Abstract
Background
Alcohol use disorder (AUD) is associated with cognitive deficits such as impaired executive functions, which are hypothesized to contribute to the progression of the disease and worsen treatment outcome. Training of working memory (WM) to improve cognitive functions and thereby reduce alcohol use has been proposed as a novel treatment strategy.
Methods
Patients with AUD (n = 50) who were recruited to an outpatient addiction clinic were randomized to receive 5 weeks of active WM training or control training. Participants had weekly follow‐up visits, and all cognitive training sessions were done online at home. Primary outcomes were WM function and change in self‐reported heavy drinking. Secondary outcomes were craving, other drinking outcomes, and performance on a range of neuropsychological tasks from the Cambridge Neuropsychological Test Automated Battery.
Results
The active training group demonstrated a significantly greater improvement in verbal WM compared with the control group. No statistically significant effect of training was found on the primary drinking outcome, but a trend was observed indicating that WM training reduces the number of drinks per drinking occasion. WM training had no statistically significant effect on any of the other neuropsychological tasks.
Conclusions
Cognitive training can improve WM function in individuals with AUD, suggesting that such interventions are feasible to administer in this patient population. The results do not support an effect of WM training on heavy drinking or transfer effects to other cognitive domains. Future studies should evaluate WM training as an adjunct to evidence‐based treatments for AUD to assess potential synergistic effects.
Keywords: Alcohol Use Disorder, Working Memory, Cognitive Training, Cogmed
Individuals with alcohol use disorder (AUD) repeatedly choose actions that are obviously disadvantageous. An inability to control drinking, repeatedly relapsing, and continued drinking despite negative physical, psychological, and social consequences are not only the diagnostic criteria of the disorder (American Psychiatric Association, 2013), but may also be evidence of a disrupted ability to make rational decisions. This is in part explained by the fact that patients with AUD exhibit impairments across a wide range of cognitive domains (Stavro et al., 2013). It has been suggested that a novel strategy in the treatment of substance use disorders (SUDs) could be to reduce substance use via improvement in cognitive dysfunction, either through pharmacological (Sofuoglu, 2010) or behavioral interventions such as cognitive training (Bickel et al., 2014).
Executive functions (EFs) refer to several cognitive functions that allow individuals to self‐regulate their behavior and select appropriate actions in accordance with their long‐term goals (Diamond, 2013; Hofmann et al., 2012; Jurado and Rosselli, 2007). Working memory (WM), defined as the ability to maintain and manipulate information during a brief period of time, is a critical EF necessary for higher order self‐regulation and decision making (Hofmann et al., 2012). There are several different theoretical models of WM, but one of the most influential models proposes that WM involves a central executive and 2 storage systems: the verbal WM (e.g., repeating a number sequence read aloud) and the visuospatial WM (e.g., remembering details/location of visual cues presented briefly on a screen; for more details, see Baddeley, 2003). Several lines of research have shown that AUD is associated with impairments in EF (for review, see Le Berre et al., 2017), including inhibition (Bjork et al., 2004; Finn et al., 2002; Lawrence et al., 2009a; Le Berre et al., 2012; Noël et al., 2012; Nowakowska‐Domagała et al., 2017), WM (Chanraud et al., 2007; Lawrence et al., 2009b; Martelli et al., 2017), cognitive flexibility (Goudriaan et al., 2006; Noël et al., 2012; Nowakowska‐Domagała et al., 2017), and more rapid discounting of future rewards (Bjork et al., 2004; Petry, 2001). These impairments in EF in AUD manifest as increased impulsive behavior (i.e., a propensity for inappropriate behavior without regard for future consequences; Dick et al., 2010; Verdejo‐García et al., 2008; de Wit, 2009). Several studies have also highlighted an association between alcohol intake, WM dysfunction, and impulsive behavior. In laboratory experimental settings, lower WM function predicts impulsive choices (Hinson et al., 2003) and is associated with greater alcohol‐induced increase in impulsive behavior (Finn et al., 1999). Furthermore, lower baseline WM capacity predicts alcohol use in adolescents, an association that was mediated by impulsivity (Khurana et al., 2013). Improvement in EF through WM training, and thereby strengthening impulse control, has therefore been proposed as a novel treatment strategy in the treatment of AUD (Bickel et al., 2014; Verdejo‐Garcia, 2016).
Computerized WM training has been shown to improve WM function in healthy volunteers (Dahlin et al., 2008; Li et al., 2008) as well as in patient populations such as stroke patients (Westerberg et al., 2007) and children with attention‐deficit/hyperactivity disorder (Klingberg et al., 2002, 2005). Furthermore, previous studies have found that WM training can induce improvement in other cognitive functions, and the most consistent finding is a decrease in inattentive symptoms (Bigorra et al., 2016; Brehmer et al., 2009; Conklin et al., 2015; Green et al., 2012; Klingberg et al., 2005). The hypothesis is based on the notion that WM is a fundamental capacity subservicing other cognitive functions, which therefore should improve if WM capacity increases. In recent years, studies have started to investigate the effect of WM training in different substance abuse populations. WM training in stimulant‐dependent individuals resulted in reduced discounting rates of future rewards, even though actual WM function did not seem to be improved by the training (Bickel et al., 2011). In a study of patients on methadone maintenance, WM training improved performance on WM tasks similar to trained tasks, and drug use remained unchanged in the treatment group while increasing in the control group (Rass et al., 2015). In heavy drinking participants recruited and tested online, training improved WM and reduced drinking (Houben et al., 2011). Recently, it was also shown that WM training improved impulse control in patients with methamphetamine use disorder (Brooks et al., 2017). Finally, in a recent study of alcohol‐dependent patients, Snider and colleagues (2018) found that WM training improved WM as well as delay discounting task of episodic future thinking in a rate‐dependent manner (i.e., only in those with greatest impairments at baseline) (Snider et al., 2018). Taken together, previous studies of WM training in SUD populations have shown mixed results, with some preliminary findings indicating an effect on WM function, substance use, and impulsive behavior. However, to our knowledge, no previous study has investigated the effects of WM training in patients with AUD.
The aim of the current study was to investigate the feasibility and efficacy of 5 consecutive weeks of computerized WM training on WM function and drinking in AUD patients. Furthermore, we wanted to investigate whether the hypothesized improvement in WM could induce transfer effects, that is, improvements in other EF related to impulsive behavior (e.g., response inhibition and risk taking), which was hypothesized to mediate the putative treatment effect on drinking behavior.
Materials and Methods
Participants
Fifty patients with AUD currently not in any form of SUD treatment were recruited through public advertising. After an initial telephone screening, potential participants were invited to the Stockholm Centre for Dependence Disorders outpatient research clinic, where the study was performed. The study physician provided each participant with detailed information regarding the study procedure before written informed consent was collected. The study was approved by the regional ethical review board in Stockholm, was independently monitored by the Karolinska Trial Alliance ( https://karolinskatrialalliance.se/), and was conducted in accordance with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
The main inclusion criteria were as follows: male or female with 18 to 60 years of age; a minimum of 9 years of education; fulfilling the DSM‐IV criteria for alcohol dependence; and having access to a home computer with an Internet connection. The main exclusion criteria were as follows: fulfilling DSM‐IV criteria for current diagnosis of abuse or dependence other than alcohol (except nicotine); fulfilling DSM‐IV criteria for any major psychiatric disorder (e.g., bipolar disorder, schizophrenia, or severe major depression); current suicidal ideation; severe somatic illness; and regular intake of psychotropic medications over the last 3 months, with the exception of selective serotonin re‐uptake inhibitors for current anxiety or depressive disorders currently in remission. See Supplementary Information for a detailed description of all the inclusion and exclusion criteria.
