Abstract
Circular RNA (circRNA) is a key regulator in the development and progression of human cancers. Previous studies confirmed circRNA‐0008717 (circABCB10) as an oncogene in osteosarcoma, but the regulatory effect of circABCB10 in nonsmall cell lung cancer (NSCLC) is still unclear. In the current study, we examined the expression of circABCB10 in different NSCLC cell lines. Bioinformatics analysis, Cell Counting Kit‐8 assays, Transwell migration, fluorescein reporting experiments, and xenografts in mice were used to detect the effect of circABCB10 on NSCLC cell proliferation and migration in vitro and tumor growth in vivo. The results showed that the expression of circABCB10 in NSCLC cell lines was increased. Downregulation of circABCB10 suppressed NSCLC cell proliferation and migration by promoting microRNA miR‐1252 expression and suppressing Forkhead box 2 (FOXR2). Fluorescein reporting experiments confirmed that circABCB10 expression increased FOXR2 levels by sponging miR‐1252, and in vivo experiments found that knockdown of circABCB10 decreased tumor growth. These data suggested that circABCB10 acted as a tumor promoter through a novel miR‐1252/FOXR2 axis, providing potential biomarkers and therapeutic targets for the management of NSCLC.
Keywords: circABCB10, FOXR2, miR‐1252, nonsmall cell lung cancer, proliferation and migration
1. INTRODUCTION
Lung cancer is a leading cause of death and is becoming more prevalent worldwide.1 The most frequent type of lung cancer is nonsmall cell lung cancer (NSCLC, approximately 85% of all lung cancers). Low‐income and middle‐income countries now account for more than 50% of lung cancer deaths each year. Although treatments are improving, the 5‐year survival rate is still only 17% for NSCLC.2, 3 Therefore, a better understanding of the molecular mechanisms associated with NSCLC pathogenesis is critical to the development of effective diagnostic and therapeutic approaches, which may lead to the identification of novel biomarkers and treatment targets for NSCLC and possibly to the development of personalized therapies for individual NSCLC patients in the future.4
As a noncoding RNA, circular RNAs (circRNAs) have become an important research topic in recent years.5 However, the biological functions of circRNAs in human diseases are still unclear, especially in cancer pathogenesis. Recently, studies have found that several circRNAs possess microRNA (miRNA) binding sites and can regulate miRNA downstream targets by sponging and arresting the activity of miRNAs.6, 7, 8 One study found that circRNA‐0008717 (circABCB10) was upregulated in osteosarcoma, suggesting its use as a prognostic biomarker for osteosarcoma patients.9 The circABCB10 is cyclized from part of the exon region in the ABCB10 gene, but its role in NSCLC remains unclear.
Therefore, in the current study, we compared circABCB10 expression between normal lung epithelial cells and lung cancer cell lines. The effects of circABCB10 expression on cell migration and proliferation of lung cancer cell lines were also investigated. Bioinformatics analysis and fluorescein reporting experiments suggested an interaction between circABCB10, miR‐1252, and Forkhead box 2 (FOXR2). We found that circABCB10 played an oncogenic role in NSCLC by sponging miR‐1252 to increase FOXR2 synthase expression and consequently promote NSCLC progression. Therefore, circABCB10 may serve as a useful biomarker for prognosis predictions and as a potential therapeutic target for NSCLC patients.
2. MATERIALS AND METHODS
2.1. Ethics statement
All animal experiments were approved and performed according to the guidelines of the Ethics Committee of Zhongshan Hospital of Fudan University, Shanghai, China. All surgical procedures were performed under anesthesia, and every effort was made to minimize suffering.
2.2. Cell culture and transfection
Breast cancer cell lines (A549, H1975, PC9, H1299, and H1650) and normal human lung epithelial cells (BEAS‐2B) were purchased from the American Type Culture Collection (Manassas, VA) and cultured in Roswell Park Memorial Institute Medium 1640 (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY) with 100 IU/mL of penicillin and 100 μg/mL of streptomycin at 37°C in 5% CO2.
