Abstract
Objectives
SS is characterized by chronic inflammation of the salivary glands leading to loss of secretory function, thereby suggesting specialized pro-resolving mediators targeting inflammation to be a viable option for treating SS. Previous studies demonstrated that aspirin-triggered resolvin D1 (AT-RvD1) prevents chronic inflammation and enhances saliva secretion in a SS-like mouse model when applied before disease onset. However, this therapy cannot be used in SS patients given that diagnosis occurs post-disease onset and no reliable screening methods exist. Therefore, we examined whether treatment with AT-RvD1 reduces SS-like features in a mouse model post-disease onset.
Methods
Tail vein injections were performed in a SS-like mouse model both with and without AT-RvD1 post-disease onset for 8 weeks, with salivary gland function and inflammatory status subsequently determined.
Results
Treatment of a SS-like mouse model with AT-RvD1 post-disease onset restores saliva secretion in both females and males. Moreover, although AT-RvD1 treatment does not reduce the overall submandibular gland lymphocytic infiltration, it does reduce the number of T helper 17 cells within the infiltrates in both sexes. Finally, AT-RvD1 reduces SS-associated pro-inflammatory cytokine gene and protein expression levels in submandibular glands from female but not male mice.
Conclusion
AT-RvD1 treatment administered post-disease onset reduces T helper 17 cells and successfully restores salivary gland function in a SS mouse model with variable effects noted by sex, thus warranting further examination of both the causes for the sex differences and the mechanisms responsible for the observed treatment effect.
Keywords: salivary glands, lipid mediators, resolvins, lipoxins, formyl peptide receptor 2, cytokines, lymphocyte, interleukin-17
Rheumatology key messages
Aspirin-triggered resolvin D1 treatment post-disease onset restores saliva secretion in SS-like mice.
Aspirin-triggered resolvin D1 treatment reduces Th17+ cell number in submandibular gland from SS-like mice.
Aspirin-triggered resolvin D1 treatment reduces pro-inflammatory cytokine expression in submandibular gland from female SS-like mice.
Introduction
SS is a systemic autoimmune disease characterized by dry eyes and dry mouth [1]. It is classified either as primary (i.e. when it occurs by itself) or secondary (i.e. when it is associated with another autoimmune disease such as lupus or rheumatoid arthritis), with both forms containing serum autoantibodies [2]. Prevalence estimates range from 2 to 10 per 100 000 [3, 4] with associated complications ranging from caries and candidiasis to infection of the parotid gland as well as parotid tumours and lymphomas [5, 6]. Together, these issues impose significant physical, psychological and economic burdens on patients with SS [7]. Although extensive investigation has been carried out to understand SS, the causes of the disease are still unknown and adequate therapies are lacking [8–10]. Specifically, current interventions include saliva substitutes with limited effectiveness and medications (e.g. pilocarpine, cevimeline) that provide only temporary relief [11]. In light of the high degree of need and the limitations of current therapies, development of novel treatments to restore salivary gland function is essential.
Uncontrolled inflammation is now recognized as a unifying component in many chronic conditions including vascular diseases, metabolic syndrome, neurological diseases, chronic pain and SS among others [12–14]. Although treatment of inflammation is nothing new, traditionally it has involved only inhibition of factors that drive inflammation, including vasoactive amines, eicosanoids, cytokines, chemokines and cell adhesion molecules, as is the case when ibuprofen is administered [15]. This form of intervention dampens inflammation but also limits the degree of healing that may occur [15]. By contrast, specialized pro-resolving lipid mediators (SPM) diminish inflammation while actually promoting natural healing processes by blocking inflammatory cytokines and at the same time accelerating tissue repair, thereby leading to more complete recovery [16]. With their discovery by Charles Serhan more than a decade ago, SPM emerged as a powerful new paradigm for inflammation resolution, with recent results demonstrating benefits for a host of conditions ranging from bacterial infection to chronic inflammation and compromised immunity [17].