Study Design
The study employed a randomized, controlled, double‐blind design. Patients were randomized (1:1 allocation ratio) to 5 weeks of active WM training or control training. An external monitor from the Karolinska Trial Alliance created the randomization list together with Cogmed® without any involvement of any research staff. Each participant was asked to perform 5 training sessions per week, and the goal was to complete 25 training sessions in total. Participants were informed that they would earn 50 Swedish crowns ($5.75 USD) for each completed training session, but would receive compensation only if they completed at least 20 training sessions and the final test day. Included participants completed a baseline battery of neuropsychological tests at the clinic and thereafter returned on a weekly basis to report drinking, craving, and mood. The trial ended with a test day at the research clinic, which comprised end‐of‐study neuropsychological testing and compensation for participation. All participants were offered referral for treatment at Stockholm Center for Dependency Disorder clinics. The primary outcome measures were heavy drinking and performance on Digit Span and Spatial Working Memory (SWM) tasks (see descriptions following).
Working Memory Training
The current study used Cogmed® software research version, which has been used in several previous studies (Klingberg et al., 2005; Rass et al., 2015) and consists of 12 different exercises of verbal and visuospatial WM. Each training session was composed of 8 exercises, of which 3 were present in all first 20 sessions, and the other 5 varied across sessions. The exercises performed at each of the first 20 sessions were grid, cube (remember sequences of visual stimuli on a grid and cube, respectively), and numbers (remember sequences of numbers read aloud). For further descriptions of the different Cogmed exercises, see the Supplementary Information. At inclusion, each participant was introduced to the software and provided with a unique login and password to use the software online at home. The participants were randomized to either active or control training, and the research staff and participants were blind to the allocation. The active training group performed 5 weeks of repeated adaptive cognitive training (5 sessions of 30 to 45 min/wk), in which the tasks become progressively more difficult based on the user's performance. The control group, however, performed the same number of training tasks, but the training was nonadaptive (i.e., the number of items to remember in each trial was 2 to 3, and there was no increase in difficulty). In accordance with previous studies (Klingberg et al., 2005), the compliance to treatment was defined as completion of a minimum 20 training sessions during the 5‐week study period. During the weekly visits, subjects also got feedback on how many trials they had completed, and encouragement to keep training. A research colleague not involved in the current study extracted information on the number of completed training sessions, without informing either the research subjects or the research staff of the treatment condition. Several measures were undertaken to minimize risk of unblinding. For instance, the randomization list was created by an external monitor, the brand name Cogmed® was never shown/mentioned to participants, and both participants and research staff were explicitly instructed not to discuss the content of the training during the weekly visits. For further details regarding blinding procedures, see the Supplementary Information.
Clinical Instruments
The psychiatric evaluation was performed by a study physician using the Structured Clinical Interview for DSM‐IV (American Psychiatric Association, 2000). Drinking outcomes were quantified by the Timeline Followback interview (Sobell and Sobell, 1992) at baseline and at the weekly visits. Heavy drinking days (HDD) were defined as a day with consumption of at least 4 or 5 standard drinks (equivalent to 12 g alcohol) for women and men, respectively. Craving at baseline and during the study was assessed using the Obsessive Compulsive Drinking Scale (Anton et al., 1995) and the Swedish shortened version of the Desire for Alcohol Questionnaire (Short‐DAQ; Khemiri et al., 2017; Love et al., 1998), respectively. Mood was assessed using the Montgomery–Asberg Depression Self‐Rating Scale (Svanborg and Asberg, 2001).
Tasks of Cognitive Function
All computerized tasks were from the Cambridge Neuropsychological Test Automated Battery (CANTAB®) and were administered using a touch‐screen tablet PC (MOTION J3500‐i7B) and press pad provided by Cambridge Cognition Ltd ( www.cambridgecognition.com). For a detailed description of all tasks of cognitive function and their outcomes, see the Supplementary Information.
Digit Span Task
The Digit Span task from the Wechsler Adult Intelligence Scale‐IV (Swedish version, 2010; Pearson assessment) was used to measure verbal WM. The participant is presented with digit sequences with increasing difficulty, and is asked to repeat each digit sequence. In the first part, participants are asked to repeat the digit sequence in the same order as presented (forward); in the second part, the digit sequences are reported in the opposite order (backward). The outcomes were total number of correctly reproduced digit sequences, as well as number of correct forward and backward digit sequences.
SWM Task
The SWM task from the CANTAB was used to asses visuospatial WM function (Owen et al., 1990). The participant is presented with a number of colored boxes on the screen and is asked to find blue tokens hidden inside these boxes and place them in an empty column on the side of the screen. Importantly, the participant is asked not to return to boxes where a token has been previously found. The outcomes were number of total errors, between‐errors (opening a box in which a token has been found previously), within‐errors (opening a box that has already been found to be empty), and a strategy score (number of times the participant begins a new search with a different box, indicating a poor choice of strategy).
Stop Signal Task
The Stop Signal Task from the CANTAB was used to measure response inhibition (i.e., the ability to inhibit a prepotent response; Logan et al., 1984). The main outcomes are the stop‐signal reaction time (SSRT), the median reaction time of go trials, and the proportion of successful stops. See the Supplementary Information for further details.
Rapid Visual Processing
The Rapid Visual Processing (RVP) task from the CANTAB measures sustained attention (Coull et al., 1995). The main outcomes are probability of hit, probability of false alarm, and mean latency. See the Supplementary Information for further details.
Cambridge Gambling Task
The Cambridge Gambling Task (CGT) from the CANTAB assesses decision making and risk taking (Rogers et al., 1999). The main outcomes are deliberation time, overall proportion bet, risk taking, and delay aversion. See the Supplementary Information for further details.
Stockings of Cambridge
The Stockings of Cambridge, a development of the Tower of London (Owen et al., 1990; Shallice, 1982), is a CANTAB test of planning and problem‐solving ability. The main outcomes are mean number of moves and number of problems solved in a minimum of moves, for the most difficult problems (i.e., 5‐move problems). See the Supplementary Information for further details.
Monetary Choice Questionnaire
The Monetary Choice Questionnaire (Kirby and Maraković, 1996; Kirby et al., 1999) is a task designed to estimate rates of delay discounting. It consists of 27 items that are presented as a choice between 2 different sums of money—either a smaller immediate reward or a larger delayed reward (e.g., “Would you…”). The rewards presented are small, medium, or large, and the time period varies across items. See the Supplementary Information for further details.
Statistical Analysis
Around the time the current study was planned, studies using Cogmed® WM training software had found effect sizes of Cohen's d of approximately 1.0 for WM tasks (Klingberg, 2010). With a total sample size of 50, an alpha level at 0.05, and power 80%, the study was powered to detect large treatment effect sizes (i.e., Cohen's d > 0.8). However, later studies examining the question of transfer effects found that effect sizes for WM training on other cognitive functions (e.g., symptoms of inattention; Spencer‐Smith and Klingberg, 2015) and clinical outcomes such as drinking (Houben et al., 2011) to be low to medium. In light of this, the results from our study on those outcomes should be viewed as “pilot.”
