Abstract
Aims
Cardiac remodelling in the ischaemic heart determines prognosis in patients with ischaemic heart disease (IHD), while enhancement of angiogenesis and cell survival has shown great potential for IHD despite translational challenges. Phosphoinositide 3-kinase (PI3K)/Akt signalling pathways play a critical role in promoting angiogenesis and cell survival. However, the effect of PI3Kβ in the ischaemic heart is poorly understood. This study investigates the role of endothelial and cardiomyocyte (CM) PI3Kβ in post-infarct cardiac remodelling.
Methods and results
PI3Kβ catalytic subunit-p110β level was increased in infarcted murine and human hearts. Using cell type-specific loss-of-function approaches, we reported novel and distinct actions of p110β in endothelial cells (ECs) vs. CMs in response to myocardial ischaemic injury. Inactivation of endothelial p110β resulted in marked resistance to infarction and adverse cardiac remodelling with decreased mortality, improved systolic function, preserved microvasculature, and enhanced Akt activation. Cultured ECs with p110β knockout or inhibition displayed preferential PI3Kα/Akt/endothelial nitric oxide synthase signalling that consequently promoted protective signalling and angiogenesis. In contrast, mice with CM p110β-deficiency exhibited adverse post-infarct ventricular remodelling with larger infarct size and deteriorated cardiac function, which was due to enhanced susceptibility of CMs to ischaemia-mediated cell death. Disruption of CM p110β signalling compromised nuclear p110β and phospho-Akt levels leading to perturbed gene expression and elevated pro-cell death protein levels, increasing the susceptibility to CM death. A similar divergent response of PI3Kβ endothelial and CM mutant mice was seen using a model of myocardial ischaemia–reperfusion injury.
Conclusion
These data demonstrate novel, differential, and cell-specific functions of PI3Kβ in the ischaemic heart. While the loss of endothelial PI3Kβ activity produces cardioprotective effects, CM PI3Kβ is protective against myocardial ischaemic injury.
Keywords: Myocardial infarction, PI3Kβ, Remodelling, Angiogenesis, Cardiomyocyte death
Graphical Abstract
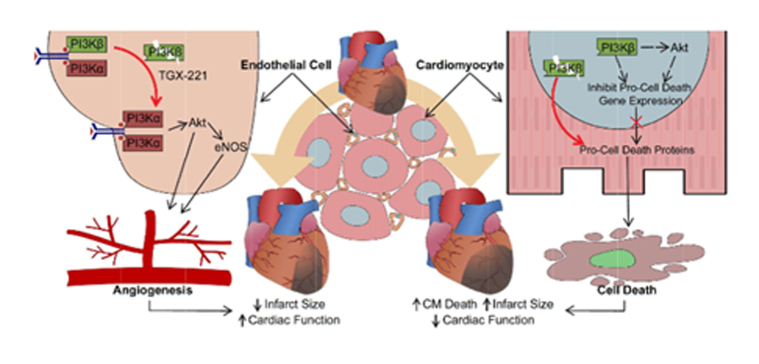
Graphical Abstract.
1. Introduction
Ischaemic heart disease has a high risk of developing heart failure (HF) with a high morbidity and mortality burden worldwide.1 Given the high metabolic demands of the heart, disruption of coronary blood flow leads to the loss of cardiomyocytes (CMs) followed by scar formation, both of which are characteristic changes of myocardial infarction (MI) and ischaemia–reperfusion (IR) injury.2,3 Hence, improving vascular supply by proangiogenic therapy and preventing CM death can protect the ischaemic myocardium. During angiogenesis, various angiogenic signals, such as vascular endothelial growth factor (VEGF), activate quiescent endothelial cells (ECs), which proliferate and differentiate to form microvascular sprouts and ultimately neovessels, rescuing peri-infarct CMs and preventing the transition to HF.4 Meanwhile, necrosis, apoptosis, and autophagy are major contributors to CM death in ischaemic hearts, and the extent of CM loss determines infarct size, cardiac function, and patient outcomes.5
Phosphoinositide 3-kinase (PI3K) signalling pathways are central determinants of cellular response to injury and play a critical role in promoting angiogenesis and cell survival.6 Members of the class IA PI3Ks are composed of a p110 catalytic subunit (α, β, and δ) and a regulatory subunit. Activation of insulin- or growth factor-receptor complexes stimulate PI3Ks which promote the production of phosphatidylinositol-3,4,5-trisphosphate (PIP3) and plasma membrane recruitment of PIP3-binding proteins, regulating various cellular responses. VEGF and its receptors signal through PI3Kα/Akt/endothelial nitric oxide synthase (eNOS) pathway in ECs, controlling cell survival, migration, vascular permeability, and vessel sprouting.7–9 In CMs, PI3Kα/Akt signalling regulates ischaemia-driven CM apoptosis, hypertrophy, and contractile function.10,11
As a member of the class IA PI3Ks, PI3K p110β isoform is ubiquitously expressed and was originally considered being functionally redundant because p110β functions are similar to other PI3Ks (p110α and p110γ).12,13 In ECs, p110β activity is lower than p110α, and mice harbouring inactivated-p110β ECs display normal vasculature, suggesting a dispensable role of p110β during embryonic vascular development.7 However, the role of p110β in response to injury has not been examined. Using genetic murine models, we examined the cell-specific function of p110β in ECs vs. CMs in response to myocardial ischaemic injury. Here, we demonstrate that inhibition of endothelial p110β protects the ischaemic heart by promoting the PI3Kα/Akt/eNOS signalling pathway and angiogenesis, while inhibition of p110β activity in CMs exacerbates the adverse cardiac remodelling post-MI by sensitizing CMs to ischaemia-triggered cell death.
2. Methods
2.1 Animal models and human explanted hearts
Mice with p110β-inactivation under the control of Tie2 promoter-driven conditionally-active Cre recombinase (p110β-Tie2) or α-MHC promoter-controlled constitutively-active Cre (p110β-αMHC) were generated as described.13,14 Homozygous littermates p110βflox/flox (p110βFlx) mice and mice expressing αMHC-driven Cre (αMHC-Cre) were used as controls. Tamoxifen (Sigma-Aldrich) was given to 10-week-old p110β-Tie2 and littermate controls to activate Cre in ECs in p110β-Tie2 mice. Cre deletes exons 21 and 22 from Pik3cb (gene encoding p110β), producing a truncated p110β which lacks catalytic activity. Animal experiments were conducted in accordance with the Canadian Council for Animal Care guidelines and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (revised 2011).
MI was achieved by permanent ligation of the proximal left anterior descending artery (LAD). The ligation or sham surgery was performed on 12-week-old male mice by a technician who was blinded to the genotype.15 Infarct size was visualized using Triphenyl Tetrazolium Chloride (TTC) (Sigma) staining and Masson’s trichrome staining. The procedures for IR surgery with 30-min occlusion protocol were similar to the MI surgery except that a piece of polyethylene tubing was placed on the LAD to minimize vessel damage.16 The IR protocol was validated by Evans Blue perfusion and electrocardiogram (ECG). Mice were sacrificed with intraperitoneal injection with ketamine (100 mg/kg) and xylazine (10 mg/kg) cocktail, and the heart tissue and bone marrow were collected.
Human tissue from non-failing control hearts and failing post-MI hearts were collected from Human Organ Procurement and Exchange program (HOPE) and Human Explanted Heart Program (HELP), respectively, with ethical approval from the Mazankowski Alberta Heart Institute and the Institutional Ethics Committee.15 Informed and signed consents were obtained from all participants; and our study conformed to the principles outlined in the Declaration of Helsinki.
2.2 Echocardiography
Noninvasive transthoracic echocardiography was performed on mice anesthetized with 1.5% isoflurane in O2 using Vevo 3100 (Visualsonics). Conventional measurements and speckle-tracking strain analysis was carried out.15,17 Global peak systolic strain was calculated as the average of 6 standard anatomical segments.
2.3 Immunofluorescence
Immunofluorescence staining was performed using established protocols.15 Wheat Germ Agglutinin (WGA, Invitrogen) staining was performed to outline CMs. Fluorescein-conjugated Lectin (Vectorlabs) intravital perfusion was performed to identify the functional vasculature.15 Fragmented DNA of apoptotic cells was detected using the DeadEnd Fluorometric Terminal Deoxynucleotidyl Transferase-mediated dUTP Nick-End Labeling (TUNEL) System (Promega) according to instructions.