Our previous studies demonstrated that mouse and human salivary glands express the functional machinery for biosynthesizing the SPM family member resolvin D1 (RvD1) [18]. Moreover, RvD1 as well as its aspirin-triggered epimer (AT-RvD1) activate their receptor, formyl peptide receptor 2 (ALX/FPR2), to increase calcium concentration and AKT signalling in salivary gland cells [19, 20]. In turn, these pathways block cytokine-mediated caspase-3 activation and increase of cell survival, tissue migration and epithelial integrity [19]. More recently, we demonstrated that AT-RvD1 prevents chronic inflammation and enhances saliva secretion in a SS-like mouse model when applied before disease onset [21]. However, this therapy cannot be used in SS patients given that diagnosis occurs post-disease onset and no reliable screening methods exist [22]. Therefore, we determined whether treatment with AT-RvD1 reduces SS-like features in a mouse model post-disease onset. Our study demonstrates that treatment of a SS-like mouse model with AT-RvD1 post-disease onset restores saliva secretion in both females and males. Moreover, although AT-RvD1 treatment does not reduce the overall submandibular gland (SMG) lymphocytic infiltration, it does reduce the number of T helper 17 cells within the infiltrates as well as SS-associated cytokine expression levels in SMG from female mice. In conclusion, AT-RvD1 treatment administered post-disease onset reduces T helper 17 cells and successfully restores salivary gland function in a SS mouse model with variable effects noted by sex, thus warranting further examination of both the causes of the sex differences and the mechanisms responsible for the observed treatment effect.
Methods
Animals
All animal studies were performed under protocols approved by the University of Utah Institutional Animal Care and Use Committee. NOD/ShiLtJ mice (SS-like mouse model) at 12 weeks (females) and 16 weeks (males) of age totalling 10 animals per group were treated twice a week for 8 weeks via tail vein injections with EtOH (3.5%, vehicle control) or AT-RvD1 (0.1 mg/kg, Cayman Chemical, Ann Arbor, MI, USA), with doses [23] and ages chosen on the basis of previous studies indicating disease onset in the SS-like mouse model to vary by sex [21]. Female mice at 20 weeks of age and male mice at 24 weeks of age were euthanized using 80–100 mg/kg ketamine + 5 mg/kg xylazine followed by abdominal exsanguination. SMG were then removed and fixed in formalin or treated with either RNAlater (Thermo Fisher Scientific, Waltham, MA, USA) or RIPA buffer with Triton X-100 (Alfa Aesar, Ward Hill, MA, USA) for the experiments described below.
Measurement of stimulated salivary flow rate
Mice were anaesthetized with 80–100 mg/kg ketamine + 5 mg/kg xylazine via an intraperitoneal injection with a combination of pilocarpine-HCl/PBS (40 mg/kg) and isoproterenol (0.4 mg/kg) to stimulate saliva secretion. Then, saliva was collected for 5 min using a 200 μl pipette. Total saliva weight was measured using a microbalance (Mettler Toledo, Columbus, OH, USA) while volume of saliva was measured using a pipette (Eppendorf AG, Hauppauge, NY, USA). Statistical results were analysed as described below.
Histopathological evaluation
Tissue sections were stained with haematoxylin and eosin (H&E) and visualized on a Leica DMI6000B inverted microscope with a grading scale of 0–4 to assess the degree of lymphocytic infiltration, as previously described [21]. Grades correspond to the number of foci (aggregate of 50 or more lymphocytes) per 4 mm2 as follows: 0: no infiltrates; 1: slight infiltrate; 2: moderate infiltrate or <1 focus; 3: one focus per 4 mm2; and 4: >1 focus per 4 mm2.
Immunofluorescence
Deparaffinization and antigen retrieval (Tris-EDTA with 0.05% Tween 20) of 5 μm-thick paraffin-embedded mouse SMG sections were performed, as previously described [24, 25]. SMG sections from various treatment groups were then blocked in 5% goat serum in phosphate buffered saline with 0.05% Tween-20 (PBST) for 1 h at room temperature and incubated overnight at 4°C with rat anti-CD4 Alexa Fluor 488 (Bio-Rad, Hercules, CA, USA) and rabbit anti-retinoic acid receptor-related orphan receptor γ (RORγ; Abcam) antibodies at 1: 250 dilutions in 5% goat serum (PBST). Specimens were then incubated for 1 h with anti-rabbit Alexa Fluor 568 secondary antibodies at 1: 250 dilutions in 5% goat serum (PBST). Subsequently, sections were stained for 5 min with TO-PRO-3 Iodide nuclear stain (Invitrogen) at 1: 1000 dilutions. Finally, specimens were analysed using a confocal Zeiss LSM 700 microscope (Carl Zeiss, Oberkochen, Germany) at ×20 magnifications. A total depth of 5 μm was acquired for each sample and a total projection was visualized in the xy planes.
