Abstract
Background and aims: Knowledge of therapeutic lifestyle interventions is one of the most important pillars of diabetes care; however, its incorporation in real-world settings is poor. This review evaluates the role of partner and family support in diabetes management. Methods: Literature searches were performed in PubMed, Medline and Embase for articles published before July 2018, using the terms “therapeutic lifestyle intervention” [MeSH Terms], OR “diet changes” [All Fields], OR “spousal participation” [All Fields], OR “lifestyle interventions” [All Fields], “lifestyle changes” [All Fields] AND “diabetes” [All Fields]. The search was not restricted to English-language literature; literature in Spanish, French and German were also evaluated. Results: A total of 66 of articles were reviewed, which included 33 original work, 21 review articles, and 12 systematic reviews and meta-analyses. Studies and meta-analyses have showed that if one partner has type-2 diabetes this increases the risk in other by 5–26%. Partner and family have similar diet, lifestyle, and micro- and macro-environments which could explain the similar increased risk of diabetes and non-communicable diseases. Studies have consistently shown that spousal and family support plays a key role in overcoming negative behaviours and optimising behaviours in diabetes control. Partner support has major role in prevention and control of diabetes distress, associated depression, and medication non-compliance which have an adverse impact in glycaemic outcomes. These data are predominantly available from observational studies. There is paucity of data from interventional trials evaluating effects of family and spousal participation on health, glycaemic control and quality of life. Conclusion: The support of family and spouse/partner is beneficial to improve adherence to the lifestyle interventions and pharmacotherapy required to achieve optimum glycaemic control and avoid associated complications.
Keywords: Diabetes, partner participation, relationships, challenges, lifestyle management
Type 2 diabetes mellitus (T2DM) is a chronic disease characterised by hyperglycaemia and compounded by insufficient production of insulin (beta cell dysfunction) to overcome the ineffective action of insulin (insulin resistance). According to International Diabetes Federation Eighth Atlas (2017), there were 425 million diabetic people (20–79 years of age) worldwide.1 The number of people affected by diabetes is expected to reach 629 million by 2045.1 Unique challenges with diabetes in India include the high prevalence of the disease (9% for diabetes and 12–15% for prediabetes), nearly 2 decade earlier onset of T2DM among Indians, as compared to the rest of the world, a more aggressive disease phenotype (highest rates of prediabetes progression to diabetes of 18% per annum), increasing problem of obesity, and primarily effecting the economically productive population (age 18–60 years) of the society.2,3
Optimal and appropriate diet, calorie restriction, physical activity and medication adherence are four key pillars of therapeutic lifestyle interventions in the management of diabetes.4,5 In spite of awareness, poor compliance to therapeutic lifestyle interventions contributes to poor glycaemic control and increased end-organ damage resulting in poor quality of life in the long run. This perpetuates a viscous cycle of poor control and further complications, which at times, becomes difficult a difficult cycle for the patient to break.6 The important factors which contribute to medication non-adherence are knowledge and perception of disease, complexity of dosing regimen and difficulty in managing complex treatment regimens or their side effects, financial constraints, psychological factors, and lack of social support.5–7
Family support and social relationships, as a part of individual treatment and healthcare, are beneficial for better glycaemic control and improved quality of life.7 Health-related behaviour change interventions influence healthier lifestyles among people with diabetes but the effectiveness and sustainability is low.8,9 The role of family support and especially the spousal relationship is an important component outside of the social networks, as the partner spends a considerable portion of his or her life in the shared environment. Joint management efforts with spousal participation are hypothesised to produce effective and sustainable results in diabetes management.10,11 Hence the aim of this review is to analyse the literature to develop better insights regarding successful implementation of lifestyle intervention programmes in diabetes. There is a special focus on evaluating the role of family and partner support in successful implementation of therapeutic lifestyle programmes.
Methods
A literature search was performed in PubMed, Medline and Embase for articles published before July 2018, using the terms “therapeutic lifestyle intervention” [MeSH Terms], OR “diet changes” [All Fields], OR “spousal participation” [All Fields], OR “lifestyle interventions” [All Fields], “lifestyle changes” [All Fields] AND “diabetes” [All Fields]. The reference lists of the articles identified were also searched. The search was not restricted to English-language literature; literature in Spanish, French and German were also evaluated.
Results
A total of 66 articles were reviewed, which included 33 original work, 21 review articles and 12 systematic reviews and meta-analysis. The key information obtained from these articles has been elaborated below.