2.4 EC culture and bead angiogenesis assay
Human umbilical vein ECs (HUVECs) and human coronary artery ECs (HCAECs, ATCC) were used between passage 3 and 7. ECs cultured to 70–80% confluence were transfected with small interfering RNA against human p110β (sip110β) or scrambled small interfering RNA (s-siRNA) for 48 h to test the effects of genetic ablation of p110β on ECs. Pharmacological inhibition of p110β using the p110β-specific inhibitor TGX-221 (500 nmol/L, Cayman Chemical) was used on ECs for 48 h. Cells were starved in basal medium for 5 h prior to stimulation with 50 ng/mL or 100 ng/mL recombinant human VEGF165 (PeproTech) for 10 min. Where indicated, cells were pre-incubated with the p110α-specific inhibitor BYL-719 (500 nmol/L, Cayman Chemical) or the Akt inhibitor MK-2206 (1 µmol/L, APExBIO) for 1 h before VEGF stimulation.
In vitro angiogenesis bead assay of HUVECs/HCAECs was performed as described.15 The number of sprouts was counted using image analysis software (OpenLab), and at least 30 beads per independent experiment were analysed.
2.5 Adult CM isolation, culture, and stretching
Adult murine left ventricular CMs were isolated from isoflurane (2%)-anaesthetized mice; and the isolated CMs were cultured and stretched as described.18 Plated CMs were cyclically stretched at 1 Hz with an elongation of 5% for 3 h by Flexcell FX-5000 Tension System (Flexcell International Corp) in serum-free culture medium under a 2% CO2 and 5% O2 atmosphere.
2.6 Immunoblotting and nuclear fractionation
Immunoblotting and nuclear fractionation were performed as previously described.15,19 Histone H3 and GAPDH (Cell Signaling) were used as nuclear and cytosolic markers, respectively.
2.7 RNA sequencing and TaqMan RT-PCR
RNA isolation and RNA sequencing were performed as described.20 Total RNAs from left ventricles (three LVs/group) were extracted. Data were analysed using WebGestalt, Protein ANalysis THrough Evolutionary Relationships (PANTHER) classification system (Pantherdb.org), and Ingenuity Pathway Analysis. RNA expression levels were examined by TaqMan real-time polymerase chain reaction (RT-PCR) as described.15 The expression levels of myocardial disease markers, including atrial natriuretic peptide (Anp), brain natriuretic peptide (Bnp), and β-myosin heavy chain (β-Mhc), were examined.
2.8 Statistical analysis
Statistical analyses were carried out using SPSS Statistics 24 software, and statistical significance was defined as P < 0.05 (two sided). Continuous data were presented in scatter plots with mean ± SEM. The differences between two groups were evaluated using independent t-test or Mann–Whitney U test after normality examination. One-way ANOVA or Kruskal–Wallis test with pairwise comparisons were used in studies with more than two groups based on the normality of the data. Survival data were presented as the Kaplan–Meier plots and the log-rank test was used to evaluate the statistical significance.
3. Results
3.1 Akt phosphorylation and p110β are elevated in ischaemic hearts
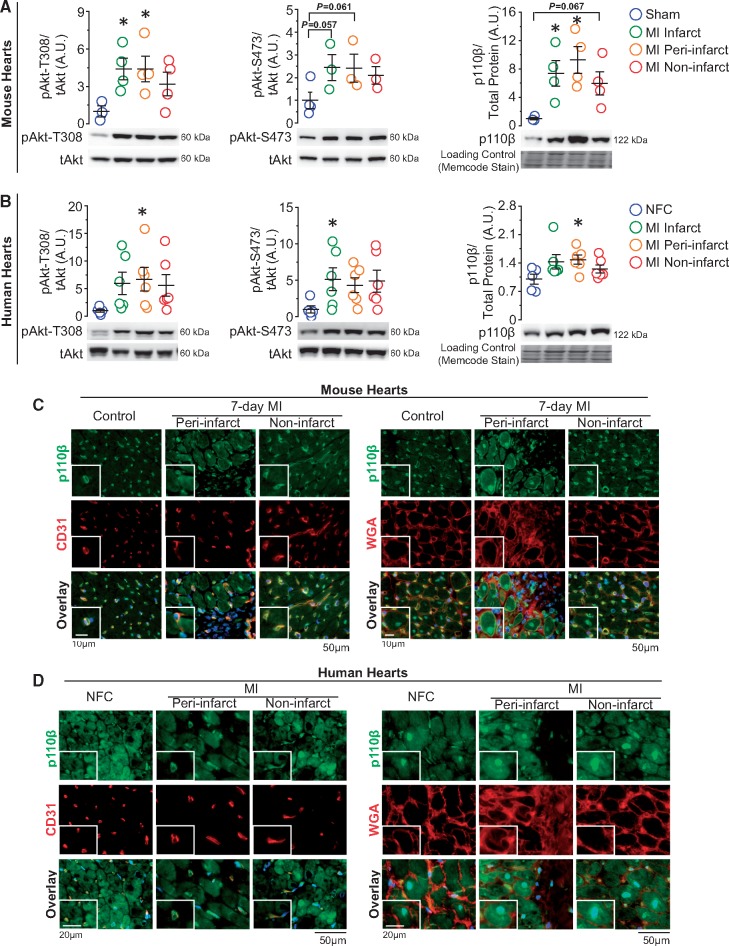
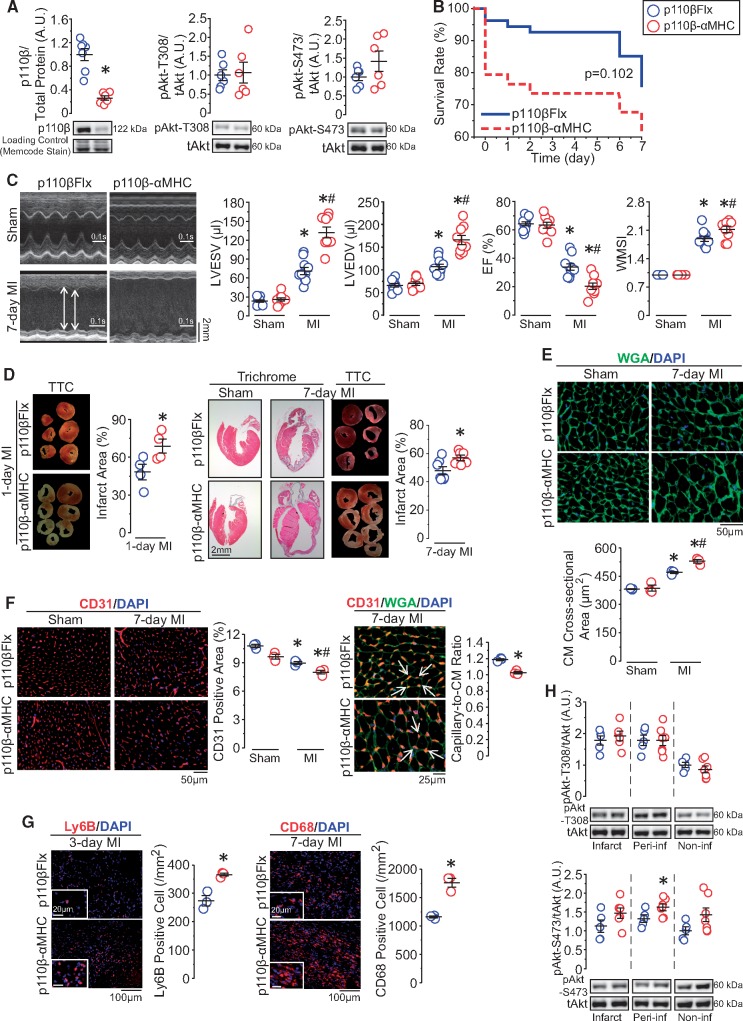
Because of the vital role of cardiac Akt in injury repair,21 we examined the protein levels of Akt in post-MI murine heart and found increased Akt phosphorylation, especially at threonine-308, in the infarct and peri-infarct area (Figure 1A). As one of the upstream regulator of Akt activation, the p110β protein level was assessed next to evaluate the effect of MI on p110β. We observed an increase in p110β level in the infarct and peri-infarct area, and to a lesser extent, in the non-infarct area (Figure 1A). Importantly, explanted human hearts showed largely similar trends of phospho-Akt and p110β levels following MI (Figure 1B), suggesting a conserved mechanism of up-regulation of p110β following myocardial ischaemia. Immunofluorescence analysis showed that in both murine and human hearts, p110β was expressed in both ECs and CMs, and while p110β was constitutively expressed in ECs, it was highly up-regulated and localized to the nuclei in CMs (Figure 1C and D). These findings suggest that p110β could have specific and distinct functions in ECs and CMs. Thus, we examined the function of p110β in ECs and CMs separately in response to MI and myocardial IR injury using genetically modified mice.