Quantitative polymerase chain reaction
A detailed cDNA preparation can be obtained from a previous study [21]. Total cDNA from SMG was diluted at 1: 50 ratios and used as templates for quantitative polymerase chain reaction (qPCR). Reactions were then carried out by adding 2.5 μl (stock 10 μm) of each primer, consistent with procedures from a previously published study [21], as follows: β-actin forward: 5′-GTAACAATGCCATGTTCAAT-3′; β-actin reverse: 5′-CTCCATCGTGGGCCGCTCTAG-3′; interferon-γ forward: 5′-TTCTTCAGCAACAGCAAGGC-3′; interferon-γ reverse: 5′-TCAGCAGCGACTCCTTTTCC-3′; interleukin-17 forward: 5′-TCTCCACCGCAATGAAGACC-3′; interleukin-17 reverse: 5′-CACACCCACCAGCATCTTCT-3′; interleukin-1β forward: 5′-GCACTACAGGCTCCGAGATGAAC-3′; interleukin-1β reverse: 5′-TTGTCGTTGCTTGGTTCTCCTTGT-3′; transforming growth factor β1 forward: 5′-CACCGGAGAGCCCTGGATA-3′; transforming growth factor β1 reverse: 5′-TGTACAGCTGCCGCACACA-3′ – with 5 μl of 1: 50 cDNA dilutions and 10 μl of 2× SYBR Green master mixes (Bio-Rad). Finally, PCR experiments were performed on 96-well plates and relative fold changes of gene expression normalized using β-actin.
Western blot analysis
Protein lysates from mouse SMG were obtained using RIPA buffer containing a cocktail of protease inhibitors (Sigma-Aldrich, St Louis, MO, USA) and dissociated using a Sonic Dismembrator (Thermo Fisher Scientific, Waltham, MA, USA). Samples were centrifuged at 12 000 g for 10 min to remove cellular debris and protein concentration determined using a detection kit (Thermo Fisher Scientific, Waltham, MA, USA) and denatured by boiling in Laemmli buffer for 7 min; 50 μg of total protein was then loaded in a gradient 4–15% SDS-PAGE gel and transferred to a nitrocellulose membrane (Bio-Rad). At this point, membranes were incubated with 30% hydrogen peroxide for 30 min and blocked for 1 h at room temperature using 3% BSA in Tris-buffered saline (0.137 M NaCl, 0.025 M Tris, pH 7.4) containing 0.1% Tween-20 (TBST) and immunoblotted overnight at 4°C with the following primary antibodies in TBST containing 3% BSA at a 1: 500 dilution: rabbit anti-β-actin, rabbit anti-TGFβ1, rabbit anti-IL-17, rabbit anti-IFNγ and rabbit anti-IL-1β (all from Abcam, Cambridge, UK). After incubation, membranes were washed three times for 10 min each with TBST, incubated with peroxidase-linked goat anti-rabbit IgG antibody (Cell Signalling Technology, Danvers, MA, USA) and diluted 1: 5000 in TBST containing 3% BSA at 4°C overnight. Membranes were then washed three times for 10 min each with TBST and treated with a Clarity™ detection reagent (Bio-Rad). The protein bands were subsequently visualized using a ChemiDoc™ MP imager and band intensities quantified using Image Lab 4.1 software (Bio-Rad), with β-actin used for protein normalization. Finally, relative protein expression level was calculated as the ratio of target proteins to β-actin.
Data handling and statistics
Data presented are mean ± S.D. and were analysed using Prism (GraphPad Software, Inc., San Diego, CA, USA) and Student’s t-test, and P-values ≤0.05 indicate significant difference.
Results
Treatment with AT-RvD1 at disease onset restores saliva flow rates
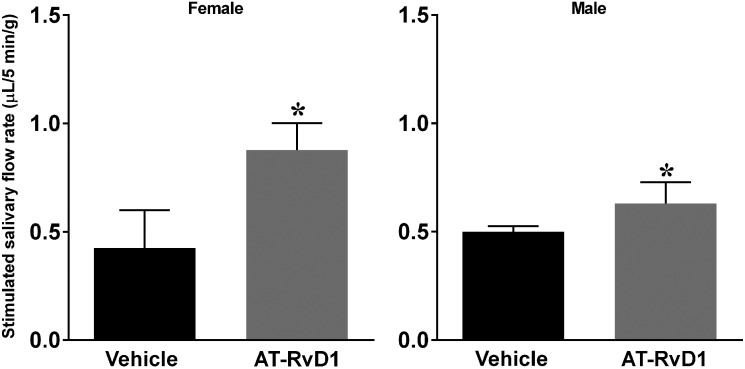
To determine the effects of AT-RvD1 treatment on saliva flow rates in the SS mouse model at disease onset (i.e. displaying salivary gland hypofunction), animals were treated as described in ‘Methods’. Results show that both female and male mice treated with AT-RvD1 showed a significant increase in saliva flow rates as compared with vehicle-treated controls (Fig. 1), thus indicating AT-RvD1 to be effective in promoting saliva secretion in both sexes.
