Abstract
The proposed advantages of intra-arterial chemotherapy (IAC) are based on the premises of local dose escalation to the tumor and reduced availability of systemic drugs. There is a lack of objective pharmacokinetic data to confirm the advantage of IAC in dogs with naturally occurring urogenital tumors. The objective of this study was to determine if IAC administration in urogenital tumors would result in decreased systemic drug exposure when compared to intravenous routes. Twenty-two dogs with naturally occurring urogenital tumors were enrolled in this prospective case-controlled study. Mitoxantrone, doxorubicin, or carboplatin were administered by IAC and intravenous routes [intravenous awake (intravenous chemotherapy — IVC) and under general anesthesia (IVGAC)] 3 weeks apart. Serum assays were used to determine the extent of systemic drug exposure. Dose-normalized peak systemic serum concentration (Cmax) and area under the serum drug concentration-time curve (AUC) were used to quantify systemic exposure. A total of 26 mitoxantrone treatments were administered to 10 dogs. While there was no significant difference in Cmax, the AUC was significantly lower after IAC compared with IVGAC. Ten doxorubicin treatments were administered to 5 dogs. There were no significant differences in Cmax or AUC. A total of 14 carboplatin treatments were administered to 7 dogs. The Cmax was significantly lower for IAC compared to IVC, while the AUC values were equivocal. This study demonstrates certain lower serum values may be achieved after IAC delivery of carboplatin and mitoxantrone. These chemotherapy agents may have a preferred pharmacological profile for regional chemotherapy delivery in dogs with urogenital tumors.
Résumé
Les avantages proposés de la chimiothérapie intra-artérielle (CIA) sont basés sur les prémisses d’une escalade de la dose locale à la tumeur et d’une disponibilité réduite des drogues systémiques. Il y a un manque de données pharmacocinétiques objectives pour confirmer l’avantage de l’administration de CIA chez les chiens avec des tumeurs urogénitales se produisant naturellement. L’objectif de la présente étude était de déterminer si l’administration de CIA lors de tumeurs urogénitales résulterait en une diminution de l’exposition systémique aux drogues lorsque comparé aux voies intraveineuses. Vingt-deux chiens avec des tumeurs urogénitales d’occurrence naturelle participèrent à cette étude cas-témoin prospective. De la mitoxantrone, de la doxorubicine, ou de la carboplatine furent administrées par CIA et voies intraveineuses [intraveineuse éveillée (chimiothérapie intraveineuse — CIV) et sous anesthésie générale (CIVAG)] à 3 sem d’intervalle. Des analyses du sérum furent utilisées afin de déterminer l’étendue de l’exposition systémique aux drogues. Le pic de la concentration sérique systémique normalisé pour la dose (Cmax) et la surface sous la courbe de la concentration sérique de la drogue-temps (SSC) furent utilisés pour quantifier l’exposition systémique. Un total de 26 traitements à la mitoxantrone fut administré à 10 chiens. Bien qu’il n’y ait pas de différence significative dans le Cmax, la SSC était significativement plus basse après la CIA comparativement à la CIVAG. Dix traitements de doxorubicine furent administrés à cinq chiens. Il n’y avait pas de différence significative dans le Cmax ou ls SSC. Un total de 14 traitements de carboplatine fut administré à sept chiens. Le Cmax était significativement plus bas pour la CIA comparativement à la CIV, alors que les valeurs de SSC étaient équivoques. Cette étude démontre que certaines valeurs sériques plus faibles peuvent être obtenues après CIA avec la carboplatine et la mitoxantrone. Ces agents de chimiothérapie pourraient avoir un profil pharmacologique préférentiel pour livraison régionale de chimiothérapie chez les chiens avec des tumeurs urogénitales.
(Traduit par Docteur Serge Messier)
Introduction
Canine malignant urogenital tumors typically carry a poor prognosis and traditional treatments include surgery and/or radiation therapy (1). The current standard-of-care therapy involves nonsteroidal anti-inflammatory drugs (NSAIDs) and chemotherapy (1). Chemotherapeutic agents are generally well-tolerated in companion animals, with standard protocols designed to result in less than 5% severe adverse events requiring hospitalization and a less than 1% mortality rate (2). Unfortunately, tumor responses to these chemotherapeutic protocols are often short-lived and inconsistent, with an approximately 30% initial tumor response rate that is not lasting (2,3). Due to these historically poor response rates, the authors and others have investigated novel approaches to improve the biological response rates to commonly available chemotherapy agents in veterinary patients (3,4).
Intra-arterial chemotherapy (IAC) is carried out to introduce an anti-cancer drug directly into the arteries that supply the tumor in the hope of providing a local dose escalation to the tumor without increasing the toxicity experienced during subsequent systemic distribution. Intra-arterial chemotherapy is conducted via super-selective catheter placement under fluoroscopic guidance and is used in humans to decrease adverse systemic side effects and achieve greater tumor chemotherapy concentrations and response rates under certain circumstances (3). This technique was first reported in veterinary patients for treating canine urinary bladder carcinomas in combination with radiotherapy (3,5). In the 2 dogs that received intra-arterial chemotherapy by way of the terminal aorta in that study, decreases in tumor dimensions were noted in reference to the pretreatment measurements. While that study was conducted in only 2 dogs and was combined with radiation therapy, further evaluations have confirmed similar findings using regional therapies with limited systemic effects (3,5–8). The authors and their colleagues have recently investigated these techniques and demonstrated initial improved tumor response rates and reduced adverse events in veterinary patients receiving IAC when compared with a similar group receiving intravenous chemotherapy (IVC) (3,7,8). The uptake of chemotherapeutics into tumors is thought to depend not only on the route of administration, but also on the rate of infusion, local blood flow, permeability of the blood vessels, and chemical structure of the compound, as well as the rate of drug metabolism and elimination (6,9).
The objective of this study was to determine from a pharmacokinetic standpoint if serum chemotherapy measurements could demonstrate an advantage of intra-arterial chemotherapy versus traditional intravenous routes of administration in terms of lower systemic drug exposure. The study used chemotherapy agents with established linear pharmacokinetic profiles and efficacy in treating urogenital tumors (6,10–14). The investigators hypothesized that measured serum chemotherapy levels would be lower with IAC than with intravenous (IVC) administration of the same chemotherapy agent and dose in the same patient.
