ABSTRACT
Amniotic suspension allografts (ASA), derived from placental tissues, contain particulated amniotic membrane and amniotic fluid cells. Recently, ASA and other placental‐derived allografts have been used in orthopaedic applications, including tendinopathies and tendon injuries. The purpose of this study was to determine the potential effects of ASA on tenocyte cell density, migration, and responses to inflammatory stimuli. Tenocyte cell density was measured using AlamarBlue over multiple time points, while migration was determined using a Boyden chamber assay. Deposition of ECM markers were measured using BioColor kits. Gene expression and protein production of cytokines and growth factors following stimulus with pro‐inflammatory IL‐1β and TNF‐α was measured using qPCR and ELISAs. Conditioned media (CM) was made from ASA and used for all assays in this study. In vitro, ASA CM treatment significantly promoted tenocyte increases in cell density and migration compared to assay media controls. ASA CM also increased the deposition of extracellular matrix (ECM) proteins, including collagen, elastin, and sGAG. Following inflammatory stimulation and treatment with ASA CM, tenocytes downregulated IL‐8 gene expression, a pro‐inflammatory cytokine normally elevated during the inflammatory phase of tendon healing. Additionally, tenocytes treated with ASA CM had significantly lower protein levels of TGF‐β1 compared to controls. This study evaluated ASA and its effect on tenocytes; specifically, treatment with ASA resulted in increased cell density, more robust migration and matrix deposition, and some alteration of inflammatory targets. © 2018 The Authors. Journal of Orthopaedic Research® Published by Wiley Periodicals, Inc. on behalf of Orthopaedic Research Society. J Orthop Res 37:412–420, 2019.
Keywords: amniotic suspension allograft, tendon, tenocytes, regenerative, repair
For generations, physicians have utilized amniotic tissues and amniotic allografts to aid in healing wounds and burns across a wide variety of tissues.1 Critical observations were made in those early patients where amniotic tissue was used to aid the healing process; following application of the allograft, the wound or burn sites had noticeably less scarring, less infection, more robust healing, and less pain.1 As decades passed, placental‐derived allografts were employed in treating an increasingly wide array of tissue injuries,1, 2 but a comprehensive understanding of the precise mechanism of repair has been elusive.
The amniotic environment is rich in biological components3, 4 that have the potential to be supportive of tissue repair following trauma. The placental membrane is comprised of multiple layers of distinct tissues with unique functions. The innermost layer of the membrane is referred to as the amnion, which provides a flexible yet strong barrier to contain the amniotic fluid within the amniotic sac. The chorionic layers of the placental membrane, which adhere to the uterine wall during gestation, are smooth and avascular. Additionally, the amniotic and chorionic membranes contain extracellular matrix (ECM) components, including collagen, proteoglycans, elastin, laminin, and fibronectin, providing a firm scaffold for cells within the tissue.4 Gene expression analysis suggests that amniotic membranes may promote an anti‐inflammatory environment in the surrounding tissue by suppressing interleukin‐1α (IL‐1α) and interleukin‐1β (IL‐1β)5 and inhibiting the activity of matrix metalloproteases (MMPs).5 Additionally, the presence of growth factors such as insulin‐like growth factor I (IGF‐I),6 transforming growth factor beta (TGF‐β),7 fibroblast growth factor (FGF),8 platelet‐derived growth factor (PDGF),8 and vascular endothelial growth factor (VEGF)9 in placental membranes may support tendon repair. While many of the studies evaluating amniotic allografts have used sheets of tissue to either cover or wrap a site of tissue trauma, an alternative approach is to utilize an amniotic suspension allograft (ASA) made up of amniotic membrane tissue and amniotic fluid cells. ASA has been clinically evaluated in various orthopaedic applications including osteoarthritis,10 spine fusion,11, 12 and talar dome lesions of the ankle13; the use of ASA in these studies resulted in improved outcomes.
There are many types of tendon injuries caused by either an acute or repetitive insult, including tendonitis (injury with inflammation of the tendon), tendinosis (chronic tendon injury with no inflammation), non‐inflammatory degeneration of the tendon (common after micro‐traumas or failure to heal following rupture), and tendon ruptures (including the partial or full tear of the tendon sheath). Tendon healing is a complex process consisting of overlapping phases and the expression of many growth factors and cytokines, including IGF‐I, TGF‐β, VEGF, PDG, and basic fibroblast growth factor (bFGF).8 These growth factors help to regulate proliferation and migration of cells needed for ECM remodeling and angiogenesis.8 Amniotic membranes contain all these growth factors,3 therefore, we hypothesize that in vitro tenocyte exposure to factors released from ASA will promote tenocyte cell responses that could support tendon healing.