Fig. 1.
Treatment with AT-RvD1 post-disease onset restored saliva flow rates in SS-like mice
Mice were treated as described in ‘Methods’. Whole saliva was then collected using an intraperitoneal injection with pilocarpine (40 mg/kg) and isoproterenol (0.4 mg/kg). Results are representative of n = 5 mice/condition and data expressed as mean ± SD, with *P < 0.05 indicating a significant difference from controls. AT-RvD1: aspirin-triggered resolvin D1.
Treatment with AT-RvD1 at disease onset does not affect lymphocytic infiltration
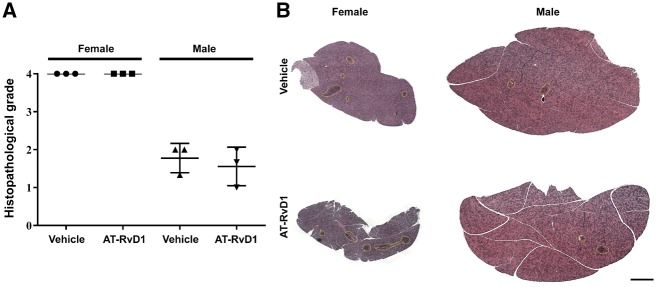
To determine the degree of lymphocytic infiltrations in AT-RvD1-treated SS-like mice at disease onset (female at 12 weeks and male at 16 weeks), SMG tissue was stained with H&E and histopathological analysis performed, as described in ‘Methods’. SMG from AT-RvD1-treated and untreated female mice showed a grade 4 lymphocytic infiltration, while male mice showed a grade 2 in both conditions (Fig. 2), thus indicating that systemic AT-RvD1 treatment has no appreciable impact on general immune cell infiltration in either female or male SS-like mouse SMG as these values remained constant in pre- and post-treatment measures.
Fig. 2.
Treatment with AT-RvD1 post-disease onset did not affect lymphocytic infiltration in SS-like mice
Mice were treated as described in ‘Methods’ and SMG were then harvested, sectioned, stained with H&E and graded as described in ‘Methods’. (A) Each point in the graph represents the average of the grades taken at three levels from a single mouse SMG (i.e. top, middle and bottom), with a grade of 3 indicating severe lymphocytic infiltration in SMG. (B) Lymphocytic foci are shown by yellow dotted lines; scale bar: 1000 μm. Results are representative of n = 3 mice/condition. AT-RvD1: aspirin-triggered resolvin D1.
Treatment with AT-RvD1 at disease onset decreases the number of Th17 cells
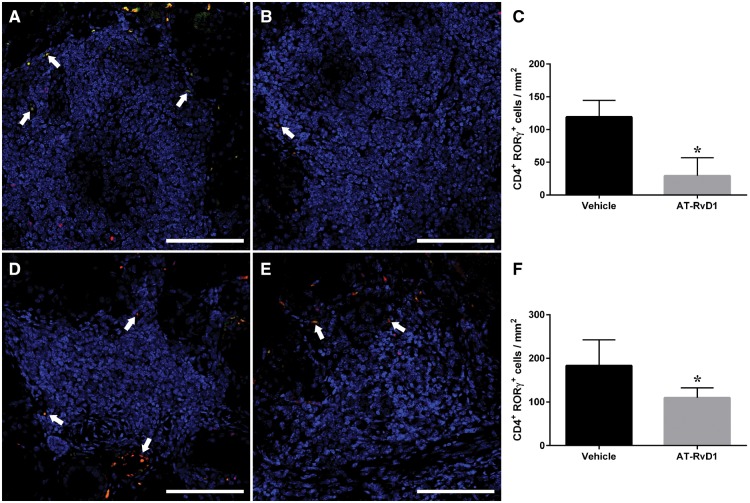
In light of the lack of general immune effect noted above, we examined the presences of specific T cell populations within SMG infiltrates as an alternative approach for demonstrating a possible effect of AT-RvD1 on immunity. In particular, we quantified the amount of Th17 cells within the infiltrates, with results indicating that expression of the Th17 cell-specific markers RORγ and CD4 was significantly reduced in SS-like mice treated with AT-RvD1 (Fig. 3B, E) as compared with the vehicle group (Fig. 3A, D). These findings suggest that treatment with AT-RvD1 limits infiltration of a specific SS-associated cell population (i.e. Th17) in SMG from both sexes.