Materials and methods
Patient selection
From October 2013 to May 2017, 22 dogs with urinary tract carcinomas (prostatic carcinoma and urinary bladder/urethral transitional cell carcinoma) considered amenable to chemotherapy treatment at the Animal Medical Center [intra-arterial chemotherapy (IAC), intravenous chemotherapy (IVC) and intravenous under general anesthesia chemotherapy (IVGAC)] and at the Hope Veterinary Specialists (IVC treatments only) were recruited to participate in the study.
Eligibility criteria for patients were as follows: a measurable tumor of the urinary tract with a cytological or histopathological diagnosis; no previous surgery or radiation therapy; no evidence of serious renal or hepatic comorbidities that would preclude the use of general anesthesia or potentially skew results; and an expected survival time of at least 3 mo. If a patient received chemotherapy before inclusion in the study, a standard washout period of 21 d was instituted as determined by the medical oncologist. Patients were not excluded if they received nonsteroidal anti-inflammatory drugs (NSAIDs) at any point.
The mean age of the dogs was 9.7 y (range: 6 to 12 y). There were 10 castrated males, 1 intact male, and 11 spayed females. Purebred dogs accounted for 68% (15/22) of the study population and the remaining 32% (7/22) were mixed-breed dogs. Purebred dogs included 5 Labrador retrievers, 3 Scottish terriers, 2 fox terriers, 1 German shorthaired pointer, 1 Belgian terrier, 1 Vizsla, 1 French bulldog, and 1 shih tzu. Median weight at diagnosis was 20.3 kg (range: 5.9 to 40.1 kg) and the mean surface area of the patient (surface area of the patient not the tumor) at diagnosis was 0.72 m2 (range: 0.32 to 1.17 m2). There were 7 dogs weighing less than 15 kg and only 1 dog weighing less than 9 kg.
Types of cancer, which included 19 transitional cell carcinomas and 3 unclassified carcinomas, were diagnosed based on cytology in 20 dogs and histopathology in 2 dogs. Thoracic radiographs were conducted before treatment in all cases and all were reported as normal by a Board-certified radiologist. Abdominal ultrasonography was carried out on all dogs in the study. A mass of the bladder/urethra was present in 16 dogs and a mass involving the prostate was found in 6 dogs. Medial iliac lymphadenopathy was confirmed on abdominal ultrasonography in 10 dogs, none of which was submitted for cytological evaluation. All dogs were receiving cyclooxygenase inhibitors, carprofen (Novox or Rimadyl), deracoxib (Deramaxx), firocoxib (Previcox), or meloxicam (Metacam) at the time of enrollment in the study.
Each dog’s medical history was obtained, a physical examination was conducted, including weight, and standard staging procedures were followed. All investigations were conducted according to the protocol described here, which was evaluated and approved by the Animal Medical Center Institutional Animal Care and Use Committee.
Procedures
Standard, routine guidelines for evaluating oncology patients and administering chemotherapy were followed. Patients were recruited into the study by either the Animal Medical Center or Hope Veterinary Specialists. If recruited by the Animal Medical Center Medical Oncology Service or Hope Veterinary Specialists, the IVC was administered first and further treatments were continued 3 wk later with the Animal Medical Center Interventional Radiology Service. If recruited by the Animal Medical Center Interventional Radiology Service, dogs received IAC first and continued treatments were administered at either the Animal Medical Center or Hope Veterinary Specialists.
Each individual patient entering the study received the same dose of chemotherapy (mg/m2) as prescribed by the medical oncologist (CC or NL) via alternate routes (IAC, IVC, or IVGAC). Serial 2-mL peripheral blood samples were taken from the forelimbs or jugular veins before treatment, immediately after chemotherapy delivery (time 0), and at subsequent predetermined times of 10, 30, 60, and 180 min after administration. Each patient therefore acted as his/her own control when comparing systemic chemotherapy levels achieved through the different routes of administration. Treatments were given approximately 3 wk apart and a complete blood count was obtained on each patient 7 to 10 d after treatment and before chemotherapy was administered.
The order of treatment (IAC, IVC, and IVGAC) was determined at the clinician’s discretion, but was typically based on clinician availability at the different testing sites. Chemotherapy drugs and doses were determined by the primary medical oncologist. Full standard systemic doses were administered using mitoxantrone (5 mg/m2), doxorubicin [30 mg/m2 or 1 mg/kg body weight (BW) if < 15 kg], or carboplatin (300 mg/m2 or 10 mg/kg BW if < 15 kg), as these substances could be readily quantified in canine serum. Intravenous chemotherapy was administered according to standard intravenous chemotherapy guidelines and the same protocol was followed during intra-arterial delivery. Adverse events were graded in accordance with the Veterinary Cooperative Oncology Group — common terminology criteria for adverse events (VCOG-CTCAE) (2) and the medical oncologist’s recommendations if grade-III to grade-IV hematologic or gastrointestinal toxicity occurred. Adverse events were defined as complications that resulted in a dose reduction or removal from the study. Dose-normalized values of peak systemic serum concentration (Cmax) and area under the serum drug concentration-time curve (AUC) were used to allow comparison of these parameters in dogs receiving different doses.
Following initial evaluation of the pilot data comparing only IAC and IVC, a third treatment arm of intravenous chemotherapy under general anesthesia (IVGAC) was added to the study to control for the potential effects of general anesthesia on drug clearance and volume of distribution. This cohort of patients, which enrolled after May 2014, received a third treatment with IVGAC. This treatment was always carried out last in order to preserve the initial study design and allow for direct comparison to participants that received only 2 of the procedure types (IAC and IVC).