METHODS
Human Amniotic Suspension Allograft
Human amniotic suspension allograft (ASA, ReNu®, Organogenesis, Canton, MA)10 was obtained from donated human placentas following planned cesarean sections with appropriate informed consent. All processing was conducted in accordance with the Food and Drug Administration's (FDA) Good Tissue Practices and the American Association of Tissue Bank (AATB) standards. ASA contains particulated human amniotic membrane and amniotic fluid cells; these components are combined and cryogenically preserved. All placental donation patients underwent medical and social history screening and were tested for infectious diseases including human immunodeficiency virus (HIV), human T‐lymphotropic virus I/II (HTLV), hepatitis B and C, and syphilis. This research has been determined to not involve human subjects as there is no intervention or interaction with individuals, and the tissues cannot be identified, directly or through identifiers linked to the participants.
Adult Tenocytes
Commercially‐available tenocytes from three male donors ages 61–81 were obtained from ZenBio (Durham, NC) and were derived from normal Achilles tendons. Prior to use, for each lot of cells, the supplier confirmed tenocytes were negative for cell surface markers CD45 and CD31, and positive for CD44 and CD90, and that cells produced collagen and expressed thrombospondin 4. Cells were shipped from the supplier on dry ice and immediately placed into liquid nitrogen vapor phase upon arrival. For the following experiments, cells were maintained as three independent populations, never refrozen, and passage numbers 3–5 were used.
Media Preparation
Tenocyte Culture Medium was purchased from ZenBio (Durham, NC) and used for maintaining and expanding tenocytes (Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin, and amphotericin B). Growth medium was used for experiments and is defined as DMEM, 10% fetal bovine serum (FBS), 1% l‐glutamine, and 1% Penicillin/Streptomycin/Amphotericin B. Assay medium was made with DMEM and a reduced concentration of FBS (2.5%), 1% l‐glutamine, and 1% Penicillin/Streptomycin/Amphotericin B . For extracellular matrix (ECM) deposition experiments, 50 mg/ml of ascorbic acid was added to assay medium as required for deposition of collagen. For all subsequent experiments, conditioned media (CM) made from placental tissue was used to model the release of growth factors and cytokines from the product in the body as previously described in a wound healing model.14 Briefly, ASA from different human donors was used to make multiple lots of CM (of note, donors were not combined). ASA was thawed and centrifuged down, and supernatant was removed. Assay media (as previously described) was added to the pellet to replace the same volume of media removed, and the solution was incubated on an orbital rocker for 3–5 days at 4°C. Following incubation, CM was made by centrifuging and only collecting the media (100% ASA CM). CM was then filter sterilized using a 0.22 μm syringe filter and stored at 4°C until use (up to 14 days later). At the time of use, 100% ASA CM was diluted for experiments. For tenocyte cell density and migration experiments, 1–50% ASA CM was used; all subsequent assays used 50% ASA CM.
Tenocyte Cell Density
Prior to all experiments, cells were counted using a Cellometer Auto 2000 (Nexcelom Bioscience, Lawrence, MA) with an acridine orange (AO)/propidium iodide (PI) stain. For tenocyte cell density experiments, cells were seeded at 3,300 cells/well in a 48 well plate and cultured using assay media, growth media, or different concentrations of ASA CM in assay media (50%, 25%, 10% v/v). Appropriate fresh media was added at day 1, 4, 7, and 10 following measurement of cell density per well using AlamarBlue (Invitrogen, Carlsbad, CA) as previously described.14 AlamarBlue measures metabolically active cells using absorbance at a single time point; by utilizing a standard curve based off known quantities of tenocytes, a conversion between the absorbance and the cell number was determined at each time point. Representative images of cells stained with 5‐chloromethylfluorescein diacetate (CMFDA) were taken at the endpoint of the experiment (14 days) using an inverted microscope (Nikon Eclipse Ti, Tokyo, Japan) and NIS Elements software (Nikon Eclipse Ti, Tokyo, Japan) at the 4× objective.
Tenocyte Migration
Migration of tenocytes with and without ASA CM was evaluated with a standard Boyden chamber migration assay14 using transwell inserts with 8 µm pore size and polyethylene terephthalate membranes (Corning Costar HTS Transwell, Corning, NY) Briefly, tenocytes in assay media were added at a concentration of 5,000 cells/insert to the top of the chamber, ASA CM was added to the bottom well of the chamber, and tenocytes were incubated for 24 h. Migrated cells were then stained with a 0.5% crystal violet solution. Quantitative assessments of cell migration were completed by imaging five representative images of each well, importing the pictures into ImageJ (National Institutes of Health, Bethesda, MD), and counting the cells for each condition. Cell migration was evaluated in response to assay media (negative control), growth media (positive control), and ASA CM (50%, 25%, 10%, 1% v/v). Qualitative assessments were made by imaging migrated cells stained with crystal violet using an inverted microscope (Nikon Eclipse Ti, Tokyo, Japan) and NIS Elements software (Nikon Eclipse Ti, Tokyo, Japan) at the 4× objective.
