Abstract
Nuclear apoptosis‐inducing factor 1 (NAIF1) acts as an oncogene and involves in tumorigenesis in several cancers. However, the expression and mechanism of NAIF1 in osteosarcoma remains unclear. In this study, we demonstrated the downregulation of NAIF1 expression in both osteosarcoma tissues and cell lines. We next explored the potential role of NAIF1 in osteosarcoma cell proliferation and migration. The result showed that overexpression of NAIF1 evidently suppressed the cell proliferation and invasion of osteosarcoma. Furthermore, we investigated the potential mechanisms accounting for dysregulation of NAIF1 in osteosarcoma. The bioinformatic prediction and luciferase reporter assay revealed that miR‐128 is a direct upstream regulator of NAIF1 and regulates NAIF1 expression by binding the 3′‐UTR of NAIF1. Consistent with previous study, we found that miR‐128 was upregulated in both osteosarcoma tissues and cell lines. Moreover, miR‐128 expression levels were inversely correlated with that of NAIF1 in osteosarcoma tissues. Finally, functional assay showed that miR‐128 significantly suppressed osteosarcoma progression partially mediated by inhibiting NAIF1 expression. These data indicate that the miR‐128 and its target gene NAIF1 played important roles by regulating OS cell proliferation and migration phenotype.
Significance of the study
Osteosarcoma (OS) is the most common malignant bone tumour and the second leading cause of cancer‐related death affecting children and adolescents. Nuclear apoptosis‐inducing factor 1 (NAIF1) plays an inhibitory role in the initial steps of different carcinomas. However, the expression and mechanism of NAIF1 in osteosarcoma remains unclear. The data of this study indicated that the miR‐128 and its target gene NAIF1 played important roles by regulating OS cell proliferation and migration phenotype. It was demonstrated that NAIF1 would demonstrate important regulative effects and may be a promising therapeutic target of OS.
Keywords: miR‐128, NAIF1, osteosarcoma
1. INTRODUCTION
Osteosarcoma (OS) is the most common malignant bone tumour and the second leading cause of cancer‐related death affecting children and adolescents.1, 2 Despite the development of therapeutic strategies (including surgery and multiagent chemotherapy), the survival rate for metastatic OS remains at 20% for the past 30 years.3, 4 Therefore, identification of biomarkers and revealing the underlying molecular mechanism of OS are critical for OS diagnosis and treatment.
The human gene encoding nuclear apoptosis‐inducing factor 1 (NAIF1) is located on chromosome 9q34.11, and reported to repress the progression of several human cancers.5, 6 NAIF1 was downregulated or lost in gastric cancer tissues, plays an inhibitory role in the initial steps of gastric cancer genesis.6, 7 Fu et al showed that overexpression of NAIF1 had an antitumor effect on prostate cancer cell proliferation and migration.5 NAIF1 expression level in NSCLC tissues was suppressed, and restoration of NAIF1 in lung cancer cell inhibited cell proliferation and anchorage‐independent survival ability.8 However, the expression and role of NAIF1 in OS remains elusive.
MicroRNA (miRNA) is an abundant group of small noncoding RNA (with about 22 nucleotides). It controls expression of target gene by binding to the 3′ untranslated region (UTR) of their target mRNAs and plays an important role in a variety of biological processes including cell proliferation, apoptosis, differentiation, invasion, migration, and so on.9, 10, 11, 12 Accumulating studies showed that miRNAs are dysregulated in a variety of cancers and play a critical role in tumorigenesis.13, 14, 15, 16, 17, 18 Recent studies demonstrated that miRNAs have been recognized as critical regulators in development and progression of cancer including OS.19, 20
In this study, we first time revealed the deregulated expression of NAIF1 in OS and investigated the function of NAIF1 on OS cell proliferation and invasion. Furthermore, we identified miR‐128 as upstream regulator of NAIF1 to involve the progression of OS. In conclusion, NAIF1 acts as a tumour suppressor and may serve as a potential therapeutic target in OS.
2. MATERIALS AND METHODS
2.1. Human tissue specimens
Paired tissue specimens of OS and matched normal tissues were obtained, with informed consent, from 30 OS patients between 2015 and 2017 at Jilin University Sino‐Japanese Friendship Hospital. All the tissues were obtained at the time of surgery and immediately stored in liquid nitrogen until use. The Institute Research Medical Ethics Committee of Jilin University granted approval for this study.