Small interfering RNA (siRNA) against hsa_circ_0008717 (circABCB10; 5′‐TAGAAGACCATGGGGGATGTCAAGAGCATCCCCCATGGTCTTCTATTTTTT‐3′), miR‐1252 mimic (5′‐AGAAGGAAAUUGAAUUCAUUUA‐3′), and miR‐1252 inhibitor (synthesized by GenePharma, Shanghai, China) were transfected into lung cancer cells using Lipofectamine 2000 Reagent (Invitrogen).
For circABCB10 overexpression, circABCB10 overexpression vector was constructed into pCDNA3.0 and transfected into BEAS‐2B cell with the help with Lipofectamine 2000.
2.3. Bioinformatics analysis
The hsa_circ_0008717 (circABCB10)‐miRNA target gene prediction was performed using the https://circinteractome.nia.nih.gov/ website. The miR‐1252–mRNA target gene prediction was performed using the http://www.targetscan.org/vert_71/ website.
2.4. Cell Counting Kit‐8 assay
A549 and PC9 cells transfected with or without siRNA against circABCB10 and a FOXR2 overexpression vector were seeded into 96‐well plates at 5 × 103 cells/well and incubated for increasing durations (0, 12, 24, 48, and 72 hours) before adding 10 μL of Cell Counting Kit‐8 (CCK8) solution per well for 1 hour. Absorbance (450 nm) was measured using a Synergy microplate reader (BioTek, Winooski, VT).
2.5. Transwell migration assays
Cell migration was analyzed using Transwell chambers (Corning, New York, NY) according to the manufacturer's protocol. After incubation for 24 hours, cells on the upper surfaces of the Transwell chambers were removed with cotton swabs, and the cells located on the lower surfaces were fixed in methanol for 10 minutes, followed by staining with crystal violet. The stained cells were photographed and counted in five randomly selected fields.
2.6. Luciferase reporter assay
For luciferase reporter assays, wild‐type (wt) and mutant (mut) promoters of circABCB10 and wt and mut sequences from the 3′‐untranslated region (3′‐UTR) of FOXR2 were synthesized and inserted into pGL3‐basic vectors (Gene‐Creat, Wuhan, China). The pGL3‐basic‐circABCB10‐wt/mut vectors, pGL3‐basic‐FOXR2‐wt/mut vectors, and pcDNA3.1‐c‐Myb vectors were cotransfected with pRL‐TK into HEK293T cells using Lipofectamine 2000. For circABCB10 and miR‐1252 luciferase reporter assays, circABCB10 and FOXR2 3′‐UTR sequences containing wt or mutated miR‐1252 binding sites were synthesized and inserted into the pmirGLO luciferase vector (Gene‐Creat) and then cotransfected with miR‐1252 mimics into HEK293T cells using Lipofectamine 2000. Cells were harvested 48 hours after transfection, and luciferase activity was detected with the dual‐luciferase reporter assay system (Promega, Madison, WI). Relative luciferase activity was normalized to the Renilla luciferase internal control.
2.7. Xenografts in mice
A549 cells were stably infected with or without circABCB10 silencing vectors. A total of 5 × 106 viable cells were injected into the right flanks of nude mice as described previously.10 Tumor sizes were measured using a vernier caliper every 5 days from the fifth day after injection, and tumor volume was calculated using the following formula: volume = 1/2 × length × width2. At 30 days after implantation, mice were euthanized for quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) and Western blot analyses.
2.8. Quantitative reverse transcription‐polymerase chain reaction
RNA was extracted from tissues or cultured cells with TRIzol reagent according to the manufacturer's protocol (Life Technologies, Scotland, UK). Reverse transcription of 200 ng of total RNA was performed in a final volume of 10 μL using random primers and standard conditions with the Prime Script RT Master Mix (Applied Biosystems, Foster City, CA). qRT‐PCR was performed using the SYBR Select Master Mix (Applied Biosystems) with 0.5 μL of complementary DNA (cDNA) on the ABI7300 system (Applied Biosystems) according to the manufacturer's instructions. We used glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) or U6 as internal controls. Relative transcription alterations were evaluated using the method as previously described.9
2.9. Western blot analysis
Protein was extracted from cells or tissues using RIPA lysis buffer (Applied Biosystems) and subjected to Western blot analysis as described previously.11 Anti‐FOXR2 and anti‐GAPDH antibodies were supplied by Cell Signaling Technology (Beverly, MA). All antibodies were used according to the manufacturer's protocol, and immunoreactivity was visualized using a chemiluminescence detection kit (ECL Western Blotting Substrate; Dongguan Biotech, Shandong, China).