Tenocyte Extracellular Matrix Deposition
To investigate the deposition of extracellular matrix molecules including collagen, elastin, and sulfated glycosaminoglycans (sGAG) by tenocytes, 50,000 cells/well were seeded into six well plates and allowed to attach overnight under standard culture conditions with growth media. The following day, tenocytes were switched to the appropriate media type: Growth media, assay media, or 50% ASA CM for 7 or 14 days. At harvest, the supernatant was removed, and plates were frozen at −80°C until use. BioColor kits (United Kingdom) were used to measure deposition of collagen (Sircol Collagen Assay kit), elastin (Fastin Elastin Assay kit), and sGAGs (Blyscan Glycosaminoglycan Assay kit). Before running assays, plates were chosen at random for use in each assay, with n = 9 per condition for each assay type (collagen, elastin, sGAG, dsDNA). All kits were run according to the manufacturer's instructions. In brief, for the collagen assay, an overnight pepsin digestion at 4°C (0.1 mg/ml 0.5 M acetic acid) was used to release deposited collagen into the solution. Following digestion and acid neutralization, Cold Isolation & Concentration Reagent (containing polyethylene glycol in a TRIS‐HCl buffer, pH 7.6, Biocolor, United Kingdom) was used to isolate and concentrate collagen. Sircol dye reagent was added and the dye was measured using a generated standard curve. For the elastin assay, insoluble elastin was converted to water‐soluble α‐elastin with oxalic acid (1.0M). Elastin precipitating reagent was added to precipitate elastin, and the elastin‐dye complex was added to quantify elastin concentration using a generated standard curve. For the sGAG assay, an overnight papain digestion at 60°C (125 µg/ml) was done to extract sGAG into solution. Blyscan dye reagent was added, and sGAG concentration was assessed using a generated standard curve. A Quant‐iT PicoGreen dsDNA Assay kit (ThermoFisher Scientific, Pittsburgh, PA) was used to quantify DNA content, and all ECM deposition targets were normalized to DNA content.
Tenocyte PCR & ELISAs
To evaluate the response of tenocytes in the context of inflammation, 40,000 cells/well were seeded into 6‐well plates and allowed to attach overnight in growth media under standard culture conditions. Media was then switched to appropriate groups: TNF‐α or IL‐1β (0.1 or 1 ng/ml) in assay media alone or in 50% ASA CM. Following a 96 h incubation period, supernatant was collected for ELISAs and stored at −80°C until use and cell monolayers were collected for RT‐qPCR using RNAzol (Molecular Research Center, Cincinnati, OH) and stored at −80°C until use.
For RT‐qPCR, RNA was extracted using RNAzol per the manufacturers instructions; in brief, samples in RNAzol were mixed with water and shaken before storing for 5–15 min, samples were spun down at 12,000 g for 15 min, and the supernatant was mixed with isopropanol to precipitate RNA. After 10 min, samples were centrifuged at 12,000 g for 8 min. Ethanol washes were done prior to elution of the RNA in RNAse‐free water. Reverse transcription to complementary DNA (cDNA) was completed per the manufacturers instructions using the Verso cDNA synthesis kit (ThermoFisher Scientific, Pittsburgh, PA) and a Veriti Thermal Cycler (Applied Biosystems, Foster City, CA). Gene expression was evaluated using commercially‐available TaqMan probes (Life Technologies, Carlsbad, CA) and a QuantStudio™ 3 Real‐Time PCR System (Applied Biosystems, Foster City, CA). A complete list of probes, including accession numbers, is highlighted in Table 1. Fold change in mRNA levels was determined using the 2−ΔΔCt method, with glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) as the housekeeping gene and assay media as the calibrator control.
Table 1.
List of TaqMan Probes Used for Gene Expression Assays
| Cytokine Name | Gene Abbreviation | Ascension Number |
|---|---|---|
| Glyceraldehyde 3‐Phosphate Dehydrogenase (GAPDH, housekeeping gene) | GAPDH | Hs02758991_g1 |
| Interleukin‐1 beta (IL‐1β) | IL1B | Hs00174097_m1 |
| Interleukin‐6 (IL‐6) | IL6 | Hs00174131_m1 |
| Interleukin‐8 (IL‐8) | CXCL8 | Hs00174103_m1 |
| Matrix Metalloproteinase 1 (MMP‐1) | MMP1 | Hs00899658_m1 |
| Transforming Growth Factor Beta‐1 (TGF‐β1) | TGFB1 | Hs00998133_m1 |
| Tumor Necrosis Factor Alpha (TNF‐α) | TNF | Hs01113624_g1 |
For ELISAs, tenocyte production of both MMP‐1 (Invitrogen, Fisher Scientific, Waltham, MA) and TGF‐β1 (R&D Systems, Minneapolis, MN) was measured following inflammatory stimulus. Supernatants from experiments were frozen until measurements with ELISA. Quantities of MMP‐1 and TGF‐β1 were evaluated using commercially‐available ELISA kits per manufacturer's instructions (samples were activated prior to TGF‐β evaluation per the manufacturers protocol). Test dilutions were used to identify appropriate dilutions for ELISAs and samples were run at a 1:3 dilution for the MMP‐1 kit and no dilution for the TGF‐β1 kit.