2.2. Cell culture and transfection
Osteosarcoma cell lines (MG‐63 and U2OS) and human normal osteoblast cell line NHOst were obtained from the American Type Culture Collection (Manassas, VA, USA). STR profiles were used in the cell line identification and detection of cross‐contamination of mycoplasma. All cells were cultured in DMEM medium supplemented with 10% fetal bovine. Cultures were maintained at 37°C in a humidified atmosphere with 5% CO2.
MG‐63 and U2OS cells were seeded in 6‐well plates and transiently transfected with pcDNA‐NAIF1 using Lipofectamine 2000 (11668‐027, Invitrogen, Carlsbad, CA, USA), or transfected with miR‐128 inhibitor, or contransfected miR‐128 inhibitor with NAIF1 siRNA using Lipofectamine 2000 for 24 hours according to the manufacturer's instructions. The miR‐128 sequence is 5′‐UCACAGUGAACCGGUCUCUUU‐3′ while the miR‐218 inhibitor is 5′‐AGUGUCACUUGGCCAGAGAAA‐3′. All the cultured cells were divided into three groups: control group (untreated cells), pc‐DNA‐NC group (mock‐transfected cells), and pcDNA‐NAIF1 group (pcDNA‐NAIF1‐transfected cells).
2.3. Quantitative real‐time polymerase chain reaction
When the cultured cells grew to 70% to 80% confluency, they were used to extract the RNA. The extracted RNA with Trizol solution (15596‐026, Invitrogen, Carlsbad, CA, USA) was subjected to reverse transcription PCR to obtain cDNA with Primescript RT Reagent kit (RR047A, Takara, Japan) according to the manufacturer's instructions. miR‐128 expression was detected using a TaqMan miRNA RT‐PCR assays (Applied Biosystem, Waltham, MA, USA) with U6 transcript as internal control. The mRNA expression of NAIF1 was detected using a SYBR Green PCR Master Mix kit (Applied Biosystem) on the ABI‐Prism 7300 System, with GAPDH as internal control.21, 22 The specific primers used for the PCR reaction were as follows: NAIF1, 5′ GGCCCAATGGAATCAGCTACAG‐3′ (forward) and 5′‐GAAGAAACTGCTTGATTCTTCG‐3′ (reverse); GAPDH, 5′ATGTCGTGGAGTCTACTGGC‐3′ (forward) and 5′‐TGACCTTGCCCACAGCCTTG‐3′ (reverse).
2.4. Luciferase reporter gene assays
The 3′‐UTR of NAIF1 was amplifies to pGL3 luciferase promoter vector (53711‐5399, Promega, Madison, WI, USA) as previously described23 as NAIF1‐WT. Next, a Site‐Directed Mutagenesis Kit (E0054S, SBS Genetech, Beijing, People's Republic of China) was used to mutant putative binding site of miR‐128 as the NAIF1‐Mut. The HEK293T cells were cotransfected with miR‐128 mimics and NAIF1‐WT and NAIF1‐Mut for 48 hours. The luciferase activity was measured using a dual‐luciferase reporter assay kit (E1910, Promega, Madison, WI, USA) according to the manufacturer's protocol.
2.5. Western blotting analysis
Cells were lysed with a lysis reagent (79306, Sigma‐Aldrich, St. Louis, MO, USA), and the protein was quantified by a BCA assay (23225, Pierce, Rockford, IL, USA) and separated by SDS‐PAGE (10%) and detected by Western blot using polyclonal (rabbit) anti‐NAIF1 antibody (STJ Ltd, London, UK). Goat anti‐rabbit IgG (Pierce, Rockford, IL, USA) secondary antibody conjugated to horseradish peroxidase and ECL detection systems (SuperSignal West Femto, Pierce) were used for detection.
2.6. Cell proliferation assay
The 3‐(4, 5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay was adopted to assess cell viability as described previously.24
2.7. Cell migration assay
The cell migration ability was examined by wound healing assay as described previously.25 Briefly, 1 × 105 MG‐63 and U2OS cells were plated in 6‐well plate at 37°C for 24 hours. And then, we used a sterilized tip drawn a line lightly. The cells incubation at 37°C for 24 hours; then, cells were put into serum‐free medium for photographing with Olympus Inverted Microscope (Olympus Optical Co., Ltd., Tokyo, Japan). All the experiments were repeated in triplicate, and the relative migration ratio was calculated through detecting the relative ratio to the untreated control cell group.