Figure 1.
Catalytic isoform of PI3Kβ-p110β is increased in post-MI murine and human hearts and expressed both in ECs and CMs. (A and B) Western blot analysis of Akt and p110β levels on 7-day post-sham/MI mouse hearts and on non-failing control (NFC) and post-MI failing human hearts. *P < 0.05 vs. sham/NFC hearts, n = 3–6 hearts/group (one-way ANOVA). (C and D) Immunofluorescence images of p110β (green) in the heart with EC marker-CD31 (red, left panels), WGA outlining CMs (red, right panels), and DAPI marking nuclei (blue) on mouse and human hearts, n = 3–4 hearts/group.
3.2 Endothelial PI3Kβ inactivation improves cardiac function and remodelling after MI
To gain insight into the role of endothelial p110β in post-MI remodelling, we generated p110β-Tie2 mice in which endothelial p110β was conditionally and partially deleted, producing truncated p110β protein lacking catalytic activity.13 Successful genetic inactivation of p110β in p110β-Tie2 was confirmed by PCR analysis with truncated p110β gene expression without affecting haematopoietic cells (Supplementary material online, Figure S1A). Both p110β-Tie2 and littermate control (p110βFlx) mice showed similar body weight, heart weight, LV weight, and levels of p110β, p110α, and phospho-Akt in LV protein extracts (Supplementary material online, Figure S1B and C).
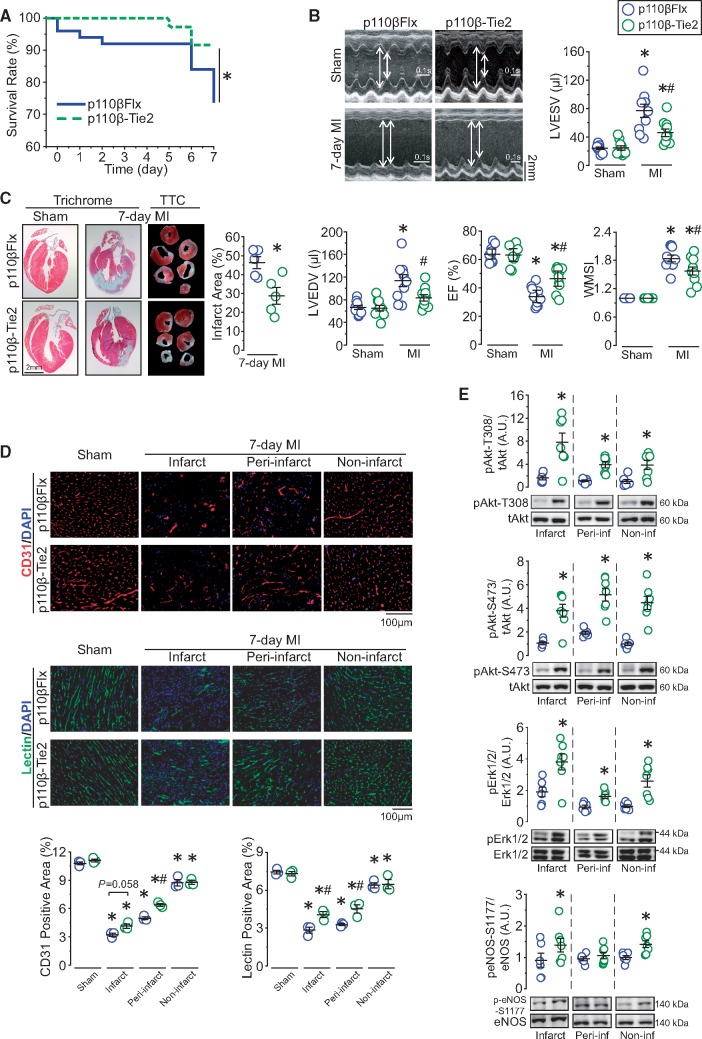
p110β-Tie2 and littermate controls were randomly and blindly assigned to sham operation or MI induction (Supplementary material online, Figure S1D). While sham-operated groups had similar cardiac function, post-MI p110β-Tie2 exhibited higher survival rate (92% vs. 74%) by Day 7, with improved post-MI cardiac function reflected by maintained left ventricular end-diastolic and end-systolic dimensions, greater ejection fraction, and enhanced regional systolic function (Figure 2A and B and Supplementary material online, Table S1). Consistent with the preservation of post-MI cardiac function, p110β-Tie2 hearts showed lower expression levels of myocardial disease markers with equivalent hypertrophy in both genotypes (Supplementary material online, Figure S1E and F). While initial post-MI infarct size and apoptotic level were comparable between genotypes, p110β-Tie2 showed reduced infarct expansion on Day 7 and absence of post-MI pulmonary oedema (Supplementary material online, Figure S1G–I and Figure 2C). Moreover, even though sham-operated hearts and the non-infarct area of post-MI hearts displayed similar vascular density between genotypes, p110β-Tie2 showed increased vascular density in the infarct and peri-infarct areas, confirmed by EC marker staining and lectin in vivo perfusion for the detection of functional vessels (Figure 2D). Since PI3K/Akt/eNOS and Erk1/2 pathways are critical mediators of cardioprotection,22 we examined the levels of phospho-Akt and phospho-Erk1/2 and found both were increased in post-MI p110β-Tie2 hearts compared with control, while phospho-eNOS was significantly increased in the infarct and remote areas (Figure 2E). Taken together, these findings demonstrate that catalytic inactivation of endothelial p110β results in marked cardioprotection following MI associated with preserved vascular density and increased Akt activation in the ischaemic area.
Figure 2.
Inactivation of endothelial p110β protects the heart against MI by preserving vasculatures and increasing Akt activity in the ischaemic areas. (A) Kaplan–Meier survival curve of p110β-Tie2 and p110βFlx mice following MI surgery. *P < 0.05, n = 36–50 mice/group (log-rank test). (B) Echocardiographic images showing ventricular morphology and analysis of left ventricular end-systolic and end-diastolic volume (LVESV and LVEDV), ejection fraction (EF), and wall motion score index (WMSI). *P < 0.05 vs. sham, #P < 0.05 vs. p110βFlx, n = 10 mice/group (one-way ANOVA). (C) Infarct size analysis from TTC-stained sections and representative images of trichrome histological staining of hearts. *P < 0.05 vs. p110βFlx, n = 5 mice/group (t-test). (D) Immunofluorescence analysis of EC marker-CD31 (red) and EC marker-lectin (green) via in vivo perfusion method on p110β-Tie2 and p110βFlx hearts, with quantification of percentage of fluorescence positive area in different areas. *P < 0.05 vs. sham, #P < 0.05 vs. p110βFlx, n = 3 mice/group (one-way ANOVA). (E) Western blot analysis of Akt, Erk1/2, and NOS protein levels in the infarct, peri-infarct, and non-infarct areas from 7-day post-MI hearts. *P < 0.05 vs. p110βFlx, n = 6–7 mice/group (t-test).