Statistics
For all experiments, at least three independent experiments were run with at least 2 different lots (donors) of human tenocytes. Additionally, multiple lots of ASA (human donors) were utilized to make conditioned media (kept independent, not pooled) for experiments. Statistical analysis was conducted using a one‐way ANOVA with a post‐hoc Bonferroni test, with p < 0.05 considered significant (Prism, GraphPad Software, San Diego, CA). Throughout the manuscript, p‐value levels are as follows unless otherwise specified: *p < 0.05, **p < 0.01, ***p < 0.001, and §p < 0.0001.
RESULTS
Promotion of Increased Cell Density and Migration
Analysis of tenocyte cell density in vitro demonstrated a significant increase in the number of tenocytes after treatment with ASA CM. When compared to tenocytes in assay media, 50% ASA CM significantly enhanced the number of cells measured at all time points (p < 0.001; Fig. 1A), while lower concentrations significantly increased cell density at 3, 7, and 10 days. Representative images highlighting tenocyte cell density in each media condition are presented in Figure 1B.
Figure 1.
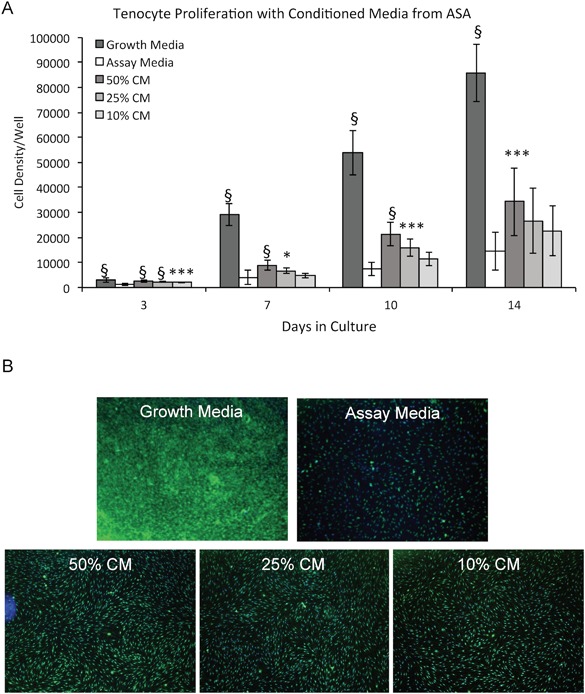
Evaluation of effects of amniotic suspension allograft (ASA) conditioned media (CM) on tenocyte cell density over 14 days. (A) Cell density was measured for cells at days 3, 7, 10, and 14. Mean ± standard deviation reported; n = 12 per group. All statistical comparisons are within the particular day and compared to assay media. *p < 0.05, ***p < 0.001, and §p < 0.0001. (B) At 14 days, cell monolayers were stained with 5‐chloromethylfluorescein diacetate (CMFDA). Representative images demonstrating cell density for each group at 4× objective are shown.
In addition to supporting cell density of tenocytes, we evaluated the promotion of migration of tenocytes by ASA CM. 50% ASA CM resulted in significantly enhanced migration of tenocytes (p < 0.0001, Fig. 2A). Representative images highlighting tenocyte cell migration in each media condition are presented in Figure 2B.
Figure 2.
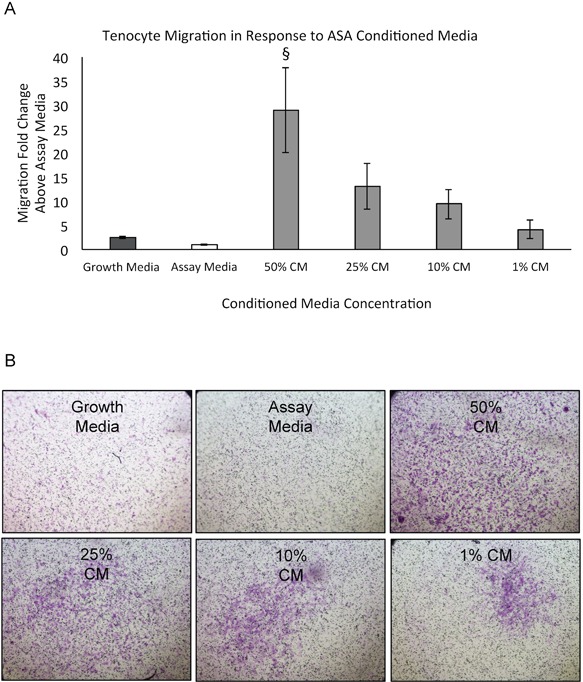
Evaluation of amniotic suspension allograft (ASA) conditioned media (CM) effects on tenocyte migration. (A) Quantitative analysis of tenocyte migration was evaluated over 24 hours. Mean ± standard deviation reported; n = 24 per group. §p < 0.0001 compared to assay media. (B) Qualitative images of cells stained with crystal violet for each group are shown at 4× objective.
