Abstract
Community health workers (CHWs) play an important role in the detection and referral of children with severe acute malnutrition (SAM) in many countries. However, distance to health facilities remains a significant obstacle for caregivers to attend treatment services, resulting in SAM treatment coverage rates below 40% in most areas of intervention. The inclusion of SAM treatment into the current curative tasks of CHWs has been proposed as an approach to increase coverage. A literature review of operational experiences was conducted to identify opportunities and challenges associated with this model. A total of 18 studies providing evidence on coverage, clinical outcomes, quality of care, and/or cost‐effectiveness were identified. The studies demonstrate that CHWs can identify and treat uncomplicated cases of SAM, achieving cure rates above the minimum standards and reducing default rates to less than 8%. Although the evidence is limited, these findings suggest that early detection and treatment in the community can increase coverage of SAM in a cost‐effective manner. Adequate training and close supervision were found to be essential to ensure high‐quality performance of CHWs. Motivation through financial compensation and other incentives, which improve their social recognition, was also found to be an important factor contributing to high‐quality performance. Another common challenge affecting performance is insufficient stock of key commodities (i.e., ready‐to‐use therapeutic food). The review of the evidence ultimately demonstrates that the successful delivery of SAM treatment via CHWs will require adaptations in nutrition and health policy and practice.
Keywords: children, community health workers (CHWs), integrated community case management (iCCM), severe acute malnutrition (SAM), treatment
Key messages.
As many as 60% of children with severe acute malnutrition (SAM) do not receive the treatment they need.
Distance, weekly follow‐up visits, and transportation‐related costs have been reported as key barriers to accessing treatment for SAM at health facilities.
Community health workers, with adequate training and supervision, can deliver high‐quality treatment for SAM at community level.
Scaling‐up management of SAM through community health workers requires that key issues regarding training, supervision, motivation, and supply chain be adequately considered in the design of such services.
Abbreviations
- CHWs
community health workers
- CMAM
community‐based management of acute malnutrition
- iCCM
integrated community case management
- MUAC
mid‐upper arm circumference
- OTP
outpatient therapeutic feeding programme
- RUTF
ready‐to‐use therapeutic food
- SAM
severe acute malnutrition
- UNICEF
United Nations children's fund
- WHO
World Health Organization
1. INTRODUCTION
According to the latest joint child malnutrition estimates, 16.9 million children under 5 years of age worldwide (2.5%) are suffering from severe acute malnutrition (SAM; United Nations children's fund [UNICEF], World Health Organization [WHO], and World Bank [WB], 2017). Children suffering from SAM are over nine times more likely to die than those who are well nourished (Black et al., 2008). As part of the global nutrition targets, the world has committed to reducing and/or maintaining the level of wasting to less than 5% by 2025 (WHO, UNICEF and World Food Programme [WFP], 2014).
Over the past two decades, there have been significant shifts in the way the world addresses SAM. The first global guidance produced in the 1990s recommended inpatient care in hospitals and the closely monitored use of therapeutic milk formulas for treatment by doctors and other experienced senior health professionals (WHO, 1999). This approach had several practical limitations, for instance, it is a fixed‐capacity approach and has high opportunity costs for families and caregivers (Rogers, Myatt, Woodhead, Guerrero, & Álvarez, 2015). As a result of these and other barriers, it is estimated that inpatient models of care for SAM rarely reached more than 4–10% of the children suffering from SAM in the areas of intervention (Collins et al., 2006).
During the early 2000s, ready‐to‐use therapeutic foods (RUTFs) were introduced. The nutritional composition of these products is similar to therapeutic milk formulas but without the need for water to be added, which significantly reduces the risk of bacterial contamination and eliminates the need for refrigeration (UNICEF, 2013a). Around the same time, the community‐based management of acute malnutrition (CMAM) model was introduced, allowing children with uncomplicated SAM to be diagnosed at the local level and referred to an outpatient therapeutic feeding programme (OTP) at health centres.
In 2006, a review of 33 outpatient programmes was published showing improved outcomes among programmes using RUTF over those that did not (Ashworth, 2006). The growing body of evidence demonstrating the effectiveness and potential for increased coverage through this facility‐based approach ultimately led to its endorsement by United Nations agencies (WHO, WFP, United Nations System Standing Committee on Nutrition [SCN], and UNICEF, 2007) and its integration into national health systems beyond emergency contexts (United States Agency for International Development [USAID], 2008a). More recent systematic reviews and meta‐analyses found that children receiving RUTF at community level were 51% more likely to achieve nutritional recovery than those who attended inpatient facilities (Lenters, Wazny, Webb, Ahmed, & Buhtta, 2013). These reviews also found that RUTF‐based protocols are more effective at reducing mortality of acute malnourished children in various emergency contexts (Akparibo, Lee, & Booth, 2017).
The success and potential of the CMAM model rested on its capacity to achieve high levels of coverage. At 90% coverage, SAM treatment was found to be the most impactful and cost‐effective nutrition intervention, capable of saving between 285,000 and 482,000 lives per year (Bhutta et al., 2013). However, in reality, few CMAM services ever achieve this level of coverage. A study carried out by Rogers et al. (2015) included data from 21 low‐middle‐income countries and found that the mean treatment coverage of CMAM programmes was less than 40%. The analysis went on to identify caregivers' awareness of the condition and/or availability of services, distance, and high opportunity costs as the most important barriers affecting coverage of outpatient, facility‐based treatment for SAM (Puett & Guerrero, 2015; Rogers et al., 2015).
These challenges are not unique to the treatment of SAM. Over the years, public health services have sought ways of making key child survival interventions more integrated and accessible to those who need it. Integrated community case management (iCCM) was introduced to improve uptake of services in areas where access to facility‐based health services is poor. The iCCM approach is based on the training of non‐medical community health workers (CHWs) to provide selected curative services, mainly to diagnose and treat diarrhoea, malaria, and pneumonia among children 2–59 months (Young, Wolfheim, Marsh, & Hammamy, 2012). Given the effect of nutritional status on the recovery of infectious diseases (Ibrahim, Zambruni, Melby, & Melby, 2017), the iCCM protocol also includes the identification and referral of SAM children 6–59 months. The CHW identifies SAM through mid‐upper arm circumference (MUAC) measurement and bilateral oedema assessment (WHO and UNICEF, 2011). However, CHWs are only able to act on SAM cases when services exist at facility level, and there is limited documented evidence on the effectiveness of these referrals. A report of interventions integrating nutrition into iCCM (Friedman & Wolfheim, 2014) describes iCCM as a logical platform, a possible missed opportunity, for increasing the coverage of uncomplicated SAM treatment, as well as an opportunity for preventing malnutrition altogether.
The aim of the present review is to contribute to the assessment of the impact of empowering CHWs to diagnose and treat SAM by summarizing the results of previous operational experiences and identifying the opportunities and challenges associated with this innovative approach.
2. METHODS
A search for peer‐reviewed articles and grey literature was conducted to identify all relevant studies published between January 2005 and February 2018. The search strategy was developed using the Population–Intervention–Comparison–Outcome framework (Schardt, Adams, Owens, Keitz, & Fontelo, 2007). This search strategy focused on the following: (a) Population: children from 6 to 59 months diagnosed with SAM, without medical complications; (b) Intervention: therapeutic food treatment provided by CHWs; (c) Comparison: outpatient treatment at health centres provided by medical staff; and (d) Outcome: clinical outcomes (cure, default, and death rates), treatment coverage, quality of care, and/or cost‐effectiveness.
The search relied on PubMed, Cochrane Library, http://ClinicalTrials.gov, LILACS, African Index Medical, and Google Scholar databases. Additional grey literature was reviewed using OpenGrey and the Emergency Nutrition Network sites. The following keywords were used during the search: “Severe Acute Malnutrition”; “Marasmus”; “Kwashiorkor”; “Community Health Worker”; “Community Nutrition Worker”; “Community Health Agent”; “Health Extension Worker”; “Lady Health Worker”; “Health Promoters”; “Health Aids”; “Health Volunteers”; “Treatment”; “Ready‐to‐Use Therapeutic Food”; “RUTF”; “local level”; “home‐based”; and “household level.”
Results of the search are shown in the PRISMA flow diagram (Figure 1). The initial search resulted in 190 peer‐reviewed articles and 188 technical reports from grey literature. Most of the peer‐review articles were excluded as they focused on facility‐based interventions without CHWs in the treatment of uncomplicated SAM. In relation to grey literature, only articles that provided outcome figures were ultimately included. A total of 18 articles on implementation experiences providing outcomes were included in the review.