Protocol for intra-arterial chemotherapy (IAC)
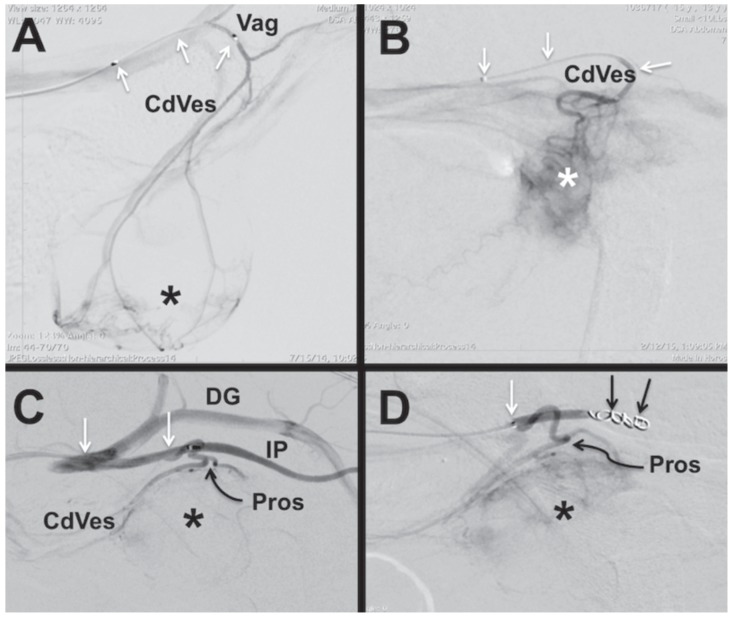
The protocol used for intra-arterial delivery was similar to that described previously (4). The patient was placed under general anesthesia and a standard aseptic preparation was made over the femoral or carotid artery. Surgical cut-down and catheterization of the femoral or external carotid artery was conducted and a combination of catheters, guide wires, microcatheters, and microwires were manipulated under fluoroscopic guidance until the catheter reached the desired position. Super-selective catheterization of the terminal feeding artery was attempted in order to deliver the chemotherapy as close to the bulk of the tumor as possible. Intraoperative fluoroscopy and angiography were used to determine positioning and blood flow through the target vessels (Figure 1). In general, for more unilateral tumors, the more tumor-burdened side was targeted. Otherwise, the better-perfused side was chosen, based on angiography. Only unilateral infusions were done, however, in order to mimic the IVC-targeted times as closely as possible. After the procedure, the access artery was ligated and the surgical site was closed routinely. Intravenous fluids were maintained at 5 mL/kg BW per hour during general anesthesia and for 3 additional hours until the final blood sample was collected.
Figure 1.
Serial lateral caudal abdominal digital subtraction (DSA) fluoroscopic images of dogs with urinary tract tumors. In all images the dog positioned with the head to the left of the image. A — Female dog with a microcatheter (white arrows) positioned in vaginal artery (Vag). A DSA image demonstrates filing of the vaginal and caudal vesical (CdVes) arteries with tumor vascular enhancement in the urinary bladder apex and body (*). B — Female dog with a microcatheter (white arrows) positioned in caudal vesical atery (CdVes). The DSA image demonstrates increased vascularity and timor blush of the trigonal tumor (*). C, D — Male dog with prostate tumor (*) and a microcatheter (white arrows) positioned in the internal pudendal artery (IP). A DSA image demonstrates filling of the IP, CdVes and prostate (Pros) arteries with reflux filling of the deep gluteal artery (DG). D — As the Pros artery was too small to be selectively catheterized, the IP artery has been coil embolized (black arrows) to divert contrast and subsequent chemotherapy into the prostatic tumor as demonstrated by the tumor blush (*).
Protocol for intravenous chemotherapy (IVC)
Intravenous chemotherapy was administered according to standard intravenous chemotherapy guidelines at the institution as a slow bolus or infusion, depending on the drug. Before intravenous chemotherapy was started, intravenous crystalloids were administered at 5 mL/kg BW per hour and continued until the 3-hour blood sample collection was obtained to help mimic the fluid volumes received during general anesthesia.
Protocol for intravenous chemotherapy under general anesthesia (IVGAC)
For the subset of patients that received the third treatment with intravenous chemotherapy under general anesthesia, the anesthetic protocol mimicked that of the IAC group, with the caveat that these patients remained under general anesthesia only for the time necessary to administer the chemotherapy agent. The infusion duration in this group was the same as for the other groups. Once the standard chemotherapy administration was complete, recovery was accomplished and blood collection and intravenous fluid rates proceeded as with the other groups. Additionally, the mean procedure times, anesthesia times, and lowest mean indirect blood pressure readings were compared between IAC and IVGAC routes of administration.
Sample evaluation
The samples were analyzed at the Pennsylvania Animal Diagnostic Laboratory System (PADLS) New Bolton Center Toxicology Laboratory, University of Pennsylvania School of Veterinary Medicine in Kennett Square, Pennsylvania, USA. Carboplatin was analyzed by measuring platinum using inductively coupled serum mass spectrometry (ICP-MS) (Nexion 300D; Perkin Elmer, Shelton, Connecticut, USA). Doxorubicin and mitoxantrone were analyzed using liquid chromatography (Shimadzu, Columbia, Maryland, USA), coupled with triple quad mass spectrometry (API 4000 LC/MS/MS; Sciex, Framingham, Massachusetts, USA).
Statistical analysis
Descriptive statistics were expressed as mean and standard deviation. The error residuals were deemed normal by visual inspection and by Kolmogorov-Smirnoff testing. A crossover design with 3 treatment routes was used to analyze the data using repeated measures analysis of variance (ANOVA) with subject nominated as a random effect and a compound symmetry covariance structure. The subject within route was used to evaluate the sequence effects, which suggested a non-significant carryover in all cases, and was subsequently omitted from the model. In the case of improper timing or missing data points, the entire procedure was removed from statistical analysis and the remaining procedures were compared for that patient. Categorical variables expressed as percentages were analyzed using chi square or Fisher’s exact test when appropriate. Power analyses from the repeated measures mixed model were analyzed as minimum, maximum, and mean standard deviations for the post-hoc analyses (10). Analyses were considered significant at P < 0.05 and were carried out using SAS software (SAS Institute, Cary, North Carolina, USA) (10).
Results
Twenty-two dogs with urinary tract carcinomas were enrolled in the study from October 2013 to May 2017. These dogs received 50 separate chemotherapy deliveries.