Sociodemographic and clinical background variables were compared between treatment groups using the Student t‐test and the chi‐square test for continuous and categorical variables, respectively. For outcomes related to WM, drinking, and other tasks of cognitive function, mixed analyses of variance (ANOVAs) were performed with Treatment (active training, control) as the between‐subject factor and Time (baseline, test day) as the within‐subject factor. Significant main effects and interactions were further analyzed using repeated‐measures ANOVA for the whole study population or within treatment condition, respectively. Measurements of craving and mood were analyzed using mixed ANOVA with Treatment as the between‐subject factor and Time (baseline, weeks 1 to 5) as the within‐subject factor. In a subgroup analysis, participants were divided into low or high baseline verbal WM function (median split of the Digit Span total score), visuospatial WM function (median split of SWM total errors), and heavy drinking (median split of percentage of heavy drinking 90 days before inclusion) before analyzing the effect of treatment on outcomes as described previously. Further, we also performed a rate dependence analysis (i.e., to what degree the intervention effect was different depending on baseline value for the 3 primary outcomes), by calculating Oldham's correlation (the correlation between change score and mean of the baseline and test day outcome) for each of the primary outcomes described. A correlation of >0.3 is indicative of a moderate effect and has been used as a cutoff in previous articles (for a full description of the method, see Quisenberry et al., 2016; Snider et al., 2016).
For each outcome, 2 separate analyses were performed. In the intention‐to‐treat (ITT) analysis, all participants were included, and missing data were imputed using baseline observations carried forward. The rationale for choosing this method was our limited study duration. We assumed that any participant who dropped out would not have undergone any significant change in drinking behavior compared to the baseline value (i.e., drinking the last 90 days before inclusion). In the per‐protocol (PP) analysis, only participants who completed the entire study (i.e., performed >20 training sessions and completed the test day) were included.
All data were analyzed using IBM SPSS software, version 24 (IBM Corp., Armonk, NY). Data were assessed for normality using the Shapiro–Wilk test and inspection of histogram plots, and transformed if severe deviations from normality were found. If the sphericity assumption was violated (evaluated using the Mauchly test), Greenhouse–Geisser corrections were applied. The alpha level was set to 0.05, uncorrected. Effect sizes were reported as partial eta squared (η p 2).
Results
Participants
As described in the Consolidated Standards of Reporting Trials (CONSORT) flowchart (Fig. 1), 227 participants were telephone‐screened, of which 64 were assessed for eligibility in person and 50 were finally randomized to active or control training. The first participants were included in October 2013, and the final participant completed the study in August 2015. There were no statistically significant differences between the treatment and control groups at baseline regarding any sociodemographic variables or clinical characteristics, including drinking, AUD severity, craving, or WM capacity in the full sample at inclusion (Table 1). In the study completers (PP sample), there were no significant differences between any of the baseline variables reported in Table 1 for the full sample, except for the number of drinks consumed in the last 90 days before inclusion, t(37) = 2.14, p = 0.039, indicating a higher consumption in the treatment group compared to the control group.
Figure 1.
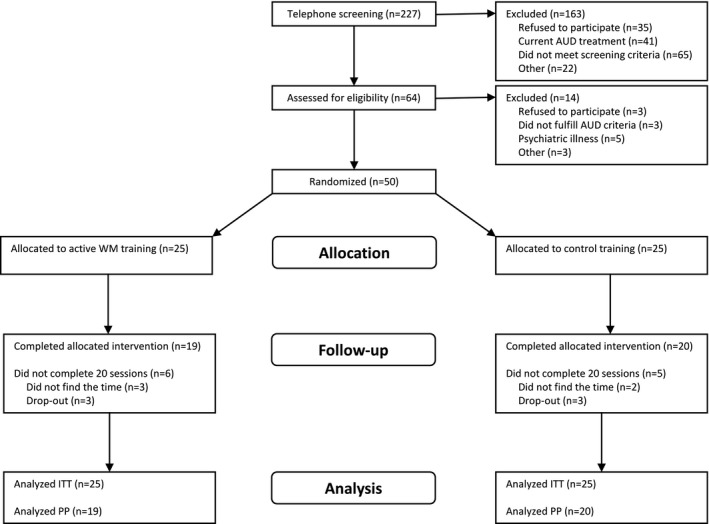
CONSORT (Consolidated Standards of Reporting Trials) flowchart illustrating the flow of study participants. AUD, alcohol use disorder; WM, working memory; ITT, intention to treat; PP, per protocol.
Table 1.
Sociodemographic and Clinical Characteristics of the Entire Sample of Study Participants at Baseline
| Active training | Control training | |
|---|---|---|
| Males/females | 13/12 | 12/13 |
| Age | 49.6 (6.1) | 49.8 (8.7) |
| Education | ||
| Elementary school | 4.0% | 8% |
| High school | 40.0% | 36% |
| University/college | 56% | 56% |
| Marital status | ||
| Never been married | 8% | 16% |
| Married/partner | 76% | 48% |
| Divorced | 16% | 32% |
| Widow | 0% | 4% |
| Daily nicotine use | 48% | 50% |
| Previous had treatment for AD | 40% | 28% |
| Age at first drink | 13.9 (1.9) | 14.8 (1.9) |
| Age when alcohol problem began | 34.0 (10.8) | 32.7 (12.7) |
| AD DSM‐IV criteria | 5.1 (1.2) | 4.8 (1.3) |
| Heredity AD | 87% | 88% |
| OCDS total | 22.7 (7.0) | 21.9 (5.4) |
| TLFB 90 drinks | 421.7 (211) | 358.1 (156) |
| TLFB 90 drinking days | 64.3 (21.6) | 63.2 (20.1) |
| TLFB 90 heavy drinking days | 49.8 (28.2) | 45.0 (28.0) |
| TLFB 90 drinks per drinking day | 6.5 (2.9) | 6.0 (2.0) |
| Alcohol‐free days before inclusion | 4.1 (2.7) | 3.6 (0.89) |
| Number of completed training sessions | 19.7 (8.3) | 20.2 (7.7) |
| Percentage of completers | 76% | 80% |
| Digit span total | 15.7 (3.6) | 16.0 (3.5) |
| Digit span forward | 9.8 (1.9) | 9.0 (2.1) |
| Digit span backward | 5.9 (2.2) | 6.9 (2.0) |
Continuous outcomes are presented as mean (standard deviation). There were no statistically significant differences between groups on any of the outcomes.
AD, alcohol dependence; OCDS, obsessive–compulsive drinking scale; TLFB, Timeline Followback.
There was no difference between treatment groups regarding the percentage of study completers (treatment 76%; control 80%; χ2(1) = 0.117, p = 0.733). Of the 11 participants who did not complete the study protocol (i.e., failed to complete 20 training sessions), 5 of them still completed the follow‐up visits including the test day. Of the remaining 6, 2 dropped out during the study and 4 dropped out immediately after inclusion and had no follow‐up visits. No serious adverse events were reported in any of the treatment groups.
The ITT and PP analyses yielded similar conclusions for all outcomes; therefore, only results from the PP analysis are presented in the main article. The complete statistical analysis including full ITT analysis is found in the Supplementary Information.
Working Memory
For Digit Span total score, there was a main effect of Time, F(1, 37) = 11.30, p = 0.002, η p 2 = 0.234, and no main effect of Treatment, F(1, 37) = 0.788, p = 0.380, η p 2 = 0.021, but a significant Treatment × Time interaction, F(1, 37) = 6.12, p = 0.018, η p 2 = 0.142. The interaction was driven by a significant improvement at test day compared with baseline only in the active training group, F(1, 18) = 14.41, p = 0.001, but not in the control group, F(1, 19) = 0.47, p = 0.50 (Fig. 2 A). Similar results were found in the Digit Span backward score, with a significant main effect of Time, F(1, 37) = 8.60, p = 0.006, η p 2 = 0.189, and no main effect of Treatment, F(1, 37) = 0.013, p = 0.911, η p 2 = 0.00, but a significant Treatment × Time interaction, F(1, 37) = 6.14, p = 0.018, η p 2 = 0.142 (Fig. 2 B). For the forward score, however, there was a main effect of Time, F(1, 37) = 4.2, p = 0.047, η p 2 = 0.102, but no significant main effect of Treatment, F(1, 37) = 2.21, p = 0.146, η p 2 = 0.056, or interaction, F(1, 37) = 1.24, p = 0.273, η p 2 = 0.032 (Fig. 2 C).