Figure 1.
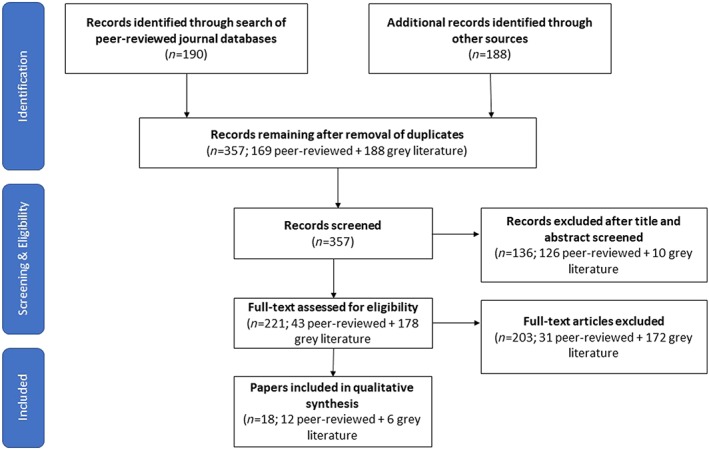
PRISMA flow diagram of literature revised and accepted for the review
3. RESULTS
The search found a total of nine countries in which CHWs were reported to have played an active role in the provision of treatment for SAM: Angola, Bangladesh, Ethiopia, India, Malawi, Mali, Pakistan, South Sudan, and Togo. In the case of Togo, the search found limited information relating to the location of the programme, and the overall number of CHWs and SAM cases involved (377 and 1,483, respectively; UNICEF, 2013b), which led to its exclusion from this review.
Apart from Ethiopia, all the examples found were small‐scale pilot projects, supported by international non‐governmental or United Nations agencies (Table 1). Ethiopia is the only programme that is implementing this model at the national level as part of its Health Extension Programme. The scaling‐up of SAM treatment through CHWs started after the 2008 drought emergency, which resulted in a dramatic increase in SAM cases. By 2012, the programme had reached 622 districts, 7,137 health posts, and 16,947 CHWs (UNICEF, 2012).
Table 1.
General description of programmes in where severe acute malnutrition treatment is provided by community health workers
| Country (region) | Organization in charge and partners | Period | SAM prevalence in the region | SAM treatment inside iCCM | No. CHWs treating SAM per study area | CHWs location distance | Therapeutic food provider | References |
|---|---|---|---|---|---|---|---|---|
| Angola (Bié, Huambo, Kwanza Sul, and Zaire) | World Vision, UNICEF, People in Need, Africare, and Ministry of Health | 2012–2013 | 4% to 5% | Nob | 1 per 2 to 5 villages | 3‐km max from communa |
RUTF UNICEF |
‐ World Vision (2014) ‐ Morgan, Bulten, and Jalipa (2015) |
| Bangladesh (Barisal) | Save the Children and Feinstein International Centre | 2009–2010 | N/F | Yes | 55 per one upazila | N/F |
RUTF UNICEF |
‐ Sadler, Puett, Mothabbir, and Myatt (2011) ‐ Puett, Coates, Alderman, Sadruddin, and Sadler (2012) ‐ Puett, Coates, et al. (2013) ‐ Puett, Sadler, et al. (2013) ‐ Puett, Alderman, Sadler, and Coates (2015) |
| Ethiopia (national) | Ministry of Health and UNICEF | Since 2010 | 2% National estimation | Yes | 16,947 national | N/F |
RUTF UNICEF |
‐ UNICEF (2012) ‐ Miller et al. (2014) ‐ Mangham‐Jefferies, Methewos, Russell, and Bekele (2014) |
| India (Melghat) | MAHAN trust (local NGO) | 2011–2012 | 7.1% | Yes | 14 | N/F | MAHAN LTFMN (local product) | ‐ Dani et al. (2016) |
|
Malawia †(Southern region) ‡(Machinga District) |
UNICEF and WFP |
†2005–2006 ‡March‐July 2006 |
N/F | N/F | N/F | N/F |
†, ‡Project peanut butter (local RUTF) |
‐ †Linneman et al. (2007) ‐ ‡Amthor et al. (2009) |
| Mali (Kita) | Action against Hunger and National Nutrition Direction | 2014–2016 | 1.2% to 2.4% | Yes | 18 per three communes | 5 km from health centre |
RUTF UNICEF |
‐ Álvarez‐Morán, Alé, Charle, et al. (2018) ‐ Álvarez‐Morán, Alé, Rogers, and Guerrero (2018) ‐ Rogers, Martínez, et al. (2018) |
| Pakistan (Dadu, Sindh) | Action against Hunger and Aga Khan University | 2015–2016 | N/F | Yes | 72 per three union council | 10‐ to 25‐min walk from households |
RUTF UNICEF |
‐ Rogers, Ali, et al. (2018) |
| South Sudan (Northern Bahr el Ghazal) | Malaria consortium | 2010–2011 | 5.3% to 9.3% | Yes | 50 per two counties | 5 km from health centre |
RUTF UNICEF |
‐ Keane (2013) |
Note. CHWs: community health workers; iCCM: integrated community case management; N/F: data not found; NGO: Non Governmental Organization; RUTF: ready‐to‐use therapeutic food; SAM: severe acute malnutrition; UNICEF: United Nations Children's Fund; WFP: World Food Programme.
In Malawi, two independent studies have been identified:
†one broader in southern region (Linneman et al., 2007) and
‡one more restricted to Machinga District of the same region (Amthor et al., 2009).
Children with SAM were referred to government mobile health clinics in each municipality because CHWs are not allowed to administer drugs.
Table 2 shows that apart from Angola, all interventions have relied primarily on female CHWs, especially in contexts where no payment was involved. All projects added SAM treatment into an existing iCCM programme, except Angola and Malawi where CHWs treat severe and moderate acute malnutrition in a more vertical or stand‐alone manner. In the case of South Sudan, there are two different cadres acting together, community nutrition workers delivering SAM treatment and community drug distributors who oversee infectious disease treatment (pneumonia, diarrhoea, and malaria).
Table 2.
Profile of the community health workers that are carrying out severe acute malnutrition treatment by country of intervention
| Country | Name of the cadre | Gender; education level | Remuneration | Duration of training | Diseases treatedb | Potential beneficiaries by CHW | CHWs per each supervisor | Additional cadre to support CHWs |
|---|---|---|---|---|---|---|---|---|
| Angola | Community health activist | Mostly men; literate | Volunteers (receiving allowance/incentives) | N/F | SAM, MAM | 100 houses | 10 to 100 | None |
| Bangladesh | CHWs | Woman; 8th grade minimum | Volunteers (receiving allowance/incentives) | 2 days for SAM | Pneumonia, diarrhoea, ARI, SAM | 150–225 houses (875 potential users) | 25 to 40 | None |
| Ethiopia | Health extension workers | Women; 10th grade minimum | Government salary | 1 year | Pneumonia, diarrhoea, ARI, malaria, severe febrile disease, measles, acute ear infection, anaemia, SAM | 2 CHWs by 5,000 potential users | N/F | Volunteer health extension workers and women development army (volunteers/to counsel, SAM screening and follow‐up) |
| India | Village health workers | Women; semi‐literate | Volunteers (receiving allowance/incentives) | N/F | Fever, diarrhoea, ARI, otitis media, malaria, SAM | 900 people | N/F | None |
| Malawia | Village health aides/community health aids | N/F | N/F |
†1 month (4‐day practice) ‡5‐day theory + 5‐day practice |
Just SAM | N/F | N/F | None |
| Mali | Agent de Santé Communautaire | Both; 9th grade minimum | Government salary | 15‐day iCCM (6 days of SAM) | Pneumonia, diarrhoea, malaria, SAM | 1 CHW per 1,500 potential users | N/F | Relais communautaire (illiterate; volunteers; to counsel and SAM screening) |
| Pakistan | Lady health workers | Women; 8th grade minimum | Government salary | 27 days (15‐day theory + 12‐day practice); 10 days of CMAM | Diarrhoea, ARI, SAM | 200 houses | N/F | None |
| South Sudan | Community nutrition workers | Both; literate | Volunteers (receiving allowance/incentives) | 5‐day specific SAM training and on‐job refreshes | Just SAM | N/F | 15 | Community drug distributors (illiterate, non‐paid iCCM providers) |
Note. ARI: acute respiratory infection; CHWs: community health workers; iCCM: integrated community case management; MAM: moderate acute malnutrition; N/F: data not found; SAM: severe acute malnutrition.