2.8. Statistical analysis
Each experiment was repeated at least three times. Data were shown as mean ± SD and analysed using SPSS 19.0. Statistical comparisons between groups were analysed using Student's t test, and a two‐tailed P < 0.05 was considered to indicate statistical significance. A paired t test was used for the paired data analyses. Bonferroni method was used in multiple comparison that was conducted.
3. RESULTS
3.1. NAIF1 expression in both osteosarcoma tissues and cell lines
NAIF1 was reported to be downregulated and functions as oncogene in several human cancers.5, 6 To reveal the potential role of NAIF1 in OS, we detected the expression levels of NAIF1 in 30 OS specimens and paired normal bone tissue (NT). As shown in Figure 1A, significantly lower levels of NAIF1 were detected by RT‐qPCR in the OS samples, compared with those in normal bone tissue (P = 0.013). Next, we detected the expression of NAIF1 in OS cell lines (MG‐63 and U2OS) and human normal osteoblast cell line NHOst. We found that NAIF1 expression in OS cell lines (MG‐63 and U2OS) was much lower than that in the human normal osteoblast cell line NHOst (P = 0.021 and P = 0.012, Figure 1B).
Figure 1.
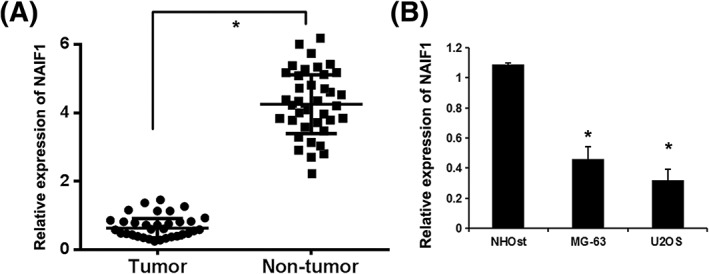
The expression levels of NAIF1 in OS tissue and cell lines. A, The expression levels of NAIF1 protein in 30 OS specimens and paired normal bone tissue (NT). *P < 0.05 vs NT group. B, The expression of NAIF1 protein in OS cell lines (MG‐63 and U2OS) and human normal osteoblast cell line NHOst. Data are presented as means ± SD from three independent experiments. *P < 0.05 vs NHOst group
3.2. NAIF1 in osteosarcoma proliferation and migration
In order to understand the molecular basis of these findings, we analysed the role of NAIF1 on OS proliferation and migration. We used MG‐63 and U2OS cell lines to transfect with pcDNA‐NAIF1 to overexpress NAIF1 in OS cells. Next, alteration of NAIF1 levels in these cells was confirmed by quantitative real‐time polymerase chain reaction (qRT‐PCR) (P < 0.001, Figure 2A). We analysed the role of NAIF1 on cell proliferation ability and migration ability. As shown in Figure 2B and 2C, cell proliferation ability was evidently repressed in pcDNA‐NAIF1 group in both MG‐63 and U2OS cells (P = 0.038 and P 0.045 in 48 hours and P = 0.012 and P = 0.021 in 72 hours). These data demonstrate that NAIF1 may repress OS cell growth in OS. As showed in Figure 2D and 2E, the wound healing assay showed that cell migration ability was evidently decreased in pcDNA‐NAIF1 group, indicating that NAIF1 may repress OS cell migration in OS (P = 0.025 and P = 0.032). In conclusion, these results indicated that NAIF1 may function as a tumour suppressor and repressed tumorigenesis in OS.
Figure 2.