Effectiveness of lifestyle interventions in people with type 2 diabetes mellitus
Lifestyle intervention such as proper diet and physical activity, can improve glycaemic control and other metabolic risk factors, which in turn, has the potential to prevent diabetic complications. Huang et al. conducted a systematic review and meta-analysis of 17 randomised clinical trials comparing lifestyle intervention with “usual care” (control) in patients with T2DM and found that dietary intervention showed an improvement in glycated haemoglobin (HbA1c), systolic/diastolic blood pressure and high-density lipoprotein (HDL) cholesterol, suggesting that nutritional intervention had a significant impact on different components of metabolic syndrome, and hence has a potential to reduce cardiovascular risk in T2DM.12
Cezaretto et al. conducted a systematic review and meta-analysis of randomised controlled trials found a significant reduction in depression scores following lifestyle interventions in the pooled analysis (standardised mean difference [SMD] -0.165; 95% confidence interval [CI] -0.265, -0.064; I[2] 67.9%) and when limited to individuals with T2DM (SMD -0.202; 95% CI -0.288, -0.079; I[2] 72.5%).13 Htoo et al. found that Southeast Asian adults with diabetes who received lifestyle modifications for 3 months had statistically significant reductions in HbA1c, compared to the control group.14 However, the effects tended to blunt out after 6 months of follow up (i.e., non-significant reduction in HbA1c in the intervention group compared to the control group beyond 6 months follow up).14,15
The above data provides evidence to support the role of therapeutic lifestyle interventions in improving glycaemia and other complications associated with diabetes. They can also be effective for other associated features such as depression. However, evidence for long-term sustainability beyond 6 months, especially in Asians, needs to be further assessed in people with diabetes.16 It may be hypothesised that poor compliance to therapeutic lifestyle interventions beyond 6 months may contribute to its decreased efficacy over long periods of time
Real-world challenges to the implementation of therapeutic lifestyle interventions
Adherence to treatment
Low compliance to prescribed treatment approaches is a common problem in patients with chronic diseases.17,18 A meta-analysis published in 2018 (including 2,491 screened records and 24 studies on 369,251 people from 20 countries) reported that target achievement rates for glycaemic control were 42.8% (95% CI 38.1–47.5%).19 The World Health Organization (WHO) has reported that approximately 50% of patients with a chronic illness are non-adherent with their treatment regimen (diet, activity, monitoring and medications); the rate is even higher in developing countries.20 A review article published in 2014 focusing on qualitative research, meta-syntheses, and meta-ethnography articles estimated more than 40% non-adherence to treatment and medical recommendations among patients with T2DM.21
The factors associated with such non-adherence could be patient related, such as understanding of the disease, coping mechanisms, motivation for control; family related factors, such as social or financial support; treatment related, such as simplicity and effectiveness of regimen; or healthcare related, for example easy accessibility to competent doctors and relationship with healthcare professionals.22–25 Evidence-based research should explore further possible factors, their operational mechanisms and possible solutions (as summarised in Tables 1 and 2) to overcome non-adherence and improve glycaemic management.26–30 The pragmatic understanding of the various reasons of non-adherence, changes in attitude and motivation along with intensive treatment and lifestyle interventions in group setting are evidenced to improve adherence to the lifestyle recommendations and glycaemic outcomes.31 Overcoming these factors with a multifaceted approach can help in achieving good treatment outcomes, with improvement in quality of life for patients.
Table 1: Factors affecting treatment adherence and strategies to overcome these.
| Possible factors | Possible solutions |
|---|---|
| Personal factors | |
|
|
| Economic factors | |
|
|
| Social factors | |
|
|
| Psychological factors | |
|
|
| Disease-related factors | |
|
|
| Healthcare-provider factors | |
|
|
Table 2: Regimen-specific factors and their coping strategies.
| Diet-specific | Personal factors | |
|
|
|
| Social factors | ||
|
|
|
| Economic factors | ||
|
|
|
| Physical activity-specific |
|
|
| BGM and awareness-specific |
|
|
| Medicine and insulin-specific |
|
|
BGM = blood glucose monitoring
Given the challenges of non-adherence, family involvement can be greatly beneficial in therapeutic lifestyle programmes, as seen in Figure 1.23,25,26,29,30
Figure 1: Approach of lifestyle interventions towards diabetes self-management.