In Malawi, two independent studies have been identified:
†one broader in southern region (Linneman et al., 2007) and
‡one more restricted to Machinga District of the same region (Amthor et al., 2009).
Only diseases diagnosed and treated by the CHWs are mentioned, although most of them also play an active role on immunization and supplementation campaigns and on counselling and demonstrations to caretakers and community for the prevention of other infectious diseases and to promote behaviour change (feeding, care, and hygiene practices). Lady health workers of Pakistan also have a main role in reproductive health (including the provision of contraceptive pills or injectable).
Olaniran, Smith, Unkels, Bar‐Zeev, and van den Broek (2017) recently published a methodological framework to categorize and define CHWs cadres with the aim to facilitate health policy formulation, programme planning, and research. According to the framework, the CHW cadres treating SAM differ widely in terms of literacy, pre‐service training, and remuneration. In most of the countries in where interventions are taking place, there is a clear policy regarding the required profile of the CHWs providing iCCM services. However, the requirements for those CHWs who treat SAM outside the health facilities are not yet defined. For example, in Malawi, iCCM is performed by health surveillance assistants, a salaried cadre included in the Public Health System structure (USAID, 2017), whereas SAM treatment is provided by volunteer cadres, or “village health aides,” whose literacy and remuneration is not clear (Amthor, Cole, & Manary, 2009; Linneman, Matilsky, Ndekha, Manary, & Maleta, 2007).
3.1. Clinical outcomes
The review found relevant differences in the treatment protocols used by the different projects (Table 3). The project in India used weight‐for‐height or weight‐for‐age as inclusion criteria but not MUAC. In contrast, five of the eight programmes used MUAC as the only anthropometric measurement for inclusion and discharge. In Angola, severe and moderate acute malnutrition were treated as one condition with a single admission criteria (MUAC < 125 mm). The Angola and Malawi programmes used a fixed dosage, rather than having CHWs calculate the dose according to the weight of each child. These discrepancies in the protocols used limits the capacity for comparative analysis.
Table 3.
Protocol for the management of severe acute malnutrition performed by community health workers in each intervention
| Country (reference) | Admission criteriaa | Systematic treatment | Discharge criteria |
|---|---|---|---|
| CMAM protocol (USAID, 2008b) |
‐ MUAC < 110 mm or ‐ WHZ < −3 Z‐score of WHO standard or ‐ Bilateral pitting oedema (+ and ++ grades) |
Follow‐up visits: Weekly or biweekly ‐ RUTF dose in relation to child's weight ‐ Amoxicillin ‐ Mebendazole/other antihelminth ‐ Vitamin A |
Depending on admission cause: ‐ No oedema in two consecutive weeks and ‐ MUAC > 110 mm (minimum 2 months of treatment) and/or ‐ WHZ > −2 Z‐score of WHO standard for two consecutive visits and/or ‐ Weight gain <20% from admission weight (discharge as non‐respondent) |
| Angola (Morgan et al., 2015) |
‐ MUAC < 125 mm and/or ‐ Bilateral pitting oedema (+ and ++ grades) |
Follow‐up visits: N/F ‐ Patients with MUAC < 115 mm supplied with two suchets of RUTF per day and those with MUAC between 115–125 with one suchet/day ‐ Antibioticb ‐ Albendazoleb ‐ Vitamin Ab |
‐ MUAC > 125 mm + no oedema ‐ Children kept in the programme for two extra weeks after discharge to prevent relapse |
| Bangladesh (Sadler et al., 2011) |
‐ MUAC < 110 mm and/or ‐ Bilateral pitting oedema (+ and ++ grades) |
Follow‐up visits: Weekly ‐ RUTF dose in relation to child's weight ‐ Antibiotic ‐ Albendazole ‐ Folic acid + vitamin A |
Depending on inclusion reason: ‐ MUAC > 110 mm and had gain at least 15% of their admission weight ‐ MUAC > 110 mm and no oedema in two consecutive weeks |
| Ethiopia (Ethiopian Federal Ministry of Health, 2007) |
‐ MUAC < 110 mm and/or ‐ Bilateral pitting oedema (+ and ++ grades) |
Follow‐up visits: N/F ‐ RUTF dose in relation to child's weight ‐ Antibiotic ‐ Albendazole/mebendazole |
No oedema for 14 days + reach target weight gain related to inclusion weight |
| India (Dani et al., 2016) |
‐ WHZ or WAZ < −3 Z‐score of WHO standard or ‐ Bilateral pitting oedema (+ and ++ grades) |
Follow‐up visits: Weekly MAHAN‐LTFMN given 4 times a day during 90 days/ration dose in relation to child's weight |
Weight gain up to 15% of inclusion weight |
|
†WHZ < −3 Z‐score of WHO standard or bilateral pitting oedema (+ and ++ grades) †MUAC < 115 mm or WHZ < −3 Z‐score of WHO standard or bilateral pitting oedema (+ and ++ grades) |
†,‡Follow‐up visits: Biweekly †, ‡One jar (260 g) of RUTF per day |
†, ‡No oedema and WHZ > −2 Z‐score of WHO standard | |
| Mali (Álvarez‐Morán, Alé, Charle, et al., 2018) |
‐ MUAC < 115 mm or ‐ WHZ < −3 Z‐score or ‐ Bilateral pitting oedema (+ and ++ grades) |
Follow‐up visits: Weekly ‐ RUTF dose in relation to child's weight ‐ Antibiotic ‐Albendazole ‐Vitamin A |
No oedema for two consecutive visits + MUAC >125 mm or weight gain > 15% |
| Pakistan (Rogers, Ali, et al., 2018) |
‐ MUAC < 115 mm or ‐ Bilateral pitting oedema (+ and ++ grades) |
Follow‐up visits: Weekly ‐ RUTF dose in relation to child's weight ‐ Antibiotic ‐ Folic acid |
No oedema + MUAC > 125 mm |
| South Sudan (Keane, 2013) |
‐ MUAC < 115 mm or ‐ Bilateral pitting oedema (+ and ++ grades) |
Follow‐up visits: N/F ‐ RUTF dose in relation to child's weight ‐ Amoxicillin ‐ Mebendazole/other antihelminth ‐ Vitamin A |
No oedema + MUAC > 125 mm in two consecutive visits |
Note. MUAC: Mid‐upper arm circumference; N/F: data not found; RUTF: ready‐to‐use therapeutic food; WHO: World Health Organization; WAZ: weight‐for‐age; WHZ: weight‐for‐height.
aAll interventions included as inclusion criteria that children had passed the appetite test and had no other medical complications. In Malawi, two independent studies have been identified: †one broader in southern region (Linneman et al., 2007) and ‡one more restricted to Machinga District of the same region (Amthor et al., 2009).
Given at government mobile health clinics because CHWs are not allowed to administer drugs.
Discharge outcomes of children admitted to these models of treatment are shown in Table 4. Apart from India, all the other cases meet the SPHERE Minimum Standard in Humanitarian Response (The Sphere Project, 2011) with more than 75% of children cured, as well as death and default rates lower than 10% and 15%, respectively. Likewise, these results are in line with those reported in a review of CMAM programmes developed in 12 countries from 2011 to 2013 (Save the Children, 2015). This report shows that from the 107,589 children receiving treatment in 24 different OTPs, 82.6% recovered, 9.2% defaulted, and 0.4% died.
Table 4.
Discharge outcomes in the experiences on SAM treated by CHWs
| Country (reference) | Children enrolled | Intervention duration | Number of CHWs treating SAM | Cure rate (%) | Death rate (%) | Default rate (%) |
|---|---|---|---|---|---|---|
| Minimum standards (The Sphere Project, 2011) | >75 | <10 | <15 | |||
| Angola (Morgan et al., 2015) | 23,865 | 12 months | Over 2,000 | 93.8 | 1.0 | 4.8 |
| Bangladesh (Sadler et al., 2011) | 724 | 8 months | 261 | 91.9 | 0.1 | 7.5 |
| Ethiopiaa (UNICEF, 2012) | 703,878 | 8 months | N/F | 82.1 | 0.7 | 5.0 |
|
India (Dani et al., 2016) |
145 | 12 weeks | 14 | 63.0 | 2.0 | N/F |
|
(Linneman et al., 2007) |
826 2,131 |
8 weeks 8 weeks |
N/F N/F |
93.7 89.0 |
0.9 1.4 |
3.6 7.4 |
| Malia (Álvarez‐Morán, Alé, Charle, et al., 2018) | 617 | 12 months | 19 | 94.2 | 0.5 | 4.5 |
| Pakistana (Rogers, Ali, et al., 2018) | 425 | 12 months | 72 | 76.0 | 0.2 | 3,8 |
| South Sudan (Keane, 2013) | 3,564 | 12 months | 45 | 89.0 | 1.0 | 6.0 |
Note. CHWs: community health workers; N/F: data not found; SAM: severe acute malnutrition.