Fig. 3.
Treatment with AT-RvD1 significantly reduced infiltrated Th17 cells in SMG from SS-like mice
(A, B, D, E) SMG from mice treated with vehicle control (A, female; D, male) and AT-RvD1 (B, female; E, male) were harvested, formalin-fixed, paraffin-embedded and sectioned. Th17 cells were then detected with the rat anti-CD4 (green) and rabbit anti-RORγ (red) antibodies and TO-PRO-3 iodide as a nucleic acid stain (blue) and images analysed using confocal microscopy. Representative fluorescence images from n = 5 are shown with white arrows indicating Th17 cells. (C, F) CD4+ and RORγ+ cells within the foci were quantified (C, female; F, male) and expressed as mean ± SD, with *P < 0.05 indicating a significant difference from vehicle controls. Scale bars: 100 μm. AT-RvD1: aspirin-triggered resolvin D1; SMG: submandibular gland.
Treatment with AT-RvD1 at disease onset decreases pro-inflammatory cytokine expression in female mice
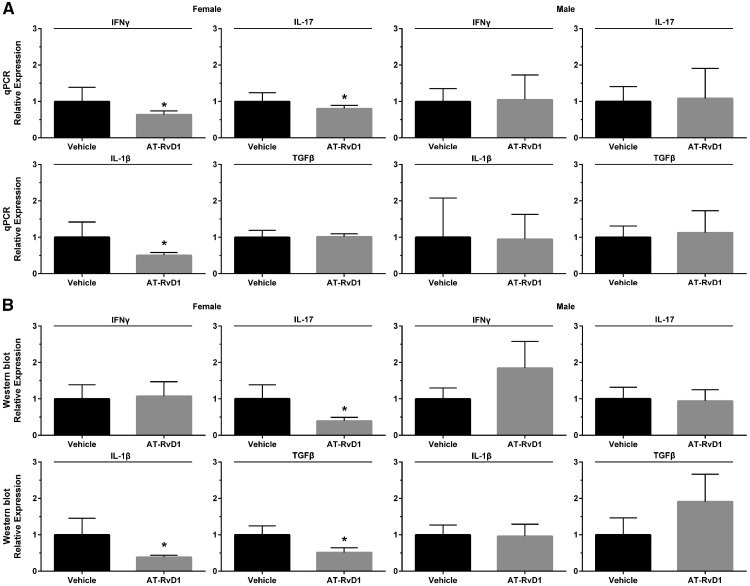
Upregulation of pro-inflammatory cytokines (e.g. IL-1β, IL-17 and IFNγ) has been previously observed in serum and salivary glands from both humans with SS and SS-like mice [26–29]. To determine whether treatment of AT-RvD1 reduced levels of these cytokines post-disease onset (female at 12 weeks and male at 16 weeks), quantitative PCR and western blot analyses were performed in SMG from both sexes of SS-like mice. As shown in Fig. 4A, SMG from 20-week female SS-like mice treated with AT-RvD1 displayed a significant gene downregulation of IL-1β, IL-17 and IFNγ as compared with vehicle-treated controls. In contrast, SMG from 24-week male NOD/ShiLtJ mice treated with AT-RvD1 show no such changes (Fig. 4). Consequently, AT-RvD1 treatment has been demonstrated to be effective in diminishing SS-associated cytokine gene expression in SMG from female mice but has as yet shown no significant effect in the male cohort. As shown in Fig. 4B, SMG from 20-week female SS-like mice treated with AT-RvD1 displayed a significant protein downregulation of IL-1β, IL-17 and TGFβ as compared with vehicle-treated controls. In contrast, SMG from 24-week male NOD/ShiLtJ mice treated with AT-RvD1 did not display these same effects (Fig. 4B). Consequently, AT-RvD1 treatment was noted to diminish SS-associated cytokine protein expression in SMG from female mice but has as yet shown no comparable effect in the male cohort.
