Summary
Electroencephalographic density spectral array monitoring has been developed to facilitate the interpretation of unprocessed electroencephalogram signals. The primary aim of this prospective observational study, performed in a tertiary children's hospital, was to identify the clinical applicability and validity of density spectral array monitoring in infants and children during sevoflurane anaesthesia. We included 104 children, aged < 6 years, undergoing elective surgery during sevoflurane anaesthesia. We investigated the correlation between non‐steady state end‐tidal sevoflurane and the expression of the four electroencephalogram frequency bands β, α, θ and δ, representing density spectral array. Patients were divided into three age groups (< 6 months, 6–12 months, > 12 months). There was a significant correlation between end‐tidal sevoflurane and density spectral array in the age groups 6–12 months (p < 0.05) and 1–6 years (p < 0.0001). In infants < 6 months of age, the relative percentages of density spectral array did not correlate with end‐tidal sevoflurane. The main finding was that different end‐tidal concentrations of sevoflurane produce age‐dependent changes in the density spectral array power spectrum. In infants younger than 6 months‐old, α and β coherence are absent, whereas θ and δ oscillations have already emerged. In cases where anaesthesia was too deep, this presented as burst suppression on the electroencephalogram, θ disappeared, leaving the electroencephalographic activity in the δ range. Future research should address this issue, aiming to clarify whether the emergence of θ oscillations in infants helps to prevent sevoflurane overdosing.
Keywords: anaesthesia, children, density spectral array, sevoflurane
Introduction
Electroencephalographic density spectral array (DSA) monitoring has been proposed to facilitate the interpretation of unprocessed electroencephalogram (EEG) signals in patients undergoing general anaesthesia 1. Encephalographic DSA is a three‐dimensional method to display EEG signals consisting of the EEG frequency (y‐axis), the power of the EEG signal (originally the z‐axis, but colour‐coded to be integrated into a two‐dimensional plot) and the development of the EEG power spectrum over time (x‐axis). The power spectrum is encoded in different colours; blue implies minimal power and red implies high or maximal power 1, 2. Compared with processed EEG monitoring, DSA provides real‐time EEG information 3. One of the major advantages of real‐time DSA EEG monitoring is the lack of any delay in detecting even subtle changes in the depth of hypnosis. However, DSA has not yet been widely implemented in the daily practice of paediatric anaesthesia. We decided to investigate the additional value of DSA in children undergoing sevoflurane anaesthesia. The primary outcome parameter of this prospective observational study in children < 6 years of age was to determine the correlation between DSA and various non‐steady‐state sevoflurane concentrations.
Methods
Following local research ethics committee approval, informed consent was obtained from the parents of all the children. We included children from birth to 6 years of age, scheduled to undergo elective surgery in the Erasmus MC‐Sophia Children's Hospital. Anaesthesia maintained with sevoflurane as the sole anaesthetic agent was the main inclusion criterion. Exclusion criteria were non‐elective procedures, neurosurgical procedures and the use of premedication. Standard monitoring, consisting of ECG, NIBP, capnography, pulse oximetry and end‐tidal sevoflurane (ETsevo) was applied.
Electroencephalographic monitoring of the depth of anaesthesia was performed using the Narcotrend EEG monitor (MT Monitortechnik, Bad Bramstedt, Germany). The Narcotrend calculates an index of hypnotic depth, ranging from 100 (fully awake) to 0 (very deep hypnosis), called the Narcotrend index. As well as the processed Narcotrend index, the Narcotrend monitor records the EEG power spectrum, relative power in β (% β: 13–25 Hz), α (% α: 9–12 Hz), θ (% θ: 5–8 Hz) and δ (% δ: 1–4 Hz), and DSA. Patients were attached to the Narcotrend monitor, according to the manufacturer's recommendations, using three electrodes positioned on the forehead.
Induction of anaesthesia was performed using a facemask with sevoflurane, or with intravenous (i.v.) propofol. The end‐tidal sevoflurane concentration was chosen by the attending anaesthetist, aiming to maintain an adequate depth of anaesthesia based on individual clinical judgement. The attending anaesthetist did not have access to the screen of the depth of hypnosis monitor. Raw EEG data were continuously recorded in each patient and exported as Excel files for subsequent analysis using the EEG Viewer™ software package (Version 1.6, MT MonitorTechnik GmbH & Co. KG, Bad Bramstedt, Germany).