Mitoxantrone group
A total of 26 mitoxantrone treatments, including 10 IAC, 9 IVC, and 7 IVGAC procedures, were conducted in 10 dogs. Three patients received mitoxantrone by IAC first, 7 received treatment with IVC first, and all IVGAC treatments were carried out last. There was no significant difference among the mean doses administered (IAC 4.48, IVC 4.7, and IVGAC 4.3 mg/m2) considering treatment type [(IAC versus IVC) P = 0.43, (IAC versus IVGAC) P = 0.57, (IVC versus IVGAC) P = 0.20]. Dose-normalized AUC for mitoxantrone was significantly lower (P = 0.04) after IAC (2999 ng/mL) when compared with IVGAC (3718 ng/mL) (Table I). This difference was not demonstrated when comparing IAC versus IVC (P = 0.86) or IVC versus IVGAC (P = 0.15). There was no significant difference in dose-normalized Cmax among the procedure types. The mean chemotherapy infusion times for the IAC (11.25 min) and IVGAC groups (11 min) were not statistically different (P = 0.95). There was a significant difference in the total anesthesia time between the IAC (82 min) and IVGAC (17 min) groups (P = 0.0001). The lowest mean indirect arterial blood pressures among groups were not statistically different (IAC = 61 mmHg, IVGAC = 70 mmHg, P = 0.14) (Table II).
Table I.
Statistical summary of comparative bioavailability data for dose-normalized mitoxantrone in 10 dogs.
| IAC versus IVC | IAC | IVC | P-value |
|---|---|---|---|
| Cmax (ng/mL) | 88.94 ± 19 | 84.63 ± 20 | 0.78 |
| AUC (min × ng/mL/mg) | 3209.71 ± 628 | 3127.25 ± 659 | 0.90 |
|
| |||
| IAC versus IVGAC | IAC | IVGAC | P-value |
|
| |||
| Cmax (ng/mL) | 84.95 ± 20 | 100.22 ± 21 | 0.34 |
| AUC (min × ng/mL/mg) | 2999.16 ± 263 | 3718.14 ± 263 | 0.04* |
|
| |||
| IVC versus IVGAC | IVC | IVGAC | P-value |
|
| |||
| Cmax (ng/mL) | 76.51 ± 20 | 104.17 ± 21 | 0.07 |
| AUC (min × ng/mL/mg) | 2759.47 ± 773 | 3669.76 ± 792 | 0.15 |
Results are presented as least square geographic means standard deviation using a repeated measures analysis of variance (ANOVA). Corrected doses of mitoxantrone (5 mg/m2) were used for least square geographic mean calculations. Statistical analysis was carried out using repeated measures ANOVA (P < 0.05).
IAC — intra-arterial chemotherapy; IVC — intravenous chemotherapy; IVGAC — intravenous under general anesthesia chemotherapy; Cmax — peak systemic serum concentration; AUC — area under the serum drug concentration-time curve.
Significant result.
Table II.
Statistical summary of comparative anesthesia data for mitoxantrone, doxorubicin, and carboplatin between procedures (IAC versus IVGAC).
| Mitoxantrone (10 dogs) | IAC (10 procedures) | IVGAC (7 procedures) | P-value |
|---|---|---|---|
| Lowest MAP (mmHg) | 61 | 70 | 0.14 |
| Total infusion time (min) | 11.25 | 11 | 0.95 |
| Total anesthesia time (min) | 82 | 17 | 0.0001* |
|
| |||
| Doxorubicin (5 dogs) | IAC (5 procedures) | IVGAC (2 procedures) | P-value |
|
| |||
| Lowest MAP (mmHg) | 65 | 73 | 0.43 |
| Total infusion time (min) | 20.8 | 15 | 0.28 |
| Total anesthesia time (min) | 65 | 31 | 0.18 |
|
| |||
| Carboplatin (7 dogs) | IAC (7 procedures) | IVGAC (4 procedures) | P-value |
|
| |||
| Lowest MAP (mmHg) | 62 | 78 | 0.04* |
| Total infusion time (min) | 14 | 12 | 0.52 |
| Total anesthesia time (min) | 90.6 | 15 | 0.00* |
|
| |||
| Combined mitoxantrone, carboplatin, and doxorubicin (22 dogs) | IAC (22 procedures) | IVGAC (13 procedures) | P-value |
|
| |||
| Lowest MAP (mmHg) | 62.7 | 73.8 | 0.023* |
| Total infusion time (min) | 15.3 | 12.5 | 0.25 |
| Total anesthesia time (min) | 79.2 | 20.1 | < 0.00* |
Results are presented as least square geographic means standard deviation. Statistical analysis was carried out using Chi-square or Fisher’s exact test when appropriate (P < 0.05). Lowest reported indirect mean arterial pressure (MAP) during anesthesia is reported in millimeters of mercury and obtained by indirect oscillometric readings.
IAC — intra-arterial chemotherapy; IVGAC — intravenous under general anesthesia chemotherapy; MAP — mean arterial pressure.
Significant result.
Doxorubicin group
A total of 10 treatments, including 5 IAC, 3 IVC, and 2 IVGAC procedures, were conducted in 5 dogs in the doxorubicin group. The first treatment was IAC in 3 patients and IVC in 2 and all patients underwent IVGAC as the last procedure. The mean IVGAC dose administered was significantly lower than the mean dose administered to the IAC group (IVGAC 23 mg/m2, IAC 27.8 mg/m2; P = 0.03) and the IVC group (IVC 29 mg/m2; P = 0.01). However, there were no significant differences in the dose-normalized Cmax or AUC values among the procedures (Table III). The mean chemotherapy infusion time was not statistically different between groups (IAC = 20.75 min, IVGAC = 15 min, P = 0.28). There was no significant difference in total anesthesia time between the groups (IAC = 65 min, IVGAC = 31 min, P = 0.18). The lowest mean indirect arterial blood pressures between the groups were not statistically different (IAC = 65 mmHg, IVGAC = 73 mmHg, P = 0.43) (Table II).
Table III.