Figure 2.
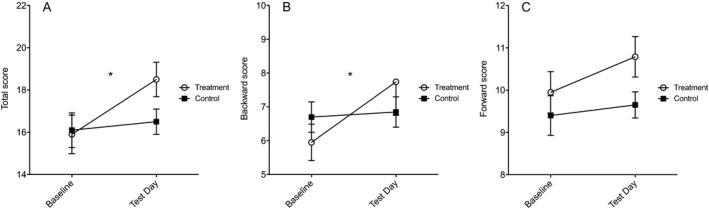
Digit Span scores at baseline and test day in participants who completed the study. The total score (A) and backward score (B) were significantly improved in the treatment group compared with controls. No statistically significant difference was found for the forward score (C). Values are presented as mean ± standard error of the mean; *p < 0.05.
For SWM total errors, there was no main effect of Time, F(1, 35) = 1.04, p = 0.316, η p 2 = 0.029, Treatment, F(1, 35) = 0.003, p = 0.960, η p 2 = 0.000, or Time × Treatment interaction, F(1, 35) = 0.057, p = 0.812, η p 2 = 0.002. Similarly, no significant main effects or interactions were found for strategy score, between‐error score, or within‐error score (Fig. 3; see the Supplementary Information for full analysis).
Figure 3.
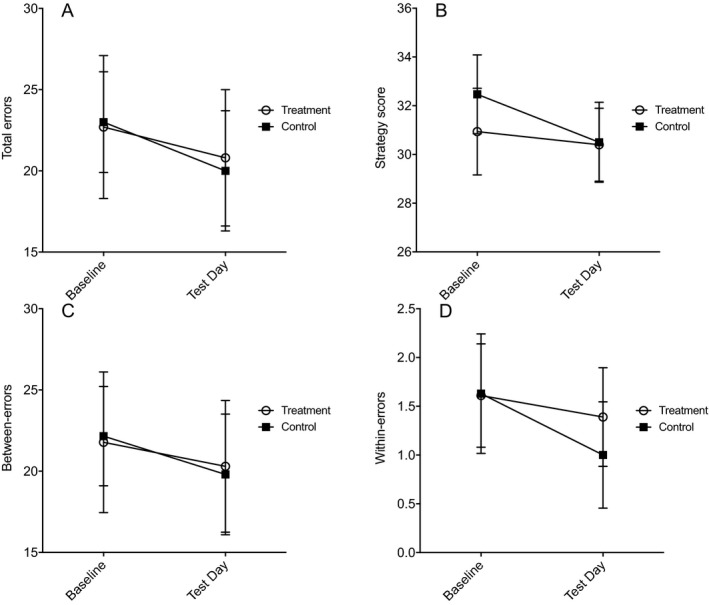
Spatial Working Memory task performance in participants who completed the study. The main outcomes were total errors (A), strategy (B), between‐errors (C), and within‐errors (D) at baseline and test day for treatment and control groups. There were no significant differences between treatment groups. Values are presented as mean ± standard error of the mean.
Drinking
For the primary outcome of percent heavy drinking days, there was a significant effect of Time, F(1, 37) = 6.278, p = 0.017, η p 2 = 0.145, indicating a general reduction over time (overall reduction from 53 to 45%), but no significant effect of Treatment, F(1, 37) = 1.82, p = 0.186, η p 2 = 0.047, or Time × Treatment interaction, F(1, 37) = 2.257, p = 0.142, η p 2 = 0.057 (Fig. 4 A). For drinks per drinking days, there was no significant effect of Time, F(1, 37) = 1.850, p = 0.182, η p 2 = 0.048, or Treatment, F(1, 37) = 1.732, p = 0.196, η p 2 = 0.045, but a trend for the Time × Treatment interaction, F(1, 37) = 3.483, p = 0.070, η p 2 = 0.086 (Fig. 4 B). The active training group significantly reduced mean number of drinks per drinking days [baseline: 7.07 (2.80); study: 6.13 (2.46)], F(1, 18) = 4.574, p = 0.046, whereas no significant change was found in the control group [baseline: 5.58 (2.06); study: 5.73 (2.31)], F(1, 19) = 0.147, p = 0.706. No significant main effects or interactions were found for percent drinking days (Fig. 4 C) or drinks per day (Fig. 4 D). See the Supplementary Information for the full analysis.
Figure 4.
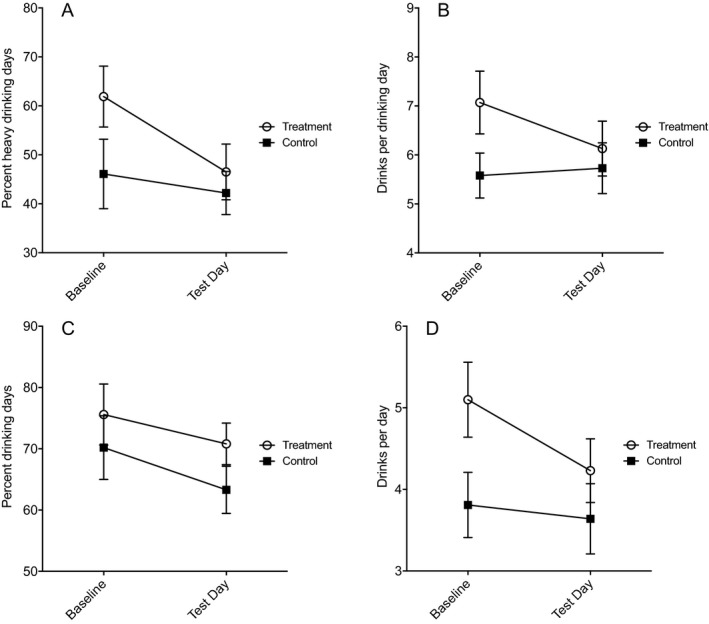
Self‐reported drinking in participants who completed the study using the Timeline Followback interview method at baseline (90 days before study) and test day (during study period). There was no significant effect of treatment on percentage of heavy drinking days (A), drinks per drinking day (B), percent drinking days (C), or drinks per day (D), but a trend (p = 0.069) was observed indicating decreased drinks per drinking day in the treatment group compared with the control group (B). Values are presented as mean ± standard error of the mean.
Craving and Mood
For the Short‐DAQ craving scale total score, there was a significant main effect of Time, F(3.6, 131.4) = 4.948, p = 0.002, η p 2 = 0.118, indicating an overall reduction in craving (pairwise comparisons showed significantly lower craving score at each time point in weeks 1 to 5 compared with baseline; p = 0.01 to 0.03 for all time points; Fig. 5 A), but no main effect of Treatment, F(1, 37) = 0.671, p = 0.418, η p 2 = 0.018, or significant Treatment × Time interaction, F(3.6, 131.4) = 0.163, p = 0.944, η p 2 = 0.004. Regarding Montgomery‐Asberg Depression Self‐Rating Scale total score, there was no significant main effect of Time, F(2, 74) = 1.544, p = 0.220, η p 2 = 0.040, Treatment, F(1, 37) = 0.266, p = 0.609, η p 2 = 0.007, or significant Treatment × Time interaction, F(2, 74) = 0.888, p = 0.416, η p 2 = 0.023 (Fig. 5 B).
