Abstract
Behavioral studies indicate that persons with Parkinson's disease have complexity dependent problems with the discrimination of auditory rhythms. Furthermore, neuroimaging studies show that rhythm processing activates many brain areas that overlap with areas affected by Parkinson's disease (PD). This study sought to investigate the neural correlates of rhythm processing in PD and healthy controls, with a particular focus on rhythmic complexity. We further aimed to investigate differences in brain activation during initial phases of rhythm processing. Functional magnetic resonance imaging was used to scan 15 persons with Parkinson's disease and 15 healthy controls while they listened to musical rhythms with two different levels of complexity. Rhythmic complexity had no significant effect on brain activations, but patients and controls showed differences in areas related to temporal auditory processing, notably bilateral planum temporale and inferior parietal lobule. We found indications of a particular sequential or phasic activation pattern of brain activity, where activity in caudate nucleus in the basal ganglia was time‐displaced by activation in the saliency network—comprised of anterior cingulate cortex and bilateral anterior insula—and cortical and subcortical motor areas, during the initial phases of listening to rhythms. We relate our findings to core PD pathology, and discuss the overall, rhythm processing related hyperactivity in PD as a possible dysfunction in specific basal ganglia mechanisms, and the phasic activation pattern in PD as a reflection of a lack of preparatory activation of task‐relevant brain networks for rhythm processing in PD.
Keywords: anterior cingulate, auditory, complexity, fMRI, insula, musical rhythm, Parkinson's disease, phasic, saliency network, temporal
1. INTRODUCTION
In Parkinson's disease (PD), the progressive death of dopaminergic neurons in the Substantia Nigra pars compacta (SNpc) in the basal ganglia disrupts several subcortico‐cortical loops in motor, associative, and limbic circuitry (Alexander, DeLong, & Strick, 1986; Lanciego, Luquin, & Obeso, 2012; Middleton & Strick, 2000), causing increasing motor and nonmotor symptoms with disease progression (Aarsland et al., 2004; Xia & Mao, 2012). Some motor symptoms in PD are responsive to dopaminergic pharmacological treatment (Mazzoni, Shabbott, & Cortes, 2012; Xia & Mao, 2012), but some gait‐specific symptoms (Knutsson, 1972) are relatively unresponsive (Blin, Ferrandez, & Serratrice, 1990; Smulders, Dale, Carlson‐Kuhta, Nutt, & Horak, 2016). However, while people with advanced stages of Parkinson's disease have problems with volitional movement, external rhythms facilitate movement (McIntosh, Brown, Rice, & Thaut, 1997), and in therapy, simple isochronous pulsed rhythms seem to be particularly effective in improving gait‐related symptoms (Thaut, McIntosh, McIntosh, & Hoemberg, 2001). Studies on rhythm perception in PD have found discrimination deficits, that is, difficulties in judging, when subsequent rhythms are identical or different. Some studies find this deficit to be more pronounced for simpler rhythms (Biswas, Hegde, Jhunjhunwala, & Pal, 2016; Grahn & Brett, 2009), while one study shows a more generalized deficit (Cameron, Pickett, Earhart, & Grahn, 2016). The effect of dopamine replacement therapies on rhythm perception indicates that medication improves beat detection in simple rhythms (Cameron et al., 2016; Geiser & Kaelin‐Lang, 2011), but has adverse effects on more complex rhythms (Cameron et al., 2016). Rhythm and rhythmic complexity modulate neural activity in areas and widespread networks across the brain and imaging studies in PD show abnormal activity in many of these areas, some of which are directly related to the pathology of PD. A better understanding of the modulatory effects of rhythmic complexity on behavior and the neural correlates, thereof could provide more specific knowledge about the neural basis for the temporal (Parker, Lamichhane, Caetano, & Narayanan, 2013; Schwartze & Kotz, 2015) and rhythm specific (Biswas et al., 2016; Cameron et al., 2016; Grahn & Brett, 2009) deficits in the disease.
Imaging studies in healthy subjects show higher basal ganglia activity for simple rhythms compared to complex rhythms (Chen, Penhune, & Zatorre, 2008a; Chen, Penhune, & Zatorre, 2008b; Geiser, Notter, & Gabrieli, 2012; Grahn, 2009; Grahn & Brett, 2007; Trost et al., 2014). Furthermore, there is evidence of complexity‐dependent, anticorrelated activity between the basal ganglia and the planum temporale (PT) in the posterior superior temporal gyrus (STG) (Geiser et al., 2012), an area sensitive to complex auditory patterns (Bengtsson et al., 2009; Chen et al., 2008a; Geiser et al., 2012; Herdener et al., 2014; Kung, Chen, Zatorre, & Penhune, 2013; Thaut, Trimarchi, & Parsons, 2014). A strong coupling of auditory and motor areas has consistently been found in imaging research on rhythm listening, involving basal ganglia, cerebellum, premotor cortex (PMC), and supplementary motor areas (SMA) (Bengtsson et al., 2009; Chen et al., 2008a; Chen et al., 2008b; Chen, Zatorre, & Penhune, 2006; Geiser et al., 2012; Grahn & Brett, 2007; Kung et al., 2013; Zatorre, Chen, & Penhune, 2007). Auditory‐motor coupling has become a dominant explanatory model for rhythm perception (Grahn, 2009; Kung et al., 2013; Todd & Lee, 2015; Zatorre et al., 2007), even in the absence of overt movement during listening (Burunat, Tsatsishvili, Brattico, & Toiviainen, 2017; Patel & Iversen, 2014). In healthy subjects, PMC and SMA show increased activity during tapping to more complex rhythms (Chen et al., 2006; Chen et al., 2008a), with increased functional coupling between PMC/SMA and the auditory cortex (Chen et al., 2006; Chen et al., 2008a; Chen, Penhune, & Zatorre, 2009) compared to simple rhythms. The auditory‐motor coupling during rhythm perception also integrates with a more widespread dorsal auditory pathway (Chapin et al., 2010; Warren, Wise, & Warren, 2005; Zatorre et al., 2007), which is somewhat right lateralized for music (Zatorre & Zarate, 2012). In this pathway, mechanisms of outcome predictions gear the PMC toward motor responses (Rauschecker, 2011; Zatorre & Zarate, 2012) through interactions of pattern recognition and segregation in STG/PT and information transformation and integration in the inferior parietal lobule (IPL) (Ragert, Fairhurst, & Keller, 2014). Like the PT and PMC, the IPL has been found to be modulated by rhythmic complexity (Bolger, Coull, & Schon, 2014; Grahn & Rowe, 2013; Lewis, Wing, Pope, Praamstra, & Miall, 2004; Thaut et al., 2014; Vuust, Roepstorff, Wallentin, Mouridsen, & Ostergaard, 2006). Studies in healthy subjects also show that more complex rhythms increase the functional connectivity between basal ganglia and more frontal executive networks (Chapin et al., 2010; Kung et al., 2013). In this context, the anterior insula (AIN) is of particular interest, as it interfaces with most of the rhythm complexity modulated areas described above and is also a part of the saliency network (SN), which plays a role in the dynamic regulation of larger network states (Goulden et al., 2014; Menon & Uddin, 2010; Sridharan, Levitin, & Menon, 2008) and upregulates brain areas needed for active cognition (Menon, 2011). Crucially, the AIN is modulated by various operationalizations of rhythmic complexity (Alluri et al., 2012; Altmann, Henning, Doring, & Kaiser, 2008; Chapin et al., 2010; Jerde, Childs, Handy, Nagode, & Pardo, 2011; Jungblut, Huber, Pustelniak, & Schnitker, 2012; Jungblut, Huber, & Schnitker, 2016; Lewis et al., 2004; Vuust et al., 2006), indicating that rhythmic complexity indeed affects large‐scale and brain wide network dynamics.
The brain areas listed above show abnormal activity in PD during various forms of rhythm processing, relative to healthy subjects. Audio‐motor coupling is integral to the underlying assumption in the literature on cue‐based therapies in PD, with external rhythms replacing a “broken clock” in PD through the effect of auditory entrainment of motor circuits (Nombela, Hughes, Owen, & Grahn, 2013). In PD, general PMC hyperactivity is found, modulated by tempo during finger‐tapping synchronization (Samuel et al., 1997; Yu, Sternad, Corcos, & Vaillancourt, 2007). Hyperactivity in the PMC in PD during movement has been explained as an externally driven compensatory recruitment of parallel motor circuits, to compensate for dysfunction in the basal ganglia (Sabatini et al., 2000; te Woerd, Oostenveld, Bloem, de Lange, & Praamstra, 2015). In PD, general IPL hyperactivity (Samuel et al., 1997) increases with complex motor tasks (Catalan, Ishii, Honda, Samii, & Hallett, 1999; Lewis et al., 2004; Wu & Hallett, 2005), and abnormal interaction between basal ganglia and widespread executive networks are found (Monchi, Petrides, Mejia‐Constain, & Strafella, 2007; Shine et al., 2013), with load‐dependent decoupling between frontal and motor areas (Rowe et al., 2002), also during timing and time perception tasks (Narayanan, Rodnitzky, & Uc, 2013; Parker et al., 2013). The insula, believed to be one of the earliest areas affected by dopamine depletion (Kish, Shannak, & Hornykiewicz, 1988; Putcha, Ross, Cronin‐Golomb, Janes, & Stern, 2015) and Lewy body accumulation (the second obligatory pathological hallmark of the disease (Poewe et al., 2017)), show abnormal activity in PD (Christopher, Koshimori, Lang, Criaud, & Strafella, 2014; Criaud et al., 2016), with hyperactivation during rhythm synchronization (Cerasa et al., 2006) and finger tapping (Caproni et al., 2013) tasks.