3.3 Loss of PI3Kβ activity promotes VEGF-induced PI3Kα/Akt signalling and angiogenic sprouting in ECs
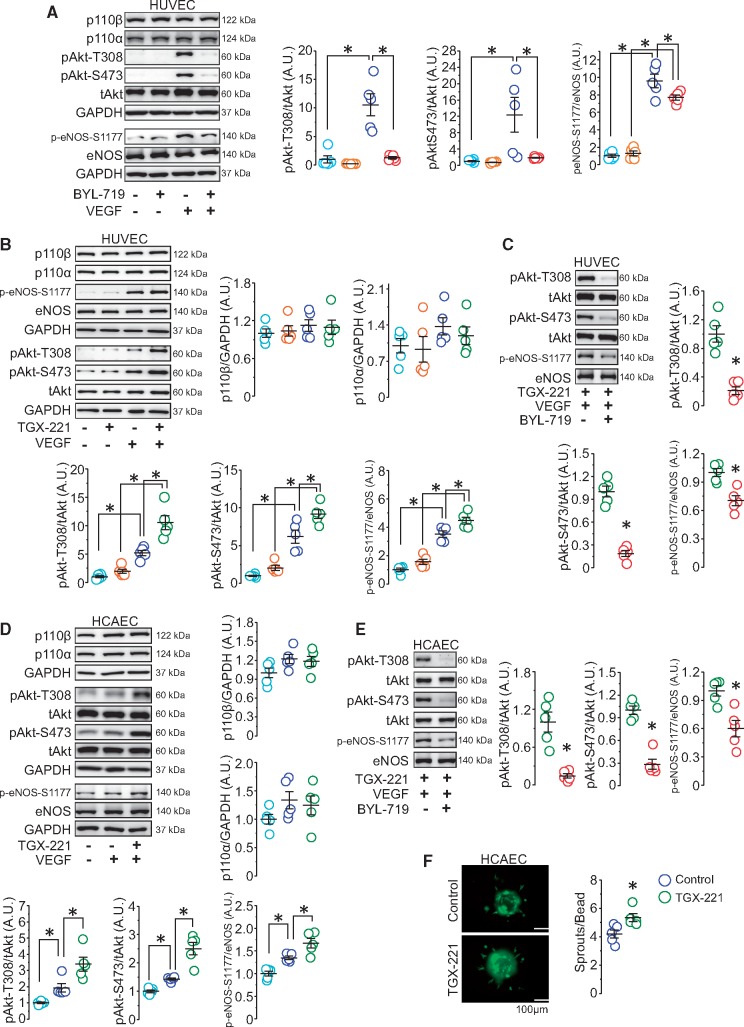
The VEGF/VEGF receptor axis plays a crucial and well-established role in vascular survival and angiogenesis through PI3Kα/Akt signalling.22,23 This was also supported by our findings in HUVECs that Akt activation was required for VEGF-induced angiogenic sprouting using Akt inhibitor (MK-2206), VEGF-stimulated Akt activation largely depended on the activity of PI3Kα as the p110α specific inhibitor (BYL-719) completely abolished the effect of VEGF-induced Akt phosphorylation without affecting p110β and p110α protein levels, and VEGF-induced eNOS activation partially relied on Akt activity (Supplementary material online, Figure S2A–C and Figure 3A). Because of the vital role of PI3Kα in VEGF-induced and Akt-mediated angiogenesis, we hypothesized that selective inactivation of p110β in ECs might up-regulate PI3K/Akt signalling via the p110α isoform. We first tested the effects of VEGF on HUVECs with p110β-specific siRNA which showed that abrogation of p110β increased Akt and eNOS phosphorylation upon VEGF stimulation which was completely blocked by BYL-719 (Supplementary material online, Figure S2D–E). These results suggest that the abrogation of p110β leads to an enhanced activation of PI3Kα/Akt signalling. Next, we studied the effect of pharmacological p110β inhibition in ECs on VEGF-induced Akt/eNOS activation as cardioprotective effects were observed in mice with endothelial-p110β inactivation (p110β-Tie2). Consistent with our animal and p110β-deleted EC data, the p110β specific inhibitor (TGX-221) potentiated VEGF-induced Akt activation in HUVECs, which was completely dependent on intact p110α signalling (Figure 3B and C).
Figure 3.
Inhibition of p110β in ECs elevates VEGF-stimulated PI3Kα/Akt/eNOS signalling, promoting angiogenesis. (A) Western blot analysis of p110β, p110α, GAPDH, Akt, and eNOS in BYL-719-treated HUVEC lysates. *P < 0.05, n = 5 independent experiments (one-way ANOVA). (B) Western blots demonstrating the effect of VEGF on p110β, p110α, GAPDH, Akt, and eNOS protein levels in TGX-221-treated HUVECs. *P < 0.05, n = 5 independent experiments (one-way ANOVA). (C) Western blot analysis of Akt and eNOS in TGX-221-treated HUVECs with and without BYL-719. *P < 0.05, n = 5 independent experiments (t-test). (D) Western blot analysis of p110β, p110α, GAPDH, Akt, and eNOS in HCAECs. *P < 0.05, n = 5 independent experiments (one-way ANOVA). (E) Western blot analysis of Akt and eNOS in TGX-221-treated HCAECs with and without BYL-719. *P < 0.05, n = 5 independent experiments (t-test). (F) Representative beads and quantification of sprout number in control and TGX-221-treated HCAECs. *P < 0.05, n = 5 independent experiments (t-test).
To address whether these effects exist in cardiac ECs, HCAECs were cultured to examine the effect of p110β inhibition on VEGF/PI3K/Akt signalling. Immunofluorescence analysis confirmed the expression of p110β in cardiac ECs (Supplementary material online, Figure S2F). Consistent with our findings in HUVECs, p110β inhibition in HCAECs resulted in a striking increase in Akt and eNOS phosphorylation upon VEGF stimulation without affecting p110α and p110β protein levels, which were suppressed by p110α inhibition (Figure 3D and E). Since the PI3K/Akt pathway is critical in angiogenesis and increased vasculature was detected in the ischaemic area in post-MI p110β-Tie2 hearts, we performed the angiogenic bead assay to test the effect of p110β inhibition on angiogenic sprouting in HCAECs. Coinciding with increased Akt activation, p110β inhibition potentiated angiogenic sprouting (Figure 3F). Taken together, these data suggest that inhibiting p110β activity enhances VEGF-mediated Akt activation via the p110α isoform resulting in increased angiogenic response in cardiac ECs.
3.4 Inactivation of PI3Kβ in CMs exacerbates cardiac dysfunction following MI
We next evaluated the role of CM-p110β in post-MI remodelling using p110β-αMHC mice which expressed kinase-dead p110β specifically in CMs (Supplementary material online, Figure S3A). Analysis of truncated p110β gene expression confirmed the success of p110β inactivation in p110β-αMHC hearts, and the adult p110β-αMHC mice were viable and fertile with comparable body weight to littermate controls-p110βFlx (Supplementary material online, Figure S3B–C). The protein level of p110β was reduced in p110β-αMHC hearts without altering whole-heart baseline phospho-Akt levels (Figure 4A). At Day 7 post-MI, p110β-αMHC mice exhibited a trend of increased mortality compared with controls (62% vs. 76%), with increased left ventricular dimensions, deteriorated systolic function, and worsened regional systolic function (Figure 4B and C and Supplementary material online, Table S2). Chamber dimensions and cardiac function did not differ between genotypes in sham-operated groups (Figure 4C and Supplementary material online, Table S2). Littermate controls (p110βFlx) exhibited similar cardiac systolic function as αMHC-Cre mice following sham operation and MI (Supplementary material online, Figure S3D), showing that the αMHC transgene expression alone did not affect post-MI remodelling.
Figure 4.