Results combined from inpatient and outpatient (health centres + health post) treatment.
The results achieved in Mali were the highest found in the review, with 94.2% of children successfully rehabilitated or cured in the intervention group. This result corresponds to a combined model of treatment with some children treated at health facilities and others by CHWs at household level, depending on the location of the households. The cure rate achieved in the control group (children treated at health facilities without any CHW delivering treatment) was 88.6%. In comparison, the probability of being cured in the intervention model was higher after adjusting for sex, oedema, and MUAC at admission (risk ratio: 1.89; 95% confidence interval [1.09, 3.27], P = 0.022; Álvarez‐Morán Alé, Charle, et al., 2018). The projects in Angola, Malawi, and Bangladesh also achieved cure rates above 90% of children enrolled.
Default rate is generally defined as the proportion of children who were absent for two consecutive weekly sessions. The Bangladesh intervention reported the highest default rate (7.5%), with distance reported as the primary barrier to access (Sadler et al., 2011). The lowest default rate was reported in Malawi where only 3.6% children defaulted from the programme (Amthor et al., 2009). In Mali, default rates were twice as high in the facility‐only control group (10.8%) compared with facility and CHWs intervention group (4.5%; risk ratio: 0.43, 95% confidence interval [0.24, 0.77], P = 0.005; Álvarez‐Morán, Alé, Charle, et al., 2018). Researchers linked improved access to treatment to the proximity of CHWs.
All interventions reviewed reported low death rates, ranging from 1.4% in Malawi to 0.1% in Bangladesh. Most interventions reported “early detection” (enrollment of children with MUAC and/or weight for height just below the admission criteria) and the associated reduction in the risk of complications and mortality among the most notable benefits of this approach. In Mali, there were more children with oedema at admission in the OTP control group (4.7%) than in the group with OTP plus CHWs (0.7%). Moreover, children in the intervention group were also twice less likely to need to be referred to stabilization centres within 24 hr of admission (Álvarez‐Morán, Alé, Charle, et al., 2018).
3.2. Treatment coverage
Even though the focus of these interventions was to increase treatment coverage, only three studies reported coverage outcomes (Bangladesh, Mali, and Angola). All coverage estimates were generated directly, using the semi‐quantitative evaluation of access and coverage/simplified lot quality assurance sampling evaluation of access and coverage methodologies (USAID, 2012). In Bangladesh, the CHW‐based intervention achieved coverage of 89%, one of the highest coverage rates recorded by a SAM treatment intervention. In contrast, its monitoring data showed that in places where treatment was provided solely on an inpatient basis, caregivers refused to attend treatment citing opportunity costs (no one to carry out household activities, transport costs, etc.) as the primary barrier (Sadler et al., 2011).
In Mali, two coverage surveys (baseline and endline) were carried out. At baseline, the treatment coverage was estimated to be 43.9% in the intervention group and 43.8% in the control group. After a year, the coverage of SAM treatment achieved in the intervention group (treatment delivered at the health facilities with CHWs) was 86.7% compared with 43.9% in the control group during the same period (P < 0.001). These results show that when CHWs offer SAM treatment, more children access health services (Álvarez‐Morán, Alé, Charle, et al., 2018). In Angola, coverage was estimated to be 82.1% in those areas where CHWs were active. However, the authors referenced the limited number of CHWs available for implementation, the lack of RUTF, and poor transport as factors that limited the capacity to achieve a level of coverage in a uniform and sustainable manner (Morgan, et al., 2015).
3.3. Quality of care
Of all the experiences reviewed, only four performed a specific study on the quality of care. In Bangladesh, the performance of 55 CHWs was assessed using an adaptation of the CMAM checklist, which included assessment, treatment, and counselling skills (Collins, 2004). The study found that 89.1% of the CHWs achieved 90% error‐free SAM case management. All diagnostic items evaluated achieved over 92% error‐free completion, all treatment‐related elements were above 89%, and all educational messages delivered were correct in over 87% of cases (Puett, Coates, et al., 2013). The authors classified this as “good quality” based on previous quality of care studies (Degefie et al., 2009). In the same study, CHWs were perceived by the caretakers as “trustworthy” because they were perceived to be close to the community but also better informed and more knowledgeable on positive feeding and hygiene practices than themselves (Puett, Coates, et al., 2013). Another qualitative study carried out to assess perceptions of 49 CHWs about the barriers for quality care found that with limited time available, CHWs found it easier to provide curative services than behaviour change advice for SAM children (Puett et al., 2015). Results following the assessment of 338 CHWs showed that the integration of SAM treatment to iCCM services did not affect the quality of care of other curative and preventive interventions provided by CHWS, although it did require an additional time every week (globally 16.7 ± 6.9 hr per week compared with 13.3 ± 4.6 hr of those CHWs not treating SAM, P < 0.001). CHWs providing SAM treatment achieved better, error‐free case management than those providing just iCCM (100% vs. 93.3%, P < 0.05; Puett et al., 2012).
In Mali, a quality of care study based on the assessment of 125 cases of uncomplicated SAM showed that 79.5% of cases received high‐quality SAM management according to a composite indicator, which included all essential tasks (Álvarez‐Morán, Alé, Rogers, & Guerrero, 2018). MUAC was correctly assessed in 96.8% of children and oedema in 78.4%. The appetite test was correctly done in 77.8% of cases. The highest result was achieved in the treatment section with a 100% correct RUTF administration. In addition, 94.3% of caretakers received essential nutritional counselling. In 75% and 100% of cases, other diseases, including malaria, pneumonia, cough, diarrhoea, fever, and vomiting, were well diagnosed and treated. The authors stipulated that the high level of accuracy could be associated with the high level of supervision provided during this pilot study.
In Ethiopia, a quality of care study found that just 64% of 137 CHWs provided correct global case management according to the Ethiopian iCCM Clinical Guidelines, which include assessment, classification, treatment, referral, and counselling (Miller et al., 2014). The study found some important differences depending on the disease: Seventy‐nine per cent of CHWs correctly treated diarrhoea, and 72% correctly treated pneumonia; however, correct SAM treatment was only 59%. In addition, only 34% of CHWs made a correct assessment of severe illness (oedema was one of the most common errors), and half of children in need were referred to a health centre. As shown in Table 2, Ethiopian CHWs have the greatest workload, managing up to 16 health services (Workie & Ramana, 2013). A study conducted by Mangham‐Jefferies et al. (2014) analysed how 131 CHWs allocated their time through all these assignments during a 1‐week period. Results indicated that 5.5% of their workload was dedicated to nutrition tasks and that time was balanced between curative and preventive actions (2.9% and 2.7%, respectively).
In Pakistan, a quality of care study assessed 17 lady health workers attending 61 cases of SAM children (Rogers et al., 2018), though this represented a smaller sample size than used in other studies in the review. These CHWs were found to have achieved a high score in assessing oedema (87.5% error‐free) but lower scores measuring MUAC and weight (57.4% and 60%, respectively). The appetite test was correctly performed in just 42% of the cases, and danger signs were accurately reported in 65% of them. Treatment with RUTF was adequate in dose and advice in 72% of cases. According to the facility survey tool (WHO, 2003), they provided correct medical and nutrition treatment to 68.0% of children. However, only 4% of cases received all the above services and key nutritional counselling messages correctly. The authors speculate that low performance could be linked to insufficient training on this component or the lack of motivation among CHWs.
With the exception of Bangladesh's project, none of the others reported impact of adding SAM treatment on the quality of services for other diseases covered by CHWs. This represents a significant evidence gap.