Statistical summary of comparative bioavailability data for dose-normalized doxorubicin in 5 dogs.
| IAC versus IVC | IAC | IVC | P-value |
|---|---|---|---|
| Cmax (ng/mL) | 40.00 ± 10 | 32.93 ± 12 | 0.75 |
| AUC (min × ng/mL/mg) | 1067.72 ± 238 | 886.03 ± 291 | 0.72 |
|
| |||
| IAC versus IVGAC | IAC | IVGAC | P-value |
|
| |||
| Cmax (ng/mL) | 40.00 ± 11 | 35.17 ± 11 | 0.55 |
| AUC (min × ng/mL/mg) | 1067.72 ± 268 | 871.91 ± 269 | 0.10 |
|
| |||
| IVC versus IVGAC | IVC | IVGAC | P-value |
|
| |||
| Cmax (ng/mL) | 0.19 ± 0 | 0.23 ± 0 | 0.46 |
| AUC (min × ng/mL/mg) | 9.99 ± 2 | 11.68 ± 2 | 0.41 |
Results are presented as least square geographic means standard deviation using a repeated measures analysis of variance (ANOVA). Corrected doses of doxorubicin (30 mg/m2) were used for least square geographic mean calculations. Statistical analysis was carried out using repeated measures ANOVA (P < 0.05).
IAC — intra-arterial chemotherapy; IVC — intravenous chemotherapy; IVGAC — intravenous under general anesthesia chemotherapy; Cmax — peak systemic serum concentration; AUC — area under the serum drug concentration-time curve.
Carboplatin group
A total of 14 carboplatin treatments, consisting of 7 IAC, 3 IVC, and 4 IVGAC procedures, were carried out in 7 dogs. Four dogs received IAC first, 3 received IVC first, and all patients underwent IVGAC as the last procedure. There was no significant difference among groups in terms of mean dose administered (IAC 270 mg/m2 versus IVC 271 mg/m2, P = 0.98; IAC 270 mg/m2 versus IVGAC 256 mg/m2, P = 0.49; IVC 271 mg/m2 versus IVGAC 256 mg/m2, P = 0.53).
Dose-normalized AUC values among treatment types were comparable (Table IV). The dose-normalized Cmax was significantly lower (P = 0.02) for the intra-arterial group (IAC 0.16 ng/mL), however, than for the intravenous group (IVC 0.19 ng/mL). This difference was not demonstrated when IAC was compared to IVGAC (P = 0.19). The mean chemotherapy infusion times for the IAC (14 min) and IVGAC groups (12 min) were not significantly different (P = 0.52). There was a significant difference in total anesthesia time among the groups (IAC = 90.6 min, IVGAC = 15 min, P < 0.001). The lowest mean indirect arterial blood pressures among the groups were significantly different (IAC = 62 mmHg, IVGAC = 78 mmHg, P = 0.04) (Table II).
Table IV.
Statistical summary of comparative bioavailability data for dose-normalized carboplatin in 7 dogs.
| IAC versus IVC | IAC | IVC | P-value |
|---|---|---|---|
| Cmax (ng/mL) | 0.16 ± 0 | 0.19 ± 0 | 0.02* |
| AUC (min × ng/mL/mg) | 9.68 ± 1 | 9.96 ± 1 | 0.73 |
|
| |||
| IAC versus IVGAC | IAC | IVGAC | P-value |
|
| |||
| Cmax (ng/mL) | 0.16 ± 0 | 0.21 ± 0 | 0.19 |
| AUC (min × ng/mL/mg) | 9.68 ± 1 | 11.34 ± 2 | 0.24 |
|
| |||
| IVC versus IVGAC | IVC | IVGAC | P-value |
|
| |||
| Cmax (ng/mL) | 0.19 ± 0 | 0.23 ± 0 | 0.46 |
| AUC (min × ng/mL/mg) | 9.99 ± 2 | 11.68 ± 2 | 0.41 |
Results are presented as least square geographic means standard deviation using a repeated measures analysis of variance (ANOVA). Corrected doses of carboplatin (300 mg/m2) were used for least square geographic mean calculations. Statistical analysis was carried out using repeated measures ANOVA (P < 0.05).
IAC — intra-arterial chemotherapy; IVC — intravenous chemotherapy; IVGAC — intravenous under general anesthesia chemotherapy; Cmax — peak systemic serum concentration; AUC — area under the serum drug concentration-time curve.
Significant result.
Procedure variables
No adverse events occurred during any types of procedures in any of the chemotherapy groups. There was no difference among the types of procedures in hematologic variables (neutrophil count P = 0.06 and platelet count P = 0.07) 7 to 10 d after treatment (Table V). Eight of the total 22 patients in the study (36%) had adverse events that resulted in a dose reduction or would have resulted in a dose reduction in the case of a treatment being administered last (Table VI). The most common adverse event was a grade-III to grade-IV febrile neutropenia (3 with IAC, 4 with IVC, and 1 with IVGAC) and grade-II or grade-IV thrombocytopenia (3 with IAC, 3 with IVC, and 1 with IVGAC) 7 to 10 d after treatment. Most serious adverse events were noted in the mitoxantrone group, which accounted for 7 of the 8 or 88% of the total adverse events. A single adverse event was noted after IAC doxorubicin due to grade-II thrombocytopenia. There were no adverse events in the carboplatin group.
Table V.
Median hematologic variables (absolute neutrophil and platelet count) 7 to 10 d after treatment with mitoxantrone, doxorubicin, and carboplatin for each procedure.
| Median absolute neutrophil count (μL) | Median grade neutropenia | Median absolute platelet count (μL) | Median grade thrombocytopenia | |
|---|---|---|---|---|
| Mitoxantrone (26 procedures in 10 dogs) | ||||
| IAC (n = 10) | 2593 | 1.7 | 184 000 | 1.7 |
| IVC (n = 9) | 2348 | 2 | 164 000 | 1.2 |
| IVGAC (n = 7) | 2600 | 1.6 | 214 000 | 1.2 |
| Total (n = 26) | 2494 | 1.8 | 182 000 | 1.4 |
| Doxorubicin (10 treatments in 5 dogs) | ||||
| IAC (n = 5) | 4488 | 1 | 261 000 | 1.3 |
| IVC (n = 3) | 2310 | 1 | 176 000 | 1 |
| IVGAC (n = 2) | 4066 | 1 | 125 000 | 1 |
| Total (n = 10) | 3968 | 1 | 216 000 | 1 |
| Carboplatin (14 treatments in 7 dogs) | ||||
| IAC (n = 7) | 4937 | 1 | 330 000 | 1 |
| IVC (n = 3) | 6383 | 1 | 281 000 | 1 |
| IVGAC (n = 4) | 3343 | 2.5 | 390 000 | 1 |
| Total (n = 14) | 4647 | 1.5 | 342 000 | 1 |
Hematologic variables are graded in accordance with the Veterinary Cooperative Oncology Group — common terminology criteria for adverse events (VCOG-CTCAE) (2).
