Abstract
Objectives
To compare retention of tocilizumab (TCZ) as monotherapy vs combination therapy with MTX in RA patients achieving clinical improvements during the first year.
Methods
We performed a multicentre cohort study using a real-life registry containing RA patients who had begun TCZ with or without MTX between April 2008 and November 2016. Among patients with ≥50% improvement of clinical disease activity index (CDAI) during the first year (CDAI50 responders), we evaluated whether MTX use may have affected TCZ discontinuation during the second and subsequent years (maintenance therapy).
Results
Among 510 patients with high or moderate CDAI, 328 (64.3%) were CDAI50 responders. The rate of MTX use was 53.0% among responders and 54.4% among non-responders. During maintenance therapy (mean follow-up 30.7 months), 43.9% of CDAI50 responders discontinued TCZ. The most common cause was efficacy loss followed by adverse events. Kaplan-Meier estimates for TCZ retention were 48.3 months (95% CI 42.0, 54.5) for monotherapy and 50.0 months (95% CI 45.9, 54.0) for combination therapy. According to Gray’s test, there was no significant impact of MTX use on cumulative incidence of efficacy loss or adverse events. In the Fine-Gray competing risk regression model, CDAI >10 at the start of maintenance therapy and age were predictive factors for TCZ discontinuation due to efficacy loss (hazard ratio 2.58, 95% CI 1.41, 4.72) and adverse events (hazard ratio 1.04, 95% CI 1.01, 1.08), respectively.
Conclusion
There was no significant difference in TCZ retention between monotherapy and combination therapy with MTX.
Keywords: rheumatoid arthritis, tocilizumab, methotrexate, drug retention, efficacy loss
Rheumatology key messages
MTX use is not necessarily important for maintaining an initial improvement during tocilizumab therapy.
Disease activity at start of maintenance therapy is an important predictor for tocilizumab discontinuation.
Elderly patients are at increased risk of tocilizumab discontinuation due to adverse events.
Introduction
Tocilizumab (TCZ), the first humanized monoclonal anti-interleukin-6 (IL-6) receptor antibody, has been approved in many countries for the treatment of moderate to severe RA. A number of important clinical trials have demonstrated the clinical and radiological efficacy of TCZ as a treatment for active RA patients, even for those who had shown inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), including MTX, and/or one or more TNF inhibitors [1–11]. The rapid effectiveness of TCZ has also been shown in prospective observational studies that are closely comparable to real-life medical care of active RA patients [12–14]. In addition, recently published open-label extension studies following clinical trials as well as real-life observational cohort studies have supported the long-term effectiveness and safety of TCZ beyond two years [15–20].
In daily practice, we often encounter cases in which MTX is contraindicated or not tolerated [21, 22]. Several clinical trials and extension studies have shown that TCZ monotherapy can induce rapid improvements in patients with active RA [1, 2, 6, 7, 10, 15, 16, 20]. Through direct comparisons, recent clinical trials have revealed no significant difference in clinical or radiological response between TCZ monotherapy and combination therapy with MTX: the ACT-RAY trial showed that, for inadequate responders to MTX, both a switch to TCZ monotherapy and an addition of TCZ to MTX therapy (combination therapy) led to meaningful clinical and radiological response [23, 24]. Similar results were obtained from the FUNCTION and U-Act-Early trials for MTX-naive patients with early RA [25–27]. Similar improvements in clinical signs and symptoms as well as comparable safety profiles were also observed in a real-life open-label study for csDMARD- and/or TNF inhibitor-resistant patients when TCZ was used as monotherapy or in combination with csDMARDs [28, 29]. In contrast, the SURPRISE study, a clinical trial conducted in Japan, indicated that, in RA patients with inadequate response to MTX, TCZ treatment in combination with MTX was clinically and radiologically superior to TCZ monotherapy [30]. The importance of MTX in achieving remission during TCZ treatment for RA with high disease activity was also indicated in a one-year observational cohort study conducted in Japan [31].
Recently, we performed a real-life registry study to examine the effectiveness and safety of TCZ in active RA patients with and without renal insufficiency (the ACTRA-RI study), in which 24 weeks of TCZ therapy had good efficacy parameters as well as stable safety and tolerability profiles, regardless of whether patients received MTX concomitantly [32]. The ACTRA-RI registry is an ongoing multicentre cohort consisting of RA patients who have started TCZ therapy since April 2008. In the present study, we used this registry to explore whether the concurrent use of MTX may contribute to prolonged retention of TCZ in RA patients who achieved and maintained ≥50% improvement of clinical disease activity index (CDAI) during the first year of treatment.
Methods
Patients and study design
The ACTRA-RI registry contains all RA patients who have started TCZ therapy since April 2008 under care of the rheumatology department at one of the following institutions in the Kyushu district of Japan: NHO Kumamoto Saishunsou National Hospital, Zenjinkai Shimin-no-Mori Hospital, Yoshitama Clinic for Rheumatic Diseases and Sasebo Chuo Hospital [32]. All registrants are required to be over 18 years of age at the time of TCZ initiation and to fulfill the 1987 Revised ACR criteria or the 2010 ACR/EULAR criteria for diagnosis of RA [33, 34]. TCZ is administered by an intravenous infusion of 8 mg/kg every four weeks or a subcutaneous injection of 162 mg every other week at one of the rheumatology departments. Data sheets for each patient are regularly submitted by the site investigators to the Data Management Center for the ACTRA-RI registry at the Clinical Research Center for Rheumatic Diseases of NHO Kumamoto Saishunsou National Hospital.