Figure 5.
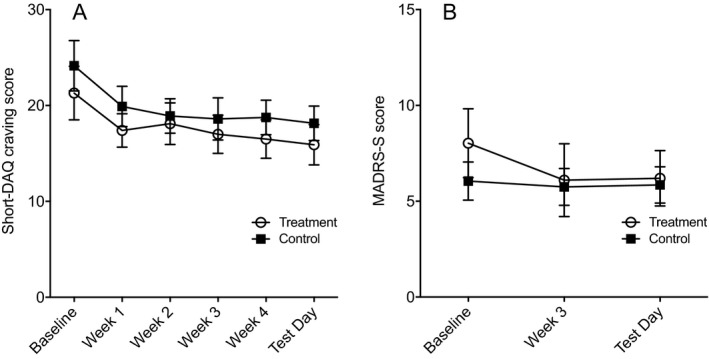
Self‐reported craving (A) and mood (B) at baseline, weekly visits, and test day for treatment group and control group in participants who completed the study. There were no significant differences between treatment groups. Values are presented as mean ± standard error of the mean. Short‐DAQ, Swedish shortened version of the Desire for Alcohol Questionnaire; MADRS‐S, Montgomery–Asberg Depression Self‐Rating Scale.
Cognitive Functions
Table 2 presents descriptive data, as well as p‐value and effect sizes for the Treatment × Time interaction for all outcomes for the different tasks of cognitive function (see Table S1 for ITT analysis). In summary, no statistically significant treatment effect of WM training on any of the neuropsychological tasks (including delay discounting) was found (all Treatment × Time interaction terms p‐value >0.1; see the Supplementary Information for full statistical analyses). However, there were significant main effects of time for some cognitive task outcomes (e.g., SSRT), F(1, 35) = 8.23, p = 0.007, η p 2 = 0.190, and RVP probability of hit, F(1, 35) = 4.560, p = 0.04, η p 2 = 0.115, indicative of overall improvement in performance at test day compared with baseline. In contrast, for CGT, the significant main effects of time for overall proportion bet, F(1, 35) = 6.688, p = 0.014, η p 2 = 0.160, and risk taking, F(1, 35) = 9.642, p = 0.004, η p 2 = 0.216, indicated more impulsive behavior at test day.
Table 2.
Main Outcomes of the Tasks of Cognitive Functions at Baseline and Test Day for Each of the Experimental Conditions
| Active training | Control training | p | η p 2 | |||
|---|---|---|---|---|---|---|
| Baseline | Test day | Baseline | Test day | |||
| Digit Span task | ||||||
| Total score | 15.9 (4.0) | 18.5 (3.6) | 16.1 (3.7) | 16.5 (2.7) | 0.018 | 0.142 |
| Backward score | 5.9 (2.4) | 7.7 (2.1) | 6.7 (2.0) | 6.9 (2.0) | 0.018 | 0.142 |
| Forward score | 9.9 (2.1) | 10.8 (2.1) | 9.4 (2.1) | 9.7 (1.4) | 0.273 | 0.032 |
| SWM task | ||||||
| Strategy | 30.9 (7.6) | 30.4 (6.4) | 32.5 (7.1) | 30.5 (7.1) | 0.452 | 0.016 |
| Within errors | 1.6 (2.3) | 1.4 (2.1) | 1.6 (2.7) | 1.0 (2.4) | 0.711 | 0.004 |
| Between errors | 21.8 (18.4) | 20.3 (17.2) | 22.2 (13.3) | 19.8 (16.2) | 0.855 | 0.001 |
| Total errors | 22.7 (18.8) | 20.8 (17.8) | 23.0 (13.4) | 19.9 (16.2) | 0.812 | 0.002 |
| Monetary choice | ||||||
| K small | 0.0188 (0.022) | 0.0183 (0.019) | 0.0080 (0.007) | 0.0127 (0.019) | 0.644 | 0.006 |
| K medium | 0.0111 (0.015) | 0.0182 (0.022) | 0.0133 (0.016) | 0.0099 (0.015) | 0.112 | 0.067 |
| K large | 0.0063 (0.007) | 0.0080 (0.010) | 0.0050 (0.006) | 0.0037 (0.003) | 0.442 | 0.016 |
| K total | 0.0098 (0.001) | 0.0128 (0.014) | 0.0073 (0.007) | 0.0065 (0.007) | 0.277 | 0.032 |
| Stop Signal Task | ||||||
| Successful stops (%) | 59.4 (13.5) | 56.4 (9.7) | 59.2 (11.1) | 56.8 (11.3) | 0.858 | 0.001 |
| SSRT | 187.4 (40.3) | 172.2 (45.4) | 195.4 (55.4) | 170.1 (35.6) | 0.478 | 0.014 |
| Median Go RT (ms) | 640 (199) | 576 (199) | 604 (187) | 592 (168) | 0.095 | 0.078 |
| Go RT variability (SD) | 245 (199) | 145 (50) | 182 (116) | 167 (83) | 0.130 | 0.064 |
| RVP | ||||||
| Mean latency (ms) | 446 (112) | 407 (69) | 446 (95) | 408 (59) | 0.967 | 0.000 |
| Probability of hit | 0.61 (0.24) | 0.64 (0.22) | 0.53 (0.25) | 0.61 (0.27) | 0.331 | 0.027 |
| Probability of false alarm | 0.005 (0.008) | 0.004 (0.005) | 0.006 (0.006) | 0.006 (0.006) | 0.342 | 0.026 |
| CGT | ||||||
| Deliberation time | 2,496 (673) | 2,332 (739) | 2,298 (576) | 2,096 (607) | 0.864 | 0.001 |
| Overall proportion bet | 0.55 (0.15) | 0.58 (0.08) | 0.50 (0.15) | 0.55 (0.14) | 0.698 | 0.004 |
| Risk taking | 0.58 (0.16) | 0.63 (0.08) | 0.53 (0.16) | 0.59 (0.14) | 0.711 | 0.004 |
| Delay aversion | 0.10 (0.15) | 0.16 (0.19) | 0.12 (0.15) | 0.09 (0.14) | 0.059 | 0.099 |
| SOC (5‐move problems) | ||||||
| Mean moves | 6.7 (1.3) | 6.3 (1.1) | 7.0 (1.3) | 7.3 (1.9) | 0.176 | 0.052 |
| Problems solved in minimum moves | 2.2 (1.3) | 2.6 (1.1) | 2.2 (1.0) | 2.0 (1.2) | 0.190 | 0.049 |
p‐Values and effect sizes are shown for the Treatment × Time interaction in the mixed ANOVA per‐protocol analysis, see Supplementary Information for full statistical analysis.
CGT, Cambridge Gambling Task; RT, response time; RVP, Rapid Visual Processing task; SOC, Stockings of Cambridge task; SSRT, stop‐signal reaction time; SWM, Spatial Working Memory. η p 2, partial eta squared.
Subgroup Analysis
In the subgroup analysis based on the median split of baseline levels of the primary outcomes (Digit Span total score; SWM total errors; percent heavy drinking), we did not find any significant Treatment × Time interactions for neither the low nor the high groups (all interaction terms p‐value >0.05) for any of the outcomes. Further, in exploratory regression analyses investigating baseline outcomes as continuous variables rather than median split, similarly no significant interactions were found (all p > 0.1). Finally, in a separate post hoc rate dependence analysis, we calculated Oldham's correlation and found no evidence of rate dependence for any primary outcome, with no correlations larger than 0.3 in the active treatment group (see the Supplementary Information for full statistical analysis).