CM-specific inactivation of p110β exacerbates cardiac dysfunction after MI, resulting in adverse ventricular remodelling. (A) Western blot analysis of p110β and Akt levels in p110β-αMHC and p110βFlx left ventricular lysates. *P < 0.05, n = 6–7 mice/group (t-test). (B) Kaplan–Meier survival analysis in post-MI p110β-αMHC and control mice, n = 34–54 mice/group (log-rank test). (C) Echocardiographic images showing left ventricular morphology and functional analysis of left ventricular end-systolic and end-diastolic volume (LVESV and LVEDV), ejection fraction (EF), and wall motion score index (WMSI). *P < 0.05 vs. sham, #P < 0.05 vs. p110βFlx, n = 9–10 mice/group (one-way ANOVA). (D) Trichrome histological-stained images and TTC-stained images and infarct size quantification on post-MI hearts. *P < 0.05, n = 4–7 mice/group (t-test). (E) Wheat Germ Agglutinin (WGA, green) immunofluorescence staining outlining CM size. *P < 0.05 vs. sham, #P < 0.05 vs. p110βFlx, n = 3 mice/group (one-way ANOVA). (F) Vascular density and capillary-to-CM ratio testing by CD31 (red) and WGA (green) immunofluorescence staining on post-surgery hearts. *P < 0.05 vs. sham/indicated group, #P < 0.05 vs. p110βFlx, n = 3 mice/group (one-way ANOVA or t-test). (G) Neutrophils identified by Ly6B (red, left panels) and macrophages marked by CD68 (red, right panels) immunofluorescence staining on post-MI hearts. *P < 0.05, n = 3 mice/group (t-test). (H) Western blot analysis of Akt protein level in 7-day post-MI hearts. *P < 0.05 vs. p110βFlx, n = 5–7 mice/group (t-test).
Consistent with worsened cardiac function, p110β-αMHC hearts showed larger infarcted area on Days 1 and 7 after MI accompanied by pulmonary oedema (Figure 4D and Supplementary material online, Figure S3E). This functional and structural deterioration in post-MI p110β-αMHC hearts was accompanied by increased pathological hypertrophy, reduced coronary microvasculature, and strikingly increased MI-related myocardial inflammation (Figure 4E–G). Importantly, analysis of the canonical Akt signalling pathway displayed comparable pAkt-T308 levels, while phosphorylation of Akt at serine-473 was slightly increased in the peri-infarct area of p110β-αMHC hearts (Figure 4H). These results demonstrate that in striking contrast to p110β function in ECs, loss of p110β activity in CMs results in increased susceptibility to ischaemic injury and adverse post-MI remodelling illustrating a novel cell-dependent role of PI3Kβ signalling.
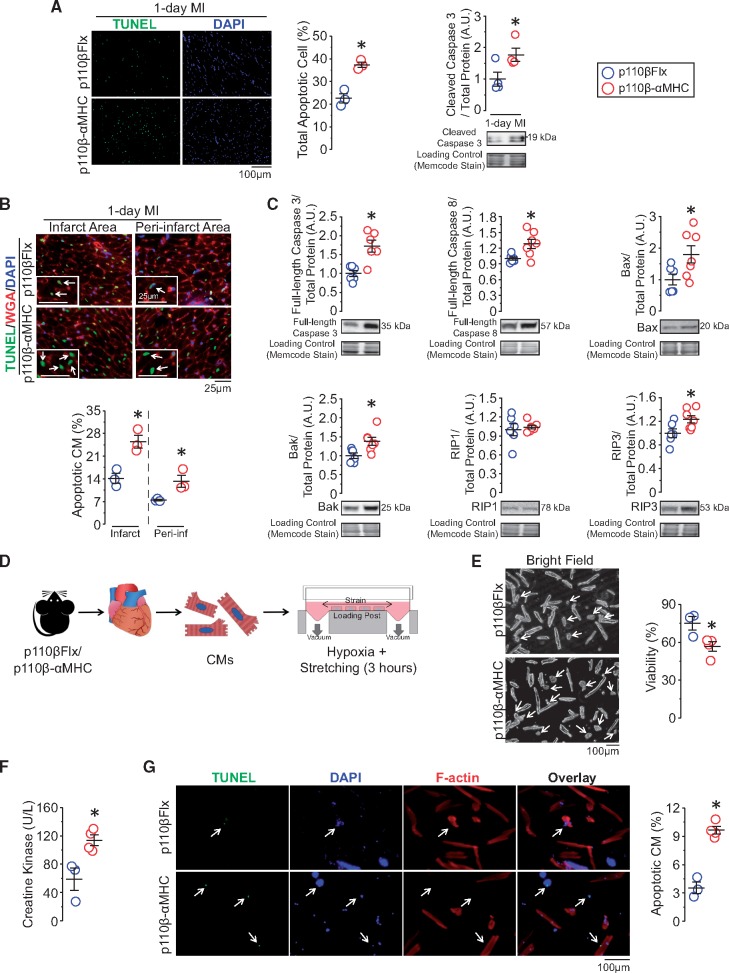
3.5 CMs with PI3Kβ deficiency are prone to hypoxia-induced cell death: roles of PI3Kβ in the regulation of myocardial gene expression
Given the increase in infarct size in p110β-αMHC hearts, we hypothesized that p110β prevents CM cell death. Immunofluorescence and western blot analysis demonstrated increased apoptosis in post-MI p110β-αMHC hearts, characterized by elevated apoptotic cell number and cleaved caspase 3 protein levels (Figure 5A). Since CMs are not the only cell types to undergo apoptosis under MI, we combined TUNEL and WGA staining to evaluate apoptotic CMs which confirmed an increase in apoptotic CMs in the infarct and peri-infarct area (Figure 5B). To elucidate the molecular mechanisms underlying the increased susceptibility of p110β-αMHC CMs to apoptosis, we analysed cell death proteins at baseline in left ventricular samples. Pro-apoptotic proteins, including full-length caspase 3, full-length caspase 8, Bax, and Bak, were up-regulated in p110β-αMHC; meanwhile, RIP3, a critical determinant of necrosis, but not RIP1, was also up-regulated (Figure 5C). To determine the effect of the loss of p110β in CMs, we isolated adult CMs and examined the role of p110β in hypoxia-induced cell death under cyclic mechanical stretch (Figure 5D). Consistent with normal cardiac structure and function in p110β-αMHC mice, p110β-αMHC and control hearts had comparable numbers of viable CMs after isolation (Supplementary material online, Figure S3F). However, cell viability was decreased in p110β-αMHC CMs compared with control CMs in response to 3-h cyclical stretch under hypoxic condition (5%O2) (Figure 5E). In line with these data, we observed higher creatine kinase level in the culture media, a marker of CM cell death, and an increase in the number of apoptotic CMs in p110β-αMHC CMs (Figure 5F–G). Thus, CMs with compromised p110β activity develop an intrinsic susceptibility to cell death in response to acute ischaemic and hypoxic stress.
Figure 5.
Inactivation of CM-p110β sensitizes CMs to cell death by increasing pro-cell death proteins expression, leading to increased post-MI cell death. (A) Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL, green) and DAPI (blue) immunofluorescence analysis for apoptotic cells (n = 3 mice/group) and western blot analysis for cleaved caspase 3 (n = 4 mice/group) on 1-day post-MI hearts. *P < 0.05 (t-test). (B) Combined wheat Germ Agglutinin (WGA, red), TUNEL, and DAPI immunofluorescence staining to highlight apoptotic CMs. *P < 0.05, n = 3 mice/group (t-test). (C) Western blot analysis for baseline protein levels of full-length caspase 3, full-length caspase 8, Bax, Bak, RIP1, and RIP3 in left ventricular lysates from p110β-αMHC and control mice. *P < 0.05, n = 6–7 mice/group (t-test). (D) Study design for isolated adult CM stretching under hypoxic condition for 3 h, n = 3–4 experiments/group (two hearts/experiment). (E) Representative bright field images and cell viability evaluation after stretching. *P < 0.05 (t-test). (F) Evaluation of creatine kinase level in the media from cultured CMs. *P < 0.05 (t-test). (G) TUNEL staining for apoptotic CMs (green) with F-actin (red) staining. *P < 0.05 (t-test).