One crucial aspect of rhythm processing, namely that rhythms unfold in time, is a somewhat neglected topic in the neuroscience of rhythm (Fitch, 2013), but a more complex view of basal ganglia activity during rhythm processing is emerging, where such phasic aspects are latent, ascribing specific roles for individual structures like the putamen and caudate nucleus during rhythm processing. Networks involving the putamen seem to have a central role in predicting and maintaining (Chapin et al., 2010; Grahn & Rowe, 2013; Jungblut et al., 2012; Lewis et al., 2004) a stable pulse percept (Chapin et al., 2010; Patel & Iversen, 2014), after a beat (i.e., perception of an underlying steady pulse) has been established (Chapin et al., 2010; Grahn & Rowe, 2013; Lewis et al., 2004), while the caudate nucleus is activated by prediction errors, that is, violations of the expected rhythmic structure (Grahn & Rowe, 2013). Percepts of more complex rhythmic patterns are harder to establish, and basal ganglia connected network activity might need more time for “activations to develop” during the processing of complex rhythms, which indicate both time and complexity‐dependent functional connectivity between basal ganglia and other brain networks during different phases of rhythm processing (Chapin et al., 2010; Chen et al., 2006; Chen et al., 2008a; Kung et al., 2013). In healthy subjects, studies show larger, network‐like changes during processing of rhythms in the span of 5–10 s (Chapin et al., 2010; Rao, Mayer, & Harrington, 2001). This time‐scale fits our own clinical observations in cue facilitation in PD, where rhythmic cues facilitate movement within the order of several seconds. If different areas within the striatum have different functional properties related to establishing and maintaining rhythmic percepts at these time‐scales, and auditory‐motor entrainment of the “broken clock” (Nombela et al., 2013) rapidly improves rhythm processing in PD, investigating phasic brain activity changes—that is, whether different brain areas are activated in different sequential stages—during the initial phases of rhythm processing could shed light on the neuronal mechanisms behind cue‐based gait facilitation in PD.
In this study, we performed whole brain fMRI scans on healthy subjects and persons with Parkinson's disease, while they listened to two different rhythms, one simple and one complex. The aim was to investigate group differences in neural activity during rhythm processing, possible group effects related to rhythmic complexity, phasic differences in brain activation during different temporal stages of rhythm processing, and finally possible interactions between phase and complexity. We hypnotized that overall group differences would reveal compensatory activity in areas outside the basal ganglia, and that there would be an interaction effect between rhythmic complexity and group. Finally, we aimed to investigate the phasic development of brain activation at a time‐scale relevant for gait facilitation. We predicted phasic activation differences in striatal areas involved in establishing rhythmic percepts as well as brain wide activity differences, particularly in areas involved in network dynamics, such as nodes in the saliency network.
2. MATERIALS AND METHODS
2.1. Participants
For this study, 15 volunteers with PD (6 female) were recruited with the help of the National Parkinson's organization of Norway. Fifteen healthy controls (8 female) were recruited, group‐matched for age, education level, as well as for musical expertise. A minimum Mini Mental Status (MMS) (Folstein, Folstein, & McHugh, 1975) test score of 24 was set as a criterion in both groups to exclude patients with cognitive impairment indicative of dementia. All participants were right handed by self‐report. The Unified Parkinson's disease rating scale III (UPDRS‐III) (Fahn et al., 1987) was administered in the PD‐group. All PD‐participants—except one newly diagnosed de novo patient—were in medication regimens (LDOPA, D2‐agonists, inhibitors) at the time of the fMRI‐scan. (See Table 1 for an overview of the groups). All procedures were approved by the Regional Committee for Medical and Health Research Ethics (REK no 2014/1915) and carried out in accordance with the code of Ethics of the World Medical Association, Declaration of Helsinki. Before the tests, all participants gave written informed consent to participate in the study. Participants were compensated with 100NOK for participation in this study.
Table 1.
Group characteristics
| N (F) | Age (SD/min/max) | Edu (SD/min/max) | MMS (SD/min/max) | |
|---|---|---|---|---|
| PD | 15 (6) | 65.6 (12.38/40/81) | 14.0 (3.14/9/18) | 28.07 (1.16/26/30) |
| HC | 15 (8) | 64.9 (11.33/40/78) | 15.2 (1.78/12/18) | 28.67 (1.35/25/30) |
| Diff. | t‐test | p < .7 | p < .21 | p < .2 |
| Parkinson's disease group: | ||||||
|---|---|---|---|---|---|---|
| Sex | UPDRS‐III | Symptoms | Diagnosis | LD | IN | D2 |
| Male | 21 | 13 | 15 | X | X | |
| Male | 17 | 4 | 6 | X | X | |
| Female | 21 | 6 | 6 | X | X | |
| Female | 11 | 3 | 4 | X | X | X |
| Female | 11 | 3 | 5 | X | X | X |
| Male | 16 | 9 | 17 | X | X | X |
| Female | 28 | 6 | 6 | X | X | |
| Male | 23 | 4 | 4 | X | X | |
| Male | 18 | 2 | 3 | X | X | |
| Male | 17 | 8 | 10 | X | X | |
| Male | 14 | 2 | 8 | X | X | |
| Female | 20 | 10 | 12 | X | X | X |
| Male | 22 | 4 | 6 | X | X | |
| Female | 13 | 1 | 1 | |||
| Male | 13 | 7 | 5 | X | X | X |
PD = Parkinson's group. HC = Healthy controls. M = Male. F = Female. Edu = Years of education. In all columns: Means (standard deviations / minimum / maximum). Bottom table: UPDRS‐III = Unified Parkinson's disease rating scale, part III. Symptoms/Diagnosis: Years since. LD = Levodopa. IN = Inhibitors. D2 = D2‐agonists.
2.2. Stimuli rating of complexity
For this study, two rhythms used were chosen from the stimulus pool of a preceding online listening survey with 19 PDs and 19 HCs (including all participants of the current study). In the online survey, participants rated the perceived complexity of a total of 60 rhythmic stimuli (10 rhythms with variations on 3 tempi × 2 modes). Based on Jeff Pressing's model for calculating cognitive complexity in rhythm (Pressing, 1999; Toussaint, 2013), 10 rhythmic patterns in 4/4 m were constructed and each pattern was repeated 8 times. (See Supporting Information Table S1 for more details on the 10 patterns and the construction of the stimuli used for the online test). For each stimulus, presented in random order, participants answered the question “How complex do you perceive this rhythm to be?” by rating the stimuli on a 11‐point Likert‐scales (ranging from “Very simple” to “Very complex”).
Group scores were analyzed using SPSS (Version 24.0.0.0/IBM). For overall complexity ratings across all stimuli, there was a significant difference between the two groups (t(35.58) = 2.45, p = .019), with the PD‐group giving higher complexity ratings than the healthy controls. Two rhythms, one simple (Rhythm#1, ranked as #1 for complexity—that is, the least complex rhythm—in both groups) and one more complex (Rhythm#5, ranked as #7 by in both groups) were chosen for the fMRI‐paradigm in the current study. Complexity ratings for the two rhythms were significantly different within both groups (both p < .01). Between group comparisons showed a significant difference for the simple rhythm (p = .003, with the PD‐group giving higher ratings), while the more complex did not (p = .092). Recalculating of the scores of the two chosen rhythms for the 15 + 15 participants in the current fMRI‐study, these values were p = .004 for the simple rhythm, and p = .189 for the more complex rhythm. (see Supporting Information Table S1 for between group differences for all 10 rhythms for the original).
2.3. Stimuli construction for fMRI
Through testing, the two rhythms from the online listening‐test were sonically reshaped to yield maximum salience in the fMRI listening situation with protective in‐ear foam plugs. A deep, multilayered bass sound in two octaves as well as a deep‐bass drum sound was used to penetrate the scanner noise and to place the general character of the stimuli in a different frequency range than the eigenfrequency of the scanner during the EPI‐sequence. In addition, the simple, isochronous stimulus was slightly altered to clearly mark the end of each pattern. Tempo was set to 120 BPM, and eight repetitions of the 2 s long patterns resulted in 16 s long stimuli presentations for the analysis.
For each stimulus, the eight repetitions contained an alternating piano chord at the first position of every bar to mark the beginning of the bar (examples of the two stimuli can be found online, see Supporting Information for further details). For variation, two versions of each rhythm were constructed, one in major and one in minor mode. As no effect of mode was found in the analysis of the online listening‐test, the choice to include these variations was made solely for the benefit of the participants, offering them a minimum amount of variation during the long scan. The final set of four different stimuli was saved as stereo, 16bit, 44.100 hz wav‐files.