Tenocyte Extracellular Matrix (ECM) Deposition
At 7 and 14 days, cells were harvested and evaluated for ECM deposition. At both time points, ASA resulted in significantly increased deposition of collagen, elastin, and sGAG compared to controls (Fig. 3A–C, p < 0.0001).
Figure 3.
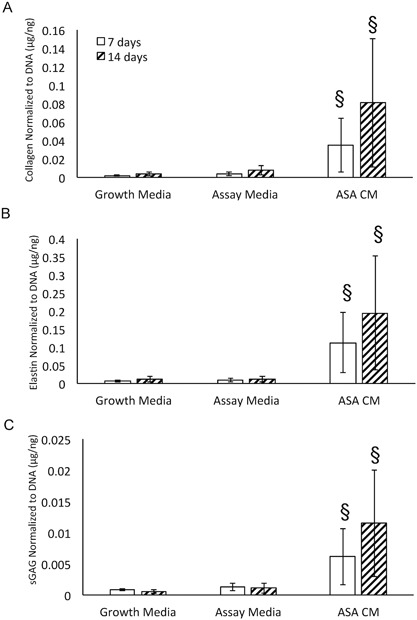
Impact of amniotic suspension allograft (ASA) conditioned media (CM) on extracellular matrix (ECM) deposition of tenocytes. Deposition of extracellular matrix molecules was evaluated including (A) collagen, (B) elastin, and (C) soluble glycosaminoglycans (sGAG). For all groups, data are presented as mean ± standard deviation; n = 9 per group. §p < 0.0001 compared to assay media at the same time point.
Tenocyte Response to Inflammatory Cytokine Stimulation
Exposure to inflammatory cytokines with addition of ASA did not result in significant changes to gene expression for IL‐1β, TNF‐α, IL‐6, MMP‐1 or TGF‐β1 compared to inflammatory cytokines alone (Fig. 4A–C, E–F). IL‐1β exposure resulted in upregulation of the expression of IL‐8 (compared to no inflammation, Fig. 4D); when 50% ASA CM was added, expression of IL‐8 was significantly downregulated compared to IL‐1β in assay media (p < 0.05, Fig. 4D).
Figure 4.
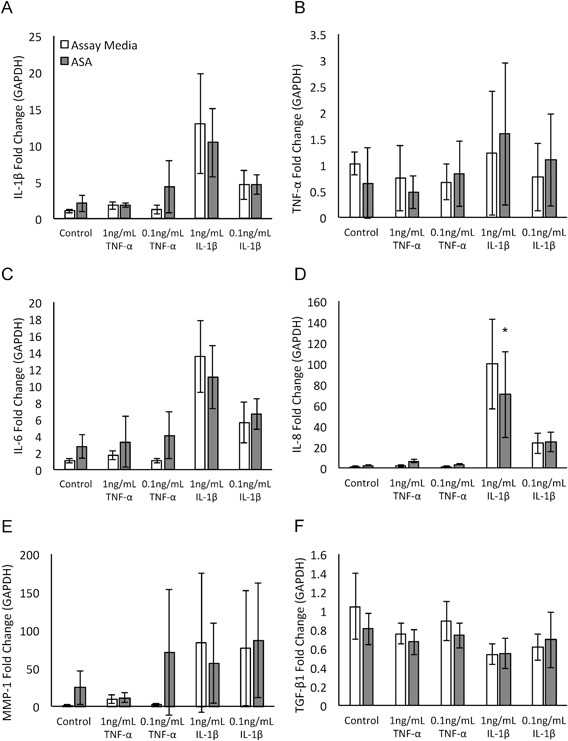
Evaluation of amniotic suspension allograft (ASA) conditioned media (CM) effect on tenocyte gene expression in pro‐inflammatory environment. Data is presented as fold change of the target of interest normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH). (A) interleukin‐1 beta (IL‐1β), (B) tumor necrosis factor alpha (TNF‐α), (C) interleukin‐6 (IL‐6), (D) interleukin‐8 (IL‐8), (E) matrix metalloproteinase 1 (MMP‐1), and (F) transforming growth factor beta 1 (TGF‐β1). Mean ± standard deviation reported; n = 12 per group. *p < 0.05 compared to untreated condition.
