going beyond being lost in transition: a decade of progress in cancer survivorship. J Clin Oncol. 2017;35:1978‐1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacobsen PB, DeRosa AP, Henderson TO, et al. Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. J Clin Oncol. 2018;36:2088‐2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boekhout AH, Maunsell E, Pond GR, et al. A survivorship care plan for breast cancer survivors: extended results of a randomized clinical trial. J Cancer Surviv. 2015;9:683‐691. [DOI] [PubMed] [Google Scholar]
- 5. Grunfeld E, Julian JA, Pond G, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol. 2011;29:4755‐4762. [DOI] [PubMed] [Google Scholar]
- 6. Brothers BM, Easley A, Salani R, Andersen BL. Do survivorship care plans impact patients' evaluations of care? A randomized evaluation with gynecologic oncology patients. Gynecol Oncol. 2013;129:554‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicolaije KA, Ezendam NP, Vos MC, et al. Impact of an automatically generated cancer survivorship care plan on patient‐reported outcomes in routine clinical practice: longitudinal outcomes of a pragmatic, cluster randomized trial. J Clin Oncol. 2015;33:3550‐3559. [DOI] [PubMed] [Google Scholar]
- 8. de Rooij BH, Ezendam NPM, Nicolaije KAH, et al. Effects of survivorship care plans on patient reported outcomes in ovarian cancer during 2‐year follow‐up—the ROGY Care Trial. Gynecol Oncol. 2017;145:319‐328. [DOI] [PubMed] [Google Scholar]
- 9. Jefford M, Gough K, Drosdowsky A, et al. A randomized controlled trial of a nurse‐led supportive care package (SurvivorCare) for survivors of colorectal cancer. Oncologist. 2016;21:1014‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emery JD, Jefford M, King M, et al. ProCare trial: a phase II randomized controlled trial of shared care for follow‐up of men with prostate cancer. BJU Int. 2017;119:381‐389. [DOI] [PubMed] [Google Scholar]
- 11. Maly RC, Liang LJ, Liu Y, Griggs JJ, Ganz PA. Randomized controlled trial of survivorship care plans among low‐income, predominantly Latina breast cancer survivors. J Clin Oncol. 2017;35:1814‐1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicolaije KA, Ezendam NP, Pijnenborg JM, et al. Paper‐based survivorship care plans may be less helpful for cancer patients who search for disease‐related information on the internet: results of the Registration System Oncological Gynecology (ROGY) Care randomized trial [serial online]. J Med Internet Res. 2016;18:e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roussi P, Miller SM. Monitoring style of coping with cancer related threats: a review of the literature. J Behav Med. 2014;37:931‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sweeny K, Melnyk D, Miller W, Shepperd JA. Information avoidance: who, what, when, and why. Rev Gen Psychol. 2010;14:340‐353. [Google Scholar]
- 15. Miller SM. Monitoring and blunting: validation of a questionnaire to assess styles of information seeking under threat [serial online]. J Pers Soc Psychol. 1987;52:345. [DOI] [PubMed] [Google Scholar]
- 16. Lerman C, Rimer B, Blumberg B, et al. Effects of coping style and relaxation on cancer chemotherapy side effects and emotional responses. Cancer Nurs. 1990;13:308‐315. [PubMed] [Google Scholar]
- 17. Miller SM, Brody DS, Summerton J. Styles of coping with threat: implications for health [serial online]. J Pers Soc Psychol. 1988;54:142. [DOI] [PubMed] [Google Scholar]
- 18. van Zuuren FJ, Grypdonck M, Crevits E, Vande Walle C, Defloor T. The effect of an information brochure on patients undergoing gastrointestinal endoscopy: a randomized controlled study. Patient Educ Counsel. 2006;64:173‐182. [DOI] [PubMed] [Google Scholar]
- 19. Miller SM, Mangan CE. Interacting effects of information and coping style in adapting to gynecologic stress: should the doctor tell all? J Pers Soc Psychol. 1983;45:223. [DOI] [PubMed] [Google Scholar]
- 20. van de Poll‐Franse LV, Nicolaije KA, Vos MC, et al. The impact of a cancer survivorship care plan on gynecological cancer patient and health care provider reported outcomes (ROGY Care): study protocol for a pragmatic cluster randomized controlled trial [serial online]. Trials. 2011;12:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Rooij BH, Ezendam NP, Nicolaije KA, et al. Factors influencing implementation of a survivorship care plan—a quantitative process evaluation of the ROGY Care trial. J Cancer Surviv. 2017;11:64‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The Netherlands Cancer Registry . Netherlands Cancer Registry (NCR). Available at: https://www.iknl.nl/over-iknl/about-iknl/what. Amsterdam, the Netherlands: The Netherlands Cancer Registry; 2015. Accessed January 30, 2015.