2.4. Experimental design
Before scanning, the participants underwent familiarization with the sound of the stimuli and the sound of the scanner. The stimuli were presented via headphones from a laptop computer. The familiarization consisted of playing a recording of scanner noise alone, musical excerpts of the rhythms used in the study (approx. 20 s) without scanner noise, and musical excerpts superimposed on top of scanner noise (approx. 20 s). This was followed by a short explanation of a covert attentional oddball omission task for a second part of the study, not further described or analyzed in this article (this analysis will be reported elsewhere and is simply referred to as “oddball” later in this article). For the second part of the familiarization period, the participants were provided with protective in‐ear foam plugs and the volume of the presentation was turned up to simulate the experience of the sound levels in the scanner, to minimize surprise for the participants in the scanner. All participants indicated that they could clearly hear the stimuli.
The participants kept the in‐ear foam plugs in place and were escorted to the scanner room, where they were placed comfortably in the scanner and fitted with MRI‐compatible headphones with additional physical noise cancelation‐foamed shells, and MRI‐compatible video goggles. During stimulus presentation of the first 16 s analyzed in this article, participants listening passively without performing any additional task. The presentation of stimuli and synchronization with the scanner was implemented in E‐Prime (Ver 2.3 Professional, Psychology Software Tools, Pittsburgh, PA).
Before each trail, a short written instruction was presented via goggles (“Get ready,” 4.5 s), followed by a blank screen and a silence period ranging (jittered) from 13 to 19 s. At the start of each music stimulus, a small fixation cross appeared in the goggles to minimize head movement. Each stimulus (two rhythms in two modalities) was presented 4 times during the scan, and the sequence of stimuli was randomized. The total scan time was 33 min ([4.5 s READ + 13~19 s jittered SILENCE + 16 s LISTENING + 88 s oddball] × 16 stimuli blocks). See Figure 1 for a schematic representation of the paradigm and stimulus presentation.
Figure 1.
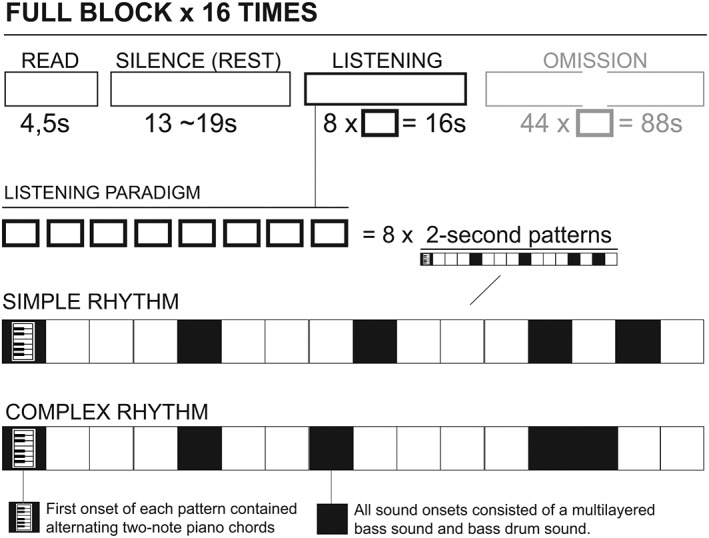
Experimental setup and the two rhythmic patterns used
2.5. Data acquisition and preprocessing
fMRI images were acquired using a 3 T scanner (GE Signa Excite 750) with a 32 channel coil. In addition to a structural scan at the beginning of the protocol, a 5 min resting‐state fMRI‐scan was performed before and after the task, and a DTI structural scan was performed after the second resting‐state scan. Repetition time (TR) for the EPI‐sequence was 1.5 s, with 28 slices interlaced. Preprocessing steps included realignment, unwarping, normalization to ICBM‐template (with 2 mm3 voxel size), smoothing with Gaussian kernel (6 mm3 voxels) and high‐pass filtering at 1/249 Hz cut‐off (calculated as the mean between onsets of the 16 main stimuli blocks). FMRI data were preprocessed and analyzed using Statistical Parametric Mapping (SPM12; Wellcome Trust Centre for Imaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm).
2.6. First‐level analysis
The blocks were first divided into simple and complex rhythm. Each block was then modeled as follows: 4.5 s of on‐screen instructions were epoched as “READ.” Silence periods (randomly assigned between 13 and 19 s) between each block were not epoched, and thus served as contrast for all other epochs (“REST”). The first 4 bars/8 s of each stimuli were epoched as “4BARS1”, and the last 4 bars/8 s epoched as “4BARS2.” Epochs were labeled according to which rhythm they belonged to, that is, ., “SIMPLE4BARS1” and “COMPLEX4BARS1” for the first 8 s of the simple and complex rhythm and “SIMPLE4BARS2” and “COMPLEX4BARS2” for the last 8 s of the simple and complex stimuli. Movement related variance (realignment parameters) was included in the model as six covariates of no interest. First level analysis produced contrasts for SIMPLE4BARS1, COMPLEX4BARS1, SIMPLE4BARS2, COMPLEX4BARS2, all contrasted to REST.
2.7. Second‐level analysis
A 2 × 2 × 2 full factorial analysis was conducted with group (PD/HC) as a between subject factor, and time (first/last 8 s) and rhythm (simple/complex) as within‐subject factors.
The PD‐group used different combinations of LDOPA, inhibitors, and D2 medication, and these were entered into the second level analysis as covariates and interaction covariates. In a follow‐up analysis, dividing the stimuli into 4 time‐bins of 4 s each, we performed a 2 × 4 × 2 full factorial analysis using the same factors as in the original analysis. All results are reported with a family‐wise error (FWE) corrected threshold of p < .05 and at least 10 voxels per cluster, with the exception of the explorative follow‐up interaction analysis (Figure 4, Table 3), which was performed with a threshold of p < .001/28 voxels, derived from two different Monte Carlo simulations. (See “Results” section for more details).
Figure 4.
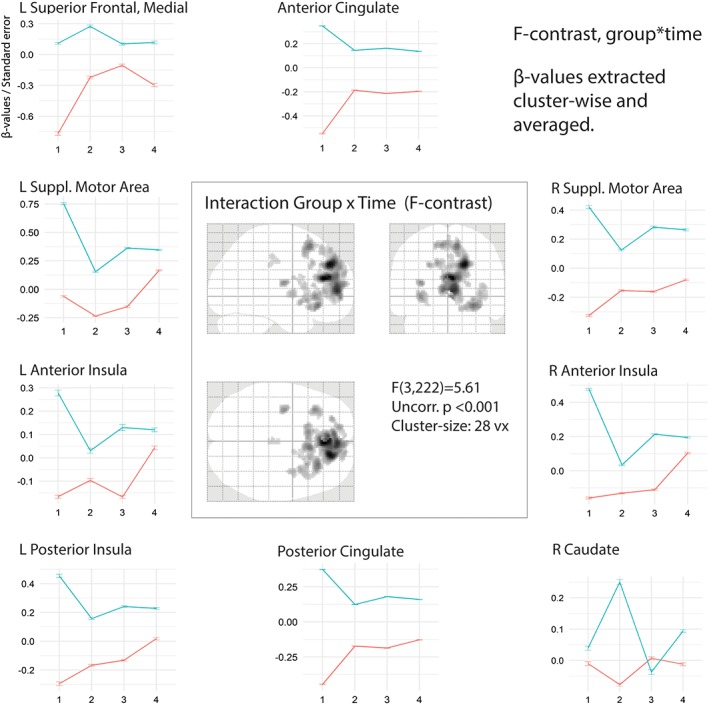
F‐contrast of interaction of group × time over four 4 s time‐bins central figure shows significant interactions in SPM at uncorrected p < .001 with a cluster size of 28 voxels. Graphs show changes in ß‐values for PD (blue) and HC (red) over four time‐windows (0–4, 4–8, 8–12, 12–16 s). L = left, R = right [Color figure can be viewed at https://wileyonlinelibrary.com]
3. RESULTS
3.1. Full factorial analysis
The full factorial analysis used group (PD/HC) as between‐subject and rhythm (simple/complex) and time (first and last 8 s) as withing‐subject factors. Counter to our hypothesis, there was no significant main effect for rhythm (complexity) or any significant interaction effects including rhythm (complexity). There were however significant main effects of group and time and an interaction effect of group and time (all effects F(1,108) > 25.7, for a complete list of significant main and interaction effects, see Supporting Information Table S2).
A between‐group t test across the two rhythms and across both time‐windows, showed significantly higher activation for the PD‐group than the HC‐group in bilateral superior temporal gyrus/planum temporale (STG/PT), (left‐lateralized) bilateral inferior parietal lobe (IPL), left‐ventromedial prefrontal cortex (vmPFC), and smaller loci in the occipital lobe (Figure 2a, Table 2a). A paired t test for the time effect of time across groups showed a decrease in activation in bilateral temporal lobe (including middle, superior, and transversal temporal gyri) from the first time‐bin to the second. On the left side, this decrease in activation extended down to the hippocampus (Table 2b). A follow up t test of the interaction effect of group and time found a group difference in the anterior cingulate cortex (ACC). We extracted cluster‐wise β‐values to visualize the changes between time bins per group. The PD‐group showed a markedly higher activation in the ACC for the first 8 s, with a downregulation of this activity in the last 8 s. For the HC‐group, the activation pattern was inverse with an upregulation of the ACC over time (shown in Figure 2b/Table 2c).