Fig. 4.
Treatment with AT-RvD1 significantly downregulated pro-inflammatory cytokine gene expression in SMG from female SS-like mice
Mice were treated as described in ‘Methods’. SMG were then removed, with RNA subsequently extracted and processed for qPCR and western blot detection of IL-1β, IL-17, IFNγ and TGFβ. Results are representative of n = 7 mice/condition for PCR and 4 mice/condition for western blot. Data are expressed as mean ± SD, with *P < 0.05 indicating a significant difference from controls. AT-RvD1: aspirin-triggered resolvin D1; SMG: submandibular gland.
Discussion
SS is characterized by uncontrolled inflammation with T and B lymphocyte infiltration into the salivary glands [30–33]. Specifically, these inflammatory cells contribute to type I interferons and toll-like receptor activation leading to disruption of salivary epithelial integrity and consequent hypofunction [34–37]. The current treatment for this condition involves the use of anti-inflammatory drugs (e.g. glucocorticoids) that inhibit the heat and pain for temporal relief; however, this therapy may suppress resolution of inflammation [38]. Therefore, targeting the natural resolution pathways using SPM [39, 40] appears to be the best alternative to control inflammation and regulate the immune system in SS.
In this study, we demonstrate that systemic treatment of both female and male SS-like mice with AT-RvD1 post-disease onset restores salivary gland functioning (Fig. 1). Regarding this effect, previous studies showed that cholinergic signalling is disrupted in salivary glands with SS [6, 41–43] and such reduction has been associated with poor SPM production. Specifically, vagotomy reduces local pro-resolving mediators, thereby delaying resolution and the ability of nerves to regulate local expression of netrin-1, an axonal guidance molecule that activates resolution of inflammation [44]. However, future studies will be necessary to determine whether AT-RvD1 increases salivary gland secretion via local neural control of resolution.
AT-RvD1 did not have a significant impact on SMG lymphocytic infiltration in either sex (Fig. 2). However, AT-RvD1 was noted to cause a reduction of a subset of infiltrating lymphocytes (i.e. Th17+ cells) in both female and male SMG (Fig. 3). This may be a significant observation in light of previous studies demonstrating that Th17 cells and their secretory product IL-17 are critical for the induction of SS-like features in mouse models [45] and are present in plasma and glandular tissue of SS patients [29]. Moreover, IL-17 derived from infiltrating lymphocytes has been shown to impair the integrity of the TJ barrier through the NF-κB signalling pathway, which in turn may contribute to salivary gland dysfunction in SS [35–37, 46].
Furthermore, AT-RvD1 treatment affected a set of pro-inflammatory cytokines that are upregulated in both plasma and salivary glands from SS mouse models and patients [26–28, 32]. Particularly, AT-RvD1 treatment diminished SS-associated cytokine gene expression (i.e. downregulation of IL-1β, IL-17 and IFNγ) in SMG from female mice but showed no comparable effect in male mice. Regarding the lack of AT-RvD1 effect on SS-associated pro-inflammatory cytokine expression levels in male mice, previous studies showed that IL-17 modulates cytokine production with a greater impact in female than in male SS-like mice [47]. As for the importance of AT-RvD1 for signalling, our previous studies demonstrated that loss of its corresponding receptor ALX/FPR2 in mice (i.e. Alx/Fpr2−/−) caused an exacerbated inflammatory response to lipopolysaccharides as compared with wild type controls [48]. More recently, we demonstrated that these mice spontaneously develop a significant reduction of saliva flow rates, upregulation of B cells and autoantibody production as compared with controls [49], thus indicating that the AT-RvD1 receptor ALX/FPR2 helps to regulate both innate and adaptive immunity in salivary glands.
In summary, the current study demonstrates that systemic treatment of SS-like mice with AT-RvD1 post-disease onset restores saliva secretion in SS-like mice and decreases a subset of immune cells (Th17). These studies build on previous studies that established our ability to prevent disease progression in a SS mouse model; however, these findings were arguably of somewhat limited utility given our current inability to screen for SS in humans. By contrast, the current findings offer the possibility of halting disease progression once SS has manifested. However, further studies are warranted to understand the varying clinical presentation by sex, to address the problem of lymphocytic infiltration in both female and male mice and better understand the mechanisms underlying the observed treatment effect.