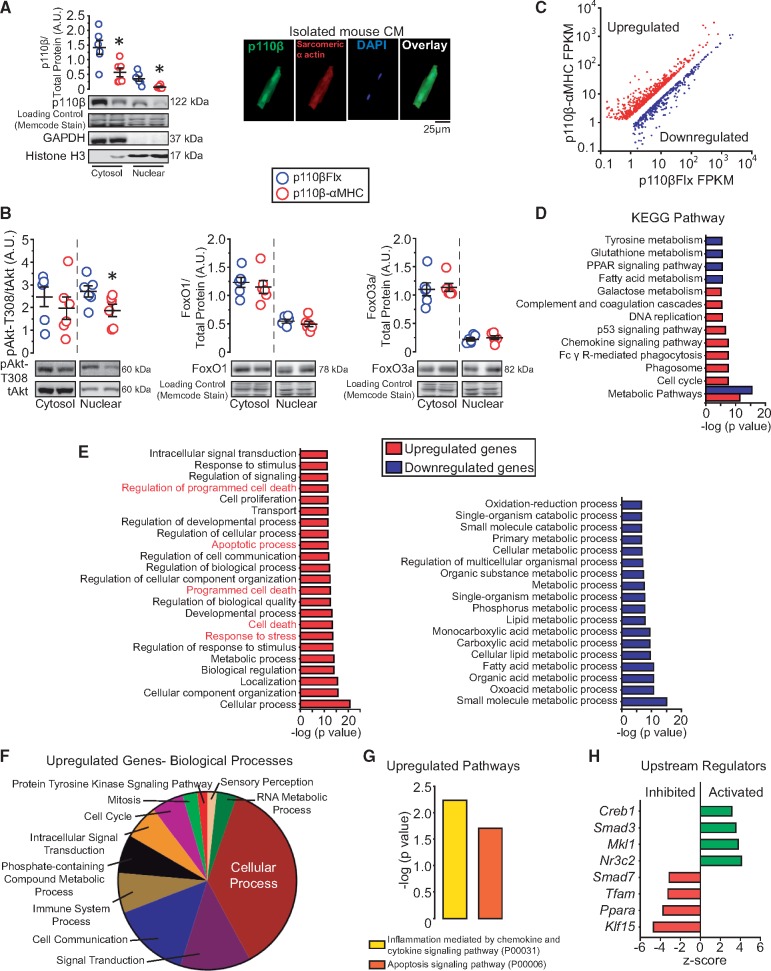
Western blot analysis demonstrated that p110β was present in the nuclear fraction of LVs, and immunostaining on isolated adult CMs confirmed the presence of p110β in the nuclei, and both cytosolic and nuclear fractions were decreased in p110β-αMHC hearts (Figure 6A). Surprisingly, in p110β-αMHC hearts, phospho-Akt level was down-regulated in the nuclear fraction, but not in the cytosolic fraction, without alteration in the protein levels of FoxO1 and FoxO3a, which are known down-stream effectors of Akt phosphorylation (Figure 6B). Taken together, these findings indicate that deficiency of CM-p110β results in decreased nuclear Akt phosphorylation which might affect nuclear activity independent of FoxO transcription factors, leading to a pro-cell death phenotype in CMs.
Figure 6.
p110β is expressed in CM nuclei and CM-p110β inactivation alters gene expression in the heart. (A) Nuclear fractionation analysis for p110β, GAPDH, and Histone H3 in p110β-αMHC and p110βFlx left ventricular lysates and immunofluorescence images of p110β (green) and sarcomeric α actin (red) on isolated murine CMs. *P < 0.05, n = 6 mice/group (t-test). (B) Fractionation analysis for Akt, FoxO1, and FoxO3a in p110β-αMHC and p110βFlx left ventricular lysates. *P < 0.05, n = 6 mice/group (t-test). (C) Scatterplot of significantly differentially-expressed genes after RNA-seq analysis on p110β-αMHC and p110βFlx left ventricles, n = 3 mice/group. (D) KEGG pathway enrichment analysis of RNA-seq results for significantly altered genes. (E) WebGestalt enrichment analysis for disrupted pathways on significantly up-regulated and down-regulated genes in biological processes. (F) Panther GO analysis showing subcategories of differential gene expression in biological processes. (G) Significantly altered signalling pathways identified by Panther signal transduction pathways analysis. (H) Potentially altered upstream transcription factors identified by Ingenuity Pathway Analysis.
To better define the involvement of p110β in transcriptional control, we performed RNA sequencing to clarify the differences in global gene expression profiles in control and p110β-αMHC LVs, which revealed 1057 up-regulated genes and 838 down-regulated genes in p110β-αMHC hearts, affecting the pathways associated with metabolism, cell cycle, and chemokine signalling (Figure 6C–D). Notably, among the affected genes in the p110β-αMHC hearts, genes associated with cell death were prominently up-regulated, while genes related to the regulation of metabolic processes were down-regulated (Figure 6E). Among the up-regulated genes related to biological processes, cellular processes were altered, while protein tyrosine kinases signalling pathway showed minimal changes (Figure 6F). Moreover, apoptosis and inflammatory signalling pathways were dramatically up-regulated in p110β-αMHC (Figure 6G), which is consistent with our observations demonstrating excessive apoptosis and inflammation in the post-MI p110β-αMHC hearts. Ingenuity Pathway Analysis identified activation of Creb1, Smad3, Mkl1, and Nr3c2 transcription factors; furthermore, Smad7, Tfam, Ppara, and Klf15 transcription factors were inhibited in p110β-αMHC hearts (Figure 6H). Several of these transcriptional regulators are known to be associated with detrimental post-infarct outcomes (Mkl1, Nr3c2, Smad, and Tfam), as well as with regulation of cardiac metabolism contributing to infarct healing (Ppara and Klf15).24–29 Our data suggest that PI3Kβ signalling in CM is required for protection against ischaemic injury and nuclear Akt contributes to this protection.
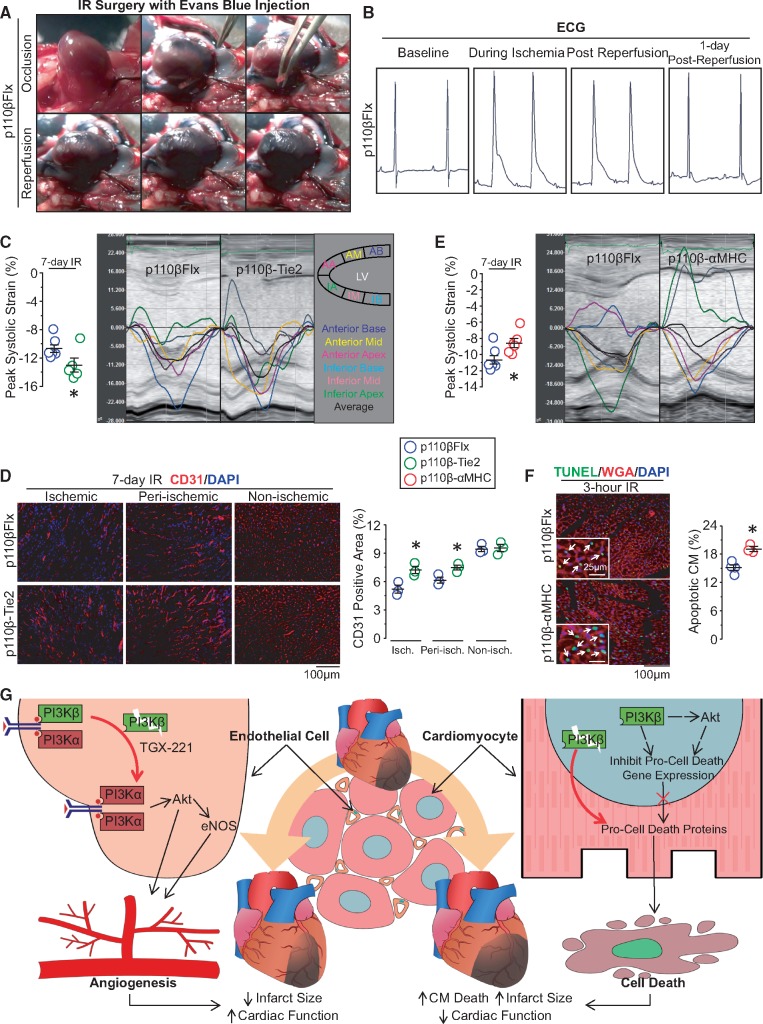
3.6 PI3Kβ has divergent effects in ECs and CMs facing IR injury
We next examined whether inactivation of p110β in ECs or CMs would affect cardiac performance after IR injury which comprises of ischaemic and reperfusion injury and commonly present in MI patients following myocardial reperfusion treatments.30 IR injury was performed using 30 minutes of LAD occlusion followed by reperfusion in p110β-Tie2, p110β-αMHC, and control mice. In vivo Evans Blue perfusion confirmed the occlusion of the LAD as the affected myocardium remained unstained, and successful reperfusion was confirmed when this area was perfused with the dark blue dye stain after the release of LAD obstruction (Figure 7A). Furthermore, bipolar surface ECG obtained from these mice showed prolonged QRS and elevated ST-segment after 30 min of LAD occlusion compared to baseline ECG (Figure 7B), indicating the presence of myocardial ischaemia. Myocardial strain analysis revealed better longitudinal peak systolic strain in the p110β-Tie2 mice at Day 7 post-IR compared with control (Figure 7C). Consistent with improved cardiac function, p110β-Tie2 mice exhibited higher coronary density in the ischaemic and peri-ischaemic myocardium (Figure 7D). In contrast, at 7-day post-IR, p110β-αMHC mice displayed deteriorated cardiac function with decreased longitudinal peak systolic strain (Figure 7E). In addition, TUNEL staining of 3-h post-reperfusion hearts revealed increased apoptotic CMs in p110β-αMHC hearts (Figure 7F). These results clearly support the use of myocardial strain analysis to detect changes in cardiac performance and demonstrate that endothelial inactivation of p110β is cardioprotective against IR injury, while p110β deficiency in CMs enhances the susceptibility to IR injury.