3.4. Cost‐effectiveness
The review found evidence on the cost‐effectiveness of delivering SAM treatment via CHWs in only two contexts. In Bangladesh, the integrated model was compared with inpatient treatment care (Puett et al., 2013). The main costs reported related to programme management (salaries and overheads related to monitoring and supervision) accounted for 53% of the total costs. However, the authors highlighted that elevated management costs reflected the substantial start‐up costs needed to establish new systems in the first year of services. They suggest that this cost structure would likely change over time due to economies of scale, as SAM treatment is integrated into ongoing non‐government organization or government services. Using an activity‐based cost model with a societal perspective that included costs incurred by providers and participants, researchers found the cost per child treated by CHWs to be US$165 and US$180 per child recovered, whereas inpatient treatment costs were estimated at US$1,344 and US$9,149, respectively. However, the authors also estimated that if coverage, recovery, and defaulting for inpatient treatment were improved by a factor of 20%, the costs would be reduced to US$520 per child treated and US$1,491 per child recovered. They also found that the community treatment cost per each disability‐adjusted life year averted was US$26, compared with US$1,344 for the inpatient model. According to the WHO criteria (Marseille, Larson, Kazi, Kahn, & Rosen, 2015), this community approach is highly cost‐effective because each disability‐adjusted life year avoided costs less than one per capita gross domestic product of the country. The authors argued that this result is comparable with the cost‐effectiveness of other basic health interventions such as immunization, insecticide‐treated bed nets, and tuberculosis treatment.
Additional cost‐effectiveness evidence is provided by the Mali study, where services provided in the facility (control group) were compared with services provided in a facility and by CHWs at household level (intervention group; Rogers et al., 2018). Authors applied a similar societal perspective with an activity‐based costing analysis. In the intervention area, the cost per child treated was US$244 and US$259 per child recovered. This was less expensive than standard outpatient care (US$442 and US$501, respectively). As in Bangladesh, supervision and monitoring was the highest cost, accounting for over half of the total costs. However, these results are based on an unequal number of children participating in each arm. If an equal scenario is modelled, outpatient care could be more cost‐effective. Authors concluded that achieving good coverage is a key factor in influencing cost‐effectiveness of CHWs delivering treatment for SAM. Considering cost to beneficiaries, households receiving treatment from CHWs spent half the amount of time on a weekly basis (2.15 vs. 3.92 hr) and three times less money on a weekly basis than households in the control group (US$0.60 vs. US$1.70).
The Ethiopia programme disaggregated costs by category, including RUTF (50%), service delivery including training and supervision (33%), logistics (12%), and other supplies and equipment (5%). Overall, it was estimated that the average cost per treated child was US$110, ranging from US$90 to US$152. This figure is based on only costs incurred by implementers, which remains sensitive to a range of factors including number of SAM cases treated, programme length, and accessibility of the programme sites (UNICEF, 2012).
4. DISCUSSION
Available evidence on the treatment of acute malnutrition by CHWs is still scarce and comes from studies designed and implemented in considerably different ways. Therefore, their results cannot be compared directly and are not generalizable. Most of the experiences found were supported by international non‐governmental organizations and were implemented in small geographic areas, limiting the ability to extrapolate the results across wider or different contexts. Ethiopia is the only country where decentralized care for acute malnutrition is implemented by the government at a national scale. Though this programme represents a unique example of what can be achieved at scale, the review found no published evidence of the coverage or clinical outcomes achieved.
This review found that operational experiences in delivering treatment for SAM through CHWs have generated a range of different results. Like much of the evidence on CHW performance, training and supervision have been identified as key determinants of success. The Mali programme has already embarked on a new study comparing three different models of training and supervision to better understand how to best support the implementation of this approach at scale (Charle, 2018). Yet the evidence found by this review already points to key determinants including incentives and motivation, literacy level, information management, supply chain of RUTF, and policy environment.
4.1. Incentives and motivation
Incentives have been widely described as a critical determinant of CHW motivation and retention (Bhutta, Lassi, Pariyo, & Huicho, 2010; Pallas et al., 2013; Zulu, Kinsman, Michelo, & Hurting, 2014) and thus a key consideration for their involvement in future scale‐up plans for the treatment of SAM. In Bangladesh, CHWs felt that their salary was inadequate for their workload, which reportedly led to increased pressure from their families to prioritize domestic responsibilities (Puett et al., 2012). In Pakistan, where lady health workers normally receive remuneration for new activities, failure to provide additional financial remuneration for adding SAM treatment to their workload was identified by the authors as a critical factor behind the low quality of care provided (Rogers, Ali, et al., 2018).
Incentives were also found to be a key determinant of programmes where CHWs focused purely on the management of acute malnutrition. In Angola, staff were initially expected to receive in‐kind incentives (e.g., mobile phone credit), but as the limitations of this approach became clear, the intervention switched to financial compensation to maintain CHW involvement. Staff emphasized the need to design a compensation scheme before implementation (Morgan et al., 2015). In South Sudan, cash incentives were made depending on the completion of timely, high‐quality reports. Additional bonuses to encourage excellence were also found to be critical (Keane, 2013).
Other experiences have reported sources of motivation beyond economic rewards, mostly based on improvement of self‐efficacy and social recognition, which is reflected in the quality of training. In Bangladesh, CHWs who had acquired skills on treating SAM had higher feelings of competence due to visible changes in children recovering from SAM. In contrast, CHWs providing only iCCM services reported frustration in encountering many malnourished children in their communities who did not respond to counselling or referral alone (Puett et al., 2012). This is consistent with previous studies that have found that CHWs who perform curative tasks, such as SAM treatment, feel more recognized by the community (Kok et al., 2015).
In addition to ensuring quality of care, regular supervision and training were also identified as a source of motivation for CHWs, in part because they reinforce a sense of belonging and connection to the programme (Strachan et al., 2012). The provision of equipment with recognizable symbols can provide a sense of pride and lead to improved status of CHWs in their communities (Bhutta et al., 2010; Strachan et al., 2012). With this in mind, the South Sudan intervention provided CHWs with branded clothing, flashlights, drug boxes, hand soap, safe water storage, and long‐lasting insecticidal nets. These items were distributed with the additional aim of helping CHWs demonstrate healthy behaviour and practices in their communities (Keane, 2013).
In Mali, the positive results achieved by the pilot were linked to the profile of the CHWs involved; they were all salaried, all had a minimum of 2 years of health training, and all had worked as CHWs between 1 and 5 years in the same community where they lived (improving their social recognition; Álvarez‐Morán, Alé, Charle, et al., 2018; Álvarez‐Morán, Alé, Rogers, & Guerrero, 2018).
4.2. Literacy and information management
Literacy was found to play a significant role in the way CHWs were ultimately involved in SAM treatment. Most of them had low literacy levels with a maximum of 10 years of primary education (Table 2). In the South Sudan intervention, the implementing team originally tried to use a single provider cadre, though finally it was deemed necessary to split functions, due to the largely illiterate community drug distributors. Therefore, a new literate cadre of community nutrition workers was formed, whose responsibility was the treatment of uncomplicated SAM cases (Keane, 2013).
The recruitment of CHWs on the basis of literacy alone would have significant implications on gender equality, especially in contexts with low rates of school attendance among women (Strachan et al., 2012). Previous reviews have proposed that in the context of scaled iCCM, monitoring systems must accommodate the wide variation in levels of literacy and skill, as programmes usually cannot invest time in testing and refining their procedures and tools (Guenther et al., 2014). Mobile phone systems (known as mHealth) have been proposed as an alternative for recording data, suggesting that it could improve accuracy and reduce time and costs (Kalländer et al., 2013). In Mali, Chad, South Sudan and India there are ongoing efforts to develop new protocols and tools to empower low‐literate CHWs to treat SAM (Tesfai, Marron, Kim, & Makura, 2016). These protocols have modified entry, follow‐up, and discharge protocols in favour of simple anthropometric criteria (MUAC), which has in turn been modified to facilitate longitudinal follow‐up by CHWs. Additional changes have been made to weighing scales, allowing low‐literacy CHWs to determine the appropriate dosage based on a child's weight using colour zones within the scale, with each colour corresponding to the number of sachets required per day. As shown in Table 3, some of the reviewed interventions had also simplified criteria for diagnosis, treatment, and discharge of SAM children compared to the standard outpatient protocol.