- 23. van Duijn C, Keij I. Sociaal‐economische status indicator op postcode niveau. Mndstat Bevolking. 2002;50:32‐35. [Google Scholar]
- 24. Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self‐Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156‐163. [DOI] [PubMed] [Google Scholar]
- 25. van Zuuren FJ, de Groot KI, Mulder NL, Muris P. Coping with medical threat: an evaluation of the Threatening Medical Situations Inventory (TMSI). Pers Individ Dif. 1996;21:21‐31. [Google Scholar]
- 26. Arraras JI, Greimel E, Sezer O, et al. An international validation study of the EORTC QLQ‐INFO25 questionnaire: an instrument to assess the information given to cancer patients. Eur J Cancer. 2010;46:2726‐2738. [DOI] [PubMed] [Google Scholar]
- 27. Bredart A, Bottomley A, Blazeby JM, et al. An international prospective study of the EORTC cancer in‐patient satisfaction with care measure (EORTC IN‐PATSAT32). Eur J Cancer. 2005;41:2120‐2131. [DOI] [PubMed] [Google Scholar]
- 28. Broadbent E, Petrie KJ, Main J, Weinman J. The Brief Illness Perception Questionnaire. J Psychosom Res. 2006;60:631‐637. [DOI] [PubMed] [Google Scholar]
- 29. Twisk JW. Applied Multilevel Analysis: A Practical Guide for Medical Researchers Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- 30. Timmermans LM, van Zuuren FJ, van der Maazen RW, Leer JW, Kraaimaat FW. Monitoring and blunting in palliative and curative radiotherapy consultations. Psychooncology. 2007;16:1111‐1120. [DOI] [PubMed] [Google Scholar]
- 31. Miller SM. Monitoring and blunting of threatening information: cognitive interference and facilitation in the coping process In: Sarason IG, Pierce GR, Sarason BR, eds. The LEA Series in Personality and Clinical Psychology. Cognitive Interference: Theories, Methods, and Findings Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1996:175‐190. [Google Scholar]
- 32. de Rooij BH, Ezendam NPM, Nicolaije KAH, et al. Survivorship care plans have a negative impact on long‐term quality of life and anxiety through more threatening illness perceptions in gynecological cancer patients: the ROGY Care Trial. Qual Life Res. 2018;27:1533‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kvale EA, Huang CS, Meneses KM, et al. Patient‐centered support in the survivorship care transition: outcomes from the Patient‐Owned Survivorship Care Plan Intervention. Cancer. 2016;122:3232‐3242. [DOI] [PubMed] [Google Scholar]
- 34. Jeppesen MM, Ezendam NPM, Pijnenborg JMA, et al. The impact of the survivorship care plan on health care use: 2‐year follow‐up results of the ROGY Care Trial. J Cancer Surviv. 2018;12:18‐27. [DOI] [PubMed] [Google Scholar]
- 35. Muris P, van Zuuren F, De Jong PJ, De Beurs E, Hanewald G. Monitoring and blunting coping styles: the Miller behavioural style scale and its correlates, and the development of an alternative questionnaire. Pers Individ Dif. 1994;17:9‐19. [Google Scholar]
- 36. Rees CE, Bath PA. Information‐seeking behaviors of women with breast cancer. Oncol Nurs Forum. 2001;28:899‐907. [PubMed] [Google Scholar]
- 37. Birken SA, Urquhart R, Munoz‐Plaza C, et al. Survivorship care plans: are randomized controlled trials assessing outcomes that are relevant to stakeholders? J Cancer Surviv. 2018;12:495‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. American Society of Clinical Oncology (ASCO) . ASCO Survivorship Care Planning Tools. Survivorship Compendium 2018. Alexandra, VA: ASCO; 2018; https://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship/survivorship-compendium. Accessed March 13, 2018. [Google Scholar]
- 39. Leeson SC, Beaver K, Ezendam NPM, et al. The future for follow‐up of gynaecological cancer in Europe. summary of available data and overview of ongoing trials. Eur J Obstet Gynecol Reprod Biol. 2017;210:376‐380. [DOI] [PubMed] [Google Scholar]
- 40. de Rooij BH, Park ER, Perez GK, et al. Cluster analysis demonstrates the need to individualize care for cancer survivors [published online ahead of print May 8, 2018]. Oncologist. doi:10.1634.theoncologist.2017-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