Cognitive Training Data
There was no difference between treatment groups in mean number of completed training sessions overall [treatment 19.7; control 20.2; t(48) = −0.194, p = 0.847] or among study completers [treatment 23.8; control 23.7; t(37) = 0.140, p = 0.889].
The active treatment group exhibited a statistically significant training index improvement from baseline to test day, F(1,18) = 169.91, p < 0.001, η p 2 = 0.904, accompanied by a significant increase in mean number of completed WM items over time, main effect: F(24, 504) = 13.75, p < 0.0001, η p 2 = 0.396. As expected, the control group had a constant training index and number of completed items across the training sessions.
Within the active treatment group, there were positive correlations between improvement in training index and improvement in Digit Span total score (r = 0.548, p = 0.008), Digit Span forward score (r = 0.409, p = 0.058), and Digit Span backward score (r = 0.503, p = 0.017).
Discussion
The present study is to our knowledge the first randomized controlled trial investigating the effect of computerized WM training in a clinical sample of patients with AUD. The main finding was that WM training significantly improved verbal, but not spatial, WM function. No significant treatment effect was found on the primary drinking outcome of heavy drinking, whereas a trend was observed indicating that WM training may reduce the number of drinks per drinking day. No effect of WM training was found on craving, mood, or other cognitive functions. The WM training was a demanding intervention (5 sessions per week) and was administered online in the homes of the participants. Despite this, more than 75% of participants completed 20 sessions of cognitive training, and no adverse events were reported, suggesting that such a cost‐effective intervention is feasible to administer in outpatient AUD patients.
Similar to previous studies in healthy control subjects (Dahlin et al., 2008; Jaeggi et al., 2008; Li et al., 2008) and other patient populations (Klingberg et al., 2005; Westerberg et al., 2007), the present study also found a statistically significant effect of repeated adaptive WM training on verbal WM capacity in AUD patients. Our results are also partly in line with the previous WM training studies indicating that WM training can improve WM function in alcohol dependence (Snider et al., 2018), heavy drinkers (Houben et al., 2011), and opioid use disorders (Rass et al., 2015). Similar to the current study, Rass and colleagues (2015) also found a significant effect of WM training on the Digit Span task manifested as improvement in backward score with no effect on forward score. Even though the Digit Span is widely used as a task of verbal WM function, it has been proposed that the backward task is a better measure of pure WM function, since it requires active manipulation of information (Gathercole et al., 2004). Within the active treatment group, we also found significant correlations between improvement in the training index and Digit Span score improvement, suggesting that the effect was mediated by the actual cognitive training performance. Taken together, our findings on WM outcomes partly corroborate previous research and further suggest that a clinical sample of AUD patients is receptive to targeted training of a specific cognitive domain such as verbal WM.
In contrast to Rass and colleagues (2015), however, we did not find a significant effect on visuospatial WM. The reason for this discrepancy is not clear and was surprising given that the majority of Cogmed training exercises are visuospatial in nature. One possible explanation for our results is that the putative negative effects of alcohol use are more severe and evident on visuospatial WM than verbal WM function, rendering the former more resistant to training for the time period observed in the present study. However, in a meta‐analysis of cognitive deficits in AUD (Stavro et al., 2013), the overall effect sizes were in a similar range (approximately Cohen's d 0.35 to 0.55) with overlapping confidence intervals for all related constructs (i.e., WM [verbal], visuospatial, visual learning/memory, and verbal learning/memory). Furthermore, all these constructs were still impaired but had recovered to a similar degree in AUD patients with long‐term abstinence (Cohen's d 0.20 to 0.25). This indicates that, in general, visuospatial WM is not more severely impaired or less prone to recovery than verbal WM in AUD patients and should therefore not explain our results. Another possibility is that our sample of AUD patients for some reason did not exhibit any impairments in SWM task performance, even at baseline. This is supported by the fact that there was no main effect of time (i.e., no significant change between baseline and test day) for any of the SWM task outcomes. Furthermore, in a previous study utilizing the same SWM task, Lawrence and colleagues (2009b) found that patients with alcohol dependence performed more errors (mean ± standard deviation [SD]: 40.3 ± 30.0) compared with healthy controls (22.8 ± 21.4), whose results on the other hand were in the same range as the AUD patients in the current study (25.2 ± 16.0). Taken together, the reason for lack of effect of WM training on visuospatial WM in our sample is not clear but can possibly be explained in part by lack of visuospatial WM impairment at baseline.
The neurobiological mechanism of the WM training in AUD patients is currently not known. Prior studies have indicated that WM training mainly affects the frontoparietal cortical regions responsible for both WM and attention (Constantinidis and Klingberg, 2016) Furthermore, positron‐emission tomography neuroimaging studies have indicated that WM improvement through cognitive training is associated with changes in dopaminergic neurotransmission, affecting cortical D1 receptor density (McNab et al., 2009) and striatal dopamine release targeting D2 receptors (Bäckman et al., 2011). Since alcohol induces dopamine release (Boileau et al., 2003; Di Chiara and Imperato, 1988) and AUD patients exhibit dysregulated dopaminergic transmission in both the striatum (Heinz et al., 2005) and frontal cortex (Narendran et al., 2014), one might hypothesize that AUD patients are resistant to dopamine‐dependent WM training. However, our findings suggest that these dopaminergic deficits in AUD patients do not hinder verbal WM improvement. Furthermore, our results indicate that it is possible to improve AUD patient's verbal WM capacity through repeated daily WM training, despite continued alcohol intake during the training period.
We found no statistically significant effect of WM training on self‐reported drinking outcomes. It is possible that there is an actual treatment effect that we failed to detect because of lack of power given our limited sample size. This is supported by the fact that the mean reduction in HDD, even though not statistically significant, indeed was greater in the treatment group (−11.5%) compared with controls (−3.4%). Furthermore, there was a trend in the secondary outcome, drinks per drinking day, suggesting a putative treatment effect in favor of WM training compared with control training. Although speculative, this may indicate that any potential clinical effect of WM training on drinking could be via improvement in impulse control when the drinking behavior is initiated, but future studies are needed to confirm this tentative finding in experimental conditions or via real‐time data collection. In contrast to our findings, a previous online study of heavy drinkers did find a significant effect of WM training on drinking outcomes (Houben et al., 2011). It is thus possible that WM training has an effect on drinking behavior in individuals with less severe substance‐related problems, whereas the effect is diminished in the more severe phenotype of AUD. Another possibility is that the putative benefits of WM training in AUD populations may be evident only if WM training is administered adjunct to evidence‐based AUD treatments, which address coping with craving and other alcohol use behaviors.