Figure 2.
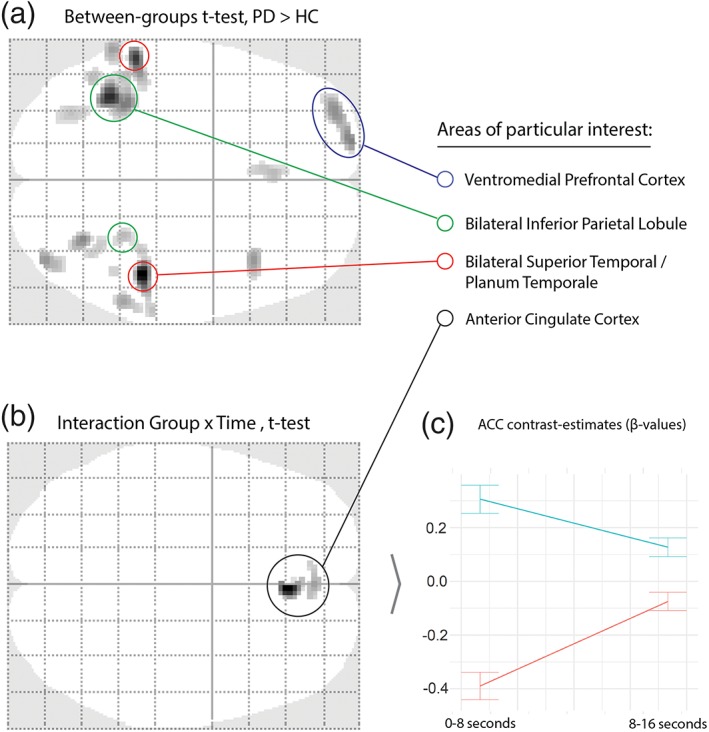
Between‐groups, across‐groups, and group × time interaction for 8 s time‐bins. Plots show activation differences through all axial planes between group for overall (a) and interaction between group and time (b). Plot (c) shows contrasts estimates of cluster‐wise β‐values from peak significant coordinate with error bars showing standard deviation. All results are FWE, p < .05, 10 voxels. See Table 2 for a complete list of activations [Color figure can be viewed at https://wileyonlinelibrary.com]
Table 2.
List of activations—To be used with Figure 3
| 2a (Figure 2a) between groups t test | ||||||
|---|---|---|---|---|---|---|
| MNI | ||||||
| Region | X | Y | Z | Size | t | |
| R | STG / PT | 46 | −36 | 18 | 214 | 11,29 |
| R | Inf. Par. | 28 | −46 | 38 | 5,93 | |
| R | STG | 64 | −32 | 16 | 5,69 | |
| L | Inf. Par. | −38 | −52 | 48 | 347 | 9,90 |
| L | Ang | −34 | −46 | 30 | 7,63 | |
| L | Inf. Par. | −36 | −44 | 38 | 7,47 | |
| L | STG /PT | −56 | −40 | 22 | 97 | 9,49 |
| L | VMPFC | −16 | 66 | −2 | 147 | 7,41 |
| L | VMPFC | −30 | 58 | 0 | 7,10 | |
| R | Calcerine | 30 | −66 | 14 | 51 | 7,25 |
| R | Operc | 40 | 18 | 18 | 64 | 6,82 |
| R | Occip | 40 | −84 | 14 | 42 | 6,82 |
| L | ITG | −60 | −52 | −6 | 41 | 6,63 |
| R | ITG | 60 | −46 | −18 | 42 | 6,53 |
| L | ITG | −58 | −52 | −18 | 24 | 6,21 |
| L | SFGmed | −2 | 28 | 54 | 63 | 5,90 |
| L | ITG | −46 | −62 | −8 | 22 | 5,85 |
| L | Fusiform | −30 | −72 | −16 | 66 | 5,71 |
| R | Angular | 34 | −58 | 32 | 25 | 5,45 |
| R | MTG/STG/ang | 44 | −50 | 10 | 11 | 5,28 |
| 2b Across‐groups, between‐times, t test | ||||||
|---|---|---|---|---|---|---|
| L | MTG / TTG | −44 | −26 | 0 | 128 | 6,5 |
| L | MTG/STG/hip | −42 | −24 | −12 | 6,4 | |
| L | STG/MTG | −50 | −14 | −4 | 5,5 | |
| R | MTG/STG | 64 | −22 | −4 | 71 | 6,3 |
| R | MTG | 62 | −14 | −8 | 5,4 | |
| R | STG/PP | 52 | −6 | −10 | 13 | 5,6 |
| R | MTG | 46 | −16 | −16 | 16 | 5,5 |
| 2c (Figure 2b) Positive interaction, groups × times, t test | ||||||
|---|---|---|---|---|---|---|
| R | Ant. Cing | 4 | 36 | 18 | 41 | 6,4 |
| R | Ant. Cing | 2 | 48 | 4 | 29 | 5,3 |
| L | Ant. Cing | −8 | 46 | 6 | 5 | |
All results are reported at FWE p < .05, voxel cluster size = 10. Abbreviations: STG = Superior Temporal Gyrus, MTG = Middle Temporal Gyrus, PT = Planum Temporale, Inf. Par = Inferior Parietal Lobule, Ang = Angular Gyrus, VMPFC = Ventromedial Prefrontal Gyrus, Operc. = Operculum, Occip = Occipital, ITG = Inferior Temporal Gyrus, SFGmed = Superior Frontal Gyrus, Hip = Hippocampus, PP = Planum Polare, Ant. Cing. = Anterior Cingulate Cortex.
3.2. Post hoc phase and interaction analyses
Based on the findings of an interaction effect between group and time in the above analysis, we decided to explore this effect further by segmenting the 16 s long stimuli into four 4 s long bins (as opposed to two 8 s long bins). A reanalysis of main effects and interaction effects reproduced the results of the original analysis. To gain more insight into the group × time interaction, we analyzed the two groups independently by contrasting each of the four time‐bins with the other three time‐bins (p < .05, FWE corrected, 10 voxels). This provided a measure of which areas were significantly more and less active in each time‐bin compared to the three others, that is, an indication of a particular “phasic role” of these areas. A side‐by‐side comparison between the two groups over these four contrasts (Figure 3) shows a strong activation in bilateral supplementary motor areas (SMA), thalamus and anterior insula (AIN), as well as in the right inferior frontal gyrus (IFG) in the first time‐bin for the Parkinson's group, not seen in the healthy controls. Furthermore, caudate nucleus was upregulated in the second time‐bin in the Parkinson‐group (with a deactivation of SMA, Thalamus and AIN in the same time‐bin), followed by a deactivation of caudate nucleus in the third time‐bin, none of which can be seen in the healthy controls.
Figure 3.