For the present study, we followed RA patients who had been enrolled in the ACTRA-RI registry during the period from April 2008 to November 2016. Eligible patients for this study were required to have a high or moderate CDAI at the start of TCZ therapy. We first examined therapeutic response during the first year of TCZ treatment. CDAI50 responders were defined as patients who had achieved and maintained a CDAI50 response at the end of the first year of treatment. We then examined TCZ retention and reasons for TCZ discontinuation in CDAI50 responders entering the second year of treatment. Follow-up started on the first day of the second year of treatment and ended with TCZ discontinuation, loss to follow-up, death or the last follow-up visit prior to December 2017, whichever was first. Reasons for TCZ discontinuation included secondary loss of efficacy, adverse events, remission and other reasons (patient preference, hospital transfer, surgery, etc.). The decision to discontinue was dependent on each treating physician at each participating hospital, and only one reason could be cited for each patient. In this study, all death cases were associated with adverse events that had caused drug discontinuation, and therefore these cases were classified as TCZ discontinuation due to adverse events. Patients who had missed at least two scheduled visits without any contact would have been classified as lost to follow-up, but no patients in this study fell into this category.
This study was conducted in accordance with the principles of the Declaration of Helsinki (2008). The protocol of this study also meets the requirements of the Ethical Guidelines for Medical and Health Research Involving Human Subjects, Japan (2014) and has been approved by the Human Research Ethics Committees of NHO Kumamoto Saishunsou National Hospital (No. 25–5/29–43). Patient informed consent was obtained from all patients prior to registration.
Baseline patient characteristics
For each patient, demographic characteristics and RA-related features such as RA duration, Steinbrocker’s radiographic stage for joint damage, anti-CCP and CDAI values were obtained within two weeks prior to the start date of TCZ treatment. BMI and comorbid diseases such as chronic kidney disease, type 2 diabetes and hypertension were also obtained at the same time. Concurrent use of MTX, other csDMARDs, and prednisolone as well as previous use of MTX and biological agents were recorded at the time of TCZ initiation. We also identified patients who had experienced failure to achieve low CDAI or remission during previous treatment with MTX and/or one or more other biological agents. Year in which TCZ was initiated was also recorded.
RA disease activity
CDAI assessments were performed to quantify RA disease activity during TCZ therapy [35–37]. Cut-off values for disease activity states were defined as follows: high disease activity, CDAI >22; moderate disease activity, CDAI >10 and ≤22; low disease activity, CDAI >2.8 and ≤10; and remission, CDAI ≤2.8 [38]. Clinical responses were evaluated according to the new CDAI improvement criteria, in which minor, moderate and major responses are defined as achieving ≥50% (CDAI50), ≥70% (CDAI70) and ≥85% (CDAI85) improvement in CDAI, respectively [39]. Secondary loss of efficacy was defined as disappearance of an initial CDAI50 improvement during follow-up after the end of the first year of TCZ treatment. In addition, CDAI improvement based on the minimum clinically important difference (MCID), defined as a CDAI reduction >12 for patients starting with a high CDAI and >6 for those starting with a moderate CDAI, was determined to evaluate clinical response to TCZ therapy [40].
Safety analysis
Type and number of adverse events that had caused TCZ discontinuation were examined. Decisions to discontinue TCZ due to adverse events were made by the treating physicians based on a comprehensive evaluation of physical findings, laboratory findings and radiological examinations.
Statistical analysis
To compare baseline clinical characteristics and therapeutic response at 6 and 12 months between CDAI50 responders and non-responders or between TCZ monotherapy and combination therapy with MTX, we calculated means, patient numbers (%), odds ratios (ORs) and 95% CIs.
Estimates for TCZ retention in CDAI50 responders from the second year of treatment were computed from life tables using the Kaplan-Meier approach. We also used the cumulative incidence function (CIF) to estimate the probability of cause-specific discontinuation over time, because we considered the presence of competing risks when estimating cause-specific discontinuation. Log-rank test for the Kaplan-Meier model and Gray’s test for CIF were used for comparisons of estimates between TCZ monotherapy and combination therapy with MTX. Cox proportional hazard regression analysis and Fine-Gray competing risks regression analysis were used to calculate hazard ratios (HRs) for cause-specific TCZ discontinuation associated with MTX use during the second and subsequent years (maintenance therapy), adjusting for confounders. Potential confounders used in the Cox and Fine-Gray regression analyses were selected based on the clinical relevance and importance of each variable, which included age, sex, RA duration, stage III/IV, CDAI values (at baseline and at 6 and 12 months), concurrent use of prednisolone or other csDMARDs, previous failure of RA treatment, previous use of biological agents, BMI, comorbid diseases and calendar year category (year of TCZ initiation), then a backward stepwise selection procedure was used with a cut-off significance level of 0.05 for deleting factors in regression models. All variables identified as true confounders were then included in regression analyses to provide estimates of the effect of MTX use on cause-specific TCZ discontinuation. Risk differences are presented as adjusted HRs with 95% CI. For Cox regression analysis, the proportional hazards assumption was checked using log-minus-log plots of log cumulative hazard curve function and scaled Schoenfeld residual plots for exposure variables over time.
For all tests, probability values (P values) <0.05 were considered to indicate statistical significance. All calculations were performed using either Excel Statistical Analysis 2010 (SSRI, Tokyo, Japan) or PASW Statistics version 22 (SPSS Japan, Tokyo, Japan) and Easy R (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [41].
Results
Baseline characteristics and therapeutic response in the first year of TCZ treatment
A total of 510 patients with high or moderate CDAI started TCZ in the participating hospitals during the period from April 2008 to November 2016. Among them, 328 patients (64.3%) achieved and maintained a CDAI50 response during the first 12 months (CDAI50 responders). The other 182 patients failed to exhibit a CDAI50 response by month 12 because of lack or loss of efficacy (98 patients) or because they dropped out before the end of the first 12 months due to adverse events (60 patients) or patient preference and hospital transfer (24 patients).
As shown in Table 1, mean CDAI values at baseline were significantly higher in CDAI50 responders compared with non-responders (26.0 vs 22.3, OR 1.03 per 1 unit more, 95% CI 1.02, 1.05). Rates of MTX use were similar between these two patient groups (53.0% vs 54.4%, OR 0.95, 95% CI 0.66, 1.36). In addition, there were no significant differences in failure of previous RA treatment, previous use of biological agents, prevalence of comorbid diseases, or TCZ initiation year (2012–2016 vs 2008–2011). After completing the first year of treatment, all CDAI50 responders started the second year with the same therapeutic strategies.