The current study found no evidence of WM training improving other cognitive functions (i.e., transfer effects), as assessed by a wide range of neuropsychological tests. Previous research has been inconsistent to what degree WM training induces such transfer effects. Some studies have indeed found that WM training can improve attention (Bigorra et al., 2016; Brehmer et al., 2009; Conklin et al., 2015; Green et al., 2012; Klingberg et al., 2005) and general fluid intelligence (Au et al., 2015; Jaeggi et al., 2008). It is, however, important to note that there are several studies that have failed to detect such effects (e.g., Owen et al., 2010), and there is an ongoing scientific debate regarding this question with conflicting results in different meta‐analyses (e.g., Au et al., 2015; Melby‐Lervåg et al., 2016). To what degree WM training can induce transfer effects in SUD patients remains, at present, an open question since very few studies have been conducted thus far. Two studies in SUD patients have indicated WM training transfer effects through improvement in delay discounting (Bickel et al., 2011) and impulse control (Brooks et al., 2017). In a recent study in opioid‐dependent patients, however, no transfer effects to other cognitive domains were found (Rass et al., 2015). This is line with the current findings, but some important considerations should be highlighted when interpreting these results. First, there was a significant main effect of time for some of the cognitive task outcomes, indicative of a spontaneous improvement in cognition or practice effects, which could conceal potential treatment effects. Second, it is possible that potential transfer effects are not possible to induce in AUD patients because of alcohol toxicity. Since acute alcohol intake impairs cognitive processes including memory function (e.g., Matthews and Silvers, 2004) and AUD is associated with widespread long‐term cognitive deficits (Stavro et al., 2013), it is possible that neurotoxic effects of both acute (during the study) and long‐term alcohol consumption diminish potential transfer effects to occur. Third, previous studies that did find transfer effects in SUD patients (Bickel et al., 2011; Brooks et al., 2017) were performed in an inpatient setting—suggesting that perhaps a controlled environment without substance intake is necessary for transfer effects to occur. Finally, it should be emphasized that the power of the present study to detect an effect with the Cohen's d effect size of 0.4, as found for inattentive symptoms in previous studies on WM training transfer effects (Spencer‐Smith and Klingberg, 2015), was only 28%. No definitive conclusions can therefore be made from the present negative finding.
There are several important limitations of the current study that are worth discussing. First, the sample size was limited, resulting in low power to detect medium‐to‐small effects. Furthermore, the follow‐up time was too short to possibly elucidate long‐term clinical benefits. Second, the AUD participants in the current study represent a subset of highly motivated patients. They are thus likely not representative of more clinically severe AUD patients, who may have more difficulties performing a demanding intervention such as 5 weeks of repeated cognitive training. Third, we did not include patients based on their baseline WM performance but rather on their DSM‐IV criteria. In a subgroup analysis, however, we did not find any significant moderating effect of verbal WM, visuospatial WM, or heavy drinking on the primary outcomes. However, our power was small given the limited sample size, and a previous study with greater sample size did find that WM training effect in alcohol dependence is different depending on baseline performance (Snider et al., 2018). Thus, it is possible that interventions such as cognitive WM training may be clinically beneficial when targeted toward patients with existing deficits in WM function. Future studies should consider stratifying patients on baseline cognitive deficits to more clearly identify phenotypical differences in treatment response. Finally, an important limitation is that all cognitive training was performed at home and not in a controlled environment. We can, thus, not exclude that other people performed the actual training or that participants were intoxicated by alcohol during training, which of course could affect the outcome of WM training. Future studies should consider conducting the WM training supervised at the research clinic or collecting self‐report data from participants on the training conditions if the training is done at home.
In summary, the current study showed preliminary data to suggest that it is possible to improve verbal WM function in AUD patients through repeated adaptive WM training performed online in the home environment. Our results did not however support an effect of WM training on drinking outcomes or transfer effects to improvement in other cognitive functions. Future studies should investigate whether administration of WM training as add‐on treatment to evidence‐based psychotherapeutic or pharmacological AUD treatments can improve treatment outcomes.
Supporting information
Supplementary Information. Material and methods.
Table S1. Sociodemographic and clinical characteristics of study participants at baseline of subjects who completed the entire study protocol (PP analysis).
Table S2. Main outcomes of the tasks of cognitive functions at baseline and test day for each of the experimental conditions.
Acknowledgments
The study was supported by project grants from the Swedish Society of Medicine (SLS500921 and SLS573691), Söderström‐Königska project grant (SLS‐313731), the foundation for Professor Bror Gadelius Memorial Fund, and the Research Council of the Swedish Alcohol Retailing Monopoly (FO2012‐0043). We thank Maija Konstenius, PhD, for extracting training progress information from Cogmed®. We also thank research nurses Margareta Gard‐Hedander and Else‐Britt Hillner, undergraduate student Maria Östman, and project coordinator Camilla Hellspong for excellent assistance in data collection.
Clinical trial registration: NCT02113618 at www.clinicaltrials.gov
References
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders 4th ed., text rev. American Psychiatric Association, Washington DC. [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders 5th ed. American Psychiatric Association, Arlington, VA. [Google Scholar]
- Anton RF, Moak DH, Latham P (1995) The Obsessive Compulsive Drinking Scale: a self‐rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res 19:92–99. [DOI] [PubMed] [Google Scholar]
- Au J, Sheehan E, Tsai N, Duncan GJ, Buschkuehl M, Jaeggi SM (2015) Improving fluid intelligence with training on working memory: a meta‐analysis. Psychon Bull Rev 22:366–377. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, Neely AS, Virta J, Laine M, Rinne JO (2011) Effects of working‐memory training on striatal dopamine release. Science 333:718. [DOI] [PubMed] [Google Scholar]
- Baddeley A (2003) Working memory: looking back and looking forward. Nat Rev Neurosci 4:829–839. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Moody L, Quisenberry A (2014) Computerized working‐memory training as a candidate adjunctive treatment for addiction. Alcohol Res Curr Rev 36:123–126. [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C (2011) Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry 69:260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigorra A, Garolera M, Guijarro S, Hervás A (2016) Long‐term far‐transfer effects of working memory training in children with ADHD: a randomized controlled trial. Eur Child Adolesc Psychiatry 25:853–867. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C (2004) Impulsivity in abstinent alcohol‐dependent patients: relation to control subjects and type 1‐/type 2‐like traits. Alcohol 34:133–150. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A (2003) Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49:226–231. [DOI] [PubMed] [Google Scholar]
- Brehmer Y, Westerberg H, Bellander M, Fürth D, Karlsson S, Bäckman L (2009) Working memory plasticity modulated by dopamine transporter genotype. Neurosci Lett 467:117–120. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Wiemerslage L, Burch KH, Maiorana SA, Cocolas E, Schiöth HB, Kamaloodien K, Stein DJ (2017) The impact of cognitive training in substance use disorder: the effect of working memory training on impulse control in methamphetamine users. Psychopharmacology 234:1911–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin H‐J, Reynaud M, Martinot J‐L (2007) Brain morphometry and cognitive performance in detoxified alcohol‐dependents with preserved psychosocial functioning. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 32:429–438. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Ogg RJ, Ashford JM, Scoggins MA, Zou P, Clark KN, Martin‐Elbahesh K, Hardy KK, Merchant TE, Jeha S, Huang L, Zhang H (2015) Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: a randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol 33:3894–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Klingberg T (2016) The neuroscience of working memory capacity and training. Nat Rev Neurosci 17:438–449. [DOI] [PubMed] [Google Scholar]
- Coull JT, Middleton HC, Robbins TW, Sahakian BJ (1995) Clonidine and diazepam have differential effects on tests of attention and learning. Psychopharmacology 120:322–332. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Nyberg L, Bäckman L, Neely AS (2008) Plasticity of executive functioning in young and older adults: immediate training gains, transfer, and long‐term maintenance. Psychol Aging 23:720–730. [DOI] [PubMed] [Google Scholar]