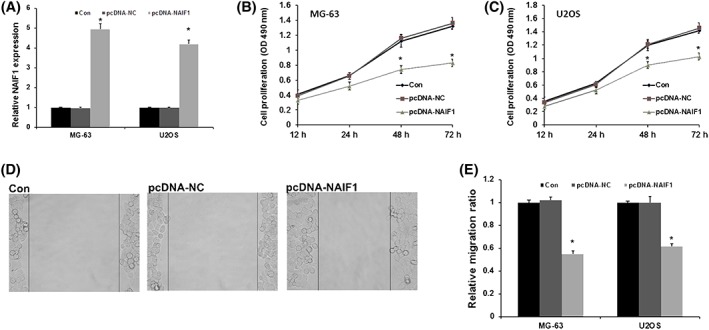
NAIF1 represses OS proliferation and migration. A, MG‐63 and U2OS cell lines to transfect with pcDNA‐NAIF1 to overexpress NAIF1 protein in OS cells. B, The role of NAIF1 on cell proliferation ability in MG‐63. C, The role of NAIF1 on cell proliferation ability in U2OS. D, The role of NAIF1 on cell migration ability. E, Data of the effect of NAIF1 on cell migration ability data are presented as means ± SD from three independent experiments. *P < 0.05 vs pc‐DNA‐NAIF1 group and NC group
3.3. NAIF1 is a direct target gene of miR‐128 in OS
Previous studies showed that miRNAs play important role in tumour progression by repressing its target gene at its 3′‐UTR. So, we performed bioinformatic analysis to predict the upstream regulator of NAIF1 in OS. In this study, TargetScan was used to predict the putative targets of miR‐128 (http://www.targetscan.org/). As shown in Figure 3A, miR‐128 could direct target the 3′‐UTR of the NAIF1. We next contransfected miR‐128 mimics and NAIF1‐WT or NAIF1‐Mut into HEK293 cell and analysed the luciferase activity a dual luciferase reporter assay. We found that miR‐128 mimics evidently repressed the luciferase activity in NAIF1‐WT group (P < 0.001) but failed to repress the luciferase activity in NAIF1‐Mut group (P = 0.865), indicating that miR‐128 specifically targets 3′‐UTR of NAIF1 mRNA to inhibit its translation in OS cells (Figure 3B). To further confirm NAIF1 as a direct target of miR‐128, we transfected miR‐128 mimics into MG‐63 and U2OS cells and then detect the expression of NAIF1 by using qRT‐PCR and western blot. As shown in Figure 3C and 3D, both mRNA and protein expression levels of NAIF1 were significantly downregulated by miR‐128 mimics in OS cells (MG‐63 and U2OS).
Figure 3.
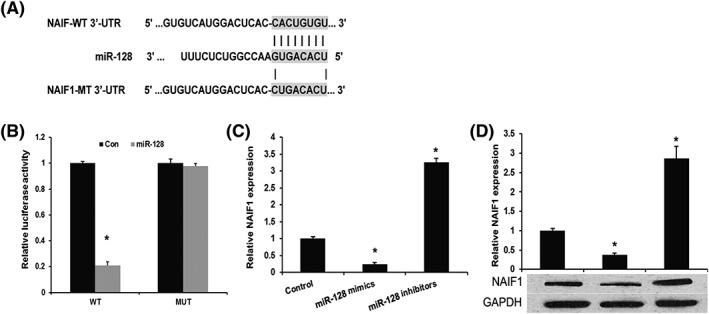
NAIF1 is a direct target gene of miR‐128 in OS. A, Sequence alignment of miR‐128, 3′‐UTR of NAIF1 and Mut‐3′‐UTR of NAIF1. B, Luciferase reporter assay. HEK293T cells were transiently cotransfected with Wt/Mut 3′‐UTR of NAIF1 with miRNAs as indicated. C, qPCR assay revealed the effects of miR‐128 on the expression levels of NAIF1 mRNA. D, Western blot assay revealed the effects of miR‐128 on the expression levels of NAIF1 protein. Data are presented as means ± SD from three independent experiments. *P < 0.05 vs control group
3.4. miR‐128 promotes OS progression by inhibiting NAIF1 expression
Previous studies showed controversial point on the expression of miR‐128 in OS. Liu et al showed that miR‐128 expression was downregulated in osteosarcoma tissues and OS cell lines,26 but Shen et al revealed upregulated miR‐128 in OS tissues compared with adjacent normal tissues.27 To further confirm the expression levels of miR‐128 in OS, we detected the miR‐128 expression in both OS tissues and cell lines. As shown in Figure 4A and 4B, miR‐128 expression was significantly increased in both OS tissues and cell lines compared with adjacent normal tissues and NHOst cell lines (P < 0.01 in each comparison). Next, we analysed the role of miR‐128‐mediated NAIF1 on OS proliferation and migration. As shown in Figure 4C and 4D, miR‐128 inhibitors evidently suppressed osteosarcoma cell proliferation and migration, which was rescued by NAIF1 siRNA. In conclusion, these results indicated that miR‐128 promotes OS progression by repressing NAIF1 expression. However, it should be noted that the expression of NAIF1 expression in the miR‐128 inhibitor and siNAIF1 group was not detected, and it is unclear if NAIF1 levels had returned to control level. Even though the results in these points were quite robust, it should also be considered in the lack of NAIF1 expression data.