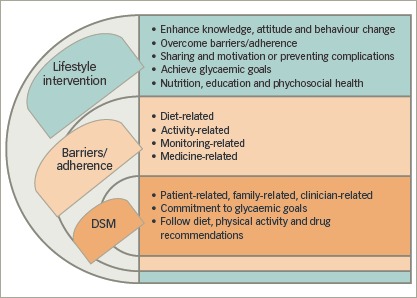
DSM = diabetes self-management
Diabetes distress
Clinical depression and diabetes distress are prevalent emotional states found among people with diabetes, which can significantly affect the glycaemic control; lower blood glucose monitoring frequency; and increase micro-vascular complications, healthcare use and expenditures.32 Lack of social support is determined to be a major risk factor for diabetes distress, and social stigma often prevents discussing or seeking help for psychological problems.33 Positive spousal support in T2DM intervention programmes has been evidenced as a moderator between psychological morbidity to promote adherence to treatment therapies, patient satisfaction with healthcare services, and compliance to therapeutic lifestyle interventions.34
Exploring family-based approach to cope with real-world challenges
It has been shown that family participation can improve the effectiveness of lifestyle interventions for enhancing diabetes self-management. People eat and enjoy meals together in routine and rituals with family members which influence dietary patterns; consistent bad dietary habits can have adverse health potentials.35 On the other hand, any cumbersome dietary changes which are difficult to accept, in society or by the people they live with, can ultimately lead to non-adherence or non-compliance. Contextually, a study conducted in 2017 assessed interconnected problem domains (knowledge, communication, support, everyday life, roles and worries) to understand how family involvement can be supported in healthcare practices.36 Similarly, a systematic review conducted in 2017, identified the behavioural influence (in terms of facilitators, barriers or equivocal behaviours) affecting an individual’s diabetes self-management.37 The important themes that emerged from these, and other studies in this context, are described as follows:
Facilitating behaviours
Positive family participation can encourage positive choices and will assist the patient to identify and overcome barriers to adherence. It can influence the patient’s psychological wellbeing, informational and financial support, and give them the confidence to follow recommendations resulting in behaviour change to adopt healthy diet, lifestyle, exercise and timely medical regimen. Family participation can also lead to improvements in glycaemic control and diabetes self-management via shared health-related activities or tasks together.38 It provides vigilance over changes in their clinical status or progressing complications.37 Finally, it enhances motivational skills for self-sufficiency, positive behaviours and independent attitudes towards disease.39–41
Negative behaviours
Despite positive influence, family participation may also lead to obstructive behaviours, refusal to share the burden and limited family support or engagement.42 The practice of unhealthy dietary habits or lifestyle routines; lack of emotional, physical and financial support; and lack of empathy, awareness, understanding and knowledge can all contribute towards non-adherence to treatment and disease management.37,43,44
Equivocal behaviours
Family behaviours such as reminders for appointments with healthcare professionals, taking medications, exercising and maintaining a healthy diet can affect as both facilitator of or barrier to diabetes self-management.37,45
Overcoming barriers
Enhancing the facilitating behaviours, overcoming the negative behaviours and optimising or reinforcing the equivocal behaviours are major facets towards effective adherence and glycaemic achievement.46,47 Family members can encourage the patient, change the types of food prepared or consumed, can enjoy similar food in a comfortable environment, engage in physical activities, attend medical visits with the patient, reprioritise family finances, and make necessary lifestyle changes. All of these supportive actions will help towards improving dietary habits, adherence to the treatment, healthcare and the patient’s clinical outcomes.40,48,49
Diabetes control and festivals
In India, numerous festivals are celebrated irrespective of caste, creed, age, and status, with different rituals irrespective of their health implications. People with diabetes are faced with multiple challenges related to diet, physical activity pattern, healthcareseeking behaviour and healthcare provision during such occasions.5 Family or spousal compliance towards the glycaemic management of their affected relative is paramount. The patient can be supported through practices such as healthy eating, medical nutrition therapy, avoiding excessive outdoor meals and erratic meal patterns, selfcare practices, blood glucose monitoring, physical activity, medication administration, and management of acute complications may; all of which will enhance compliance for the effective management of diabetes during the cultural and religious occasions.50
Spousal participation in diabetes care plan
A collaborative project from the UK Department of Health and the Royal Pharmaceutical Society of Great Britain in the mid-1990s yielded a better understanding of the factors affecting compliance, which conceptualised the evolution of ‘‘concordance’’.48,49 With a shift from compliance to concordance in diabetes and its complications, spousal concordance is defined by researchers as “shared similarity of habits, behaviours and health statuses among the partners or spouses”.51,52
In this view, the concept of dyadic coping refers to the collective efforts of the partners to manage stress, develop problem-focused or emotion-focused strategies for better glycaemic control and improved quality of life together (Figure 2). It leads to the emergence of empirical and theoretical evidence of illness-specific spousal support in better health outcomes of chronic illness, with assumption of sustained effects with regards to the patient’s adherence to diet and exercise programmes. Spousal support is associated with better patient diabetes self-efficacy, both with regards to diet and exercise.53,54
Figure 2: Challenges in diabetes self-management and effect of social support.