- Diamond A (2013) Executive functions. Annu Rev Psychol 64:135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O'Malley SS, Sher K (2010) Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol 15:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Justus A, Mazas C, Steinmetz JE (1999) Working memory, executive processes and the effects of alcohol on Go/No‐Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology 146:465–472. [DOI] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J (2002) Early‐onset alcoholism with conduct disorder: go/no go learning deficits, working memory capacity, and personality. Alcohol Clin Exp Res 26:186–206. [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H (2004) The structure of working memory from 4 to 15 years of age. Dev Psychol 40:177–190. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W (2006) Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addict Abingdon Engl 101:534–547. [DOI] [PubMed] [Google Scholar]
- Green CT, Long DL, Green D, Iosif A‐M, Dixon JF, Miller MR, Fassbender C, Schweitzer JB (2012) Will working memory training generalize to improve off‐task behavior in children with attention‐deficit/hyperactivity disorder? Neurother J Am Soc Exp Neurother 9:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Gründer G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rösch F, Mann K, Bartenstein P (2005) Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry 162:1515–1520. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P (2003) Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn 29:298–306. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ, Baddeley AD (2012) Executive functions and self‐regulation. Trends Cogn Sci 16:174–180. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW, Jansen A (2011) Getting a grip on drinking behavior: training working memory to reduce alcohol abuse. Psychol Sci 22:968–975. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ (2008) Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA 105:6829–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M (2007) The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev 17:213–233. [DOI] [PubMed] [Google Scholar]
- Khemiri L, Jayaram‐Lindström N, Hammarberg A (2017) Psychometric evaluation of a Swedish version of the Shortened Desires for Alcohol Questionnaire (Shortened‐DAQ). J Subst Abuse Treat 79:61–66. [DOI] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H (2013) Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsivity. Addict Abingdon Engl 108:506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Maraković NN (1996) Delay‐discounting probabilistic rewards: rates decrease as amounts increase. Psychon Bull Rev 3:100–104. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK (1999) Heroin addicts have higher discount rates for delayed rewards than non‐drug‐using controls. J Exp Psychol Gen 128:78–87. [DOI] [PubMed] [Google Scholar]
- Klingberg T (2010) Training and plasticity of working memory. Trends Cogn Sci (Regul Ed) 14:317–324. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, Gillberg CG, Forssberg H, Westerberg H (2005) Computerized training of working memory in children with ADHD–a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 44:177–186. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H (2002) Training of working memory in children with ADHD. J Clin Exp Neuropsychol 24:781–791. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L (2009a) Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology 207:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L (2009b) Problem gamblers share deficits in impulsive decision‐making with alcohol‐dependent individuals. Addict Abingdon Engl 104:1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre A‐P, Fama R, Sullivan EV (2017) Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res 41:1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre A‐P, Vabret F, Cauvin C, Pinon K, Allain P, Pitel A‐L, Eustache F, Beaunieux H (2012) Cognitive barriers to readiness to change in alcohol‐dependent patients. Alcohol Clin Exp Res 36:1542–1549. [DOI] [PubMed] [Google Scholar]
- Li S‐C, Schmiedek F, Huxhold O, Röcke C, Smith J, Lindenberger U (2008) Working memory plasticity in old age: practice gain, transfer, and maintenance. Psychol Aging 23:731–742. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA (1984) On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform 10:276–291. [DOI] [PubMed] [Google Scholar]
- Love A, James D, Willner P (1998) A comparison of two alcohol craving questionnaires. Addict Abingdon Engl 93:1091–1102. [DOI] [PubMed] [Google Scholar]
- Martelli C, Petillion A, Brunet‐Lecomte M, Miranda Marcos R, Chanraud S, Amirouche A, Letierce A, Kostogianni N, Lemaitre H, Aubin H‐J, Blecha L, Reynaud M, Martinot J‐L, Benyamina A (2017) Neuropsychological impairment in detoxified alcohol‐dependent subjects with preserved psychosocial functioning. Front Psychiatry 8:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Silvers JR (2004) The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiol Learn Mem 82:299–308. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T (2009) Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science 323:800–802. [DOI] [PubMed] [Google Scholar]
- Melby‐Lervåg M, Redick TS, Hulme C (2016) Working memory training does not improve performance on measures of intelligence or other measures of “Far Transfer”: evidence from a meta‐analytic review. Perspect Psychol Sci 11:512–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Mason NS, Paris J, Himes ML, Douaihy AB, Frankle WG (2014) Decreased prefrontal cortical dopamine transmission in alcoholism. Am J Psychiatry 171:881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël X, Van der Linden M, Brevers D, Campanella S, Hanak C, Kornreich C, Verbanck P (2012) The contribution of executive functions deficits to impaired episodic memory in individuals with alcoholism. Psychiatry Res 198:116–122. [DOI] [PubMed] [Google Scholar]
- Nowakowska‐Domagała K, Jabłkowska‐Górecka K, Mokros Ł, Koprowicz J, Pietras T (2017) Differences in the verbal fluency, working memory and executive functions in alcoholics: short‐term vs. long‐term abstainers. Psychiatry Res 249:1–8. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW (1990) Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28:1021–1034. [DOI] [PubMed] [Google Scholar]
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, Howard RJ, Ballard CG (2010) Putting brain training to the test. Nature 465:775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM (2001) Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology 154:243–250. [DOI] [PubMed] [Google Scholar]
- Quisenberry AJ, Snider SE, Bickel WK (2016) The return of rate dependence. Behav Anal (Wash DC) 16:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass O, Schacht RL, Buckheit K, Johnson MW, Strain EC, Mintzer MZ (2015) A randomized controlled trial of the effects of working memory training in methadone maintenance patients. Drug Alcohol Depend 156:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW (1999) Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci Off J Soc Neurosci 19:9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T (1982) Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 298:199–209. [DOI] [PubMed] [Google Scholar]
- Snider SE, Deshpande HU, Lisinski JM, Koffarnus MN, LaConte SM, Bickel WK (2018) Working memory training improves alcohol users' episodic future thinking: a rate‐dependent analysis. Biol Psychiatry Cogn Neurosci Neuroimaging 3:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Quisenberry AJ, Bickel WK (2016) Order in the absence of an effect: identifying rate‐dependent relationships. Behav Processes 127:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow‐back: a technique for assessing self‐reported alcohol consumption, in Measuring Alcohol Consumption: psychosocial and Biological Methods (Litten RZ, Allen JP. eds), pp 41–72. Humana Press, Totowa, NJ. [Google Scholar]
- Sofuoglu M (2010) Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addict Abingdon Engl 105:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer‐Smith M, Klingberg T (2015) Benefits of a working memory training program for inattention in daily life: a systematic review and meta‐analysis. PLoS One 10:e0119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S (2013) Widespread and sustained cognitive deficits in alcoholism: a meta‐analysis. Addict Biol 18:203–213. [DOI] [PubMed] [Google Scholar]
- Svanborg P, Asberg M (2001) A comparison between the Beck Depression Inventory (BDI) and the self‐rating version of the Montgomery Asberg Depression Rating Scale (MADRS). J Affect Disord 64:203–216. [DOI] [PubMed] [Google Scholar]
- Verdejo‐Garcia A (2016) Cognitive training for substance use disorders: neuroscientific mechanisms. Neurosci Biobehav Rev 68:270–281. [DOI] [PubMed] [Google Scholar]
- Verdejo‐García A, Lawrence AJ, Clark L (2008) Impulsivity as a vulnerability marker for substance‐use disorders: review of findings from high‐risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 32:777–810. [DOI] [PubMed] [Google Scholar]
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Ostensson M‐L, Bartfai A, Klingberg T (2007) Computerized working memory training after stroke—a pilot study. Brain Inj 21:21–29. [DOI] [PubMed] [Google Scholar]
- de Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information. Material and methods.
Table S1. Sociodemographic and clinical characteristics of study participants at baseline of subjects who completed the entire study protocol (PP analysis).
Table S2. Main outcomes of the tasks of cognitive functions at baseline and test day for each of the experimental conditions.