Figure 7.
Divergent roles of p110β in ECs and CMs in response to myocardial IR injury. (A) Sequential images of IR surgery with Evans Blue perfusion showing the blue dye rushes into the infarcted area after the release of occlusion. (B) Representative ECG of mice with IR surgery. (C) Echocardiographic longitudinal strain analysis on 7-day post-IR p110β-Tie2 and control mice with representative longitudinal strain curve images. *P < 0.05, n = 5–6 mice/group (t-test). (D) Vascular density evaluated by staining EC marker-CD31 (red) on 7-day post-IR hearts. *P < 0.05 vs. p110βFlx, n = 3 mice/group (t-test). (E) Echocardiographic longitudinal strain analysis and representative longitudinal strain curve on 7-day post-IR p110β-αMHC and control hearts. *P < 0.05, n = 6–7 mice/group (t-test). (F) Representative immunofluorescence images of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL, green) staining with Wheat Germ Agglutinin (WGA, red) showing apoptotic CMs on 3-h post-IR hearts. *P < 0.05, n = 3–4 mice/group (t-test). (G) Schematic diagram depicting the functions of p110β in cardiac ECs and CMs in response to ischaemic injury. Disruption of EC-p110β signalling enhances PI3Kα/Akt activation in the ischaemic heart, promoting angiogenesis, while nuclear PI3Kβ/Akt in CMs is required to maintain cellular homeostasis to prevent cell death facing ischaemic stress.
4. Discussion
Our findings reveal a novel, critical, and cell-specific role of p110β in the regulation of endothelial sprouting and CM survival in ischaemic hearts. Using EC- and CM-specific p110β-inactivated mice which display comparable cardiac function under physiological conditions, we demonstrate that p110β plays distinct cell-specific roles in the ischaemic heart. Specifically, inactivation of endothelial p110β enhances VEGF-stimulated PI3Kα/Akt/eNOS signalling and angiogenesis, reducing myocardial ischaemic injury in vivo, whereas CM-specific p110β ablation disrupts cellular homeostasis with a pro-cell death profile, sensitizing CMs to cell death following myocardial ischaemia (Figure 7G). In the heart, p110β is expressed in ECs and CMs and is up-regulated following MI in both murine and human hearts confirming the PI3Kβ signalling pathway is altered in heart disease.
In striking contrast to the dispensable role of endothelial p110β in adult quiescent cardiac vasculature,7 EC-specific p110β inactivation leads to increased Akt phosphorylation, myocardial microvasculature preservation, cardiac function maintenance, and reduced mortality after ischaemic injury. Activation of endothelial Akt/eNOS pathway is essential in VEGF-induce postnatal angiogenesis by regulating cell survival, migration, and NO release.8,31,32 Interestingly, despite that endothelial p110α drives the VEGF-induced Akt phosphorylation and following angiogenesis, the reduction or inhibition of p110β in HUVECs enhances VEGF-induced PI3Kα/Akt activation and, to a lesser extent, eNOS activation, improving angiogenic sprouting. The short-term endothelial p110β inhibition has no influence on VEGF-induced Akt activation.7,33 However, studies on p110α- or p110β-dependent cancer cells have revealed time-dependent activation of PI3K/Akt signalling in response to dominant-PI3K isoform inhibition by relieving the feedback inhibition of dominant-PI3K isoform on another isoform.34,35 Similarly, our results suggest that inhibition of p110β in ECs relieves feedback inhibition of p110β on p110α amplifying VEGF-induced Akt activation and angiogenic sprouting after MI, leading to reduced infarct size, protected cardiac function, and reduced mortality.
Similarly to EC-p110β, CM-p110β is not required for postnatal cardiac development.36 However, in response to myocardial ischaemia, in contrast to the cardioprotective effects of endothelial p110β inactivation, we observe that inactivation of p110β in myocytes sensitizes them to cell death, leading to adverse cardiac remodelling and deteriorated cardiac function. In line with our observation that considerable nuclear-p110β were present in the CMs, cytosolic p110β/p85β complexes are known to enter the nucleus where they play a role in protecting cells against oxidative stress-induced apoptosis and regulating DNA replication and repair.37–39 In this framework, inactivation and/or reduction in levels of nuclear p110β should promote oxidative stress-induced apoptosis and hinder DNA repair, facilitating cell death and increasing the infarct size. In addition, CM-p110β deficiency significantly affects the transcriptional profile of the myocardium characterized by the altered expression of metabolic genes and the increased expression of programmed cell death genes, leading to an increase in pro-cell death protein levels, including caspase 3, caspase 8, Bax, Bak, and RIP3, which are associated with adverse outcomes in ischaemic hearts by promoting CM death and increasing infarct size.5,40,41 In line with this framework, cardiac overexpression of p110β mediates cardioprotective effects in mice with MI by reducing hypoxia-induced CM apoptosis with increased Akt activation.42 In this study, we find that overall Akt activation is largely unaffected by p110β deficiency either at quiescent or infarcted state, which is in line with the prevailing view that p110β is not required for postnatal cardiac development.36 However, nuclear Akt activation is diminished in p110β deficient mice, suggesting that the nuclear fraction of p110β/Akt contributes to the adverse post-MI outcomes in the model. The cardioprotective function of nuclear p110β may be mediated via Akt, which is known to have multiple roles in the nucleus, such as promoting cell survival and regulating cell cycle.43 Specifically, in the heart, nuclear accumulation of Akt inhibits CM apoptosis, protecting the heart against IR injury.44
Overall, our study highlights that p110β is a versatile PI3K isoform in the heart where it has distinct roles in cardiac endothelium vs. CMs. Inactivation of p110β in the cardiac endothelium protects the heart from ischaemic injury by promoting PI3Kα/Akt signalling and angiogenesis, whereas inactivation of p110β in CMs promotes ischaemia-induced cell death by disrupting gene programs and increasing pro-cell death protein levels.
Conflict of interest: none declared.
Funding
This work was supported by operating grants from the Canadian Institutes of Health Research (CIHR) and Heart and Stroke Foundation (to G.Y.O.); the American Heart Association (AHA 13SDG14660064 to C.E.G.) and NIH (R01 HL125436 to C.E.G.). X.C. is funded by Li Ka Shing Sino-Canadian Exchange Program.