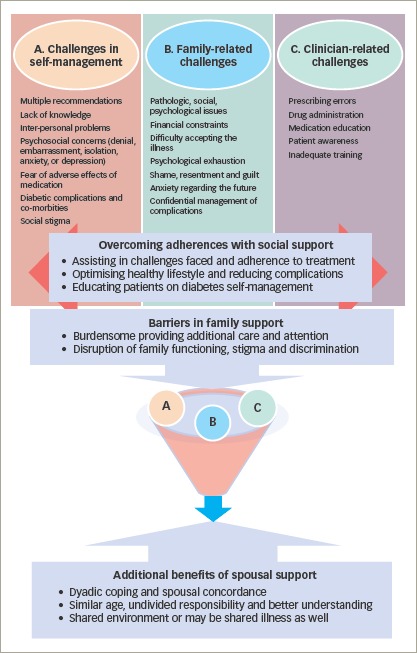
Some of the explored factors with regards to better glycaemic control include age, family environment, emotional support, social status, positive relationship, shared expectations, motivation and responsibility, effective communication, sense of responsibility and common family eating patterns.55–57 A sense of coherence also seems to be established among older populations.58 Table 3 highlights the domains of spousal concordance in diabetes.44,54,57,59–64
Table 3: Domains of spousal concordance in diabetes.
| Study and year | Domains | Effects |
|---|---|---|
| Johnson et al., 201544 | Spousal overprotection |
|
| Pereira et al., 201554 | Patients’ and partners’ variables regarding adherence to self-care |
|
| Henry et al., 201359 | Spousal tempting and undermining of the diabetic regimen |
|
| Johnson et al., 201360 | Diabetes efficacy |
|
| Franks et al., 201261 | Association of diet-related interactions with partner’s adjustment to the illness |
|
| August et al., 201157 | Social control |
|
| Stephens et al., 201062 | Spousal control strategies |
|
| Beverly et al., 200863 | Food-related behaviour change |
|
| Garay-Sevilla et al., 199564 | Adherence to diet and medication, knowledge on diabetes, social support, structure and functioning |
|
A systematic review and meta-analysis conducted in 2014 has shown, with a pooled estimate, that partners have a 26% increased risk of developing diabetes which highlights the importance of collective efforts to optimise healthy eating and physical activity patterns.65 An article published by Wang et al., reported a significantly higher risk (5.19%, p<0.0001) of diabetes concordance in couples, associated with old age, middle levels of urbanisation, and high comorbidities (all p<0.05).66 A systematic review published in 2017 by Dimova et al. identified shared risk of diabetes among relatives of people with T2DM.62 It examined various randomised controlled trials to identify behaviour change strategies focussed on diet and physical activity to delay or prevent T2DM among the relatives or partners of people with T2DM to enhance effectiveness in their own health. It found sustained modifications in participant’s behaviour, which resulted in decreased daily calorie intake, increased physical activity, weight loss, decreased waist circumference and sagittal diameter, with significant correlations between the behaviour of patients and their partners.67
Only a few interventional studies have been conducted so far to explore the direct influence of partners of diabetic partners on lifestyle interventions for the improvement of adherence to the treatment (Table 4).68–70 Spousal involvement has been associated with improved support or care in chronic pain outcomes such as pain severity, pain behaviours and psychological distress.10,71 This may be especially relevant in patients with painful diabetic neuropathy or musculoskeletal complications of diabetes like frozen shoulder among others. Spousal participation can be a potential source of health-enhancing support (Figure 1).23,26,31,36,38,41,49,51,68
Table 4: Spousal interventional studies among people with diabetes.
| Study and year | Sample size | Domains/parameters | Interventions, scales or tools | Results | Conclusion |
|---|---|---|---|---|---|
| Trief et al., 201668 | 280 couples | Glycaemic control and secondary outcomes: BMI, waist circumference, blood pressure, depressive symptoms, diabetes self-efficacy, and diabetes distress | Four-month intervention among three arms: CC n=104, IC n=94; DE n=82 |
|
A collaborative couples intervention resulted in significant, lasting improvements in HbA1C levels, obesity measures, and some psychosocial outcomes |
| Trief et al., 201169 | Couples (n=44), in which one partner had T2DM | Telephone goal-setting, dietary behaviour change, and a focus on emotions | Couple intervention, individual intervention, individual DE |
|
DE resulted in improved blood glucose control |
| Gilden et al., 198970 | Older male patients (aged 65–82 years) and their partners | Scored questionnaires | Six-week DE programme |
|
DE intervention is effective for both patients and their partners |
BMI = body mass index; CC = couples call; DE = diabetes education; HbA1C = glycated haemoglobin; IC = individual calls; QoL = quality of life; T2DM = type 2 diabetes mellitus; WC = waist circumference
Role of partner and family support – evidence from the clinical trials
The Look AHEAD (Action for Health in Diabetes) trial is the longest trial published, to date (8 years’ data), to evaluate the impact of intensive lifestyle interventions on weight loss, glycaemic control and cardiovascular outcomes in diabetes.72 Weight loss in Look AHEAD trial was -8.6% at 1-year follow-up and continued to be an impressive -4.7% at 8 years of follow-up.72 Unlike previous studies, the Look AHEAD trial did not involve intensive one-to-one sessions with dieticians/diabetes educators. Instead, in this study a group-counselling approach was used. A total of 19 group-counselling sessions took place over a period of 8 years where the patient and family members/ partners were given general advice on therapeutic lifestyle interventions.72,73 The study’s “toolbox” technique of group counselling, motivational interviews, improving self-image among family and friends was found to be highly effective. It was not only found to be cost effective, but also as efficacious in regards to weight loss and glycaemic control, which has been traditionally used in diabetes management.73 Hence the Look AHEAD study highlighted the importance of the positive impact of lifestyle changes in the entire family/community group rather than the individual alone.