In the presence of TNF‐α and IL‐1β, tenocytes treated with 50% ASA CM resulted in non‐significant decreases in MMP‐1 production (Fig. 5A). Tenocytes cultured with 0.1 ng/ml of TNF‐α or IL‐1β in 50% ASA CM produced significantly less TGF‐β1 protein (p < 0.0001, p < 0.05; Fig. 5B). Overall, 50% ASA CM resulted in reduction of gene expression of IL‐8 and reduction of protein levels of TGF‐β1.
Figure 5.
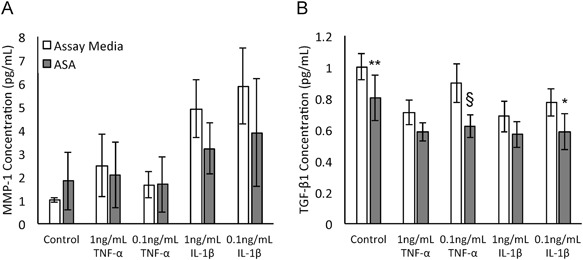
Evaluation of effect of amniotic suspension allograft (ASA) conditioned media (CM) on protein production of tenocytes in a pro‐inflammatory environment. Concentrations of (A) matrix metalloproteinase 1 (MMP‐1) and (B) transforming growth factor beta 1 (TGF‐β1) were measured. Mean ± standard deviation reported; n = 9 per group. *p < 0.05, **p < 0.01, and §p < 0.0001 compared to untreated condition.
DISCUSSION
The primary goal of this work was to better understand the potential role of amniotic suspension allograft (ASA) in supporting tendon healing. In this study, we saw that 50% ASA conditioned media (CM) resulted in significantly greater cell density at 14 days, cell migration at 24 hours, and deposition of collagen, elastin, and sGAGs at both 7 and 14 days. Furthermore, 50% ASA CM significantly downregulated IL‐8 levels following stimulation with IL‐1β and significantly decreased TGF‐β1 protein production following stimulation with both IL‐1β and TNF‐α. These results provide evidence supporting the use of ASA for supporting tendon healing.
In aging, the number of tenocytes per unit of surface area in a tendon decrease as well as their overall metabolic activity.15 Improving the inefficient healing process in tendinopathies may require increasing the number of tenocytes at the injury site, as these are the cells responsible for repairing the cellular scaffold that tendons require.16 In the current study, tenocytes exposed to 50% ASA CM grew and migrated at significantly higher rates than those in assay media alone. Placental‐derived tissues such as ASA are known to contain growth factors, including aFGF, bFGF, PDGF‐BB, IGF‐I, TGF‐β1, and TGF‐β3.3 Interestingly, others have shown that exogenous growth factors, including aFGF,17 bFGF,17, 18, 19 TGF‐β3,20 VEGF,19 human recombinant epidermal growth factor (hrEGF),21 and PRP,21 have enhanced tenocyte migration and/or proliferation in preclinical models of tendon injury. Furthermore, evidence suggests that a combination of factors, including bFGF, PDGF‐BB, and IGF‐I, were superior to each factor individually in promoting tenocyte proliferation.22 The current study evaluated the effects of a combination of factors released from ASA. Similarly, others have shown increases in tenocyte number and migration as a result of conditioned media made from rat mesenchymal stem cells18 and PRP.21 Conditioned media from ASA was used throughout the current study; however, in future studies, we will evaluate whether placing ASA in physical contact with the cells of interest results in stronger effects.
Structural changes to tendons are known to occur during the aging process23 and also in certain diseases, including diabetes.24 Exogenous delivery of bFGF in a rabbit model of Achilles tendon repair resulted in enhanced cell maturation and improvements to the structural organization of the tendon.25 Others have seen increased collagen deposition as a result of bFGF treatment via gene therapy19 and increased deposition of elastin as a result of IGF‐I in other tissue types.26 In the current study, treatment of tenocytes with 50% ASA CM significantly increased collagen and elastin deposition at both 7 and 14 days. While sGAGs are not directly related to mechanical strength of the tendon, elastin signaling pathways, along with FGFs, TGF‐βs, IGF, TIMPs, TNF‐α, PDGF, and collagen pathways, are highly regulated by sGAGs.27 The presence of these factors in ASA CM could explain the significant increase in sGAG deposition in ASA CM group compared to assay media group.