2.10. Statistical analysis
Data were analyzed using the SPSS software package (IBM, Armonk, NY). Statistical significance was determined with the Student t test or analysis of variance, and P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Knockdown of circABCB10 suppressed NSCLC cell proliferation and migration
The qRT‐PCR detection found that circABCB10 was increased in lung cancer cell lines, including the A549, PC9, H1299, H1975, and H1650 cell lines when compared with normal BEAS‐2B lung epithelial cells, with the highest expression in A549 and PC9 cells (Figure 1A). Consequently, A549 and PC9 cells were selected for the following experiment. To determine if circABCB10 played a role in lung cancer cell proliferation and migration, siRNA against circABCB10 was constructed and transfected into A549 and PC9 cells. The qRT‐PCR results showed that circABCB10 expression was decreased after knockdown of circABCB10 for 48 hours compared with the negative control (NC) or nontransfected control cells (Figure 1B). CCK8 assays showed that knockdown of circABCB10 suppressed cell proliferation in both A549 and PC9 cells (Figure 1C and 1D). Transwell analysis found that downregulating circABCB10 expression decreased the migratory capacity of both A549 and PC9 cells (Figure 1E and 1F), suggesting that circABCB10 plays a role in lung cancer cell proliferation and migration.
Figure 1.
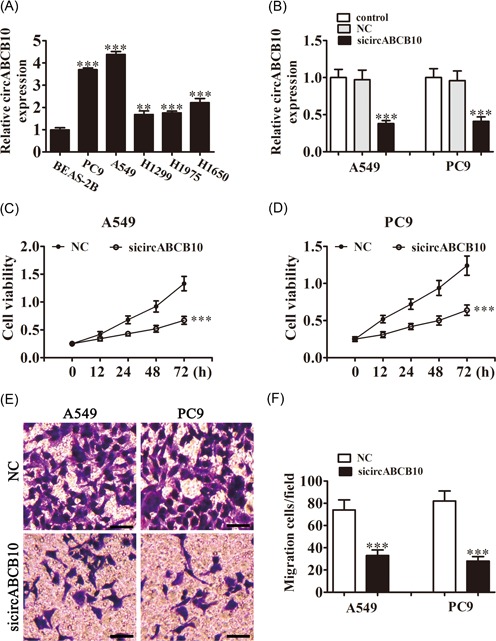
Downregulation of circABCB10 suppressed NSCLC proliferation and migration. A, Relative expression levels of circABCB10 were measured by qRT‐PCR in the lung cancer cell lines A549, PC9, H1299, H1975, H1650, and in normal BEAS‐2B lung epithelial cells. B, CircABCB10 expression in A549 and PC9 cells was measured by qRT‐PCR after transfection with siRNA against circABCB10 (sicircABCB10) or negative control (NC). C, D, CCK8 assays were used to detect the proliferation of A549 and PC9 cells. E, F, Cell migration of A549 and PC9 cells was measured by Transwell assay. Each experiment was repeated at least three times; *P < 0.05, **P < 0.01, and ***P < 0.001. CCK8, Cell Counting Kit‐8; NSCLC, nonsmall cell lung cancer; qRT‐PCR, quantitative reverse transcription‐polymerase chain reaction; siRNA, small interfering RNA
3.2. Overexpression circABCB10 promotion BEAS‐2B cell proliferation and migration
To identify if circABCB10 has an opposite effect on low expressed cell, circABCB10 overexpression vector was constructed and transfected into BEAS‐2B cells. The results show that after transfected with circABCB10 overexpression vector for 48 hours, the expression of circABCB10 was significantly increased compared with the NC group (Figure. 2A). CCK8 assays showed that overexpression circABCB10 promotion cell proliferation in BEAS‐2B cells (Figure 2B). Transwell analysis found that upregulating circABCB10 expression increased the migratory capacity of BEAS‐2B cells (Figure 2C), which confirmed that circABCB10 plays a promotion effect in cell proliferation and migration.