IAC — intra-arterial chemotherapy; IVC — intravenous chemotherapy; IVGAC — intravenous under general anesthesia chemotherapy.
Table VI.
Hematologic adverse events that led to dose reduction or removal from study.
| Adverse events | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| First procedure | Second procedure | Third procedure | ||||||
|
|
|
|
||||||
| Dose (mg/m2) | Grade neutropenia | Grade thrombocytopenia | Dose (mg/m2) | Grade neutropenia | Grade thrombocytopenia | Dose (mg/m2) | Grade neutropenia | Grade thrombocytopenia |
| Mitoxantrone (10 dogs, 26 procedures) | ||||||||
| Patient 1 | ||||||||
| IAC | IVC | |||||||
|
|
|
|||||||
| 5 | 3 | 4 | 3.75 | 1 | 1 | |||
|
|
|
|||||||
| Patient 2 | ||||||||
| IVC | IAC | IVGAC | ||||||
|
|
|
|
||||||
| 4.6 | 3 | 1 | 3.68 | 1 | 1 | 3.68 | 1 | 1 |
|
|
|
|
||||||
| Patient 3 | ||||||||
| IVC | IAC | IVGAC | ||||||
|
|
|
|
||||||
| 4 | 4 | 1 | 3.4 | 4 | 1 | 3 | 1 | 1 |
|
|
|
|
||||||
| Patient 4 | ||||||||
| IVC | IAC | IVGAC | ||||||
|
|
|
|
||||||
| 5 | 4 | 2 | 4 | 1 | 2 | 4 | 4 | 2 |
|
|
|
|
||||||
| Patient 5 | ||||||||
| IVC | IAC | IVGAC | ||||||
|
|
|
|
||||||
| 5 | 4 | 2 | 3.75 | 1 | 1 | 3.75 | 1 | 1 |
|
|
|
|
||||||
| Patient 6 | ||||||||
| IAC | ||||||||
|
| ||||||||
| 4.5 | 4 | 4 | ||||||
|
| ||||||||
| Patient 7 | ||||||||
| IVC | IAC | IVGAC | ||||||
|
|
|
|
||||||
| 5 | 4 | 2 | 3.75 | 1 | 1 | 3.75 | 1 | 1 |
|
|
|
|
||||||
| Doxorubicin (5 dogs, 10 treatments) | ||||||||
| Patient 8 | ||||||||
| IAC | IVGAC | |||||||
|
|
|
|||||||
| 30 | 1 | 2 | 24 | 1 | 1 | |||
Hematologic adverse events are graded in accordance with the Veterinary Cooperative Oncology Group — common terminology criteria for adverse events (VCOG-CTCAE) (2). Adverse events were defined as complications that resulted in a dose reduction (grade-III to grade-IV neutropenia and/or grade-II to grade-IV thrombocytopenia) or removal from the study.
IAC — intra-arterial chemotherapy; IVC — intravenous chemotherapy; IVGAC — intravenous under general anesthesia chemotherapy.
Discussion
The results of this study demonstrate weakly significant differences in peak serum concentrations between intravenous and intra-arterial carboplatin treatments. These results were not consistently seen across all tumor types or across all chemotherapy agents. This is not overly surprising as systemic drug concentrations do not necessarily represent the local tumor environment. Unfortunately, from a clinical standpoint, obtaining local tumor concentration values is not a realistic goal in client-owned animals. As previously stated, the way in which a tumor takes up a chemotherapeutic compound is governed by many factors that were not directly investigated in this study and therefore definitive conclusions about drug concentration cannot be made. This point is further strengthened by the limitations of the patient population. This study should therefore be considered as a pilot study on which to base further studies.
The peak serum concentrations of carboplatin were weakly but significantly higher in the awake intravenous group than in the intra-arterial group (P = 0.02). Serial peripheral blood sampling was carried out at consistent times and Cmax was determined as the measured concentration immediately after infusion for all chemotherapeutic agents. These results were not consistent across all intravenous groups (awake and under general anesthesia) as would be expected from our hypothesis. While lower means were seen with IAC (0.16 ng/dL) when compared with IVGAC (0.21 ng/dL), these results were not significant (P = 0.19). Additionally, the duration of anesthesia before delivery of chemotherapy was considerably shorter for the IVGAC group than for the IAC group, as it takes time to gain vascular access and for catheter placement before IAC is administered. Even in light of this consideration, which would be expected to increase serum levels in the IAC group due to reduced systemic clearance and smaller volume of distribution, IAC consistently demonstrated lower dose-normalized Cmax values. These results suggest that regional delivery of carboplatin should be investigated further if Cmax values correlate with enhanced tumor response and/or reduced adverse events.
Interestingly, much of the published data about carboplatin refers to the AUC as it correlates to efficacy and toxicity. Joerger et al (11) reported that toxicity of carboplatin, thrombocytopenia in this case, was best correlated with carboplatin AUC, but could also correlate with overall exposure when Cmax was included as well. A target AUC has been established in humans to produce better outcomes and studies have shown that the number of platinum-DNA adducts increases with increasing plasma AUC. As platinum-DNA adducts are the described mechanism of action for carboplatin for most cancers, a link can be drawn between plasma AUC and efficacy and toxicity (11,15). In a phase-I evaluation of a dosing strategy provided by AUC, a targeted/maximum tolerated AUC for carboplatin could be determined in cats (16). In humans, efficacy of carboplatin in ovarian and testicular cancer was described using a targeted AUC of 5 to 7 mg/mL per minute and was associated with better treatment outcomes (11). It may be more reasonable to consider AUC as a parameter to guide further studies, although a lower Cmax has also been linked to a reduction in adverse events during administration in humans (11).