One problematic aspect in some tendinopathies is the persistent inflammatory response; this process must be tightly regulated in order to prevent aberrant tissue damage.28 Two of the hallmark cytokines responsible for inflammation in tendons are IL‐1β and TNF‐α.29 In a tendon, IL‐1β is primarily produced by invading immune cells and leads to a breakdown of the surrounding ECM, triggering a switch from the production of collagen I to collagen III.28, 30 Similarly, TNF‐α reduces overall collagen production and is thought to increase the inflammatory positive feedback loop.28 When tenocytes were stimulated with inflammatory cytokines in the presence of ASA CM, tenocytes expressed significantly less IL‐8 in response to 1 ng/ml IL‐1β. IL‐8 specifically activates neutrophils, which has been shown to lead to the destruction of connective tissue.31 In a previous study, stimulation of human tendon cells with IL‐1β led to increases in gene expression of IL‐1β, MMP‐1, and IL‐6, along with increases in protein production of MMP‐1.30 In the current study, we also saw trends for increased expression in those genes when tenocytes were exposed to IL‐1β, and trends towards decreased MMP‐1 protein production. Interestingly, when ASA CM was included along with IL‐1β or TNF‐α, the protein production of TGF‐β1 was significantly downregulated. While some TGF‐β1 is thought to be favorable to tendon healing, a reduction in TGF‐β1 levels may be beneficial as studies have suggested that high levels of TGF‐β1 expression promote scar and adhesion formation32 and reduced levels of TGF‐β1 results in improved range of motion.33
Overall, ASA promoted increases in cell density, migration, and deposition of ECM from tenocytes; interestingly, in an inflammatory model, exposure to ASA resulted in downregulation of IL‐8 gene expression and reduced protein production of TGF‐β1, suggesting an overall anti‐inflammatory effect. In sum, this study has suggested a benefit of placental‐derived tissues in the context of tendon healing. While some early clinical work has been completed, large randomized clinical trials are necessary to evaluate the clinical benefit of ASA in tendon repair.
AUTHORS' CONTRIBUTIONS
KAK‐ Designed Study, Completed Experiments, and Wrote Manuscript JPM: Designed Study, Completed Experiments, and Wrote Manuscript MCS: Interpreted Data, Wrote Manuscript KCM: Designed Study, Wrote Manuscript, and Edited Manuscript
ACKNOWLEDGEMENTS
This study was supported and funded by Organogenesis, Inc, Canton, MA. KAK, JPM, and KCM are employees of Organogenesis, Inc.
Kelly A. Kimmerling and John P. McQuilling are co‐first authors.
REFERENCES
- 1. Sabella N. 1913. Use of fetal membranes in skin grafting. Med Rec 83:478–480. [Google Scholar]
- 2. Riboh JC, Saltzman BM, Yanke AB, et al. 2016. Human amniotic membrane‐derived products in sports medicine: basic science, early results, and potential clinical applications. Am J Sports Med 44:2425–2434. [DOI] [PubMed] [Google Scholar]
- 3. McQuilling JP, Vines JB, Kimmerling KA, et al. 2017. Proteomic comparison of amnion and chorion and evaluation of the effects of processing on placental membranes. Wounds 29:E36–E40. [PMC free article] [PubMed] [Google Scholar]
- 4. Bryant‐Greenwood GD. 1998. The extracellular matrix of the human fetal membranes: structure and function. Placenta 19:1–11. [DOI] [PubMed] [Google Scholar]
- 5. Hao Y, Ma DH‐K, Hwang DG, et al. 2000. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 19:348–352. [DOI] [PubMed] [Google Scholar]
- 6. Schlabritz‐loutsevitch N, Li CUN, Nathanielsz PW. 2007. Insulin‐Like growth factors and placental function. Science 18:201–224. [Google Scholar]
- 7. Lyall F, Simpson H, Bulmer JN, et al. 2001. Transforming growth factor‐beta expression in human placenta and placental bed in third trimester normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol 159:1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molloy T, Wang Y, Murrell GAC. 2003. The roles of growth factors in tendon and ligament healing. Sport Med 33:381–394. [DOI] [PubMed] [Google Scholar]
- 9. Vuorela P, Hatva E, Lymboussaki A, et al. 1997. Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol Reprod 56:489–494. [DOI] [PubMed] [Google Scholar]
- 10. Vines JB, Aliprantis AO, Gomoll AH, et al. 2016. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg 29:443–450. [DOI] [PubMed] [Google Scholar]