Figure 2.
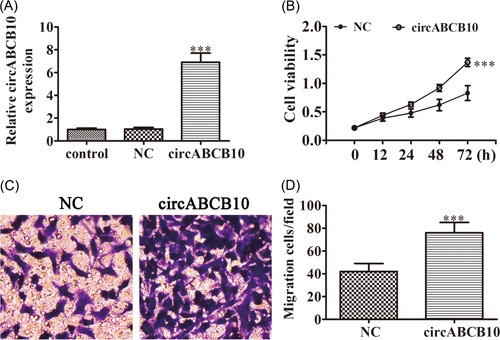
Overexpression of circABCB10 promotion BEAS‐2B proliferation and migration. A, Relative expression levels of circABCB10 were measured by qRT‐PCR in BEAS‐2B after transfected with circABCB10 overexpression vector or negative control (NC). B, CCK8 assays were used to detect the proliferation of BEAS‐2B cells. C, Cell migration of BEAS‐2B cells was measured by Transwell assay. Each experiment was repeated at least three times; *P < 0.05, **P < 0.01, and ***P < 0.001. CCK8, Cell Counting Kit‐8; qRT‐PCR, quantitative reverse transcription‐polymerase chain reaction
3.3. MiR‐1252 was the target of circABCB10
Increasing evidence has indicated that there is novel functional crosstalk between circRNAs and miRNAs.6 Therefore, in this study, we analyzed and predicted the interaction between circRNAs and miRNAs using a bioinformatics analysis website. The results showed that miR‐1252 was a potential target of circABCB10. To prove this prediction, we conducted luciferase reporter assays (Figure 3A) and showed that miR‐1252 overexpression significantly inhibited luciferase activity of the circABCB10‐wt reporter in both A549 and PC9 cells (Figure 3B and 3C), indicating a direct interaction between circABCB10 and miR‐1252. The qRT‐PCR assays showed that knockdown of circABCB10 promoted miR‐1252 expression in both A549 and PC9 cells (Figure 3D), suggesting that circABCB10 could sponge miR‐1252.
Figure 3.
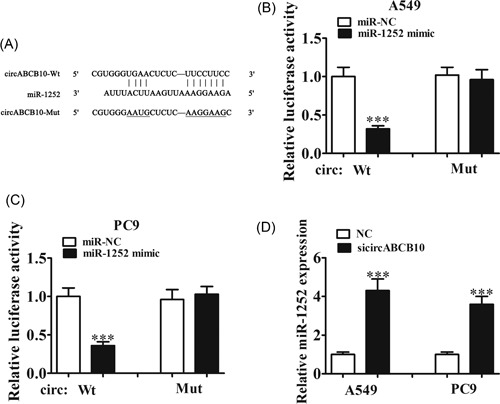
The miR‐1252 was the target of circABCB10. A, Predicted binding sites of miR‐1252 and circABCB10. B, C, Luciferase assay of A549 and PC9 cells cotransfected with miR‐1252 mimics and a luciferase reporter containing circABCB10‐wt or mutant constructs (circABCB10‐mut). D, CircABCB10 knockdown promoted miR‐1252 expression in both A549 and PC9 cells. Each experiment was repeated at least three times; ***P < 0.001. Mut, mutant; NC, negative control; Wt, wild type
3.4. CircABCB10 promoted FOXR2 expression by sponging miR‐1252
Our bioinformatics analysis further suggested that miR‐1252 could interact with the 3′‐UTR of FOXR2 (Figure 4A). In confirmation, luciferase reporter assays showed that miR‐1252 overexpression significantly inhibited luciferase activity of the FOXR2 3′‐UTR‐wt reporter in both A549 and PC9 cells (Figure 4B and 4C), indicating a direct interaction between the 3′‐UTR of FOXR2 and miR‐1252. The qRT‐PCR and Western blot analysis confirmed that miR‐1252 overexpression suppressed the expression of FOXR2 messenger RNA (mRNA) and protein, respectively (Figure 4D and 4E) and demonstrated that circABCB10 knockdown also decreased FOXR2 mRNA and protein expression in both A549 and PC9 cells (Figure 4F and 4G).