Figure 4.
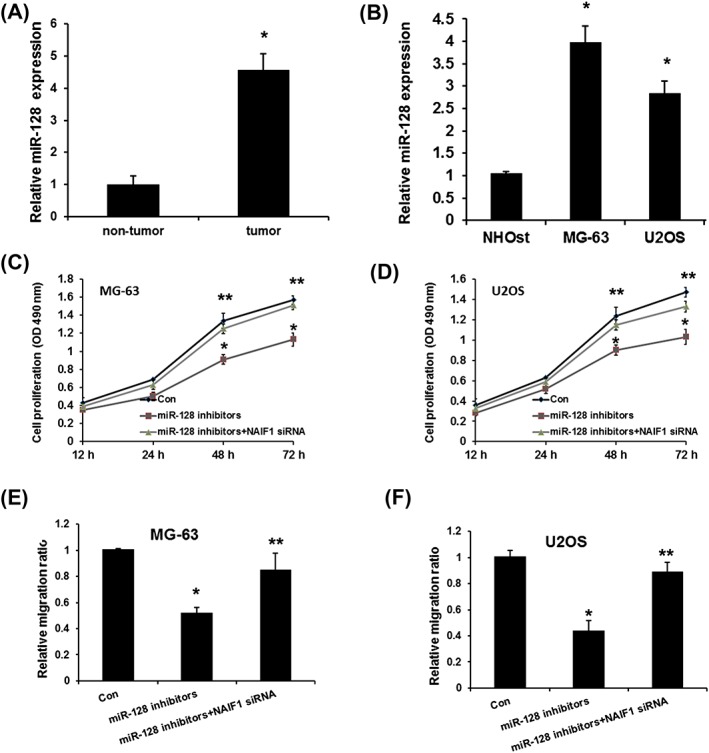
miR‐128 promotes OS proliferation and migration by inhibiting NAIF1 expression. A, Expression levels of miR‐128 in 30 OS specimens and paired normal bone tissue. B, miR‐128 expression in OS cell lines. C, Effect of miR‐128 on cell proliferation assay in MG‐63. D, Effect of miR‐128 on cell proliferation assay in U2OS. E, Effect of miR‐128 on cell migration assay in MG‐63. F, Effect of miR‐128 on cell migration assay in U2OS. Data are presented as means ± SD from three independent experiments. *P < 0.05 vs control group, *P < 0.05 vs control group, and ***P < 0.001 vs control group
4. DISCUSSION
The underlying mechanism of OS carcinogenesis is critical for predicting prognosis and developing therapeutic strategy. Therefore, it is urgent to investigate the potential molecule mechanism of osteosarcoma progression. In the present study, we indicated NAIF1, as a tumour suppressor, regulated by miR‐128, involving OS progression by repressing cell proliferation and migration.
NAIF1 functions as an tumour suppressor and represses tumour progression of several human cancers,5, 6 including gastric cancer genesis,6, 7 prostate cancer,5 and NSCLC.8 However, the expression and role of NAIF1 in OS remains unclear. In this study, we revealed the upregulation of NAIF1 expression in OS tissues and cell lines. Furthermore, pcDNA‐NAIF1 was used to overexpress NAIF1 expression in both MG‐63 and U2OS cell and found that overexpressed NAIF1 may repress OS cell proliferation and migration in OS. In conclusion, these results indicated that NAIF1 may function as a tumour suppressor and repressed tumorigenesis in OS. However, there was one limitation that should be noticed in this study. The detection of cell proliferation was based on MTT methods, and it cannot distinguish it from cell death events. The cells in different vitality status that were measured in equality might lead to bias. However, the conclusion of this study might not be influenced by this point.