4.3. Supply chain management
Under the current iCCM protocol, CHWs dispense oral rehydration salts solution, zinc, amoxicillin, and an antimalarial drug (Young et al., 2012). The current protocol for treatment of SAM generally involves two additional products: albendazole/mebendazole for deworming and RUTF (USAID, 2008b). It is estimated that RUTF purchasing and distribution represent about half of the total cost of SAM treatment (Emergency Nutrition Network, 2012). In the cost‐effectiveness studies carried out in Mali and Bangladesh, RUTF reflected 13% and 24% of the total budget, respectively (Puett, Sadler, et al., 2013; Rogers, Martínez, et al., 2018). The proven effectiveness of RUTF on CMAM programmes had led to an increase in product demand (UNICEF, 2017). UNICEF is currently the major global purchaser and provider of RUTF and, as can be seen in Table 1, is also the provider of supplies for many interventions. The high product cost, high weight, and volume of RUTF needed for treatment make its integration into national supply chains more challenging (Komrska, Rock‐Kopczak, & Swaminathan, 2013).
In most of the interventions in which CHWs treat SAM, running out of RUTF stock over the course of the study or intervention was one of the most important barriers cited for scaling‐up implementation. This issue has been identified as one of the top five barriers of access to treatment in countries with less than 50% SAM coverage, as it negatively impacts default rates (Puett, Swan, & Guerrero, 2013). Running out of stock can have direct and indirect consequences for these programmes: In South Sudan, staff reported that running out of stock was affecting community perceptions of the intervention and impacting future attendance and uptake (Keane, 2013). Apart from India, all the reviewed studies supplied CHWs with RUTF in the health centres. In Angola, the intervention aimed to address the challenge of RUTF distribution by providing CHWs with bicycles to facilitate the transport of supplies. This approach was ultimately replaced by hiring local, three‐wheeled motorbikes, as it proved more efficient and effective in distributing larger amounts of RUTF across difficult terrain (Morgan et al., 2015; World Vision, 2014). The review found no documented evidence of the efficiency or effectiveness of different modalities for supplying CHWs with RUTF, making it an important evidence gap to be addressed by future research projects.
4.4. Policies and protocols
As the projects reviewed have shown, provisioning of SAM treatment by CHWs demands adaptations to national and global policies and guidelines. Some authors have speculated that the inclusion of SAM treatment into this package could cause tensions between government directorates of health and nutrition, mainly due to management structures and funding availability (Friedman & Wolfheim, 2014). In some contexts, such as Angola, this would require a revision of national guidelines concerning the distribution of antibiotics and other essential drugs by CHWs. National nutrition guidelines would also have to be modified to simplify protocols. A report on the policy and programme options to improve nutrition in Ethiopia suggests that to achieve full integration of SAM treatment into the Health Extension Programme, modifications of the CMAM protocol should be explored to find ways to reduce cost and dependency on (imported) RUTF products (Ljungqvist, 2015). The Combined Protocol for Acute Malnutrition Study (ComPAS), currently implemented in South Sudan and Kenya, is exploring some of these potential protocol modifications. The study aims to assess the effectiveness and cost‐effectiveness of a reduced and standardized dose of RUTF to treat severe and moderate acute malnutrition (Bailey et al., 2018; Lelijveld et al., 2018). Reduced dosage of RUTF has already shown potential in trials in Myanmar, where a reduced dosage protocol used to treat over 3,000 children with SAM achieved 90.2% recovery with 2.0% of default rates, 0.9% of non‐respondents, and no deaths registered (James et al., 2015).
A review of policy challenges facing iCCM in sub‐Saharan Africa has shown that the most critical actors for new policy development are technical officers within the Ministry of Health supported by key development partners, particularly WHO, UNICEF, the USAID, and its collaborating agencies (Bennett, George, Rodríguez, Shearer, & Diallo, 2014). This review also showed that senior policymakers, particularly those with a clinical background, are initially resistant to changes due to concerns about CHWs treating more complex conditions, and experience has shown that shifting these perceptions takes time. It is therefore crucial that major nutrition and health agencies support the introduction of SAM treatment into iCCM mainly through the development of statement papers, standardized protocols, tools, and training courses as done previously for CMAM (USAID, 2008b; WHO, WFP, SCN and UNICEF, 2007). This would encourage governments to generate their own context‐specific evidence by learning and supporting the identification of key policy changes necessary to support interventions at scale.
5. CONCLUSIONS
Most of interventions reached cure rates around 90% or above, and all achieved default and death rates below 7% and 1.5%, respectively. These results are similar or better than those reported by facility‐based interventions, suggesting that CHWs have the potential to improve early detection and treatment of SAM children, thereby reducing the risk of medical complications. However, these results have generally been achieved by small projects supported by international non‐governmental organizations, and there is very limited evidence on the implementation of these approaches at scale.
Three studies have assessed treatment coverage, achieving figures above 80% in all cases. However, more studies carried out at scale are needed to ascertain whether the integration of CHWs in the treatment of SAM could increase the current coverage of treatment. The review also found evidence to suggest that this approach can be more cost‐effective compared with conventional inpatient and outpatient treatment models.
Ensuring high quality of care was found to be dependent on various key factors including the level of literacy, training, and supervision of CHWS, as well as the ability to keep them motivated through financial compensation and social recognition in the community. These and other determinants ultimately suggest that effectively scaling‐up this approach will require various adaptations to health and nutrition policies and practice.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
NLE, PCC, AV, and SG performed the research. PCC, AV, and SG designed the research study and contributed essential reagents. NLE, PCC, and AV analysed the data. NLE and SG wrote the paper.
ACKNOWLEDGMENTS
The authors wish to thank Cathy Wolfheim and Lynette Friedman for allowing them to draw extensively from their unpublished analysis of enabling factors for severe acute malnutrition treatment by community health workers. The authors also acknowledge the contribution of Grace Funnell in the preparation of this manuscript.
López‐Ejeda N, Charle Cuellar P, Vargas A, Guerrero S. Can community health workers manage uncomplicated severe acute malnutrition? A review of operational experiences in delivering severe acute malnutrition treatment through community health platforms. Matern Child Nutr. 2019;15:e12719 10.1111/mcn.12719
[The copyright line for this article was changed on 12 March 2019 after original online publication.]
REFERENCES
- Akparibo, R. , Lee, A. C. K. , & Booth, A. (2017). Recovery, relapse and episodes of default in the management of acute malnutrition in children in humanitarian emergencies: A systematic review. Humanitarian evidence Programme. Available at: http://fic.tufts.edu/assets/Acute-Malnutrition-Systematic-Review.pdf
- Álvarez‐Morán, J. L. , Alé, F. G. B. , Charle, P. , Sessions, N. , Doumbia, S. , & Guerrero, S. (2018). The effectiveness of treatment for Severe Acute Malnutrition (SAM) delivered by Community Health Workers compared to a traditional facility based model. BMC Health Services Research, 18(1), 207 10.1186/s12913-018-2987-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez‐Morán, J. L. , Alé, F. G. B. , Rogers, E. , & Guerrero, S. (2018). Quality of care for treatment of uncomplicated severe acute malnutrition delivered by community health workers in a rural area of Mali. Maternal and Child Nutrition, 18, e12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor, R. E. , Cole, S. M. , & Manary, M. J. (2009). The use of home‐based therapy with ready‐to‐use therapeutic food to treat malnutrition in a rural area during a food crisis. Journal of American Dietetic Association, 109, 464–467. 10.1016/j.jada.2008.11.028 [DOI] [PubMed] [Google Scholar]