Figure 4.
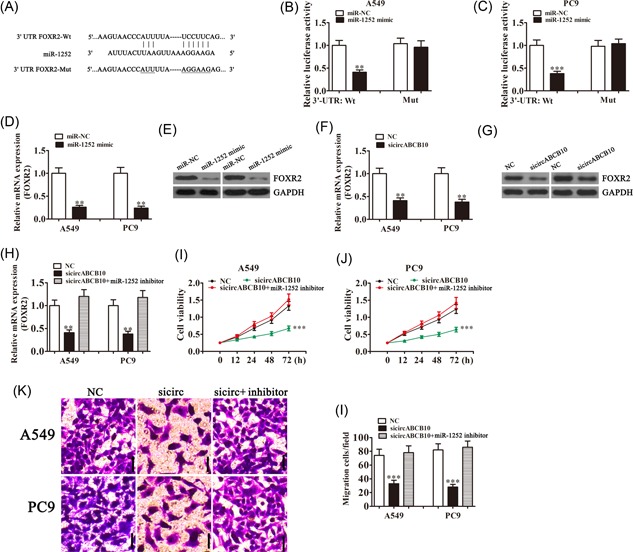
The circABCB10 promoted FOXR2 expression by sponging miR‐1252. A, Predicted binding sites of miR‐1252 in the 3′‐UTR of FOXR2. B, C Luciferase assay of A549 and PC9 cells cotransfected with miR‐1252 mimics and a luciferase reporter containing wt or mutant 3′‐UTR of FOXR2 (FOXR2‐Mut). D, E, The miR‐1252 overexpression suppressed FOXR2 expression at both the mRNA and protein levels in both A549 and PC9 cells. F, G, The circABCB10 knockdown suppressed FOXR2 expression at both the mRNA and protein levels in both A549 and PC9 cells. H, FOXR2 expression was rescued by transfection with an miR‐1252 inhibitor in A549 and PC9 cells. I, J, Cell proliferation was determined by CCK8 assays in A549 and PC9 cells. K, L, Transwell assays were used to measure cell migration of A549 and PC9 cells. Each experiment was repeated at least three times; **P < 0.01 and ***P < 0.001. CCK8, Cell Counting Kit‐8; FOXR2, Forkhead box 2; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; mRNA, messenger RNA; mut, mutant; NC, negative control; 3′‐UTR, 3′‐untranslated region; wt, wild type
The qRT‐PCR experiments showed that circABCB10 silencing significantly suppressed FOXR2 expression, whereas inhibition of miR‐1252 rescued FOXR2 levels in both A549 and PC9 cells (Figure 4H). CCK8 analysis found that miR‐1252 inhibitor treatment reversed the inhibition of cell proliferation induced by circABCB10 silencing in both A549 and PC9 cells (Figure 4I and 4J). Transwell analysis also found that downregulation of miR‐1252 rescued the loss of migration induced by circABCB10 silencing in both A549 and PC9 cells (Figure 4K and 4J). These results suggested that circABCB10 promoted FOXR2 expression by sponging miR‐1252, which inhibited lung cancer cell proliferation and migration.
3.5. Knockdown circABCB10 inhibited lung cancer growth in vivo
Finally, we sought to analyze the effects of circABCB10 on tumor growth in vivo. We performed a xenograft experiment by injecting A549 cells into nude recipient mice. Tumor size was measured and calculated every 5 days. The results show that circABCB10 knockdown significantly inhibited tumor growth (Figure 5A). Furthermore, after 30 days postinjection, we measured tumor weights and found that circABCB10 silencing led to smaller tumors (Figure 5B). The qRT‐PCR detection found that the expression of miR‐1252 was increased more in tumor tissues from the circABCB10‐silenced group than that in the NC group (Figure 5C). Western blot analyses revealed that FOXR2 expression in tumor tissues from the circABCB10‐silenced group was decreased when compared with the NC group (Figure 5D).