Area under the curve (AUC) is often considered the most important calculation in terms of systemic drug exposure and subsequently the most valuable in predicting toxicity after exposure to chemotherapeutic drugs. The AUC has been demonstrated to correlate most significantly with bone marrow suppression and, more specifically, the degree of neutropenia (16).
Mitoxantrone AUC concentrations were significantly different for IAC than for IVGAC, but not for any other route. A higher AUC for IVGAC would support our hypothesis that IVGAC provides higher systemic drug concentrations based on logical assumptions of drug distribution under general anesthesia, although we cannot make this determination. While the mitoxantrone group experienced the most adverse events, the population was too small to interpret statistically significant differences between the routes of administration. There are few data in terms of serum mitoxantrone levels (Cmax and AUC) regarding efficacy and toxicity (adverse events). For mitoxantrone, information pertaining to Cmax relates to acute toxicity, whereas AUC has traditionally correlated better with efficacy and, in some reports, with adverse events such as gastrointestinal signs and bone marrow suppression (13). Information provided by human studies regarding Cmax and AUC is reported after standard intravenous routes of delivery. From a toxicity standpoint, it would be important to determine if targeted delivery permitted similar efficacy with lower systemic toxicity due to the reduced AUC. While IVGAC would not be routinely administered clinically, the comparison to IAC reduces the conflicting effects of general anesthesia. The information gained from this comparison could suggest that placement of the intra-arterial port for IAC in awake patients is superior to IVC. This requires future investigation.
Anti-cancer drugs have a narrow therapeutic index and the impact of general anesthesia on metabolism, distribution, and clearance is largely unknown. After intravenous administration, the peak blood concentration of the chemotherapeutic agent is determined by the dose, rate of administration, and cardiac output. Higher cardiac output and subsequent higher volume of blood delivered to the tissues results in lower peak systemic levels. General anesthesia often reduces glomerular filtration rate and therefore clearance of chemotherapeutic agents that rely on renal clearance, such as carboplatin, which may result in higher AUC. This effect may not pertain to drugs cleared through metabolism by hepatic mixed function oxidase enzymes (9,17). The third arm of the study incorporating the IVGAC group was ultimately added to control for these factors as much as possible.
For carboplatin, the lower Cmax values after intra-arterial chemotherapy (IAC) might be explained by higher initial local uptake by the tumor tissue. While this supports the hypothesis that IAC would result in lower systemic chemotherapy levels, similar statistically significant values were not found for the mitoxantrone or doxorubicin groups. The failure to identify a statistical difference could be complicated by the relatively small tumors being perfused comparable to the volume of distribution of the entire systemic circulation, systemic circulatory changes under anesthesia over time, tumor vascularity and microenvironment, or physiochemical properties of the individual drugs. In order to determine if intra-arterial chemotherapy resulted in greater total drug exposure to the tumor itself, a preferable approach would be to take tumor samples after chemotherapy is administered in order to obtain tissue drug concentrations, although this would have been difficult in this client-owned patient population.
A previous study validated the use of AUC for tumor tissue as being far more sensitive an indicator of drug absorption than plasma concentrations (17). A research study conducted with laboratory rabbits with bladder tumors determined that internal iliac artery infusion of carboplatin and pirarubicin resulted in significantly higher intratumoral chemotherapy levels, although no significant differences in the plasma levels were demonstrated (17). In a study by Chen et al (6), while no significant difference was noted in plasma AUC for dogs administered carboplatin by intravenous or internal iliac intra-arterial delivery, platinum AUC for uterine tissue was significantly higher for the intra-arterial group. The Cmax for the peripheral vein administration group was significantly higher than the abdominal aorta and internal iliac artery administration groups at time zero, 15 and 30 min after carboplatin was administered (6). That study did not use super-selective arterial catheterization as we did in the current study. In-vivo pharmacokinetic studies in humans have used dynamic positron emission tomography (PET) as an alternative to invasive tissue sampling, although this modality is not commonly used in the veterinary clinical setting (18).
As blood flow in tumors is the primary determinant of exposure to the chemotherapeutic agent, it will be maximized if the tumor is well-vascularized and blood flow is slow (9,17). The degree of tumor perfusion among patients was not evaluated or characterized in the current study. Intra-arterial chemotherapy uses super-selective catheters that can deliver chemotherapy to the arteries feeding the tumor (3). In the current study, the chemotherapy agent within the local arteries was visualized by use of iodinated contrast and fluoroscopic guidance-conferring exposure. It would be reasonable to speculate that longer exposure times would result in more chemotherapy drugs being retained by the tumor itself.
Although not used in this study, methods to increase temporal exposure of the chemotherapy being infused could result in additional uptake by the tumor. This could be accomplished by temporary balloon occlusion of proximal arteries while chemotherapy was being administered or by vascular embolization with chemotherapy (chemoembolization). In a study by Song et al (17), transarterial chemotherapy (TAC) with carboplatin was compared with a transarterial chemoembolization (TACE) procedure using a lipiodol-carboplatin mixture (TACE group) administered to the internal iliac arteries in normal dogs. This study found that TACE had a pharmacokinetic advantage compared to TAC in the urogenital system as there was a 2-fold increase in uterine AUC concentrations and lower peak plasma concentrations in the TACE group (17). These animals were not recovered to determine if any detrimental effects of embolization of this vessel resulted in morbidity.
It is not clear if the dogs with naturally occurring tumors that received super-selective delivery in the present study could safely tolerate a chemoembolization procedure, as non-target embolization would likely occur to the caudal vesical artery, internal pudendal artery, and possibly, the middle rectal arteries in certain circumstances. In a canine prostatic hyperplasia model, super-selective catheterization of the prostatic branches of the internal pudendal arteries was successfully carried out using a 3-French microinfusion catheter, which was shown to induce prostatic infarction and subsequent reduction in prostate size (3). Chemoembolization for liver tumors has been demonstrated to result in 10- to 50-fold increases in intra-tumoral drug concentrations and to be generally well-tolerated, presumably due to the dual blood supply present in this organ (7,8).