- Ashworth, A. (2006). Efficacy and effectiveness of community‐based treatment of severe malnutrition. Food Nut Bull, 27(3), S24–S48. 10.1177/15648265060273S303 [DOI] [PubMed] [Google Scholar]
- Bailey, J. , Lelijveld, N. , Marron, B. , Onyoo, P. , Ho, L. S. , Manary, M. , … Kerac, M. (2018). Combined Protocol for Acute Malnutrition Study (ComPAS) in rural South Sudan and urban Kenya: study protocol for a randomized controlled trial. Trials, 19, 251 10.1186/s13063-018-2643-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, S. , George, A. , Rodríguez, D. , Shearer, J. , Diallo, B. , Konate, M. , … Cliff, J. (2014). Policy challenges facing integrated community case management in sub‐Saharan Africa. Tropical Medicine & International Health, 19(7), 872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Das, J. K. , Rizvi, A. , Gaffey, M. F. , Walker, N. , Horton, S. , … the Maternal and Child Nutrition Study Group (2013). Evidence‐based interventions for improvement of maternal and child nutrition: What can be done and at what cost? The Lancet, 382(9890), 452–477. [DOI] [PubMed] [Google Scholar]
- Bhutta, Z.A. , Lassi, Z.S. , Pariyo, G. & Huicho, L. (2010). Global experience of community health workers for delivery of health related millennium development goals: A systematic review, country case studies, and recommendations for integration into national health systems. WHO & Global Health workforce Alliance. Available at: http://www.who.int/workforcealliance/knowledge/resources/chwreport/en
- Black, R. E. , Allen, L. H. , Bhutta, Z. A. , Caulfield, L. E. , de Onis, M. , Ezzati, M. , … The Maternal and Child Undernutrition Study Group (2008). Maternal and child undernutrition: Global and regional exposures and health consequences. The Lancet, 371(9608), 243–260. [DOI] [PubMed] [Google Scholar]
- Charle, P. (2018). Comparison of three models of training and supervision with community health workers providing treatment of children with severe acute malnutrition in Mali. Trial Registration (ISRCTN14990746). BMC ISRCTN registry. Available at: http://www.isrctn.com/ISRCTN14990746?q=PilarCharle&filters=&sort=&offset=2&totalResults=2&page=1&pageSize=10&searchType=basic-search
- Collins, S. (2004). Community‐based therapeutic care: A new paradigm for selective feeding in nutritional crises. Humanitarian practice network paper. Available at: https://reliefweb.int/report/world/community-based-therapeutic-care-new-paradigm-selective-feeding-nutritional-crises
- Collins, S. , Dent, N. , Binns, P. , Bahwere, P. , Sadler, K. , & Hallam, A. (2006). Management of severe acute malnutrition children. Lancet, 368(9551), 1992–2000. 10.1016/S0140-6736(06)69443-9 [DOI] [PubMed] [Google Scholar]
- Dani, V. , Satav, A. , Pendharkar, M. J. , Satav, K. , Sadanshiv, A. , Adhav, A. S. , & Thakare, B. S. (2016). Community‐based management of severe malnutrition: SAM and SUW in children under five in the Melghat tribal area. Central India. Field Exchange, 52, 27. [Google Scholar]
- Degefie, T. , Marsh, D. , Gebremariam, A. , Tefera, W. , Osborn, G. , & Waltensperger, K. (2009). Community case management improves use of treatment for childhood diarrhea, malaria and pneumonia in a remote district of Ethiopia. Ethiopian Journal of Health Development, 23, 120–126. [Google Scholar]
- Emergency Nutrition Network . (2012). Government experiences of scale‐up of community‐based management of acute malnutrition (CMAM). A synthesis of lessons. CMAM Conference, Addis Ababa, 2011. Available at: https://www.ennonline.net/cmamgovernmentlessons
- Ethiopian Federal Ministry of Health (2007). Protocol for the management of severe acute malnutrition. Available at: https://www.ennonline.net/attachments/897/ethiopia-sam-guideline-march-2007.pdf
- Friedman, L. & Wolfheim, C. (2014). Linking nutrition & (integrated) community case management. A review of operational experiences. Available at: https://www.ennonline.net/linkingnutritionintegratedcommunitycasemanagementareviewofoperationalexperiences
- Guenther, T. , Barberá‐Laínez, Y. , Oliphant, N. P. , Dale, M. , Raharison, S. , Miller, L. , … Diaz, T. (2014). Routine monitoring systems for integrated community case management programs: Lessons from 18 countries in sub‐Saharan Africa. Journal of Global Health, 4(2), 020301 10.7189/jogh-04-020301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, M. K. , Zambruni, M. , Melby, C. L. , & Melby, P. C. (2017). Impact of childhood malnutrition on host defense and infection. Clinical Microbiology Reviews, 30(4), 919–971. 10.1128/CMR.00119-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P. T. , Van den Briel, N. , Rozet, A. , Israel, A. D. , Fenn, B. , & Navarro‐Colorado, C. (2015). Low‐dose RUTF protocol and improved service delivery lead to good programme outcomes in the treatment of uncomplicated SAM: A programme report from Myanmar. Maternal and Child Nutrition, 11, 859–869. 10.1111/mcn.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalländer, K. , Tibenderana, J. K. , Akpogheneta, O. J. , Strachan, D. L. , Hill, Z. , ten Asbroek, A. H. A. , … Meek, S. R. (2013). Mobile health (mHealth) approaches and lessons for increased performance and retention of community health workers in low‐ and middle‐income countries: A review. Journal of Medical Internet Research, 15(1), e17 10.2196/jmir.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane, E. (2013). Integrating severe acute malnutrition into the management of childhood diseases at community level in South Sudan. Malaria consortium learning paper series. Available at: http://www.malariaconsortium.org/media-downloads/248/
- Kok, M. C. , Dieleman, M. , Taegtmeyer, M. , Broerse, J. E. W. , Kane, S. S. , Ormel, H. , … Koning, A. M. (2015). Which intervention design factors influence performance of community health workers in low‐ and middle‐income countries? A systematic review. Health Policy and Planning, 30, 1207–1227. 10.1093/heapol/czu126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komrska, J. , Rock‐Kopczak, L. , Swaminathan, J.M. (2013). When supply chains save lives. Supply Chain Management Review. Available at: https://www.unicef.org/supply/files/SCMR1301_Unicef.pdf
- Lelijveld, N. , Bailey, J. , Mayberry, A. , Trenouth, L. , N'Diaye, D. , Haghparast‐Bidgoli, H. , & Puett, C. (2018). The "ComPAS Trial" combined treatment model for acute malnutrition: study protocol for the economic evaluation. Trials, 19, 252 10.1186/s13063-018-2594-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenters, L. M. , Wazny, K. , Webb, P. , Ahmed, T. , & Buhtta, Z. A. (2013). Treatment of severe and moderate acute malnutrition in low‐ and middle‐income settings: A systematic review, meta‐analysis and Delphi process. BMC Public Health, 13(Suppl 3), S23 10.1186/1471-2458-13-S3-S23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linneman, Z. , Matilsky, D. , Ndekha, M. , Manary, M. J. , Maleta, K. , & Manary,M.J. (2007). A large‐scale operational study of home‐based therapy with ready‐to‐use therapeutic food in childhood malnutrition in Malawi. Maternal and Child Nutrition, 3 (3), 206–215. 10.1111/j.1740-8709.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungqvist, B. (2015). A situation analysis of nutrition in Ethiopia—Policy and program options. Final Report. Available at: https://eeas.europa.eu/sites/eeas/files/a_situation_analysis_of_nutrition_in_ethiopia_-_policy_and_programme_options.pdf
- Mangham‐Jefferies, L. , Methewos, B. , Russell, J. , & Bekele, A. (2014). How do health extension workers in Ethiopia allocate their time? Human Resources for Health, 12, 61 10.1186/1478-4491-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseille, E. , Larson, B. , Kazi, D. S. , Kahn, J. G. , & Rosen, S. (2015). Thresholds for the cost–effectiveness of interventions: alternative approaches. Bulletin of the World Health Organization, 93, 118–124. 10.2471/BLT.14.138206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, N. P. , Amouzou, A. , Tafesse, M. , Hazel, E. , Hailemariam, L. , Degefie, T. , … Bryce, J. (2014). Integrated community case management of childhood illness in Ethiopia: Implementation strength and quality of care. American Journal of Tropical Medicine and Hygiene, 91(2), 424–434. 10.4269/ajtmh.13-0751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, S. , Bulten, R. , & Jalipa, H. (2015). Community case management approach to SAM treatment in Angola. Field Exchange, 49, 3 Available at: http://www.ennonline.net/fex/49/angola [Google Scholar]