Table 1.
Comparisons of baseline characteristics and therapeutic response between CDAI50 responders and non-responders
| Total (n = 510) | CDAI50 respondersa (n = 328) | Non-respondersa (n = 182) | OR (95% CI)b | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years, mean (95% CI) | 62.8 (61.7, 63.9) | 62.5 (61.1, 64.0) | 63.4 (61.7, 65.0) | 1.00 (0.98, 1.01) |
| Male/female | 109/401 | 61/267 | 48/134 | 0.64 (0.41, 0.98) |
| RA duration, months, mean (95% CI) | 117.2 (107.3, 127.0) | 109.0 (97.2, 120.8) | 131.9 (114.2, 149.5) | 0.99 (0.99, 1.00) |
| Radiographic stages III/IV, number (%) | 309 (60.6) | 191 (58.2) | 118 (64.8) | 0.76 (0.52, 1.10) |
| Anti-CCP (+), number (%) | 439 (86.1) | 288 (87.8) | 151 (83.0) | 1.48 (0.89, 2.46) |
| CDAI at the start of TCZ treatment, mean (95% CI) | 24.7 (23.7, 25.6) | 26.0 (24.7, 27.2) | 22.3 (21.0, 23.7) | 1.03 (1.02, 1.05) |
| High CDAI (CDAI > 22), number (%) | 254 (49.8) | 180 (54.9) | 74 (40.7) | 1.78 (1.23, 2.56) |
| Failure of previous RA treatmentc, number (%) | 373 (73.1) | 240 (73.2) | 133 (73.1) | 1.01 (0.67, 1.51) |
| Previous use of biological DMARDs, number (%) | 287 (56.3) | 176 (53.7) | 111 (61.0) | 0.74 (0.51, 1.07) |
| Concurrent use of MTX, number (%) | 273 (53.5) | 174 (53.0) | 99 (54.4) | 0.95 (0.66, 1.36) |
| Dose, mg/week, mean (95% CI) | 8.9 (8.6, 9.2) | 8.9 (8.5, 9.2) | 8.9 (8.4, 9.5) | 0.98 (0.89, 1.08) |
| Concurrent use of other csDMARDsd, number (%) | 150 (29.4) | 93 (28.4) | 57 (31.3) | 0.87 (0.59, 1.29) |
| Concurrent use of prednisolone, number (%) | 218 (42.7) | 135 (41.2) | 83 (45.6) | 0.83 (0.58, 1.20) |
| Dose, mg/day, mean (95% CI) | 5.8 (5.4, 6.3) | 5.9 (5.3, 6.5) | 5.7 (5.1, 6.4) | 1.01 (0.95, 1.08) |
| Hypertension, number (%) | 187 (36.7) | 116 (35.4) | 71 (39.0) | 0.86 (0.59, 1.24) |
| Type 2 diabetes, number (%) | 75 (14.7) | 49 (14.9) | 26 (14.3) | 1.05 (0.63, 1.76) |
| CKD, number (%) | 98 (19.2) | 61 (18.6) | 37 (20.3) | 0.90 (0.57, 1.41) |
| Year of TCZ initiation (since 2012), number (%) | 308 (60.4) | 196 (59.8) | 112 (61.5) | 0.93 (0.64, 1.35) |
| CDAI at 6 monthse, mean (95% CI) | 10.4 (9.6, 11.2) | 7.9 (7.2, 8.7) | 14.8 (13.2, 16.4) | 0.91 (0.89, 0.93) |
| CDAI85 (major response), number (%) | 102 (20) | 91 (27.7) | 11 (6.0) | 5.97 (3.10, 11.50) |
| CDAI70 (moderate response), number (%) | 194 (38.0) | 173 (52.7) | 21 (11.5) | 8.56 (5.17, 14.17) |
| High and moderate CDAI (CDAI > 10), number (%) | 221 (43.3) | 90 (27.4) | 131 (72.0) | 0.23 (0.16, 0.34) |
| Remission (CDAI ≤ 2.8), number (%) | 83 (16.3) | 74 (22.6) | 9 (4.9) | 5.60 (2.73, 11.49) |
| CDAI50 response, number (%) | 320 (62.7) | 272 (82.9) | 48 (26.4) | 13.6 (8.8, 21.0) |
| MCID-based CDAI improvement, number (%) | 315 (61.8) | 287 (87.5) | 28 (15.4) | 38.5 (22.9, 64.7) |
| CDAI at 12 monthse, mean (95% CI) | 9.0 (8.2, 9.7) | 5.0 (4.6, 5.4) | 16.1 (14.5, 17.6) | 0.75 (0.71, 0.79) |
| CDAI85 (major response), number (%) | 135 (26.5) | 135 (41.2) | 0 | — |
| CDAI70 (moderate response), number (%) | 245 (48.0) | 245 (74.7) | 0 | — |
| High and moderate CDAI (CDAI > 10), number (%) | 188 (36.9) | 26 (7.9) | 162 (89.0) | — |
| Remission (CDAI ≤ 2.8), number (%) | 107 (21.0) | 107 (32.6) | 0 | — |
| MCID-based CDAI improvement, number (%) | 341 (66.9) | 325 (99.1) | 16 (8.8) | — |
aCDAI50 responders were defined as patients who had achieved and maintained a CDAI50 response during the first 12 months of TCZ treatment.
bORs (95% CI) are presented for CDAI50 responders compared with non-responders based on binominal logistic regression analysis. For continuous data, ORs are shown per 1 year more of age, 1 month more of RA duration, 1 mg more of MTX and prednisolone, and 1 unit more of CDAI values.
cPrevious RA treatment includes treatment with biologics and/or MTX.
dOther csDMARDs included tacrolimus, salazosulfapyridine, bucillamine and mizoribine. No patients used leflunomide or targeted synthetic DMARDs.
eFor discontinuation patients, missing data were replaced by the last observed values.