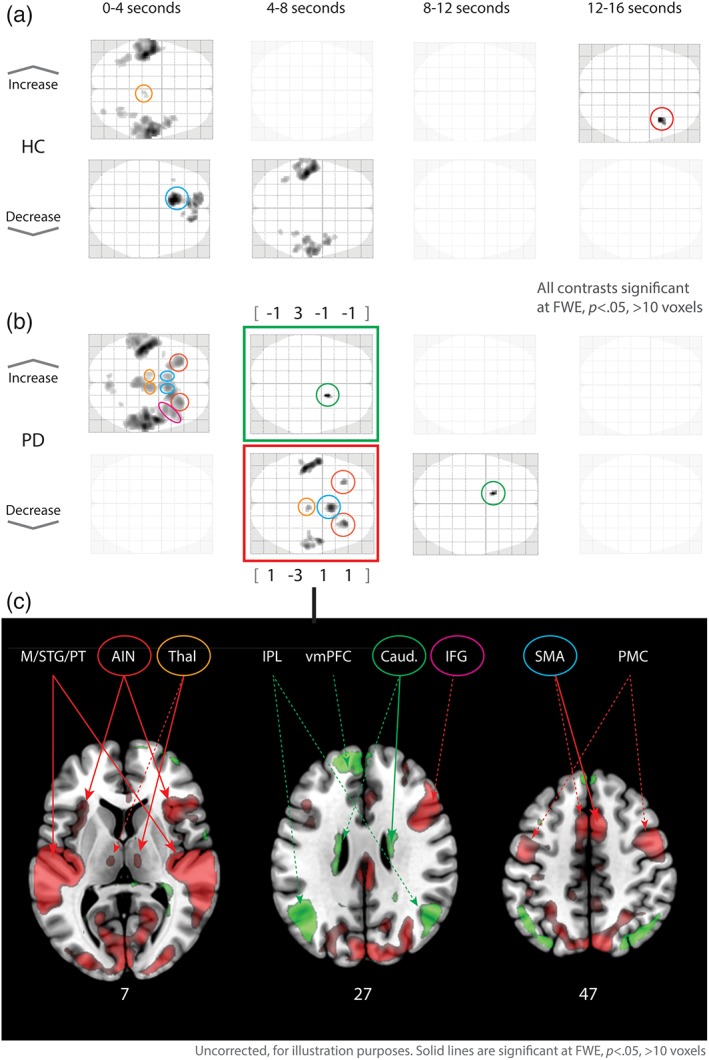
Within‐group phasic contrasts for four time‐windows. Upper panels show phasic increased and decreased activation differences in the four 4 s time‐windows for healthy controls (upper) and the Parkinson's group (lower). Bottom panel shows (uncorrected) upregulated (green) and down‐regulated (red) areas in the second time‐window (4–8 seconds). Ring color corresponds to upper panels. Full lines show significant, dashed lines not‐significant contrasts at FWE, p < .05. M/STG/PT = medial/superior temporal gyrus/planum temporale, AIN = anterior inula, Thal = thalamus, IPL = inferior parietal lobule, vmPFC = ventromedial prefrontal cortex, Caud = caudate nucleus, IFG = inferior frontal gyrus, SMA = supplementary motor area, PMC = premotor cortex. Bottom numbers indicate axial coordinates
This pattern of phasic activation and deactivation of particularly the AIN (as one node in the saliency network) and the caudate nucleus, combined with the significant interaction effect of group and time in the ACC (as the other node in the saliency network), made us reanalyze the group/time interaction. To increase sensitivity in the analysis, we performed two different Monte Carlo simulations (Forman et al., 1995; Slotnick, Moo, Segal, & Hart, 2003). As a less conservative alternative to FWE‐correction, Monte Carlo‐simulations identify activation patterns in the fMRI‐data and calculates the minimal cluster‐size activation threshold of how many adjacent voxels could be active without being random activations. Both simulations yielded a cluster‐size threshold of 14 voxels at uncorrected p < .001, and to further minimize type‐2 errors, we chose to double this threshold to 28 voxels. With this threshold, several brain areas showed a significant interaction effect of group × time, as seen in the central plot in Figure 4 (see Table 3 for list of peak values). For these areas, cluster‐wise β‐values were extracted and averaged within groups. The differences in values in both groups across the four time‐bins are shown in surrounding plots in Figure 4. As can be seen from the different plots, most envelopes replicate the findings in the within‐group, side‐by‐side comparison above, that is, that many regions are more active for the first part of listening to a stimulus for the PD‐group compared to the HC‐group, and that the difference between the groups diminishes over time. A closer look reveals how the different areas have distinct temporal envelopes for the two groups in the different areas, and in particular the envelope of the caudate nucleus seems to be time displaced relative to the other areas (ACC, AIN, and SMA notably).
Table 3.
Interaction group × time for four time bins. To be used with Figure 4
| Interaction group × time | ||||||
|---|---|---|---|---|---|---|
| MNI | ||||||
| Region | X | Y | Z | Size | F | |
| R | Anterior cing | 4 | 34 | 18 | 2,235 | 15,3 |
| R | SFG | 18 | 42 | 34 | 14,7 | |
| R | MSFG | 4 | 50 | 4 | 12,9 | |
| R | Anterior insula | 28 | 28 | −12 | 479 | 10,2 |
| L | 28 | 14 | −6 | 8,05 | ||
| R | 34 | 8 | −8 | 7,94 | ||
| R | SMA | 10 | 18 | 56 | 377 | 10,2 |
| L | −8 | 16 | 56 | 9,03 | ||
| C | 0 | 16 | 66 | 6,37 | ||
| L | Anterior insula | −34 | 24 | −2 | 183 | 9,16 |
| L | Posterior insula | −34 | −12 | 14 | 65 | 8,86 |
| L | SFG | −14 | 52 | 36 | 110 | 8,35 |
| L | −24 | 44 | 34 | 8,03 | ||
| L | −4 | 60 | 30 | 6,45 | ||
| L | SMA | −10 | 22 | 36 | 32 | 7,63 |
| R | Anterior insula | 36 | −2 | 0 | 59 | 7,62 |
| R | Caudate | 22 | 12 | 12 | 49 | 6,65 |
| R | 14 | −2 | 18 | 6,48 | ||
| R | 14 | 10 | 20 | 6,28 | ||
| R | Posterior cingulate | 2 | −26 | 44 | 56 | 6,37 |
| R | 4 | −20 | 38 | 6,25 | ||
| R | 4 | −28 | 34 | 6,13 | ||
All results are reported uncorrected p < .001, cluster size = 28 voxels. Lines in red also significant at FWE p < .05, cluster size = 10 voxels. Ant Cing = Anterior Cingulate Cortex, SFG = Superior Frontal Gyrus, MSFG = Medial Superior Frontal Gyrus, Ant. Insula = Anterior Insula, SMA = Supplementary Motor Areas, Post Insula = Posterior Insula, Post. Cing. = Posterior Cingulate, Caudate = Caudate Nucleus.
4. DISCUSSION
This study set out to answer the following questions: whether persons with Parkinson's disease differed in their processing of rhythms and whether there were differences in brain activity during different phases of rhythm perception. Counter to our hypothesis, there was no min effect of complexity, that is, differences between simple and complex rhythms—neither across nor between within the two groups. Complexity also did not interact with other factors. We did however find overall hyperactivity in bilateral PT and IPL, as well as left vmPFC, in the PD group, and hyperactivity in these areas could be related to basal ganglia dysfunction during rhythm processing. If basic mechanisms in the basal ganglia—such as the processing of temporal patterns (Chapin et al., 2010; Graybiel, 1997; Kung et al., 2013; Lewis et al., 2004) and the signal‐to‐noise gating (Boecker et al., 1999; Gruber, Dayan, Gutkin, & Solla, 2006; Kotz, Schwartze, & Schmidt‐Kassow, 2009; Steriade & Llinas, 1988) during processing interactions with other cortical areas (Cohen & Frank, 2009)—fail to correctly reduce the perceptual complexity of the rhythm in the back‐projection to cortical areas in the cortico‐basal ganglia‐thalamo‐cortical loop, then an inherently more complex signal must be processed in the rest of the processing chain. This could explain the hyperactivity in the PT, an area sensitive to complex rhythms (Bengtsson et al., 2009; Chen et al., 2008a; Geiser et al., 2012; Herdener et al., 2014; Kung et al., 2013; Thaut et al., 2014), and with a potentially anticorrelated relationship with the basal ganglia for rhythmic complexity (Geiser et al., 2012). The PT segregates and gates processed temporal patterns to the IPL (Griffiths & Warren, 2002; Ragert et al., 2014; Zatorre et al., 2007), another area sensitive to rhythmic complexity (Bolger et al., 2014; Grahn & Rowe, 2013; Lewis et al., 2004; Thaut et al., 2014; Vuust et al., 2006), and a more complex signal processed in the PT could therefore increase activity in the IPL. If the processing of more complex rhythms is more dependent on recruiting frontal areas (Chapin et al., 2010; Kung et al., 2013), an overall more complex signal in the PD‐group could also explain the increased activity in the left‐PFC in the PD‐group. Hyperactivity in PT, IPL, and left‐PFC in the PD group could therefore be due to dysfunction in the basal ganglia, with rhythms being processed by the PD‐group as if they were perceived as overall more complex than by the healthy subjects, a finding that is consistent with the results of our online listening test and previous behavioral studies on PD (Biswas et al., 2016; Cameron et al., 2016; Grahn & Brett, 2009).
The interaction analysis of group and time, and our phasic within‐group analyses of the PD‐group, showed initial hyperactivity in widespread auditory, cortical and subcortical motor areas, and notably ACC and bilateral AIN (which constitute the saliency network). In the PD group, this initial hyperactivity was followed by a downregulation of these same areas and a simultaneous upregulation of the caudate nucleus. This activation pattern is consistent with previous findings in healthy subjects, where sudden activity in motor systems has been found to suppress basal ganglia output (Wessel & Aron, 2017). This could explain the subsequent upregulation of the caudate nucleus in the PD‐group, possibly indicating higher prediction error activity in the caudate nucleus (Grahn & Rowe, 2013; Haruno & Kawato, 2006) in secondary stages of rhythm processing, as the PD group adapt to the stimuli. In this context, auditory and basal ganglia activity might not be anticorrelated with activity in other brain areas (Geiser et al., 2012), but rather time‐displaced.