Supplementary Material
Time for primary review: 23 days
References
- 1. Roth GA, Nguyen G, Forouzanfar MH, Mokdad AH, Naghavi M, Murray CJ.. Estimates of global and regional premature cardiovascular mortality in 2025. Circulation 2015;132:1270–1282. [DOI] [PubMed] [Google Scholar]
- 2. Sutton MG, Sharpe N.. Left ventricular remodeling after myocardial infarction—pathophysiology and therapy. Circulation 2000;101:2981–2988. [DOI] [PubMed] [Google Scholar]
- 3. Yellon DM, Hausenloy DJ.. Myocardial reperfusion injury. N Engl J Med 2007;357:1121–1135. [DOI] [PubMed] [Google Scholar]
- 4. Ferrara N, Kerbel RS.. Angiogenesis as a therapeutic target. Nature 2005;438:967–974. [DOI] [PubMed] [Google Scholar]
- 5. Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA, Lavandero S.. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis 2011;2:e244.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Datta SR, Brunet A, Greenberg ME.. Cellular survival: a play in three Akts. Genes Dev 1999;13:2905–2927. [DOI] [PubMed] [Google Scholar]
- 7. Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B.. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature 2008;453:662–666. [DOI] [PubMed] [Google Scholar]
- 8. Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC.. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 2005;115:2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dimmeler S, Dernbach E, Zeiher AM.. Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett 2000;477:258–262. [DOI] [PubMed] [Google Scholar]
- 10. Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A.. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 2001;104:330–335. [DOI] [PubMed] [Google Scholar]
- 11. Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HM, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM.. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 2002;110:737–749. [DOI] [PubMed] [Google Scholar]
- 12. Heller R, Chang Q, Ehrlich G, Hsieh SN, Schoenwaelder SM, Kuhlencordt PJ, Preissner KT, Hirsch E, Wetzker R.. Overlapping and distinct roles for PI3Kbeta and gamma isoforms in S1P-induced migration of human and mouse endothelial cells. Cardiovasc Res 2008;80:96–105. [DOI] [PubMed] [Google Scholar]
- 13. Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, Meek S, Smith AJ, Okkenhaug K, Vanhaesebroeck B.. The p110β isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110γ. Proc Natl Acad Sci USA 2008;105:8292–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McLean BA, Zhabyeyev P, Patel VB, Basu R, Parajuli N, DesAulniers J, Murray AG, Kassiri Z, Vanhaesebroeck B, Oudit GY.. PI3Kalpha is essential for the recovery from Cre/tamoxifen cardiotoxicity and in myocardial insulin signalling but is not required for normal myocardial contractility in the adult heart. Cardiovasc Res 2015;105:292–303. [DOI] [PubMed] [Google Scholar]
- 15. Wang W, McKinnie SM, Patel VB, Haddad G, Wang Z, Zhabyeyev P, Das SK, Basu R, McLean B, Kandalam V, Penninger JM, Kassiri Z, Vederas JC, Murray AG, Oudit GY.. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic Apelin analogues. J Am Heart Assoc 2013;2:e000249.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu Z, Alloush J, Beck E, Weisleder N.. A murine model of myocardial ischemia-reperfusion injury through ligation of the left anterior descending artery. J Vis Exp 2014;86:51329.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin F, Liao R.. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res 2011;108:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li D, Wu J, Bai Y, Zhao X, Liu L.. Isolation and culture of adult mouse cardiomyocytes for cell signaling and in vitro cardiac hypertrophy. J Vis Exp 2014;87:e51357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dimauro I, Pearson T, Caporossi D, Jackson MJ.. A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res Notes 2012;5:513.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall DD, Ponce JM, Chen B, Spitler KM, Alexia A, Oudit GY, Song LS, Grueter CE.. Ectopic expression of Cdk8 induces eccentric hypertrophy and heart failure. JCI Insight 2017;2:e92476.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, Gude N, Avitabile D, Alvarez R, Sundararaman B, Quijada P, Mason M, Konstandin MH, Malhowski A, Cheng Z, Khan M, McGregor M.. Myocardial AKT: the omnipresent nexus. Physiol Rev 2011;91:1023–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simons M, Gordon E, Claesson-Welsh L.. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 2016;17:611–625. [DOI] [PubMed] [Google Scholar]
- 23. Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML.. Autocrine VEGF signaling is required for vascular homeostasis. Cell 2007;130:691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Small EM, Thatcher JE, Sutherland LB, Kinoshita H, Gerard RD, Richardson JA, Dimaio JM, Sadek H, Kuwahara K, Olson EN.. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res 2010;107:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fraccarollo D, Berger S, Galuppo P, Kneitz S, Hein L, Schutz G, Frantz S, Ertl G, Bauersachs J.. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 2011;123:400–408. [DOI] [PubMed] [Google Scholar]
- 26. Euler-Taimor G, Heger J.. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc Res 2006;69:15–25. [DOI] [PubMed] [Google Scholar]
- 27. Kunkel GH, Chaturvedi P, Tyagi SC.. Mitochondrial pathways to cardiac recovery: TFAM. Heart Fail Rev 2016;21:499–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Finck BN. The PPAR regulatory system in cardiac physiology and disease. Cardiovasc Res 2007;73:269–277. [DOI] [PubMed] [Google Scholar]
- 29. McConnell BB, Yang VW.. Mammalian Kruppel-like factors in health and diseases. Physiol Rev 2010;90:1337–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hausenloy DJ, Yellon DM.. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013;123:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morello F, Perino A, Hirsch E.. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc Res 2008;82:261–271. [DOI] [PubMed] [Google Scholar]
- 32. Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, Simons M, Kyriakides TR, Morales-Ruiz M, Sessa WC.. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci USA 2014;111:12865–12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haddad G, Zhabyeyev P, Farhan M, Zhu LF, Kassiri Z, Rayner DC, Vanhaesebroeck B, Oudit GY, Murray AG.. Phosphoinositide 3-kinase β mediates microvascular endothelial repair of thrombotic microangiopathy. Blood 2014;124:2142–2149. [DOI] [PubMed] [Google Scholar]
- 34. Costa C, Ebi H, Martini M, Beausoleil SA, Faber AC, Jakubik CT, Huang A, Wang Y, Nishtala M, Hall B, Rikova K, Zhao J, Hirsch E, Benes CH, Engelman JA.. Measurement of PIP3 levels reveals an unexpected role for p110beta in early adaptive responses to p110alpha-specific inhibitors in luminal breast cancer. Cancer Cell 2015;27:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, Will M, Yellen P, de Stanchina E, Baselga J, Scher HI, Barry ST, Sawyers CL, Chandarlapaty S, Rosen N.. Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer Cell 2015;27:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu Z, Jiang YP, Wang W, Xu XH, Mathias RT, Entcheva E, Ballou LM, Cohen IS, Lin RZ.. Loss of cardiac phosphoinositide 3-kinase p110 alpha results in contractile dysfunction. Circulation 2009;120:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumar A, Redondo-Muñoz J, Perez-García V, Cortes I, Chagoyen M, Carrera AC.. Nuclear but not cytosolic phosphoinositide 3-kinase beta has an essential function in cell survival. Mol Cell Biol 2011;31:2122–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marques M, Kumar A, Poveda AM, Zuluaga S, Hernandez C, Jackson S, Pasero P, Carrera AC.. Specific function of phosphoinositide 3-kinase beta in the control of DNA replication. Proc Natl Acad Sci USA 2009;106:7525–7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumar A, Fernandez-Capetillo O, Fernadez-Capetillo O, Carrera AC.. Nuclear phosphoinositide 3-kinase beta controls double-strand break DNA repair. Proc Natl Acad Sci USA 2010;107:7491–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Condorelli G, Roncarati R, Ross J Jr, Pisani A, Stassi G, Todaro M, Trocha S, Drusco A, Gu Y, Russo MA, Frati G, Jones SP, Lefer DJ, Napoli C, Croce CM.. Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc Natl Acad Sci USA 2001;98:9977–9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F, Hippe H-J, Linkermann A, Wolf MJ, Rose-John S, Lüllmann-Rauch R, Adam D, Flögel U, Heikenwalder M, Luedde T, Frey N.. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res 2014;103:206–216. [DOI] [PubMed] [Google Scholar]
- 42. Lin Z, Zhou P, von Gise A, Gu F, Ma Q, Chen J, Guo H, van Gorp PR, Wang DZ, Pu WT.. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res 2015;116:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martelli AM, Tabellini G, Bressanin D, Ognibene A, Goto K, Cocco L, Evangelisti C.. The emerging multiple roles of nuclear Akt. Biochim Biophys Acta 2012;1823:2168–2178. [DOI] [PubMed] [Google Scholar]
- 44. Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA.. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res 2004;94:884–891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.