The DiRECT (DIabetes REmission Clinical Trial) study has further highlighted the importance of weight loss in diabetes management.74 It demonstrated that in overweight and obese patients with T2DM of up to 6 years of disease duration and on multiple oral anti-diabetes medications, aggressive weight loss strategies with very low-calorie diet can result in remission of diabetes – defined as stoppage of all anti-diabetes medications and HbA1c <6.5% for at least 2 months.74 The greater the weight loss, the better were the chances of diabetes remission as evidenced by 7% remission with 0–5 kg weight loss, 34% remission with 5–10 kg loss, 57% remission with 10–15 kg loss, and 86% remission with >15 kg weight loss.74
Future implications
There is a paucity of data on the effects of spousal participation on health, glycaemic control, diabetes management and quality of life in lifestyle intervention studies and therefore more research is needed in this area. Further interventional studies evaluating the role of partner and family support in weight loss and diabetes management are urgently warranted.
Conclusion
Social support from family members and spouses/partners is beneficial to improve adherence to the lifestyle interventions and diabetes self-management required to achieve optimum glycaemic control and prevent or delay associated complications. There is paucity of data regarding the mechanism behind such support. Clinical and research interventions should consider implementing dyadic educational (e.g. nutrition, exercise, medication adherence) and motivational skills training (e.g. problem solving, emotional expressiveness training) to facilitate and optimise spousal support resources.
Funding Statement
Support: No funding was received in the publication of this article.
References
- 1.IDF Diabetes Atlas –. 8th Edition. 2017. www.diabetesatlas.org/resources/2017-atlas.html Available at: (accessed 24 August 2018)
- 2.Dutta D, Mukhopadhyay S. Intervening at prediabetes stage is critical to controlling the diabetes epidemic among Asian Indians. Indian J Med Res. 2016;143:23–6. doi: 10.4103/0971-5916.184281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dutta D, Mukhopadhyay S. et al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the chennai urban rural epidemiology study (CURES). Diabetes Care. Diabetes Care. 2015;2015 Sep;3838((9)):1441–1448.. doi: 10.2337/dc15-0908. Comment on Anjana. e146. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4.NDTV, World Diabetes Day 2017: Number of Diabetics to Double In India by 2023, 2017. www.ndtv.com/food/world-diabetes-day-2017-number-of-diabetics-to-double-in-india-by-2023-1775180 Available at: (accessed 24 August 2018).