Funding: This study was supported by the National Institutes of Health-National Institute of Dental and Craniofacial Research Grants R56DE021697, R01DE022971 and R01DE027884 (to O.J.B.).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Grossman S, Tagliavini LB. Managing Sjogren’s syndrome. Home Healthc Now 2015;33:487–92. [DOI] [PubMed] [Google Scholar]
- 2. Baldini C, Talarico R, Tzioufas AG, Bombardieri S. Classification criteria for Sjogren’s syndrome: a critical review. J Autoimmun 2012;39:9–14. [DOI] [PubMed] [Google Scholar]
- 3. Maciel G, Crowson CS, Matteson EL, Cornec D. Incidence and mortality of physician-diagnosed primary Sjogren syndrome: time trends over a 40-year period in a population-based US cohort. Mayo Clin Proc 2017;92:734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maciel G, Crowson CS, Matteson EL, Cornec D. Prevalence of primary Sjogren’s syndrome in a US population-based cohort. Arthritis Care Res (Hoboken) 2017;69:1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. González S, Sung H, Sepúlveda D, González M, Molina C. Oral manifestations and their treatment in Sjogren’s syndrome. Oral Dis 2014;20:153–61. [DOI] [PubMed] [Google Scholar]
- 6. Mathews SA, Kurien BT, Scofield RH. Oral manifestations of Sjogren’s syndrome. J Dent Res 2008;87:308–18. [DOI] [PubMed] [Google Scholar]
- 7. Segal B, Bowman SJ, Fox PC, et al. Primary Sjogren’s syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes 2009;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fox RI, Stern M. Sjogren’s syndrome: mechanisms of pathogenesis involve interaction of immune and neurosecretory systems. Scand J Rheumatol Suppl 2002;116:3–13. [PubMed] [Google Scholar]
- 9. Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjogren’s syndrome. Nat Rev Rheumatol 2013;9:544–56. [DOI] [PubMed] [Google Scholar]
- 10. Sandhya P, Kurien BT, Danda D, Scofield RH. Update on pathogenesis of Sjogren’s syndrome. Curr Rheumatol Rev 2017;13:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daniels TE, Fox PC. Salivary and oral components of Sjogren’s syndrome. Rheum Dis Clin North Am 1992;18:571–89. [PubMed] [Google Scholar]
- 12. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 2018;128:2657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med 2017;58:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J 2017;31:1273–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haupt MT, Jastremski MS, Clemmer TP, Metz CA, Goris GB. Effect of ibuprofen in patients with severe sepsis: a randomized, double-blind, multicenter study. The Ibuprofen Study Group. Crit Care Med 1991;19:1339–47. [DOI] [PubMed] [Google Scholar]
- 16. Keinan D, Leigh NJ, Nelson JW, De Oleo L, Baker OJ. Understanding resolvin signaling pathways to improve oral health. Int J Mol Sci 2013;14:5501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leigh NJ, Nelson JW, Mellas RE, Aguirre A, Baker OJ. Expression of resolvin D1 biosynthetic pathways in salivary epithelium. J Dent Res 2014;93:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson JW, Leigh NJ, Mellas RE, et al. ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am J Physiol Cell Physiol 2014;306:C178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin D1 prevents TNF-alpha-mediated disruption of salivary epithelial formation. Am J Physiol Cell Physiol 2012;302:C1331–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang CS, Maruyama CL, Easley JT, Trump BG, Baker OJ. AT-RvD1 promotes resolution of inflammation in NOD/ShiLtJ mice. Sci Rep 2017;7:45525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fox RI, Tornwall J, Michelson P. Current issues in the diagnosis and treatment of Sjogren’s syndrome. Curr Opin Rheumatol 1999;11:364–71. [DOI] [PubMed] [Google Scholar]
- 23. Easley JT, Nelson JW, Mellas RE, et al. Aspirin-triggered resolvin D1 versus dexamethasone in the treatment of Sjogren’s syndrome-like NOD/ShiLtJ mice – a pilot study. J Rheum Dis Treat 2015;1:027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nam K, Maruyama CL, Wang CS, et al. Laminin-111-derived peptide conjugated fibrin hydrogel restores salivary gland function. PLoS One 2017;12:e0187069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nam K, Wang CS, Maruyama CLM, et al. L1 peptide-conjugated fibrin hydrogels promote salivary gland regeneration. J Dent Res 2017;96:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren’s syndrome. J Immunol 1994;152:5532–9. [PubMed] [Google Scholar]