CDAI: clinical disease activity index; CKD: chronic kidney disease; MCID: minimum clinically important difference; TCZ: tocilizumab; DMARDs: disease-modifying antirheumatic drugs; csDMARDs: conventional synthetic DMARDs; OR: odds ratio.
Comparison of baseline characteristics and therapeutic response between CDAI50 responders on TCZ monotherapy and those on combination therapy with MTX
As shown in Table 2, CDAI50 responders receiving TCZ monotherapy were significantly older (66.6 years vs 58.9 years, OR 1.05 per 1 year more, 95% CI 1.03, 1.07) and were less likely to have previously used biological agents (40.9% vs 64.9%, OR 0.37, 95% CI 0.24, 0.59) compared with those receiving combination therapy. Rates of previous treatment failure were also lower in the monotherapy group compared with the combination therapy group (67.5% vs 78.2%, OR 0.58, 95% CI 0.36, 0.95). In addition, patients on TCZ monotherapy had higher rates of chronic kidney disease (27.3% vs 10.9%, OR 3.06, 95% CI 1.69, 5.54) compared with those on combination therapy. Patients receiving monotherapy were more likely to have initiated TCZ since 2012 compared with combination therapy patients (70.8% vs 50.0%, OR 2.42, 95% CI 1.53, 3.83).
Table 2.
Comparisons of baseline characteristics and therapeutic response between TCZ monotherapy patients and combination therapy patients
| CDAI50 respondersa | |||
|---|---|---|---|
| TCZ monotherapy (n = 154) | TCZ + MTX (n = 174) | OR (95% CI)b | |
| Baseline characteristics | |||
| Age, years, mean (95% CI) | 66.6 (64.6, 68.6) | 58.9 (56.9, 60.9) | 1.05 (1.03, 1.07) |
| Male/female | 28/126 | 33/141 | 0.95 (0.54, 1.66) |
| RA duration, months, mean (95% CI) | 114.1 (95.0, 133.2) | 104.5 (89.8, 119.1) | 1.00 (0.99, 1.01) |
| Radiographic stages III/IV, number (%) | 90 (58.4) | 102 (58.0) | 1.02 (0.66, 1.58) |
| Anti-CCP (+), number (%) | 137 (89.0) | 151 (86.8) | 1.23 (0.63, 2.39) |
| CDAI at the start of TCZ treatment, mean (95% CI) | 27.5 (25.5, 29.4) | 24.6 (23.0, 26.3) | 1.02 (1.00, 1.04) |
| High CDAI (CDAI > 22), number (%) | 92 (59.7) | 88 (50.6) | 1.45 (0.94, 2.25) |
| Failure of previous RA treatmentc, number (%) | 104 (67.5) | 136 (78.2) | 0.58 (0.36, 0.95) |
| Previous use of biological DMARDs, number (%) | 63 (40.9) | 113 (64.9) | 0.37 (0.24, 0.59) |
| Concurrent use of other csDMARDsd, number (%) | 50 (32.5) | 43 (24.7) | 1.47 (0.90, 2.37) |
| Concurrent use of prednisolone, number (%) | 63 (40.9) | 72 (41.4) | 0.98 (0.63, 1.52) |
| Hypertension, number (%) | 61 (39.6) | 55 (31.6) | 1.42 (0.90, 2.24) |
| Type 2 diabetes, number (%) | 27 (17.5) | 22 (12.6) | 1.47 (0.80, 2.70) |
| CKD, number (%) | 42 (27.3) | 19 (10.9) | 3.06 (1.69, 5.54) |
| BMI, mean (95% CI) | 22.2 (21.6, 22.7) | 22.5 (21.8, 23.2) | 0.98 (0.93, 1.03) |
| Year of TCZ initiation (since 2012), number (%) | 109 (70.8) | 87 (50.0) | 2.42 (1.53, 3.83) |
| CDAI at 6 months, mean (95% CI) | 7.9 (6.9, 8.8) | 8.0 (6.9, 9.1) | 1.00 (0.97, 1.03) |
| High and moderate CDAI (CDAI > 10), number (%) | 42 (27.3) | 48 (27.6) | 0.95 (0.61, 1.60) |
| Remission (CDAI ≤ 2.8), number (%) | 34 (22.1) | 40 (23.0) | 0.95 (0.57, 1.60) |
| MCID-based CDAI improvement, number (%) | 139 (90.3) | 148 (85.1) | 1.63 (0.83, 3.20) |
| CDAI at 12 months of TCZ treatment, mean (95% CI) | 5.2 (4.5, 5.8) | 4.9 (4.4, 5.5) | 1.02 (0.96, 1.08) |
| High and moderate CDAI (CDAI > 10), number (%) | 14 (9.1) | 12 (6.9) | 1.35 (0.60, 3.02) |
| Remission (CDAI ≤ 2.8), number (%) | 49 (31.8) | 58 (33.3) | 0.93 (0.56, 1.48) |
| MCID-based CDAI improvement, number (%) | 154 (100) | 171 (98.3) | — |
aCDAI50 responders were defined as patients who had achieved and maintained a CDAI50 response during the first 12 months of TCZ treatment.
bORs (95% CI) are presented for monotherapy patients compared with combination therapy patients based on binominal logistic regression analysis. For continuous data, ORs are shown per 1 year more of age, 1 month more of RA duration, 1 mg more of MTX or prednisolone, 1 unit more for CDAI values and 1 unit more of BMI.
cPrevious RA treatment includes treatment with biologics and/or MTX.
dOther csDMARDs included tacrolimus, salazosulfapyridine, bucillamine and mizoribine. No patients used leflunomide or targeted synthetic DMARDs.
CDAI: clinical disease activity index; CKD: chronic kidney disease; MCID: minimum clinically important difference; TCZ: tocilizumab; DMARDs: disease-modifying antirheumatic drugs; OR: odds ratio.