- 5.Gupta L, Khandelwal D, Singla R. et al. Pragmatic dietary advice for diabetes during Navratris. Indian J Endocrinol Metab. 2017;21:231–7. doi: 10.4103/2230-8210.196009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delamater AM. Improving patient adherence. Clinical Diabetes. 2006;24:71–7. [Google Scholar]
- 7.Miller TA, Dimatteo MR. Importance of family/social support and impact on adherence to diabetic therapy. Diabetes Metab Syndr Obes. 2013;6:421–6. doi: 10.2147/DMSO.S36368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesla CA, Fisher L, Skaff MM. et al. Family predictors of disease management over one year in Latino and European American patients with type 2 diabetes. Fam Process. 2003;42:375–90. doi: 10.1111/j.1545-5300.2003.00375.x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher L, Weihs KL. Can addressing family relationships improve outcomes in chronic disease? Report of the National Working Group on family-based interventions in chronic disease. J Fam Pract. 2000;49:561–6. [PubMed] [Google Scholar]
- 10.Seidel AJ, Franks MM, Stephens MA, Rook KS. Spouse control and type 2 diabetes management: moderating effects of dyadic expectations for spouse involvement. Fam Relat. 2012;61:698–709. doi: 10.1111/j.1741-3729.2012.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black DR, Gleser LJ, Kooyers KJ. A meta-analytic evaluation of couples weight-loss programs. Health Psychol. 1990;9:330–47. doi: 10.1037//0278-6133.9.3.330. [DOI] [PubMed] [Google Scholar]
- 12.Huang XL, Pan JH, Chen D. et al. Efficacy of lifestyle interventions in patients with type 2 diabetes: A systematic review and meta-analysis. Eur J Intern Med. 2016;27:37–47. doi: 10.1016/j.ejim.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Cezaretto A, Ferreira SR, Sharma S. et al. Impact of lifestyle interventions on depressive symptoms in individuals at-risk of, or with, type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2016;26:649–62. doi: 10.1016/j.numecd.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Htoo ZW, Hsu WW, Rosenkranz R. Systematic review and meta-analysis: Is lifestyle modification effective for glycemic control among adults with type II diabetes in Southeast Asia? Diabetes Res Clin Pract. 2016;122:148–53.. doi: 10.1016/j.diabres.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Norris SL, Lau J, Smith SJ. et al. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–71. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 16.Schellenberg ES, Dryden DM, Vandermeer B. et al. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:543–51. doi: 10.7326/0003-4819-159-8-201310150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Vermeire E, Wens J, Van Royen P. et al. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;((2)) doi: 10.1002/14651858.CD003638.pub2. CD003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mumu SJ, Saleh F, Ara F. et al. Non-adherence to life-style modification and its factors among type 2 diabetic patients. Indian J Public Health. 2014;58:40–4. doi: 10.4103/0019-557X.128165. [DOI] [PubMed] [Google Scholar]
- 19.Khunti K, Ceriello A, Cos X, De Block C. Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: A meta-analysis. Diabetes Res Clin Pract. 2018;137:137–48. doi: 10.1016/j.diabres.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Amaltinga APM. Non adherence to diabetic medication among diabetic patients, a case study of Dormaa Hospital Ghana. Science Journal of Public Health. 2017;5:88–97. [Google Scholar]
- 21.Debussche X. Is adherence a relevant issue in the self-management education of diabetes? A mixed narrative review. Diabetes Metab Syndr Obes. 2014;7:357–67. doi: 10.2147/DMSO.S36369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299–307. doi: 10.2147/PPA.S106821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahola AJ, Groop PH. Barriers to self-management of diabetes. Diabet Med. 2013;30:413–20. doi: 10.1111/dme.12105. [DOI] [PubMed] [Google Scholar]
- 24.Vermeire E, Hearnshaw H, Rdtsep A. et al. Obstacles to adherence in living with type-2 diabetes: an international qualitative study using meta-ethnography (EUROBSTACLE). Prim Care Diabetes. 2007;1:25–33. doi: 10.1016/j.pcd.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Hilliard ME, Powell PW, Anderson BJ. Evidence-based behavioral interventions to promote diabetes management in children, adolescents, and families. Am Psychol. 2016;71:590–601. doi: 10.1037/a0040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohal T, Sohal P, King-Shier KM, Khan NA. Barriers and facilitators for type-2 diabetes management in South Asians: a systematic review. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136202. e0136202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venditti EM, Tan K, Chang N. et al. TODAY Study Group. Barriers and strategies for oral medication adherence among children and adolescents with Type 2 diabetes. Diabetes Res Clin Pract. 2018;139:24–31. doi: 10.1016/j.diabres.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trevisan DD, Sco-Joco TM, Cornilio ME. et al. A randomized controlled trial on the effect of behavioral strategies for adherence to oral antidiabetic drugs: study protocol. Contemp Nurse. 2017;53:658–68. doi: 10.1080/10376178.2017.1421862. [DOI] [PubMed] [Google Scholar]