Figure 5.
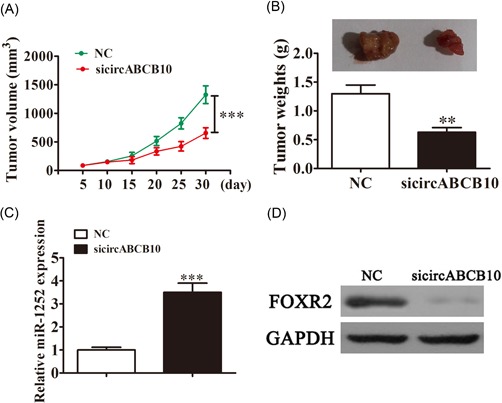
Knockdown of circABCB10 inhibited lung cancer growth in vivo. A, Tumor sizes were measured every 5 days. B, Tumor weight was analyzed 30 days postinjection. C, The qRT‐PCR assays showed expression of miR‐1252. D, Western blot analysis detection showed the expression of FOXR2 in tumor tissues. Each experiment was repeated at least three times; **P < 0.01 and ***P < 0.001. FOXR2, Forkhead box 2; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; NC, negative control; qRT‐PCR, quantitative reverse transcription‐polymerase chain reaction
4. DISCUSSION
Lung cancer is the leading cause of cancer deaths worldwide. Previous studies have found that miRNAs and long noncoding RNAs play an important role in lung cancer development and progression.12, 13, 14 Whether circRNAs exert similar roles in tumorigenesis remains unclear. In the current study, we found that circABCB10 expression was increased in lung cancer cells. Knockdown of circABCB10 suppressed proliferation and migration of both A549 and PC9 cells. Bioinformatics analysis found that miR‐1252 was the target of circABCB10, which was confirmed by fluorescein reporting experiments. The results also found that circABCB10 silencing promoted miR‐1252 expression, suggesting that circABCB10 may act as an miR‐1252 sponge. Further bioinformatics analysis found that FoxR2 was the target of miR‐1252. Fluorescein reporting experiments confirmed that miR‐1252 interacted with the 3′‐UTR of FoxR2 and suppressed FoxR2 expression posttranscriptionally.
Human Fox genes belong to the family of winged/forkhead transcription factors, including at least 43 members from FoxA1 to FoxQ1.15 FoxR2 is a more recently discovered member of the Fox transcription factor family, first identified in 2004.16 An increasing number of studies have found that FoxR2 plays an important role in the development of many human tumors, including glioma,11 prostate cancer,17 hepatocellular carcinoma,18 and even NSCLC.19 Knockdown of FOXR2 suppressed tumorigenesis, growth, and metastasis.19 In the current study, we found that overexpression of miR‐1252 suppressed FOXR2 expression. Silencing circABCB10 suppressed FOXR2 expression, suggesting that circABCB10 expression promoted FOXR2 by sponging miR‐1252. Silencing of miR‐1252 reversed circABCB10 knockdown‐induced proliferation and inhibited migration of lung cancer cells by promoting FOXR2 expression, which suggested that circABCB10 promoted FOXR2‐mediated NSCLC proliferation and migration by regulating miR‐1252. Our in vivo studies also confirmed that knockdown of circABCB10 suppressed tumor growth.
In summary, our study has for the first time to our knowledge investigated the role of circABCB10 and identified it as an oncogene in NSCLC. Our findings demonstrated that circABCB10 contributed to NSCLC progression by enhancing FOXR2 expression via sponging of miR‐1252, suggesting that circABCB10 may be a potential target for NSCLC therapy.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
Tian X, Zhang L, Jiao Y, Chen J, Shan Y, Yang W. CircABCB10 promotes nonsmall cell lung cancer cell proliferation and migration by regulating the miR‐1252/FOXR2 axis. J Cell Biochem. 2019;120:3765‐3772. 10.1002/jcb.27657
Contributor Information
Xufeng Tian, Email: xufeng120_120@163.com.