The high initial activity in widespread motor areas and the ACC and AIN also taps into another question that remains unanswered in relation to PD, namely, whether persons with PD can internally generate rhythms without external stimuli. Research shows that persons with PD can maintain a rhythm already entrained to (Cerasa et al., 2006; te Woerd et al., 2015), potentially tapping into predictive mechanisms in basal ganglia‐premotor interactions, and not bypassing them (te Woerd et al., 2015). Other studies show that expecting particular stimuli activates brain areas relevant for the upcoming stimuli (Osnes, Hugdahl, Hjelmervik, & Specht, 2012; SanMiguel, Widmann, Bendixen, Trujillo‐Barreto, & Schroger, 2013). The ACC and the AIN, which constitute the saliency network, are triggered by novelty or salient stimuli, where particularly the AIN serves as a “network‐switch” (Menon & Uddin, 2010) to upregulate brain areas needed for task‐related action (Menon, 2011). The hyperactivity in the saliency network and widespread motor areas in the PD‐group could therefore reflect a lack of internal preparation of circuitries relevant for rhythm processing. The lack of similar activation differences in these areas in the healthy control group could mean that they are prepared, and are maintaining the brain state necessary to process rhythms also in the silent periods between stimuli. Conversely, the PD‐group needs time for every new stimulus presentation to activate “rhythm relevant” brain areas, in a specific phasic sequence of events involving the saliency network, several cortical motor areas, as well as the basal ganglia. The activity patterns of the AIN and the caudate nucleus should be of particular interest to future studies, as these two areas relate directly to core PD pathology (Kish et al., 1988; Poewe et al., 2017; Putcha et al., 2015).
4.1. Limitations and outlook
One important limitation of this study is the fact that passive listening yields lower levels of activation in the basal ganglia than active paradigms that include motor responses (Chen et al., 2008b; Kung et al., 2013): Basal‐ganglia activation in beat‐processing has been predominantly linked to the preparation, production or imagining motor response (Kung et al., 2013). This might account for the lack of overall basal ganglia activation differences between the two groups and also between the two rhythms.
The lack of differences between the rhythms could also be due to the use of only two different rhythms, presented several times during scanning, decreasing rhythmic complexity, and cognitive load through simple learning effects in both groups. We also did not investigate whether medication states (“on”/“off”) would modulate the results, as all our participants with PD were in medication regimens during the study, and future studies should investigate de novo patients before medication or patients in “off”‐periods. We also recognize that the limited number of trails as well as small group sizes limit the strength of our results, especially for the explorative post hoc “phasic” interaction analysis, were cluster‐wise thresholds were used instead of FWE‐corrections. Future studies should expand and change the paradigm in such a way that the changes over time in the two groups could be more fully explored, and in such a way that it also takes into consideration the preparatory part of the listening task. Future studies should also include more than just two rhythms, extend the time‐frame, use more time‐bins, and use a variety of tempi. Learning and repetition effects should also be investigated, and the use of more ecological valid music, as well as including participants' musical preferences in the design, should be considered. Investigating the overall effect of music listening on larger network functionality in PD through resting state and functional connectivity studies would be yet another important avenue to explore in future studies.
5. CONCLUSION
We found rhythm processing related group differences that could be related to PD‐pathophysiology on different levels. On one level, basal‐ganglia dysfunction in the basic processing of rhythm patterns could lead to an overall more complex signal for the PD‐brain to process, increasing activity in task‐relevant areas, such as the PT and the IPL. On another level, rhythm onset led to high activity in the saliency network and widespread motor areas in the PD‐group, with subsequent upregulation of the caudate nucleus in the basal ganglia. Despite the limitations of this study, we believe that it constitutes an important contribution to the exploration of the neural basis of rhythm perception and processing in Parkinson's disease, using fMRI. In particular, the combination of overall hyperactivity in task‐relevant areas, and the phasic, sequential activation in brain areas crucial affected by PD, merits further investigation.
CONFLICT OF INTERESTS
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
KV, GOS, and KS contributed to the conception of the research project. KV recruited participants and organized the study, GOS performed all physical examinations, and KV supervised neuropsychological tests and constructed and administered the online‐survey. KV and KS conducted statistical analyses. KV wrote the first draft. All contributed to the final version of the manuscript.
Supporting information
Supplementary Table 1 Overview over the 10 rhythmic patterns used in the online‐test A) Black boxes on top signifies quarter notes of each 4/4 bar, and x's signifies sound onsets. R#: Rhythm number. Each square represents on interval of 166.67 ms @90 bpm, 125 ms @120 bpm and 100 ms @150 bpm, and with 8 repetitions of the pattern, stimuli lasted 22, 16 and 13 seconds for the three tempi respectively. Stimuli were constructed using Steinberg Cubase 7 (http://www.steinberg.net), using drum, bass and piano samples. PS: The complexity scores according to Jeff Pressing's model (Pressing, 1999). B) Results of the online‐test per rhythm, 1‐tailed t‐tests. PD: Mean of Parkinsons‐group score. HC: Mean of healthy controls score. (SD): Standard Deviation. p: Between‐group P‐value.
Supplementary Table 2 List of main and interaction effects MNI: Montreal Neurological Institute coordinates. Size: Voxel size of cluster. F: F‐value at peak, F[1,108]. p: P‐values reported with FWE.
ACKNOWLEDGMENTS
The authors wish to thank all persons with PD and healthy controls who took part in this study. We are grateful for the support of the Hordaland branch of the National Parkinson's organization of Norway in recruiting participants, especially the late John Axel Sundal. The first author would also like to thank particularly nurse Marit Elise Arnevik Renså at the department of Neurology, University Hospital of Haukeland, Bergen, for her practical help in setting up examination rooms and aiding in performing the neuropsychological tests. The first author would also like to thank Ulvhild Helena Færøvik, for her help during data collection in her role as research assistant. Finally, the staff at the department of radiology at Haukeland Universitetssjukehus deserves special mention, as this study would not have been possible without them. Kjetil Vikene has received traveling grants from Meltzer's Fund, University of Bergen, Norwegian Research School of Neuroscience, Department of Biological and Medical Psychology, University of Bergen, and financial support through GC Rieber Foundations, and the Research Council of Norway. Grant Number: 217932/F20. Karsten Specht has received financial support and funding through the Research Council of Norway. Grant Number: 217932/F20. Geir Olve Skeie has received no financial support and funding.
Vikene K, Skeie G‐O, Specht K. Abnormal phasic activity in saliency network, motor areas, and basal ganglia in Parkinson's disease during rhythm perception. Hum Brain Mapp. 2019;40:916–927. 10.1002/hbm.24421
Funding information: GC Rieber Foundations; L. Meltzers Høyskolefond; Norwegian Research School of Neuroscience; Universitetet i Bergen; The Norwegian Research Council, Grant/Award Number: 217932/F20
REFERENCES
- Aarsland, D. , Andersen, K. , Larsen, J. P. , Perry, R. , Wentzel‐Larsen, T. , Lolk, A. , & Kragh‐Sorensen, P. (2004). The rate of cognitive decline in Parkinson disease. Archives of Neurology, 61, 1906–1911. [DOI] [PubMed] [Google Scholar]
- Alexander, G. E. , DeLong, M. R. , & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–381. [DOI] [PubMed] [Google Scholar]
- Alluri, V. , Toiviainen, P. , Jaaskelainen, I. P. , Glerean, E. , Sams, M. , & Brattico, E. (2012). Large‐scale brain networks emerge from dynamic processing of musical timbre, key and rhythm. NeuroImage, 59, 3677–3689. [DOI] [PubMed] [Google Scholar]
- Altmann, C. F. , Henning, M. , Doring, M. K. , & Kaiser, J. (2008). Effects of feature‐selective attention on auditory pattern and location processing. NeuroImage, 41, 69–79. [DOI] [PubMed] [Google Scholar]
- Bengtsson, S. L. , Ullen, F. , Ehrsson, H. H. , Hashimoto, T. , Kito, T. , Naito, E. , … Sadato, N. (2009). Listening to rhythms activates motor and premotor cortices. Cortex, 45, 62–71. [DOI] [PubMed] [Google Scholar]
- Biswas, A. , Hegde, S. , Jhunjhunwala, K. , & Pal, P. K. (2016). Two sides of the same coin: Impairment in perception of temporal components of rhythm and cognitive functions in Parkinson's disease. Basal Ganglia, 6, 63–70. [Google Scholar]