- 29.Jaworski M, Panczyk M, Cedro M, Kucharska A. Adherence to dietary recommendations in diabetes mellitus: disease acceptance as a potential mediator. Patient Prefer Adherence. 2018;12:163–74. doi: 10.2147/PPA.S147233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kavitha S, Nalini GK, Suresh RM. et al. Treatment adherence and factors contributing to non adherence among type 2 diabetes mellitus patients in a tertiary care hospital: a cross sectional study. Int J Basic Clin Pharmacol. 2017;6:689–94. [Google Scholar]
- 31.Pereira MG, Pedras S, Ferreira G, Machado JC. Family and couple variables regarding adherence in type 2 diabetes patients in the initial stages of the disease. J Marital Fam Ther. 2019;45:134–48. doi: 10.1111/jmft.12281. [DOI] [PubMed] [Google Scholar]
- 32.Van Buren DJ, Wilfley DE, Marcus MD. et al. TODAY Study Group. Depressive symptoms and glycemic control in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Res Clin Pract. 2018;135:85–7. doi: 10.1016/j.diabres.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seamark D, Gabriel L. Barriers to support: a qualitative exploration into the help-seeking and avoidance factors of young adults. Br J Guid Counc. 2018;46:120–31. [Google Scholar]
- 34.Pereira MG, Ferreira G, Machado JC. Spousal support and satisfaction with healthcare services as moderators between psychological morbidity and adherence to diet in type 2 diabetes patients. Health Behav Policy Rev. 2014;1:278–89. [Google Scholar]
- 35.Denham SA. Family routines: a structural perspective for viewing family health. Adv Nurs Sci. 2002;24:60–74. doi: 10.1097/00012272-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Grabowski D, Andersen TH, Varming A. et al. Involvement of family members in life with type 2 diabetes: Six interconnected problem domains of significance for family health identity and healthcare authenticity. SAGE Open Med. 2017;5 doi: 10.1177/2050312117728654. 2050312117728654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vongmany J, Luckett T, Lam L, Phillips JL. Family behaviours that have an impact on the self-management activities of adults living with Type 2 diabetes: a systematic review and meta-synthesis. Diabet Med. 2018;35:184–94. doi: 10.1111/dme.13547. [DOI] [PubMed] [Google Scholar]
- 38.Baig AA, Benitez A, Quinn MT, Burnet DL. Family interventions to improve diabetes outcomes for adults. Ann N Y Acad Sci. 2015;1353:89–112.. doi: 10.1111/nyas.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markle-Reid M, Ploeg J, Fraser KD. et al. The ACHRU-CPP versus usual care for older adults with type-2 diabetes and multiple chronic conditions and their family caregivers: study protocol for a randomized controlled trial. Trials. 2017;18:55.. doi: 10.1186/s13063-017-1795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wichit N, Mnatzaganian G, Courtney M. et al. Randomized controlled trial of a family-oriented self-management program to improve self-efficacy, glycemic control and quality of life among Thai individuals with type 2 diabetes. Diabetes Res Clin Pract. 2017;123:37–48. doi: 10.1016/j.diabres.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Pamungkas RA, Chamroonsawasdi K, Vatanasomboon P.. A systematic review: family support integrated with diabetes self-management among uncontrolled type II diabetes mellitus patients. Behav Sci (Basel). 2017;7:62.. doi: 10.3390/bs7030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennich BB, Rxder ME, Overgaard D. et al. Supportive and non-supportive interactions in families with a type 2 diabetes patient: an integrative review. Diabetol Metab Syndr. 2017;9:57.. doi: 10.1186/s13098-017-0256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayberry LS, Osborn CY. Family support, medication adherence, and glycemic control among adults with type 2 diabetes. Diabetes Care. 2012;35:1239–45. doi: 10.2337/dc11-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson MD, Anderson JR, Walker A. et al. Spousal overprotection is indirectly associated with poorer dietary adherence for patients with type 2 diabetes via diabetes distress when active engagement is low. Br J Health Psychol. 2015;20:360–73. doi: 10.1111/bjhp.12105. [DOI] [PubMed] [Google Scholar]
- 45.Keogh KM, White P, Smith SM. et al. Changing illness perceptions in patients with poorly controlled type 2 diabetes, a randomised controlled trial of a family-based intervention: protocol and pilot study. BMC Fam Pract. 2007;8:36.. doi: 10.1186/1471-2296-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vissenberg C, Nierkens V, Uitewaal PJM. et al. Development of the social network-based intervention “powerful together with diabetes” using intervention mapping. Front Public Health. 2017;5:334.. doi: 10.3389/fpubh.2017.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samuel-Hodge CD, Cene CW, Corsino L. et al. Family diabetes matters: a view from the other side. J Gen Intern Med. 2013;28:428–35. doi: 10.1007/s11606-012-2230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. Int J Obes (Lond). 2010;34:217–26. doi: 10.1038/ijo.2009.195. TODAY Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed Z, Yeasmeen F. Active family participation in diabetes self-care: a commentery. Diabetes Management. 2016;6:104–7. [Google Scholar]
- 50.Dutta D K, Biswas M, Sharma. et al. Managing diabetes during Navratris with special focus on Durga pujas. Journal of Social Health & Diabetes. 2015;3:84–8. [Google Scholar]