Reasons for discontinuation of TCZ in CDAI50 responders entering the second treatment year with and without MTX
Among CDAI50 responders, we examined TCZ retention during maintenance therapy and compared discontinuation rates among responders with and without MTX. As shown in Table 3, the mean follow-up duration for TCZ treatment from the start of the second year was 30.7 months (95% CI 28.5, 33.0). Discontinuation was seen in 144 out of 328 patients (43.9%), including 21.0% due to secondary loss of efficacy, 9.5% due to adverse events, 5.8% due to remission and 7.9% due to other reasons. Adverse events that caused TCZ discontinuation are shown in the supplementary material, Results section, available at Rheumatology online. Five patients died from adverse events. Neither rates of overall discontinuation nor rates of discontinuation for any specific causes were significantly different between CDAI50 responders receiving TCZ monotherapy and those receiving combination therapy with MTX (Table 3).
Table 3.
Retention of TCZ in CDAI50 responders entering second treatment year: comparisons between monotherapy and combination therapy
| Overall (n = 328) | TCZ monotherapy (n = 154) | TCZ + MTX (n = 174) | OR (95% CI)a | |
|---|---|---|---|---|
| Follow-upb, months, mean (95% CI) | 30.7 (28.5, 33.0) | 27.7 (24.5, 30.9) | 33.4 (30.3, 36.5) | 0.99 (0.98, 1.00) |
| Discontinuation | ||||
| Any reason, number (%) | 144 (43.9) | 63 (40.9) | 81 (46.6) | 0.80 (0.51, 1.23) |
| Secondary loss of efficacy, number (%) | 69 (21.0) | 31 (20.1) | 38 (21.8) | 0.90 (0.53, 1.54) |
| Adverse events, number (%) | 31 (9.5) | 16 (10.4) | 15 (8.6) | 1.22 (0.59, 2.58) |
| Remission, number (%) | 19 (5.8) | 5 (3.2) | 14 (8.0) | 0.38 (0.13, 1.09) |
| Other reasons, number (%) | 25 (7.6) | 11 (7.1) | 14 (8.0) | 0.88 (0.39, 2.00) |
| Kaplan-Meier estimates for retention (duration)c | (P values)d | |||
| Any reason, months, mean (95% CI) | 50.0 (45.9, 54.0) | 48.3 (42.0, 54.5) | 50.0 (45.9, 54.0) | 0.57 |
| Secondary loss of efficacy, months, mean (95% CI) | 65.9 (61.3, 70.4) | 64.7 (57.3, 72.1) | 65.4 (60.0, 70.8) | 0.50 |
| Adverse events, months, mean (95% CI) | 76.9 (72.8, 81.0) | 74.1 (72.8, 81.0) | 76.9 (72.5, 81.3) | 0.25 |
| Remission/other reasons, months, mean (95% CI) | 74.2 (70.2, 78.3) | 74.7 (68.0, 81.3) | 71.2 (66.3, 76.2) | 0.44 |
aORs (95% CI) are presented for TCZ monotherapy compared with combination therapy based on binominal logistic regression analysis.
bFollow-up was measured from the start of the second year of TCZ treatment.
cRetention was defined as the length of time between the start of the second treatment year and the date of TCZ discontinuation. In all participants (n = 510), the mean retention from the time of TCZ initiation was 45.2 months (95% CI 40.2, 50.3) for monotherapy and 48.9 months (95% CI 44.2, 53.9) for combination therapy (P = 0.51 by log-rank test).
d P values were determined using log-rank test for comparisons of estimates between monotherapy and combination therapy. CDAI: clinical disease activity index; TCZ: tocilizumab; OR, odds ratio.
Kaplan-Meier estimates for TCZ retention and cumulative incidences of cause-specific TCZ discontinuation
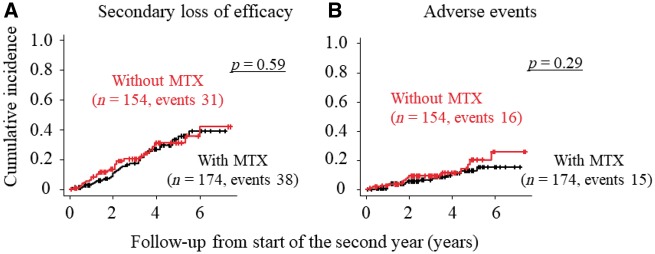
According to the Kaplan-Meier approach, mean duration of overall TCZ retention was 50.0 months (95% CI, 45.9, 54.0) from the start of the second year; the mean duration was 48.3 months (95% CI 42.0, 54.5) in monotherapy patients and 50.0 months (95% CI 45.9, 54.0) in combination therapy patients (Table 3). There was no significant difference in retention probability over time between monotherapy and combination therapy with MTX (Fig. 1). The probability of the occurrence of cause-specific TCZ discontinuation was estimated by CIF analysis based on a competing risks model. As shown in Fig. 2, the cumulative incidence of cause-specific TCZ discontinuation was also similar between the two treatment groups.
Fig. 1.
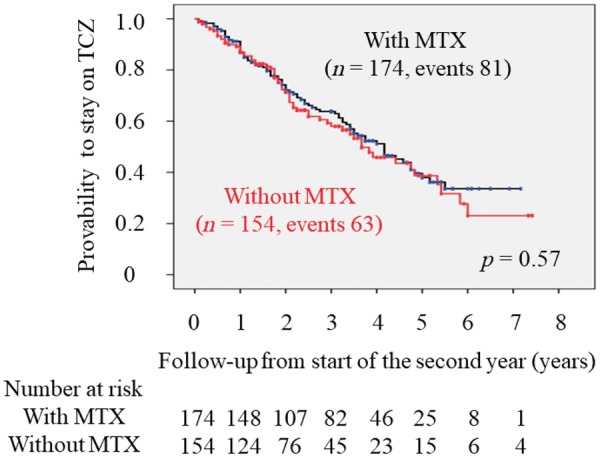
Kaplan-Meier plots of TCZ retention grouped by concurrent MTX use
Retention probabilities of TCZ in CDAI50 responders entering the second treatment year are shown grouped according to concurrent MTX use. Numbers below these figures represent the numbers of CDAI50 responders remaining on TCZ treatment. TCZ retention between monotherapy and combination therapy with MTX was compared using the log-rank test. TCZ: tocilizumab; CDAI, clinical disease activity index.