- 11. Nunley P, Kerr E, Utter P, et al. 2016. Preliminary results of bioactive amniotic suspension with allograft for achieving one and two‐Level lumbar interbody fusion. Int J Spine Surg 10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sclafani JA, Liang K, Mosley D, et al. 2016. A retrospective chart assessment of clinical outcomes after amniotic suspension allograft is used during spinal arthrodesis procedures. Sci Res 7:150–156. [Google Scholar]
- 13. Anderson JJ, Swayzee Z. 2015. The use of human amniotic allograft on osteochondritis dissecans of the talar dome: a comparison with and without allografts in arthroscopically treated ankles. Sci Res 6:412–417. [Google Scholar]
- 14. McQuilling JP, Vines JB, Mowry KC. 2017. In vitro assessment of a novel, hypothermically stored amniotic membrane for use in a chronic wound environment. Int Wound J 14:993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kannus P, Paavola M, Józsa L. 2005. Aging and degeneration of tendons In: Maffulli N, Renstrom P, Leadbetter WB, editors. Tendon Injuries. London: Springer. [Google Scholar]
- 16. Liu C‐F, Aschbacher‐Smith L, Barthelery NJ, et al. 2011. What we should know before using tissue engineering techniques to repair injured tendons: a developmental biology perspective. Tissue Eng Part B Rev 17:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tokunaga T, Shukunami C, Okamoto N, et al. 2015. FGF‐2 stimulates the growth of tenogenic progenitor cells to facilitate the generation of Tenomodulin‐Positive tenocytes in a rat rotator cuff healing model. Am J Sports Med 43:2411–2422. [DOI] [PubMed] [Google Scholar]
- 18. Chen Q, Liang Q, Zhuang W, et al. 2017. Tenocyte proliferation and migration promoted by rat bone marrow mesenchymal stem cell‐derived conditioned medium. Biotechnol Lett 40:215–224. [DOI] [PubMed] [Google Scholar]
- 19. Tang JB, Wu YF, Cao Y, et al. 2016. Basic FGF or VEGF gene therapy corrects insufficiency in the intrinsic healing capacity of tendons. Sci Rep 6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huegel J, Mauck R, Soslowsky L, et al. 2015. Differential effects of growth factors on neonatal and adult achilles tenocytes. Uni Pennsylvania Ortho J (UPOJ) 3:165–168. [Google Scholar]
- 21. Sarıkaya B. 2017. Comparison of the effects of human recombinant epidermal growth factor and platelet‐rich plasma on healing of rabbit patellar tendon. Jt Dis Relat Surg 28:92–99. [DOI] [PubMed] [Google Scholar]
- 22. Costa MA, Wu C, Pham BV, et al. 2006. Tissue engineering of flexor tendons: optimization of tenocyte proliferation using growth factor supplementation. Tissue Eng 12:1937–1943. [DOI] [PubMed] [Google Scholar]
- 23. Godinho MSC, Thorpe CT, Greenwald SE, et al. 2017. Elastin is localised to the interfascicular matrix of energy storing tendons and becomes increasingly disorganised with ageing. Sci Rep 7:9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Oliveira RR, Martins CS, Rocha YR, et al. 2013. Experimental diabetes induces structural, inflammatory and vascular changes of achilles tendons. PLoS ONE 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Najafbeygi A, Fatemi MJ, Lebaschi AH, et al. 2017. Effect of basic fibroblast growth factor on achilles tendon healing in rabbit. World J Plast Surg 6:26–32. [PMC free article] [PubMed] [Google Scholar]
- 26. Rosa RG, Joazeiro PP, Bianco J, et al. 2014. Growth factor stimulation improves the structure and properties of scaffold‐free engineered auricular cartilage constructs. PLoS ONE 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L. 2010. Glycosaminoglycan (GAG) biosynthesis and GAG‐Binding proteins. Prog Mol Biol Transl Sci 93:1–17. [DOI] [PubMed] [Google Scholar]
- 28. Millar NL, Murrell GAC, McInnes IB. 2017. Inflammatory mechanisms in tendinopathy‐towards translation. Nat Rev Rheumatol 13:110–122. [DOI] [PubMed] [Google Scholar]
- 29. Müller SA, Todorov A, Heisterbach PE, et al. 2015. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surgery, Sport Traumatol Arthrosc 23:2097–2105. [DOI] [PubMed] [Google Scholar]
- 30. Tsuzaki M, Guyton G, Garrett W, et al. 2003. IL‐1b induces COX2, MMP‐1, ‐3 and ‐13, ADAMTS‐4, IL‐1b and IL‐6 in human tendon cells. J Orthop Res 21:256–264. [DOI] [PubMed] [Google Scholar]
- 31. Ackermann PW, Domeij‐Arverud E, Leclerc P, et al. 2013. Anti‐inflammatory cytokine profile in early human tendon repair. Knee Surgery, Sport Traumatol Arthrosc 21:1801–1806. [DOI] [PubMed] [Google Scholar]
- 32. Katzel EB, Wolenski M, Loiselle AE, et al. 2011. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res 29:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xia C, Yang X, Wang Y, et al. 2010. Tendon healing in vivo and in vitro: neutralizing antibody to TGF‐β improves range of motion after flexor tendon repair. Orthopedics 33:809. [DOI] [PubMed] [Google Scholar]