Wenfeng Yang, Email: lwyangwf@163.com.
References
REFERENCES
- 1. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1‐19. [DOI] [PubMed] [Google Scholar]
- 2. Wu K, House L, Liu W, Cho WC. Personalized targeted therapy for lung cancer. Int J Mol Sci. 2012;13:11471‐11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma PC. Personalized targeted therapy in advanced non‐small cell lung cancer. Cleve Clin J Med. 2012;79(Electronic Suppl 1):eS56‐eS60. [DOI] [PubMed] [Google Scholar]
- 4. Zhang S, Zeng X, Ding T, et al. Microarray profile of circular RNAs identifies hsa_circ_0014130 as a new circular RNA biomarker in non‐small cell lung cancer. Sci Rep. 2018;8:2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333‐338. [DOI] [PubMed] [Google Scholar]
- 6. Tian F, Yu CT, Ye WD, Wang Q. Cinnamaldehyde induces cell apoptosis mediated by a novel circular RNA hsa_circ_0043256 in non‐small cell lung cancer. Biochem Biophys Res Commun. 2017;493:1260‐1266. [DOI] [PubMed] [Google Scholar]
- 7. Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384‐388. [DOI] [PubMed] [Google Scholar]
- 8. Lasda E, Parker R. Circular RNAs: diversity of form and function. RNA. 2014;20:1829‐1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou X, Natino D, Qin Z, et al. Identification and functional characterization of circRNA‐0008717 as an oncogene in osteosarcoma through sponging miR‐203. Oncotarget. 2018;9:22288‐22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y, Lu C, Zhou Y, Zhang Z, Sun L. Circular RNA hsa_circ_0008039 promotes breast cancer cell proliferation and migration by regulating miR‐432‐5p/E2F3 axis. Biochem Biophys Res Commun. 2018;502:358‐363. [DOI] [PubMed] [Google Scholar]
- 11. Liu X, Liu N, Yue C, et al. FoxR2 promotes glioma proliferation by suppression of the p27 pathway. Oncotarget. 2017;8:56255‐56266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grelet S, Link LA, Howley B, et al. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol. 2017;19:1105‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seiler J, Breinig M, Caudron‐Herger M, Polycarpou‐Schwarz M, Boutros M, Diederichs S. The lncRNA VELUCT strongly regulates viability of lung cancer cells despite its extremely low abundance. Nucleic Acids Res. 2017;45:5458‐5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Zeng X, Wang N, et al. Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1‐mediated proliferation and metastasis via decoying of miR‐335‐5p and miR‐1972 in osteosarcoma. Mol Cancer. 2018b;17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katoh M, Igarashi M, Fukuda H, Nakagama H, Katoh M. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328:198‐206. [DOI] [PubMed] [Google Scholar]
- 16. Katoh M. Identification and characterization of human FOXN6, mouse Foxn6, and rat Foxn6 genes in silico. Int J Oncol. 2004;25:219‐223. [PubMed] [Google Scholar]
- 17. Xu W, Chang J, Liu G, Du X, Li X. Knockdown of FOXR2 suppresses the tumorigenesis, growth and metastasis of prostate cancer. Biomed Pharmacother. 2017;87:471‐475. [DOI] [PubMed] [Google Scholar]
- 18. Wang X, He B, Gao Y, Li Y. FOXR2 contributes to cell proliferation and malignancy in human hepatocellular carcinoma. Tumour Biol. 2016;37:10459‐10467. [DOI] [PubMed] [Google Scholar]
- 19. Wang XH, Cui YX, Wang ZM, Liu J. Down‐regulation of FOXR2 inhibits non‐small cell lung cancer cell proliferation and invasion through the Wnt/beta‐catenin signaling pathway. Biochem Biophys Res Commun. 2018a;500:229‐235. [DOI] [PubMed] [Google Scholar]