Fig. 2.
Cumulative incidence of cause-specific discontinuation of TCZ in CDAI50 responders
Using CIF, the cumulative incidence of cause-specific TCZ discontinuation in CDAI50 responders entering the second treatment year are shown grouped according to concurrent MTX use: (A) secondary loss of efficacy and (B) adverse events. Provability of TCZ discontinuation between monotherapy and combination therapy with MTX was compared using Gray’s test. TCZ: tocilizumab; CDAI, clinical disease activity index; CIF: cumulative incidence function.
Cox and Fine-Gray regression models
In both the Cox and Fine-Gray regression models, CDAI >10 at 12 months and age were significant predictors for increased risk of TCZ discontinuation due to secondary loss of efficacy and for adverse events, respectively (Table 4). After adjusting the HRs for these true cofounders using both regression models, the concurrent use of MTX had no significant impact on the risk of TCZ discontinuation during maintenance therapy. The results did not change when TCZ discontinuations due to remission or other reasons were included in the CIF or Fine-Gray regression analysis as competing events. No predictive factor was identified for discontinuation due to these reasons.
Table 4.
Predictive factors for TCZ discontinuation in CDAI50 responders entering the second treatment year
| Cox regression model | Fine-Gray regression model | |||
|---|---|---|---|---|
| Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Due to secondary loss of efficiency | ||||
| High and moderate CDAI at 12 months | 2.35 (1.21, 4.54) | 0.01 | 2.58 (1.41, 4.72) | 0.002 |
| High and moderate CDAI at 6 months | 1.77 (1.07, 2.94) | 0.03 | — | — |
| Due to adverse events | ||||
| Age per 1 year more | 1.05 (1.02, 1.09) | 0.01 | 1.04 (1.02, 1.08) | 0.01 |
All confounders listed in the table were factors identified as true confounders and were included in the final regression model.
TCZ: tocilizumab; CDAI: clinical disease activity index; HR: hazard ratio.
Sensitive analyses for MCID-based CDAI responders and biologic-experienced CDAI50 responders
Patient selection based on CDAI50 response might have caused bias in the analysis of TCZ retention. In this study, patients with higher CDAI values at baseline were more likely to achieve CDAI50 response after one year of TCZ treatment. In addition, 341 patients were MCID-based CDAI responders during the first year, but 16 of these were classified as CDAI50 non-responders (Table 1). To address this issue, we performed additional analyses for patients who had achieved MCID-based CDAI response during the first treatment year, using the same statistical approach described above. We found that regarding the effect of MTX use on TCZ retention and cause-specific discontinuation, data obtained from MCID-based CDAI responders were consistent with those obtained from CDAI50 responders (Supplementary Table S1 and Table S2, and Fig. S1, available at Rheumatology online).
Besides the limitation of CDAI50 response, the use of a mixed population of biologic-naïve and experienced patients may have been another confounder in the analysis of TCZ retention during maintenance therapy. To reduce the effect of this confounding, we performed statistical analyses for TCZ retention and cause-specific discontinuation in CDAI50 responders after stratifying patients based on previous use of biological agents. As shown in Supplementary Table S3, Table S4, and Fig. S2, available at Rheumatology online, data obtained from biologic-experienced CDAI50 responders were consistent with those obtained from all CDAI50 responders in terms of the effect of MTX use on TCZ retention and cause-specific discontinuation. Similar data were also obtained from biologic-naïve CDAI50 responders (data not shown). In these sensitive analyses, there was no significant difference in TCZ retention between monotherapy and combination therapy with MTX.
Discussion
In this multicentre cohort study using the ACTRA-RI registry, the estimated mean durations of TCZ retention in first-year CDAI50 responders were 48.3 months for monotherapy and 50.0 months for combination therapy with MTX. The adjusted risks for TCZ discontinuation due to secondary loss of efficacy and adverse events were similar between monotherapy and combination therapy with MTX.
In a European collaborative study for RA (the TOCERRA registry study), Gabay et al. showed that clinical response as assessed by CDAI change as well as the likelihood of remission were not significantly different between patients receiving TCZ as monotherapy and patients receiving it in combination with csDMARDs, but TCZ retention was more prolonged in combination therapy patients than in monotherapy patients (retention time 3.7 years vs 2.3 years from the start of treatment). There was no significant effect of MTX on the risk of TCZ discontinuation during the first 1.5 years; after that period, however, the risk of discontinuation in monotherapy significantly increased over time (adjusted HRs 1.54 at 2 years, 3.00 at 3 years and 5.86 at 4 years) [42]. Using the same registry, however, Lauper et al. showed that the risk of TCZ discontinuation was similar between monotherapy and combination therapy with csDMARDs in patients who had shown inadequate response to one or more biological DMARDs. Kaplan-Meier estimates for TCZ retention were 2.31 years for monotherapy and 1.98 years for combination therapy [43]. Compared with the TOCERRA registry, TCZ retention was longer in the Japanese ACTRA-RI registry, either as monotherapy or combination therapy. Retention time from the start of TCZ treatment in all participants was also longer compared with the TOCERRA registry. TCZ retention can be influenced by many factors. A recent study using California Medicaid data showed a race/ethnicity difference in rates of biological DMARD use among RA patients with relatively homogeneous socioeconomic status and healthcare benefits, suggesting that the mechanism underlying DMARD choice may be related to patient preference and/or patient trust of physician recommendations [44]. It is unclear whether such factors may account for the difference in TCZ retention between the European and Japanese registries.