- Blin, O. , Ferrandez, A. M. , & Serratrice, G. (1990). Quantitative analysis of gait in Parkinson patients: Increased variability of stride length. Journal of the Neurological Sciences, 98, 91–97. [DOI] [PubMed] [Google Scholar]
- Boecker, H. , Ceballos‐Baumann, A. , Bartenstein, P. , Weindl, A. , Siebner, H. R. , Fassbender, T. , … Conrad, B. (1999). Sensory processing in Parkinson's and Huntington's disease ‐ investigations with 3D (H2O)‐O‐15‐PET. Brain, 122, 1651–1665. [DOI] [PubMed] [Google Scholar]
- Bolger, D. , Coull, J. T. , & Schon, D. (2014). Metrical rhythm implicitly orients attention in time as indexed by improved target detection and left inferior parietal activation. Journal of Cognitive Neuroscience, 26, 593–605. [DOI] [PubMed] [Google Scholar]
- Burunat, I. , Tsatsishvili, V. , Brattico, E. , & Toiviainen, P. (2017). Coupling of action‐perception brain networks during musical pulse processing: Evidence from region‐of‐interest‐based independent component analysis. Frontiers in Human Neuroscience, 11, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, D. J. , Pickett, K. A. , Earhart, G. M. , & Grahn, J. A. (2016). The effect of dopaminergic medication on beat‐based auditory timing in Parkinson's disease. Frontiers in Neurology, 7, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caproni, S. , Muti, M. , Principi, M. , Ottaviano, P. , Frondizi, D. , Capocchi, G. , … Tambasco, N. (2013). Complexity of motor sequences and cortical reorganization in Parkinson's disease: A functional MRI study. PLoS One, 8, e66834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan, M. J. , Ishii, K. , Honda, M. , Samii, A. , & Hallett, M. (1999). A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain, 122, 483–495. [DOI] [PubMed] [Google Scholar]
- Cerasa, A. , Hagberg, G. E. , Peppe, A. , Bianciardi, M. , Gioia, M. C. , Costa, A. , … Sabatini, U. (2006). Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson's disease. Brain Research Bulletin, 71, 259–269. [DOI] [PubMed] [Google Scholar]
- Chapin, H. L. , Zanto, T. , Jantzen, K. J. , Kelso, S. J. , Steinberg, F. , & Large, E. W. (2010). Neural responses to complex auditory rhythms: The role of attending. Frontiers in Psychology, 1, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. L. , Penhune, V. B. , & Zatorre, R. J. (2008a). Moving on time: Brain network for auditory‐motor synchronization is modulated by rhythm complexity and musical training. Journal of Cognitive Neuroscience, 20, 226–239. [DOI] [PubMed] [Google Scholar]
- Chen, J. L. , Penhune, V. B. , & Zatorre, R. J. (2008b). Listening to musical rhythms recruits motor regions of the brain. Cerebral Cortex, 18, 2844–2854. [DOI] [PubMed] [Google Scholar]
- Chen, J. L. , Penhune, V. B. , & Zatorre, R. J. (2009). The role of auditory and premotor cortex in sensorimotor transformations. Annals of the New York Academy of Sciences, 1169, 15–34. [DOI] [PubMed] [Google Scholar]
- Chen, J. L. , Zatorre, R. J. , & Penhune, V. B. (2006). Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. NeuroImage, 32, 1771–1781. [DOI] [PubMed] [Google Scholar]
- Christopher, L. , Koshimori, Y. , Lang, A. E. , Criaud, M. , & Strafella, A. P. (2014). Uncovering the role of the insula in non‐motor symptoms of Parkinson's disease. Brain, 137, 2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M. X. , & Frank, M. J. (2009). Neurocomputational models of basal ganglia function in learning, memory and choice. Behavioural Brain Research, 199, 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud, M. , Christopher, L. , Boulinguez, P. , Ballanger, B. , Lang, A. E. , Cho, S. S. , … Strafella, A. P. (2016). Contribution of insula in Parkinson's disease: A quantitative meta‐analysis study. Human Brain Mapping, 37, 1375–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn, S. , Elton, R. L. , UPDRS program members (1987). Unified Parkinsons Disease Rating Scale In Fahn S., Marsden C. D., M. Goldstein, Calne D. B., editors, Recent developments in Parkinsons disease, Vol. 2, Florham Park, NJ: Macmillan Health Care Information, p. 153–163. [Google Scholar]
- Fitch, W. T. (2013). Rhythmic cognition in humans and animals: Distinguishing meter and pulse perception. Frontiers in Systems Neuroscience, 7, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein, M. F. , Folstein, S. E. , & McHugh, P. R. (1975). Mini‐mental state. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Forman, S. D. , Cohen, J. D. , Fitzgerald, M. , Eddy, W. F. , Mintun, M. A. , & Noll, D. C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magnetic Resonance in Medicine, 33, 636–647. [DOI] [PubMed] [Google Scholar]
- Geiser, E. , & Kaelin‐Lang, A. (2011). The function of dopaminergic neural signal transmission in auditory pulse perception: Evidence from dopaminergic treatment in Parkinson's patients. Behavioural Brain Research, 225, 270–275. [DOI] [PubMed] [Google Scholar]
- Geiser, E. , Notter, M. , & Gabrieli, J. D. (2012). A corticostriatal neural system enhances auditory perception through temporal context processing. The Journal of neuroscience : the official journal of the Society for Neuroscience, 32, 6177–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden, N. , Khusnulina, A. , Davis, N. J. , Bracewell, R. M. , Bokde, A. L. , McNulty, J. P. , & Mullins, P. G. (2014). The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. NeuroImage, 99, 180–190. [DOI] [PubMed] [Google Scholar]
- Grahn, J. A. (2009). The Role of the Basal Ganglia in Beat Perception. Annals of the New York Academy of Sciences, 1169, 35–45. [DOI] [PubMed] [Google Scholar]
- Grahn, J. A. , & Brett, M. (2007). Rhythm and beat perception in motor areas of the brain. Journal of Cognitive Neuroscience, 19, 893–906. [DOI] [PubMed] [Google Scholar]
- Grahn, J. A. , & Brett, M. (2009). Impairment of beat‐based rhythm discrimination in Parkinson's disease. Cortex, 45, 54–61. [DOI] [PubMed] [Google Scholar]
- Grahn, J. A. , & Rowe, J. B. (2013). Finding and feeling the musical beat: Striatal dissociations between detection and prediction of regularity. Cerebral Cortex, 23, 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel, A. M. (1997). The basal ganglia and cognitive pattern generators. Schizophrenia Bulletin, 23, 459–469. [DOI] [PubMed] [Google Scholar]
- Griffiths, T. D. , & Warren, J. D. (2002). The planum temporale as a computational hub. Trends in Neurosciences, 25, 348–353. [DOI] [PubMed] [Google Scholar]
- Gruber, A. J. , Dayan, P. , Gutkin, B. S. , & Solla, S. A. (2006). Dopamine modulation in the basal ganglia locks the gate to working memory. Journal of Computational Neuroscience, 20, 153–166. [DOI] [PubMed] [Google Scholar]
- Haruno, M. , & Kawato, M. (2006). Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus‐action‐reward association learning. Journal of Neurophysiology, 95, 948–959. [DOI] [PubMed] [Google Scholar]
- Herdener, M. , Humbel, T. , Esposito, F. , Habermeyer, B. , Cattapan‐Ludewig, K. , & Seifritz, E. (2014). Jazz drummers recruit language‐specific areas for the processing of rhythmic structure. Cerebral Cortex, 24, 836–843. [DOI] [PubMed] [Google Scholar]
- Jerde, T. A. , Childs, S. K. , Handy, S. T. , Nagode, J. C. , & Pardo, J. V. (2011). Dissociable systems of working memory for rhythm and melody. NeuroImage, 57, 1572–1579. [DOI] [PubMed] [Google Scholar]
- Jungblut, M. , Huber, W. , Pustelniak, M. , & Schnitker, R. (2012). The impact of rhythm complexity on brain activation during simple singing: An event‐related fMRI study. Restorative Neurology and Neuroscience, 30, 39–53. [DOI] [PubMed] [Google Scholar]
- Jungblut, M. , Huber, W. , & Schnitker, R. (2016). Rhythm structure influences auditory‐motor interaction during anticipatory listening to simple singing. Journal of Speech Pathology and Therapy, 1, 108. [Google Scholar]
- Kish, S. J. , Shannak, K. , & Hornykiewicz, O. (1988). Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. The New England Journal of Medicine, 318, 876–880. [DOI] [PubMed] [Google Scholar]
- Knutsson, E. (1972). An analysis of Parkinsonian gait. Brain, 95, 475–486. [DOI] [PubMed] [Google Scholar]
- Kotz, S. A. , Schwartze, M. , & Schmidt‐Kassow, M. (2009). Non‐motor basal ganglia functions: A review and proposal for a model of sensory predictability in auditory language perception. Cortex, 45, 982–990. [DOI] [PubMed] [Google Scholar]
- Kung, S. J. , Chen, J. L. , Zatorre, R. J. , & Penhune, V. B. (2013). Interacting cortical and basal ganglia networks underlying finding and tapping to the musical beat. Journal of Cognitive Neuroscience, 25, 401–420. [DOI] [PubMed] [Google Scholar]
- Lanciego, J. L. , Luquin, N. , & Obeso, J. A. (2012). Functional neuroanatomy of the basal ganglia. Cold Spring Harbor Perspectives in Medicine, 2, a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, P. A. , Wing, A. M. , Pope, P. A. , Praamstra, P. , & Miall, R. C. (2004). Brain activity correlates differentially with increasing temporal complexity of rhythms during initialisation, synchronisation, and continuation phases of paced finger tapping. Neuropsychologia, 42, 1301–1312. [DOI] [PubMed] [Google Scholar]