To the authors’ knowledge, this is the first study to compare the pharmacokinetic profiles of intra-arterial chemotherapy with intravenous routes, awake and under general anesthesia, in a canine urogenital tumor model. Intra-arterial administration of carboplatin and mitoxantrone demonstrated potentially lower Cmax and AUC values, respectively, when compared to certain intravenous counterparts. This points to a possible advantageous “first pass effect” after regional delivery of carboplatin and mitoxantrone and both should be considered for future larger comparative pharmacokinetic investigations in dogs with naturally occurring urogenital tumors. A natural next step for investigation would be to confirm the pharmacokinetic advantages of using arterial ports to deliver these agents intra-arterially in awake patients (19).
Acknowledgment
The study was supported by a grant from the Puppy Up Foundation.
Footnotes
Dr. Weisse and Dr. Berent are consultants for Infiniti Medical, LLC. Dr. Clifford and Dr. Weisse were members of the scientific advisory board for the Puppy Up Foundation. The grant from Puppy Up to support this study was awarded before they became members of its advisory board.
The authors declare no off-label use of antimicrobials.
References
- 1.Knapp DW, Ramos-Vara JA, Moore GE, Dhawan D, Bonney PL, Young KE. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014;55:100–118. doi: 10.1093/ilar/ilu018. [DOI] [PubMed] [Google Scholar]
- 2.Veterinary cooperative oncology group. Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2016;14:417–446. doi: 10.1111/vco.283. [DOI] [PubMed] [Google Scholar]
- 3.Culp WTN, Weisse C, Berent AC, et al. Early tumor response to intraarterial or intravenous administration of carboplatin to treat naturally occurring lower urinary tract carcinoma in dogs. J Vet Intern Med. 2015;29:900–907. doi: 10.1111/jvim.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha TA, Mauldin GN, Patnaik AK, Bergman PJ. Prognostic factors in dogs with urinary bladder carcinoma. J Vet Intern Med. 2000;14:486. doi: 10.1892/0891-6640(2000)014<0486:pfidwu>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 5.McCaw DL, Lattimer JC. Radiation and cisplatin for treatment of canine urinary bladder carcinoma: A report of two case histories. Vet Radiol. 1988;29:264–268. [Google Scholar]
- 6.Chen C, Wang W, Zhou H, et al. Pharmacokinetic comparison between systemic and local chemotherapy by carboplatin in dogs. Reprod Sci. 2009;16:1097–1102. doi: 10.1177/1933719109341999. [DOI] [PubMed] [Google Scholar]
- 7.Weisse C. Hepatic chemoembolization: A novel regional therapy. Vet Clin North Am Small Anim Pract. 2009;39:627–630. doi: 10.1016/j.cvsm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Weisse C, Clifford CA, Holt D, Solomon JA. Percutaneous arterial embolization and chemoembolization for treatment of benign and malignant tumors in three dogs and a goat. J Am Vet Med Assoc. 2002;221:1430–1436. doi: 10.2460/javma.2002.221.1430. [DOI] [PubMed] [Google Scholar]
- 9.Chen HS, Gross JF. Intra-arterial infusion of anticancer drugs: Theoretic aspects of drug delivery and review of responses. Cancer Treat Rep. 1980;64:31–40. [PubMed] [Google Scholar]
- 10.Shieh G. A comparative study of power and sample size calculations for multivariate general linear models. Multivar Behav Res. 2003;38:285–307. doi: 10.1207/S15327906MBR3803_01. [DOI] [PubMed] [Google Scholar]
- 11.Joerger M, Huitema ADR, Richel DJ, et al. Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: A study by the European organization for research and treatment of cancer-pharmacology and molecular mechanisms group and new drug development group. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13:6410–6418. doi: 10.1158/1078-0432.CCR-07-0064. [DOI] [PubMed] [Google Scholar]
- 12.Gustafson DL, Rastatter JC, Colombo T, Long ME. Doxorubicin pharmacokinetics: Macromolecule binding, metabolism, and excretion in the context of a physiologic model. J Pharm Sci. 2002;91:1488–1501. doi: 10.1002/jps.10161. [DOI] [PubMed] [Google Scholar]
- 13.An G, Morris ME. A physiologically based pharmacokinetic model of mitoxantrone in mice and scale-up to humans: A semi-mechanistic model incorporating DNA and protein binding. AAPS J. 2012;14:352–364. doi: 10.1208/s12248-012-9344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frazier DL, Price GS. Use of body surface area to calculate chemotherapeutic drug dose in dogs: II. Limitations imposed by pharmacokinetic factors. J Vet Intern Med. 1998;12:272–278. doi: 10.1111/j.1939-1676.1998.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 15.Duffull SB, Robinson BA. Clinical pharmacokinetics and dose optimisation of carboplatin. Clin Pharmacokinet. 1997;33:161–183. doi: 10.2165/00003088-199733030-00002. [DOI] [PubMed] [Google Scholar]
- 16.Bailey DB, Rassnick KM, Dykes NL, Pendyala L. Phase I evaluation of carboplatin by use of a dosing strategy based on a targeted area under the platinum concentration-versus-time curve and individual glomerular filtration rate in cats with tumors. Am J Vet Res. 2009;70:770–776. doi: 10.2460/ajvr.70.6.770. [DOI] [PubMed] [Google Scholar]
- 17.Song T, Wang W, Liu P, Chen C. Pharmacokinetic comparison between pelvic transarterial chemoembolization and transcatheter arterial chemotherapy in an animal model. Mol Med Rep. 2009;2:663–667. doi: 10.3892/mmr_00000154. [DOI] [PubMed] [Google Scholar]
- 18.Cho SY, Szabo Z. Molecular imaging of urogenital diseases. Semin Nucl Med. 2014;44:93–109. doi: 10.1053/j.semnuclmed.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinn M, Nicolaou A, Ricke J, et al. Interventionally implanted port catheter systems for hepatic arterial infusion of chemotherapy in patients with primary liver cancer: A phase II-study ( NCT00356161) BMC Gastroenterol. 2013;13:125. doi: 10.1186/1471-230X-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]