Growing evidence shows that miRNAs have been linked to various types of cancers and play a variety of crucial regulatory functions related to cell growth, development, and differentiation, by repressing the target gene expression.28, 29 miR‐128 was reported as oncogene or tumour suppressor in various cancers. For example, miR‐128 was reported to be downregulated in cancer tissues and represses growth and metastasis of bladder cancer, lung cancer, and glioblastoma multiforme.30, 31, 32 However, different studies of the miR‐128 in carcinoma reported discordant conclusions. In this study, we found that miR‐128 expression was significantly increased in both OS tissues and cell lines; the further functional analysis revealed that miR‐128 promotes OS progression by inhibiting NAIF1 expression.
In conclusion, this study showed for the first time that NAIF1 was downregulated in OS tissue and cell lines. We also demonstrated that NAIF1 can suppress the proliferation and invasion of OS cells. Furthermore, we also showed miR‐24 as oncogenic effects by negatively regulating NAIF1 expression in OS, revealing a new avenue for treatment of OS.
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
Kong D, Zhang Z. NAIF1 suppresses osteosarcoma progression and is regulated by miR‐128. Cell Biochem Funct. 2018;36:443–449. 10.1002/cbf.3365
REFERENCES
- 1. Wang Z, Liu Z, Wu S. Long non‐coding RNA CTA sensitizes osteosarcoma cells to doxorubicin through inhibition of autophagy. Oncotarget. Mar 18. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ying B, Huang H, Li H, Song M, Wu S, Ying H. Procaine inhibits proliferation and migration and promotes cell apoptosis in osteosarcoma cells by upregulation of MicroRNA‐133b. Oncol Res. Mar 02. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Wang XX, Liu J, Tang YM, Hong L, Zeng Z, Tan GH. MicroRNA‐638 inhibits cell proliferation by targeting suppress PIM1 expression in human osteosarcoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. Jan 03. 2017. [DOI] [PubMed] [Google Scholar]
- 4. Wang Q, Cai J, Wang J, Xiong C, Zhao J. MiR‐143 inhibits EGFR‐signaling‐dependent osteosarcoma invasion. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine Dec. 2014;35(12):12743‐12748. [DOI] [PubMed] [Google Scholar]
- 5. Fu Y, Cao F. MicroRNA‐125a‐5p regulates cancer cell proliferation and migration through NAIF1 in prostate carcinoma. OncoTargets and Therapy. 2015;8:3827‐3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo Q, Zhao M, Zhong J, et al. NAIF1 is down‐regulated in gastric cancer and promotes apoptosis through the caspase‐9 pathway in human MKN45 cells. Oncol Rep Apr. 2011;25(4):1117‐1123. [DOI] [PubMed] [Google Scholar]
- 7. Yang M, Gu YY, Peng H, et al. NAIF1 inhibits gastric cancer cells migration and invasion via the MAPK pathways. J Cancer Res Clin Oncol. Jun 2015;141(6):1037‐1047. [DOI] [PubMed] [Google Scholar]
- 8. Zhao G, Liu L, Zhao T, et al. Upregulation of miR‐24 promotes cell proliferation by targeting NAIF1 in non‐small cell lung cancer. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine May. 2015;36(5):3693‐3701. [DOI] [PubMed] [Google Scholar]
- 9. Li Z, Lei H, Luo M, et al. DNA methylation downregulated mir‐10b acts as a tumor suppressor in gastric cancer. Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association Jan. 2015;18(1):43‐54. [DOI] [PubMed] [Google Scholar]
- 10. Xiao X, Tang C, Xiao S, Fu C, Yu P. Enhancement of proliferation and invasion by MicroRNA‐590‐5p via targeting PBRM1 in clear cell renal carcinoma cells. Oncol Res. 2013;20(11):537‐544. [DOI] [PubMed] [Google Scholar]
- 11. Yin WZ, Li F, Zhang L, Ren XP, Zhang N, Wen JF. Down‐regulation of microRNA‐205 promotes gastric cancer cell proliferation. Eur Rev Med Pharmacol Sci. 2014;18(7):1027‐1032. [PubMed] [Google Scholar]
- 12. Yang X, Ni W, Lei K. miR‐200b suppresses cell growth, migration and invasion by targeting Notch1 in nasopharyngeal carcinoma. Cell Physiol Biochem. 2013;32(5):1288‐1298. [DOI] [PubMed] [Google Scholar]