- 51.Chatterjee JS. From compliance to concordance in diabetes. J Med Ethics. 2006;32:507–10. doi: 10.1136/jme.2005.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Sharbatti SS, Abed YI, Al-Heety LM, Basha SA. Spousal concordance of diabetes mellitus among women in Ajman, United Arab Emirates. Sultan Qaboos Univ Med J. 2016;16:e197–202.. doi: 10.18295/squmj.2016.16.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis MA, McBride CM, Pollak KI. et al. Understanding health behavior change among couples: an interdependence and communal coping approach. Soc Sci Med. 2006;62:1369–80. doi: 10.1016/j.socscimed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Pereira MG, Costa V, Oliveira D. et al. Patients’ and spouses’ contribution toward adherence to self-care behaviors in type 2 diabetes. Res Theory Nurs Pract. 2015;29:276–96. doi: 10.1891/1541-6577.29.4.276. [DOI] [PubMed] [Google Scholar]
- 55.Lin EH, Katon W, Von Korff M. et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–60. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 56.Trief PM, Sandberg J, Greenberg RP. et al. Describing support: A qualitative study of couples living with diabetes. Families, Systems, & Health. 2003;21:57–67. [Google Scholar]
- 57.August KJ, Rook KS, Stephens MA, Franks MM. Are spouses of chronically ill partners burdened by exerting health-related social control? J Health Psychol. 2011;16:1109–19.. doi: 10.1177/1359105311401670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Potier F, Degryse JM, Henrard S. et al. A high sense of coherence protects from the burden of caregiving in older spousal caregivers. Arch Gerontol Geriatr. 2018;75:76–82. doi: 10.1016/j.archger.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 59.Henry SL, Rook KS, Stephens MA, Franks MM. Spousal undermining of older diabetic patients’ disease management. J Health Psychol. 2013;18:1550–61. doi: 10.1177/1359105312465913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson MD, Anderson JR, Walker A. et al. Common dyadic coping is indirectly related to dietary and exercise adherence via patient and partner diabetes efficacy. J Fam Psychol. 2013;27:722–30. doi: 10.1037/a0034006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franks MM, Sahin ZS, Seidel AJ. Table for two: diabetes distress and diet-related interactions of married patients with diabetes and their spouses. Fam Syst Health. 2012;30:154–65. doi: 10.1037/a0028614. [DOI] [PubMed] [Google Scholar]
- 62.Stephens MA, Rook KS, Franks MM. et al. Spouses use of social control to improve diabetic patients’ dietary adherence. Fam Syst Health. 2010;28:199–208. doi: 10.1037/a0020513. [DOI] [PubMed] [Google Scholar]
- 63.Beverly EA, Miller CK, Wray LA. Spousal support and food-related behavior change in middle-aged and older adults living with type 2 diabetes. Health Educ Behav. 2008;35:707–20. doi: 10.1177/1090198107299787. [DOI] [PubMed] [Google Scholar]
- 64.Garay-Sevilla ME, Nava LE, Malacara JM. et al. Adherence to treatment and social support in patients with non-insulin dependent diabetes mellitus. J Diabetes Complications. 1995;9:81–6. doi: 10.1016/1056-8727(94)00021-f. [DOI] [PubMed] [Google Scholar]
- 65.Leong A, Rahme E, Dasgupta K. Spousal diabetes as a diabetes risk factor: a systematic review and meta-analysis. BMC Med. 2014;12:12.. doi: 10.1186/1741-7015-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang JY, Liu CS, Lung CH. et al. Investigating spousal concordance of diabetes through statistical analysis and data mining. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183413. e0183413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dimova ED, Mohan ARM, Swanson V, Evans JMM. Interventions for prevention of type 2 diabetes in relatives: A systematic review. Prim Care Diabetes. 2017;11:313–26. doi: 10.1016/j.pcd.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Trief PM, Fisher L, Sandberg J. et al. Health and psychosocial outcomes of a telephonic couples behavior change intervention in patients with poorly controlled type 2 diabetes: a randomized clinical trial. Diabetes Care. 2016;39:2165–73. doi: 10.2337/dc16-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trief P, Sandberg JG, Ploutz-Snyder R. et al. Promoting couples collaboration in type 2 diabetes: the diabetes support project pilot data. Fam Syst Health. 2011;29:253–61. doi: 10.1037/a0024564. [DOI] [PubMed] [Google Scholar]
- 70.Gilden JL, Hendryx M, Casia C, Singh SP. The effectiveness of diabetes education programs for older patients and their spouses. J Am Geriatr Soc. 1989;37:1023–30. doi: 10.1111/j.1532-5415.1989.tb06915.x. [DOI] [PubMed] [Google Scholar]
- 71.Leonard MT, Cano A, Johansen AB. Chronic pain in a couples context: a review and integration of theoretical models and empirical evidence. J Pain. 2006;7:377–90. doi: 10.1016/j.jpain.2006.01.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wadden TA, Bantle JP, Blackburn GL. et al. Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2014;22:5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salvia MG. The look AHEAD trial: translating lessons learned into clinical practice and further study. Diabetes Spectr. 2017;30:166–170. doi: 10.2337/ds17-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lean ME, Leslie WS, Barnes AC. et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541–51. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]