- 27. Garcic-Carrasco M, Font J, Filella X, et al. Circulating levels of Th1/Th2 cytokines in patients with primary Sjogren’s syndrome: correlation with clinical and immunological features. Clin Exp Rheumatol 2001;19:411–5. [PubMed] [Google Scholar]
- 28. Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren’s syndrome: findings in humans and mice. Arthritis Rheum 2008;58:734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjogren’s syndrome immunopathogenesis. Am J Pathol 2009;175:1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luciano N, Valentini V, Calabro A, et al. One year in review 2015: Sjogren’s syndrome. Clin Exp Rheumatol 2015;33:259–71. [PubMed] [Google Scholar]
- 31. Price EJ, Venables PJ. The etiopathogenesis of Sjogren’s syndrome. Semin Arthritis Rheum 1995;25:117–33. [DOI] [PubMed] [Google Scholar]
- 32. Robinson CP, Cornelius J, Bounous DE, et al. Characterization of the changing lymphocyte populations and cytokine expression in the exocrine tissues of autoimmune NOD mice. Autoimmunity 1998;27:29–44. [DOI] [PubMed] [Google Scholar]
- 33. Talal N, Sylvester RA, Daniels TE, Greenspan JS, Williams RC Jr. T and B lymphocytes in peripheral blood and tissue lesions in Sjogren’s syndrome. J Clin Invest 1974;53:180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nandula SR, Scindia YM, Dey P, Bagavant H, Deshmukh US. Activation of innate immunity accelerates sialoadenitis in a mouse model for Sjogren’s syndrome-like disease. Oral Dis 2011;17:801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang LW, Cong X, Zhang Y, et al. Interleukin-17 impairs salivary tight junction integrity in Sjogren’s syndrome. J Dent Res 2016;95:784–92. [DOI] [PubMed] [Google Scholar]
- 36. Baker OJ, Camden JM, Redman RS, et al. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am J Physiol Cell Physiol 2008;295:C1191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baker OJ, Schulz DJ, Camden JM, et al. Rat parotid gland cell differentiation in three-dimensional culture. Tissue Eng Part C Methods 2010;16:1135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 2011;335:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elajami TK, Colas RA, Dalli J, et al. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J 2016;30:2792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fredman G, Serhan CN. Specialized pro-resolving mediators: wiring the circuitry of effector immune and tissue homeostasis. Endodontic Topics 2011;24:39–58. [Google Scholar]
- 41. Imrich R, Alevizos I, Bebris L, et al. Predominant glandular cholinergic dysautonomia in patients with primary Sjögren’s syndrome. Arthritis Rheumatol 2015;67:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nauntofte B. Regulation of electrolyte and fluid secretion in salivary acinar cells. Am J Physiol 1992;263:G823–37. [DOI] [PubMed] [Google Scholar]
- 43. Rhodus NL, Schuh MJ. Effects of pilocarpine on salivary flow in patients with Sjogren’s syndrome. Oral Surg Oral Med Oral Pathol 1991;72:545–9. [DOI] [PubMed] [Google Scholar]
- 44. Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med 2014;211:1037–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verstappen GM, Corneth OBJ, Bootsma H, Kroese FGM. Th17 cells in primary Sjogren’s syndrome: pathogenicity and plasticity. J Autoimmun 2018;87:16–25. [DOI] [PubMed] [Google Scholar]
- 46. Ewert P, Aguilera S, Alliende C, et al. Disruption of tight junction structure in salivary glands from Sjogren’s syndrome patients is linked to pro-inflammatory cytokine exposure. Arthritis Rheum 2010;62:1280– 9. [DOI] [PubMed] [Google Scholar]
- 47. Voigt A, Esfandiary L, Wanchoo A, et al. Sexual dimorphic function of IL-17 in salivary gland dysfunction of the C57BL/6.NOD-Aec1Aec2 model of Sjogren’s syndrome. Sci Rep 2016;6:38717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang CS, Wee Y, Yang CH, Melvin JE, Baker OJ. ALX/FPR2 modulates anti-inflammatory responses in mouse submandibular gland. Sci Rep 2016;6:24244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang C-S, Baker OJ. The G-protein-coupled receptor ALX/Fpr2 regulates adaptive immune responses in mouse submandibular glands. Am J Pathol 2018;188:1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]