The development of antidrug antibodies (ADAs) can lead to secondary loss of efficacy. The appearance of ADAs is commonly observed in RA patients receiving biological agents, especially with anti-TNF monoclonal antibodies such as adalimumab and infliximab [45]. The concurrent use of MTX appears to decrease the risk of production of ADAs in RA patients. In a 26-week clinical trial, Maini et al. showed that ADAs were observed in 21% of patients receiving 3 mg/kg of infliximab alone but in only 7% of patients receiving the same dose of infliximab in combination with MTX [46]. Through immunogenicity assays on blood samples obtained from previous clinical trials of TCZ, Burmester et al. indicated that the rate of anti-TCZ antibody development was low, regardless of whether TCZ was used as monotherapy or in combination with csDMARDs (incidence rates 0.7–2.0%) [47]. Similar incidence rates of anti-TCZ antibody detection were reported in the ACT-RAY study, in which immunogenic effects were compared between patients who added TCZ to MTX therapy and patients who switched to TCZ monotherapy (1.5% vs 2.2%) [24]. The low immunogenic risk of TCZ may explain the observed similarity between the efficacy of TCZ monotherapy and that of combination therapy [23–29, 48]. In addition, recent clinical trials have shown that, after achieving low disease activity or good/moderate EULAR response during 24-week subcutaneous or intravenous TCZ therapy with MTX, patients can discontinue or taper MTX without significant worsening of disease activity in the following 16 or 36 weeks [49, 50]. In the present study, we showed that concurrent use of MTX is not necessarily important for maintaining an initial CDAI50 response during a mean follow-up duration of 30.7 months. These findings may also be explained by the low immunogenicity of TCZ.
The present study was an observational cohort study providing important information regarding long-term retention of TCZ in a real-world medical care setting. However, because studies of this type adhere to less restrictive methodological standards than randomized controlled clinical trials do, they can be limited by inherent methodological problems that make it difficult to interpret the results. Confounding by indication might have resulted in biased estimates for the effect of TCZ monotherapy and that of TCZ in combination with MTX. To address this problem, we performed Fine-Gray regression analysis including the possible confounders and calculated adjusted HRs for cause-specific discontinuation. In spite of our efforts, however, we cannot entirely exclude the presence of residual confounding by other unmeasured confounders.
In conclusion, the concurrent use of MTX had no significant impact on either CDAI50 achievement during the first year of TCZ treatment or TCZ retention during maintenance therapy after the end of the first year. The most common cause of TCZ discontinuation was efficacy loss followed by adverse events. The cumulative incidence of cause-specific TCZ discontinuation was similar between monotherapy and combination therapy. Considering that aging is associated with increased prevalence of renal insufficiency and other comorbid conditions in RA patients, it is reasonable to assume that the rate of MTX intolerance will also increase in this patient population. TCZ monotherapy can be an important option for the initiation and maintenance of favorable responses in RA patients who cannot be treated with MTX.
Supplementary Material
Acknowledgements
S.M. and Y.U. contributed to the study concept and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. T.Y., T.H. and N.H. participated in data collection and drafted the manuscript. Y.A. and K.A. contributed to the analysis and interpretation of data as well as drafting of the manuscript. All authors read and approved the final manuscript.
Funding: This study was supported by research funds from the National Hospital Organization, Japan.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Maini RN, Taylor PC, Szechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006;54:2817–29. [DOI] [PubMed] [Google Scholar]
- 2. Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis 2007;66:1162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968–80. [DOI] [PubMed] [Google Scholar]
- 4. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- 6. Nishimoto N, Miyasaka N, Yamamoto K, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol 2009;19:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 2010;69:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011;63:609–21. [DOI] [PubMed] [Google Scholar]
- 9. Yazici Y, Curtis JR, Ince A, et al. Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease-modifying antirheumatic drugs: the ROSE study. Ann Rheum Dis 2012;71:198–205. [DOI] [PubMed] [Google Scholar]
- 10. Ogata A, Tanimura K, Sugimoto T, et al. Phase III study of the efficacy and safety of subcutaneous versus intravenous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res 2014;66:344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burmester GR, Rubbert-Roth A, Cantagrel A, et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis 2014;73:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burmester GR, Feist E, Kellner H, et al. Effectiveness and safety of the interleukin 6-receptor antagonist tocilizumab after 4 and 24 weeks in patients with active rheumatoid arthritis: the first phase IIIb real-life study (TAMARA). Ann Rheum Dis 2011;70:755–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bykerk VP, Ostor AJ, Alvaro-Gracia J, et al. Tocilizumab in patients with active rheumatoid arthritis and inadequate responses to DMARDs and/or TNF inhibitors: a large, open-label study close to clinical practice. Ann Rheum Dis 2012;71:1950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iking-Konert C, von Hinuber U, Richter C, et al. ROUTINE-a prospective, multicentre, non-interventional, observational study to evaluate the safety and effectiveness of intravenous tocilizumab for the treatment of active rheumatoid arthritis in daily practice in Germany. Rheumatology 2016;55:624–35. [DOI] [PubMed] [Google Scholar]
- 15. Nishimoto N, Miyasaka N, Yamamoto K, et al. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis 2009;68:1580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogata A, Atsumi T, Fukuda T, et al. Sustainable efficacy of switching from intravenous to subcutaneous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res 2015;67:1354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kremer JM, Blanco R, Halland AM, et al. Clinical efficacy and safety maintained up to 5 years in patients with rheumatoid arthritis treated with tocilizumab in a randomised trial. Clin Exp Rheumatol 2016;34:625–33. [PubMed] [Google Scholar]