- Olaniran, A. , Smith, H. , Unkels, R. , Bar‐Zeev, S. , & van den Broek, N. (2017). Who is a community health worker?—A systematic review of definitions. Global Health Action., 10(1), 1272223 10.1080/16549716.2017.1272223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas, S. W. , Minhas, D. , Pérez‐Escamilla, R. , Taylor, L. , Curry, L. , & Bradley, E. H. (2013). Community health workers in low‐ and middle‐income countries: What do we know about scaling up and sustainability? American Journal of Public Health, 109(7), e74–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett, C. , Alderman, H. , Sadler, K. , & Coates, J. (2015). Sometimes they fail to keep their faith in us': community health worker perceptions of structural barriers to quality of care and community utilisation of services in Bangladesh. Maternal and Child Nutrition, 11, 1011–1022. 10.1111/mcn.12072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett, C. , Coates, J. , Alderman, H. , & Sadler, K. (2013). Quality of care for severe acute malnutrition delivered by community health workers in southern Bangladesh. Maternal and Child Nutrition, 9, 130–142. 10.1111/j.1740-8709.2012.00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett, C. , Coates, J. , Alderman, H. , Sadruddin, S. , & Sadler, K. (2012). Does greater workload lead to reduced quality of preventive and curative care among community health workers in Bangladesh? Food and Nutrition Bulletin, 33(4), 273–285. 10.1177/156482651203300408 [DOI] [PubMed] [Google Scholar]
- Puett, C. , & Guerrero, S. (2015). Barriers to access for severe acute malnutrition treatment services in Pakistan and Ethiopia: a comparative qualitative analysis. Public Health Nutrition, 18(10), 1873–1882. 10.1017/S1368980014002444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett, C. , Sadler, K. , Alderman, H. , Coates, J. , Fledler, J. L. , & Myatt, M. (2013). Cost‐effectiveness of the community‐based management of severe acute malnutrition by community health workers in southern Bangladesh. Health Policy and Planning, 28, 386–399. 10.1093/heapol/czs070 [DOI] [PubMed] [Google Scholar]
- Puett, C. , Swan, H. , & Guerrero, S. (2013). Access for all, volume 2: What factors influence access to community‐based treatment of severe acute malnutrition? Available at: http://www.coverage-monitoring.org/wp-content/uploads/2013/11/Access-for-All-Volume-2.pdf
- Rogers, E. , Ali, M. , Fazal, S. , Kumar, D. , Guerrero, S. , Hussain, I. , … Álvarez‐Morán, J. L. (2018). Quality of care of treatment for uncomplicated severe acute malnutrition provided by lady health workers in Pakistan. Public Health Nutrition, 21(2), 385–390. 10.1017/S1368980017002610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, E. , Martínez, M. , Álvarez‐Morán, J. L. , Alé, F. G. B. , Charle, P. , Guerrero, S. , & Puett, C. (2018). Cost‐effectiveness of the treatment of uncomplicated severe acute malnutrition by community health workers compared to treatment provided at an outpatient facility in rural Mali. Human Resources for Health, 16, 12 10.1186/s12960-018-0273-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, E. , Myatt, M. , Woodhead, S. , Guerrero, S. , & Álvarez, J. L. (2015). Coverage of community‐based management of severe acute malnutrition programmes in twenty‐one countries, 2012–2013. PLoS One, 10(6), e0128666 10.1371/journal.pone.0128666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler, K. , Puett, C. , Mothabbir, G. & Myatt, M. (2011). Community case management of severe acute malnutrition in Southern Bangladesh. Save the Children, Feinstein International Center, Tufts University.
- Save the Children (2015). Review of current community management of acute malnutrition (CMAM) practice and outcomes in 12 countries using the minimum reporting package. Available at: https://www.elrha.org/wp-content/uploads/2015/01/Appendix-1-MRP-analysis-report-2015-Final.pdf
- Schardt, C. , Adams, M. B. , Owens, T. , Keitz, S. , & Fontelo, P. (2007). Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Medical Informatics & Decision Making, 7, 16 10.1186/1472-6947-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan, D. L. , Källander, K. , ten Asbroek, A. H. A. , Kirkwood, B. , Meek, S. R. , Benton, L. , … Hill, Z. (2012). Interventions to improve motivation and retention of community health workers delivering integrated community case management (iCCM): Stakeholder perceptions and priorities. American Journal of Tropical Medicine Ang Hygiene, 87(suppl 5), 111–119. 10.4269/ajtmh.2012.12-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfai, C. , Marron, B. , Kim, A. , & Makura, I. (2016). Enabling low‐literacy community health workers to treat uncomplicated SAM as part of community case management: Innovation and field tests. Field Exchange, 52, 3. [Google Scholar]
- The Sphere Project . (2011). Minimum standards in food security and nutrition. In: Humanitarian charter and minimum standards in humanitarian response. Sphere Handbook. Available at: http://www.spherehandbook.org/, 10.3362/9781908176202.005 [DOI]
- UNICEF . (2012). Evaluation of community management of acute malnutrition (CMAM). Ethiopia country case study. Evaluation report. Available at: https://www.unicef.org/evaldatabase/files/CMAM_Final_Ethioia.pdf
- UNICEF . (2013a). Ready‐to‐use therapeutic food for children with severe acute malnutrition. Position paper. Available at: https://www.unicef.org/media/files/Position_Paper_Ready-to-use_therapeutic_food_for_children_with_severe_acute_malnutrition__June_2013.pdf
- UNICEF . (2013b). UNICEF annual report 2013 – Togo. Available at: https://www.unicef.org/about/annualreport/files/Togo_COAR_2013.pdf
- UNICEF . (2017). ready‐to‐use therapeutic food: Current outlook. UNICEF Supply Division. February 2017. Available at: https://www.unicef.org/supply/files/Ready-to-use_Therapeutic_Food_Current_Outlook.pdf
- UNICEF, WHO & WBG . (2017). Levels and trends in child malnutrition. Key findings of the 2017 edition. Available at: http://www.who.int/nutgrowthdb/estimates/en/
- USAID . (2008a). Review of community‐based management of acute malnutrition (CMAM) in the post‐emergency context: Synthesis of lessons on integration of CMAM into national health systems. Ethiopia, Malawi and Niger April–June 2007. Available at: https://www.fantaproject.org/sites/default/files/resources/CMAM%20Review%20Synthesis%20Report_v2_0.pdf
- USAID . (2008b). Training guide for community‐based management of acute malnutrition (CMAM). Module 4. Outpatient care for the management of SAM without complications. Food and Nutrition Technical Assistance (FANTA III). Available at: https://www.fantaproject.org/focus-areas/nutrition-emergencies-mam/cmam-training
- USAID (2012). Semi‐quantitative evaluation of access and coverage (SQUEAC)/simplified lot quality assurance sampling evaluation of access and coverage (SLEAC) technical reference. Food and Nutrition Technical Assistance (FANTA III). Available at: https://www.fantaproject.org/monitoring-and-evaluation/squeac-sleac
- USAID . (2017). Malawi's community‐based health system model: Structure, strategies and learning. Community‐based health system model series – Brief two. Available at: https://www.advancingpartners.org/sites/default/files/technical-briefs/apc_malawi_brief_508.pdf
- WHO (1999). Management of severe malnutrition: A manual for physicians and other senior health workers. Geneva: World Health Organization; Available at: http://apps.who.int/iris/bitstream/10665/41999/1/a57361.pdf [Google Scholar]
- WHO . (2003). Facility survey: Tool to evaluate the quality of care delivered to sick children attending outpatient facilities. Available at: http://apps.who.int/iris/handle/10665/42643?locale=es
- WHO & UNICEF . (2011). Caring newborns and children in the community. A training course for community health workers. Participant's manual. Available at: http://www.who.int/maternal_child_adolescent/documents/caring-for-the-sick-child/en/
- WHO, UNICEF & WFP . (2014). Global nutrition targets 2025: Wasting policy brief (WHO/NMH/NHD/14.8). Available at: http://www.who.int/nutrition/publications/globaltargets2025_policybrief_wasting/en
- WHO, WFP, SCN & UNICEF . (2007). Joint statement on community‐based management of severe acute malnutrition. Available at: https://www.unicef.org/publications/index_39468.html
- Workie, N. W. , & Ramana, G. N. V. (2013). The health extension program in Ethiopia. Universal Health Coverage Studies Series No. 10. Human Development Network, World Bank Group Available at: https://openknowledge.worldbank.org/handle/10986/13280
- World Vision . (2014). Community‐based management of acute malnutrition using community health activist in Angola. November 2012‐December 2013 Report. Available at: https://www.wvi.org/publication/cmam-using-community-health-activists-angola
- Young, M. , Wolfheim, C. , Marsh, D. R. , & Hammamy, D. (2012). World Health Organization/United Nations Children's Fund Joint Statement on Integrated Community Case Management: An equity‐focused strategy to improve access to essential treatment services for children. American Journal of Tropical Medicine and Hygiene, 87(suppl 5), 6–10. 10.4269/ajtmh.2012.12-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulu, J. M. , Kinsman, J. , Michelo, C. , & Hurting, A. K. (2014). Integrating national community‐based health worker programmes into health systems: A systematic review identifying lessons learned from low‐ and middle‐income countries. BCM Public Health, 14, 987. [DOI] [PMC free article] [PubMed] [Google Scholar]