- Mazzoni, P. , Shabbott, B. , & Cortes, J. C. (2012). Motor control abnormalities in Parkinson's disease. Cold Spring Harbor Perspectives in Medicine, 2, a009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, G. C. , Brown, S. H. , Rice, R. R. , & Thaut, M. H. (1997). Rhythmic auditory‐motor facilitation of gait patterns in patients with Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 62, 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. (2011). Large‐scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton, F. A. , & Strick, P. L. (2000). Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research, 31, 236–250. [DOI] [PubMed] [Google Scholar]
- Monchi, O. , Petrides, M. , Mejia‐Constain, B. , & Strafella, A. P. (2007). Cortical activity in Parkinson's disease during executive processing depends on striatal involvement. Brain, 130, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan, N. S. , Rodnitzky, R. L. , & Uc, E. Y. (2013). Prefrontal dopamine signaling and cognitive symptoms of Parkinson's disease. Reviews in the Neurosciences, 24, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela, C. , Hughes, L. E. , Owen, A. M. , & Grahn, J. A. (2013). Into the groove: Can rhythm influence Parkinson's disease? Neuroscience and Biobehavioral Reviews, 37, 2564–2570. [DOI] [PubMed] [Google Scholar]
- Osnes, B. , Hugdahl, K. , Hjelmervik, H. , & Specht, K. (2012). Stimulus expectancy modulates inferior frontal gyrus and premotor cortex activity in auditory perception. Brain and Language, 121, 65–69. [DOI] [PubMed] [Google Scholar]
- Parker, K. L. , Lamichhane, D. , Caetano, M. S. , & Narayanan, N. S. (2013). Executive dysfunction in Parkinson's disease and timing deficits. Frontiers in Integrative Neuroscience, 7, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, A. D. , & Iversen, J. R. (2014). The evolutionary neuroscience of musical beat perception: The action simulation for auditory prediction (ASAP) hypothesis. Frontiers in Systems Neuroscience, 8, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe, W. , Seppi, K. , Tanner, C. M. , Halliday, G. M. , Brundin, P. , Volkmann, J. , … Lang, A. E. (2017). Parkinson disease. Nature Reviews Disease Primers, 3, 17013. [DOI] [PubMed] [Google Scholar]
- Pressing, J. (1999). Cognitive complexity and the structure of musical patterns. In Proceedings of the 4th Conference of the Australasian Cognitive Science Society. [Google Scholar]
- Putcha, D. , Ross, R. S. , Cronin‐Golomb, A. , Janes, A. C. , & Stern, C. E. (2015). Altered intrinsic functional coupling between core neurocognitive networks in Parkinson's disease. NeuroImage. Clinical, 7, 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert, M. , Fairhurst, M. T. , & Keller, P. E. (2014). Segregation and integration of auditory streams when listening to multi‐part music. PLoS One, 9, e84085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, S. M. , Mayer, A. R. , & Harrington, D. L. (2001). The evolution of brain activation during temporal processing. Nature Neuroscience, 4, 317–323. [DOI] [PubMed] [Google Scholar]
- Rauschecker, J. P. (2011). An expanded role for the dorsal auditory pathway in sensorimotor control and integration. Hearing Research, 271, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe, J. , Stephan, K. E. , Friston, K. J. , Frackowiak, R. , Lees, A. , & Passingham, R. (2002). Attention to action in Parkinson's disease ‐ impaired effective connectivity among frontal cortical regions. Brain, 125, 276–289. [DOI] [PubMed] [Google Scholar]
- Sabatini, U. , Boulanouar, K. , Fabre, N. , Martin, F. , Carel, C. , Colonnese, C. , … Rascol, O. (2000). Cortical motor reorganization in akinetic patients with Parkinson's disease: A functional MRI study. Brain, 123(Pt 2), 394–403. [DOI] [PubMed] [Google Scholar]
- Samuel, M. , Ceballos‐Baumann, A. O. , Blin, J. , Uema, T. , Boecker, H. , Passingham, R. E. , & Brooks, D. J. (1997). Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain, 120(Pt 6), 963–976. [DOI] [PubMed] [Google Scholar]
- SanMiguel, I. , Widmann, A. , Bendixen, A. , Trujillo‐Barreto, N. , & Schroger, E. (2013). Hearing silences: Human auditory processing relies on Preactivation of sound‐specific brain activity patterns. Journal of Neuroscience, 33, 8633–8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartze, M. , & Kotz, S. A. (2015). Regional interplay for temporal processing in Parkinson's disease: Possibilities and challenges. Frontiers in Neurology, 6, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine, J. M. , Matar, E. , Ward, P. B. , Frank, M. J. , Moustafa, A. A. , Pearson, M. , … Lewis, S. J. (2013). Freezing of gait in Parkinson's disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain, 136, 3671–3681. [DOI] [PubMed] [Google Scholar]
- Slotnick, S. D. , Moo, L. R. , Segal, J. B. , & Hart, J. (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research, 17, 75–82. [DOI] [PubMed] [Google Scholar]
- Smulders, K. , Dale, M. L. , Carlson‐Kuhta, P. , Nutt, J. G. , & Horak, F. B. (2016). Pharmacological treatment in Parkinson's disease: Effects on gait. Parkinsonism & Related Disorders, 31, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan, D. , Levitin, D. J. , & Menon, V. (2008). A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proceedings of the National Academy of Sciences, 105, 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade, M. , & Llinas, R. R. (1988). The functional states of the thalamus and the associated neuronal interplay. Physiological Reviews, 68, 649–742. [DOI] [PubMed] [Google Scholar]
- te Woerd, E. S. , Oostenveld, R. , Bloem, B. R. , de Lange, F. P. , & Praamstra, P. (2015). Effects of rhythmic stimulus presentation on oscillatory brain activity: The physiology of cueing in Parkinson's disease. NeuroImage. Clinical, 9, 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaut, M. H. , McIntosh, K. W. , McIntosh, G. C. , & Hoemberg, V. (2001). Auditory rhythmicity enhances movement and speech motor control in patients with Parkinson's disease. Functional Neurology, 16, 163–172. [PubMed] [Google Scholar]
- Thaut, M. H. , Trimarchi, P. D. , & Parsons, L. M. (2014). Human brain basis of musical rhythm perception: Common and distinct neural substrates for meter, tempo, and pattern. Brain Sciences, 4, 428–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, N. P. , & Lee, C. S. (2015). The sensory‐motor theory of rhythm and beat induction 20 years on: A new synthesis and future perspectives. Frontiers in Human Neuroscience, 9, 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint, G. T. (2013). The geometry of musical rhythm : What makes a "good" rhythm good? Boca Raton, Fla.: CRC Press. [Google Scholar]
- Trost, W. , Fruhholz, S. , Schon, D. , Labbe, C. , Pichon, S. , Grandjean, D. , & Vuilleumier, P. (2014). Getting the beat: Entrainment of brain activity by musical rhythm and pleasantness. NeuroImage, 103, 55–64. [DOI] [PubMed] [Google Scholar]
- Vuust, P. , Roepstorff, A. , Wallentin, M. , Mouridsen, K. , & Ostergaard, L. (2006). It don't mean a thing… Keeping the rhythm during polyrhythmic tension, activates language areas (BA47). NeuroImage, 31, 832–841. [DOI] [PubMed] [Google Scholar]
- Warren, J. E. , Wise, R. J. , & Warren, J. D. (2005). Sounds do‐able: Auditory‐motor transformations and the posterior temporal plane. Trends in Neurosciences, 28, 636–643. [DOI] [PubMed] [Google Scholar]
- Wessel, J. R. , & Aron, A. R. (2017). On the Globality of motor suppression: Unexpected events and their influence on behavior and cognition. Neuron, 93, 259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, T. , & Hallett, M. (2005). A functional MRI study of automatic movements in patients with Parkinson's disease. Brain, 128, 2250–2259. [DOI] [PubMed] [Google Scholar]
- Xia, R. , & Mao, Z. H. (2012). Progression of motor symptoms in Parkinson's disease. Neuroscience Bulletin, 28, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H. , Sternad, D. , Corcos, D. M. , & Vaillancourt, D. E. (2007). Role of hyperactive cerebellum and motor cortex in Parkinson's disease. NeuroImage, 35, 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre, R. J. , Chen, J. L. , & Penhune, V. B. (2007). When the brain plays music: Auditory‐motor interactions in music perception and production. Nature Reviews. Neuroscience, 8, 547–558. [DOI] [PubMed] [Google Scholar]
- Zatorre, R. J. , & Zarate, J. M. (2012). Cortical processing of music In Poeppel O. T., Popper A. N., & Fay R. R. (Eds.), In the human auditory cortex springer handbook of auditory research (pp. 261–294). New York, NY: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Overview over the 10 rhythmic patterns used in the online‐test A) Black boxes on top signifies quarter notes of each 4/4 bar, and x's signifies sound onsets. R#: Rhythm number. Each square represents on interval of 166.67 ms @90 bpm, 125 ms @120 bpm and 100 ms @150 bpm, and with 8 repetitions of the pattern, stimuli lasted 22, 16 and 13 seconds for the three tempi respectively. Stimuli were constructed using Steinberg Cubase 7 (http://www.steinberg.net), using drum, bass and piano samples. PS: The complexity scores according to Jeff Pressing's model (Pressing, 1999). B) Results of the online‐test per rhythm, 1‐tailed t‐tests. PD: Mean of Parkinsons‐group score. HC: Mean of healthy controls score. (SD): Standard Deviation. p: Between‐group P‐value.
Supplementary Table 2 List of main and interaction effects MNI: Montreal Neurological Institute coordinates. Size: Voxel size of cluster. F: F‐value at peak, F[1,108]. p: P‐values reported with FWE.