- 18. Hirabayashi Y, Munakata Y, Miyata M, et al. Clinical and structural remission rates increased annually and radiographic progression was continuously inhibited during a 3-year administration of tocilizumab in patients with rheumatoid arthritis: a multi-center, prospective cohort study by the Michinoku Tocilizumab Study Group. Mod Rheumatol 2016;26:828–35. [DOI] [PubMed] [Google Scholar]
- 19. Burmester GR, Rubbert-Roth A, Cantagrel A, et al. Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA). Ann Rheum Dis 2016;75:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones G, Wallace T, McIntosh MJ, et al. safety of tocilizumab monotherapy in patients with rheumatoid arthritis who were methotrexate- and biologic-naive or free of methotrexate for 6 months: the AMBITION study. J Rheumatol 2017;44:142–6. [DOI] [PubMed] [Google Scholar]
- 21. Mori S, Hidaka M, Kawakita T, et al. Factors associated with myelosuppression related to low-dose methotrexate therapy for inflammatory rheumatic diseases. PLoS One 2016;11:e0154744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mori S, Yoshitama T, Hirakata N, Ueki Y. Prevalence of and factors associated with renal dysfunction in rheumatoid arthritis patients: a cross-sectional study in community hospitals. Clin Rheumatol 2017;36:2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dougados M, Kissel K, Sheeran T, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis 2013;72:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dougados M, Kissel K, Conaghan PG, et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study. Ann Rheum Dis 2014;73:803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis 2016;75:1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016;388:343–55. [DOI] [PubMed] [Google Scholar]
- 27. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab combination therapy or monotherapy or methotrexate monotherapy in methotrexate-naive patients with early rheumatoid arthritis: 2-year clinical and radiographic results from the randomised, placebo-controlled FUNCTION trial. Ann Rheum Dis 2017;76:1279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weinblatt ME, Kremer J, Cush J, et al. Tocilizumab as monotherapy or in combination with nonbiologic disease-modifying antirheumatic drugs: twenty-four-week results of an open-label, clinical practice study. Arthritis Care Res 2013;65:362–71. [DOI] [PubMed] [Google Scholar]
- 29. Bykerk VP, Ostor AJ, Alvaro-Gracia J, et al. Comparison of tocilizumab as monotherapy or with add-on disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and inadequate responses to previous treatments: an open-label study close to clinical practice. Clin Rheumatol 2015;34:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaneko Y, Atsumi T, Tanaka Y, et al. Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study). Ann Rheum Dis 2016;75:1917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kojima T, Yabe Y, Kaneko A, et al. Importance of methotrexate therapy concomitant with tocilizumab treatment in achieving better clinical outcomes for rheumatoid arthritis patients with high disease activity: an observational cohort study. Rheumatology 2015;54:113–20. [DOI] [PubMed] [Google Scholar]
- 32. Mori S, Yoshitama T, Hidaka T, Hirakata N, Ueki Y. Effectiveness and safety of tocilizumab therapy for patients with rheumatoid arthritis and renal insufficiency: a real-life registry study in Japan (the ACTRA-RI study). Ann Rheum Dis 2015;74:627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 34. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 35. Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum 2011;63:43–52. [DOI] [PubMed] [Google Scholar]
- 36. Schoels M, Alasti F, Smolen JS, Aletaha D. Evaluation of newly proposed remission cut-points for disease activity score in 28 joints (DAS28) in rheumatoid arthritis patients upon IL-6 pathway inhibition. Arthritis Res Ther 2017;19:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mori S, Yoshitama T, Ueki Y. Tofacitinib therapy for rheumatoid arthritis: a direct comparison study between biologic-naïve and experienced patients. Internal Medicine 2018;57:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23:S100–8. [PubMed] [Google Scholar]
- 39. Aletaha D, Martinez-Avila J, Kvien TK, Smolen JS. Definition of treatment response in rheumatoid arthritis based on the simplified and the clinical disease activity index. Ann Rheum Dis 2012;71:1190–6. [DOI] [PubMed] [Google Scholar]
- 40. Curtis JR, Yang S, Chen L, et al. Determining the minimally important difference in the clinical disease activity index for improvement and worsening in early rheumatoid arthritis patients. Arthritis Care Res 2015;67:1345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gabay C, Riek M, Hetland ML, et al. Effectiveness of tocilizumab with and without synthetic disease-modifying antirheumatic drugs in rheumatoid arthritis: results from a European collaborative study. Ann Rheum Dis 2016;75:1336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lauper K, Nordstrom DC, Pavelka K, et al. Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biologic disease-modifying antirheumatic drug: analyses from the pan-European TOCERRA register collaboration. Ann Rheum Dis 2018;77:1276–82. [DOI] [PubMed] [Google Scholar]
- 44. Chu LH, Portugal C, Kawatkar AA, Stohl W, Nichol MB. Racial/ethnic differences in the use of biologic disease-modifying antirheumatic drugs among California Medicaid rheumatoid arthritis patients. Arthritis Care Res 2013;65:299–303. [DOI] [PubMed] [Google Scholar]
- 45. Schaeverbeke T, Truchetet ME, Kostine M, et al. Immunogenicity of biologic agents in rheumatoid arthritis patients: lessons for clinical practice. Rheumatology 2016;55:210–20. [DOI] [PubMed] [Google Scholar]
- 46. Maini RN, Taylor PC, Paleolog E, et al. Anti-tumour necrosis factor specific antibody (infliximab) treatment provides insights into the pathophysiology of rheumatoid arthritis. Ann Rheum Dis 1999;58 Suppl 1:I56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burmester GR, Choy E, Kivitz A, et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:1078–85. [DOI] [PubMed] [Google Scholar]
- 48. Teitsma XM, Marijnissen AK, Bijlsma JW, Lafeber FP, Jacobs JW. Tocilizumab as monotherapy or combination therapy for treating active rheumatoid arthritis: a meta-analysis of efficacy and safety reported in randomized controlled trials. Arthritis Res Ther 2016;18:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kremer JM, Rigby W, Singer NG, et al. Sustained response following discontinuation of methotrexate in patients with rheumatoid arthritis treated with subcutaneous tocilizumab: results from a randomized, controlled trial. Arthritis Rheumatol 2018;70:1200–8. [DOI] [PubMed] [Google Scholar]
- 50. Edwards CJ, Ostor AJK, Naisbett-Groet B, Kiely P. Tapering versus steady-state methotrexate in combination with tocilizumab for rheumatoid arthritis: a randomized, double-blind trial. Rheumatology 2018;57:84–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.