- 13. Liu Z, Mai C, Yang H, et al. Candidate tumour suppressor CCDC19 regulates miR‐184 direct targeting of C‐Myc thereby suppressing cell growth in non‐small cell lung cancers. J Cell Mol Med Aug. 2014;18(8):1667‐1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Q, Wang Y, Lu X, et al. MiR‐125b regulates epithelial‐mesenchymal transition via targeting Sema4C in paclitaxel‐resistant breast cancer cells. Oncotarget Feb 202015;6(5):3268‐3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gong B, Hu H, Chen J, et al. Caprin‐1 is a novel microRNA‐223 target for regulating the proliferation and invasion of human breast cancer cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. Sep. 2013;67(7):629‐636. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Raimondo M, Guha S, et al. Circulating microRNAs in pancreatic juice as candidate biomarkers of pancreatic cancer. J Cancer. 2014;5(8):696‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duan HF, Li XQ, Hu HY, et al. Functional elucidation of miR‐494 in the tumorigenesis of nasopharyngeal carcinoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. Mar 26. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu J, He ML, Wang L, et al. MiR‐26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. Jan 1. 2011;71(1):225‐233. [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Zhang S, Xu Y, et al. Upregulation of miR‐192 inhibits cell growth and invasion and induces cell apoptosis by targeting TCF7 in human osteosarcoma. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine Nov. 2016;37(11):15211‐15220. [DOI] [PubMed] [Google Scholar]
- 20. Wang M, Xie R, Si H, Shen B. Integrated bioinformatics analysis of miRNA expression in osteosarcoma. Artificial cells, nanomedicine, and biotechnology. Jun 17. 2016;1‐8. [DOI] [PubMed] [Google Scholar]
- 21. Yu H, Sun H, Bai Y, et al. MEF2D overexpression contributes to the progression of osteosarcoma. Gene. Jun 1. 2015;563(2):130‐135. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Q, Tang Q, Qin D, et al. Role of microRNA 30a targeting insulin receptor substrate 2 in colorectal tumorigenesis. Mol Cell Biol. Mar 2015;35(6):988‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Song L, Li D, Zhao Y, et al. miR‐218 suppressed the growth of lung carcinoma by reducing MEF2D expression. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. Sep 26. 2015. [DOI] [PubMed] [Google Scholar]
- 24. Han K, Zhao T, Chen X, et al. microRNA‐194 suppresses osteosarcoma cell proliferation and metastasis in vitro and in vivo by targeting CDH2 and IGF1R. Int J Oncol. Oct 2014;45(4):1437‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kong LY, Xue M, Zhang QC, Su CF. In vivo and in vitro effects of microRNA‐27a on proliferation, migration and invasion of breast cancer cells through targeting of SFRP1 gene via Wnt/beta‐catenin signaling pathway. Oncotarget. Feb 28. 2017;8(9):15507‐15519. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Liu X, Liang Z, Gao K, et al. MicroRNA‐128 inhibits EMT of human osteosarcoma cells by directly targeting integrin alpha2. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine Jun. 2016;37(6):7951‐7957. [DOI] [PubMed] [Google Scholar]
- 27. Shen L, Chen XD, Zhang YH. MicroRNA‐128 promotes proliferation in osteosarcoma cells by downregulating PTEN. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine Mar. 2014;35(3):2069‐2074. [DOI] [PubMed] [Google Scholar]
- 28. Zheng Q, Chen C, Guan H, Kang W, Yu C. Prognostic role of microRNAs in human gastrointestinal cancer: a systematic review and meta‐analysis. Oncotarget. Mar 29. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moridikia A, Mirzaei H, Sahebkar A, Salimian J. MicroRNAs: potential candidates for diagnosis and treatment of colorectal cancer. J Cell Physiol. Jan 16. 2017. [DOI] [PubMed] [Google Scholar]
- 30. Zhou XU, Qi L, Tong S, et al. miR‐128 downregulation promotes growth and metastasis of bladder cancer cells and involves VEGF‐C upregulation. Oncol Lett Nov. 2015;10(5):3183‐3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shan ZN, Tian R, Zhang M, et al. miR128‐1 inhibits the growth of glioblastoma multiforme and glioma stem‐like cells via targeting BMI1 and E2F3. Oncotarget. Nov 29. 2016;7(48):78813‐78826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao D, Han W, Liu X, Cui D, Chen Y. MicroRNA‐128 promotes apoptosis in lung cancer by directly targeting NIMA‐related kinase 2. Thoracic Cancer. Jul 2017;8(4):304‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]