Intra‐operative use of propofol, opioids, other analgesics and locoregional techniques were recorded. If induction of anaesthesia was performed with i.v. propofol, then DSA monitoring was commenced 15 min after induction. If a bolus of propofol was given during maintenance of anaesthesia, DSA measurements were postponed for 15 min after the bolus in order to exclude the effect of propofol on the DSA relative power percentages.
Based on previously performed studies 4, 5, patients were divided into three different age groups (< 6 months; 6–12 months; and 1–6 years) for analysis.
In order to investigate variations in total power between different age groups, we performed a one‐way ANOVA with a Games–Howell post‐hoc test. Spearman correlation was performed to analyse the correlation between ETsevo and the relative EEG power percentages. The relationship between DSA relative power and ETsevo was displayed using a heatmap, a graphical tool representing grouped data. Continuous variables were tested for normality using the D'Agostino–Pearson omnibus test and the Shapiro–Wilk normality test. Statistical analysis was performed using SPSS for Mac (version 22.0 SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7 for Mac (version 7.0, GraphPad Software Inc., San Diego, CA, USA). A p value of < 0.05 was considered statistically significant.
Results
We recorded data in 108 children. Data from four patients had to be excluded; one patient was not included due to the use of premedication (group 3) and three due to technical issues with data collection (two who were < 6 months old and one who was between 1–6 years of age). This resulted in 104 datasets being available for analysis. Baseline characteristics of patients included in the study are shown in Table 1.
Table 1.
Baseline characteristics of patients included in the study. Values are median (IQR [range]) or number (proportion)
| 0–6 months old | 6–12 months old | 1–6 years old | |
|---|---|---|---|
| n = 23 | n = 30 | n = 51 | |
| Age; months | 3 (2–4 [0–5]) | 9 (7–10 [6–11]) | 27 (14–46 [12–70]) |
| Weight; kg | 4.8 (3.8–6.0 [2.0–8.3]) | 8.0 (7.4–9.0 [6.2–10.0]) | 12.0 (10.0–15.0 [7.0–30.0]) |
| Sex; female | 5 (22%) | 2 (7%) | 19 (37%) |
There was a significant difference in total power between the three different age groups (Table 2). There was a significant correlation between ETsevo and relative EEG power in β, α, θ and δ in children aged 6–12 months and 1–6 years. (Table 3). From 6 months of age, the ETsevo concentration had a significant negative correlation with the relative percentage of β and α, and a significantly positive correlation with θ and δ. In infants < 6 months of age, no significant correlation was found.
Table 2.
Multiple comparisons between different age groups using Games–Howell non‐parametric post‐hoc test
| SE | p value | Mean difference (95%CI) | ||
|---|---|---|---|---|
| 0–6 months | 6–12 months | 84.7 | 0.001 | −322.7 (−528.0 to −117.4) |
| 1–6 years | 244.7 | <0.001 | −1806.3 (−2395.0 to −1217.7) | |
| 6–12 months | 1–6 years | 241.5 | <0.001 | −1483.6 (−2065.3 to −901.9) |
SE, standard error.
Table 3.
Spearman correlation analysis for the three groups included in the study. Values are r (95%CI)
| ETsevo | ETsevo | ETsevo | |
|---|---|---|---|
| 0–6 months | 6–12 months | 1–6 years | |
| Rel. β % | 0.17 (−0.18 to 0.47) | −0.82 (−0.91 to −0.65)** | −0.77 (−0.88 to −0.60)** |
| Rel. α % | 0.25 (−0.09 to 0.54) | −0.54 (−0.75 to −0.22)* | −0.68 (−0.83 to −0.45)** |
| Rel. θ % | 0.33 (−0.00 to 0.60) | 0.81 (0.65 to 0.91)** | 0.80 (0.64–0.90)** |
| Rel. δ % | −0.24 (−0.53 to 0.10) | 0.74 (0.52 to 0.87)** | 0.70 (0.47–0.83)** |
*p < 0.050.
**p < 0.001.
Increasing ETsevo caused augmentation in frontal θ and δ activity and a decrease in β and α oscillations in children > 6 months. In infants < 6 months, the EEG power spectrum consisted mainly of δ oscillations and minimal θ oscillations. The β and α oscillations were absent in this age group. This effect of ETsevo on DSA is illustrated using heatmaps (Fig. 1).
Figure 1.
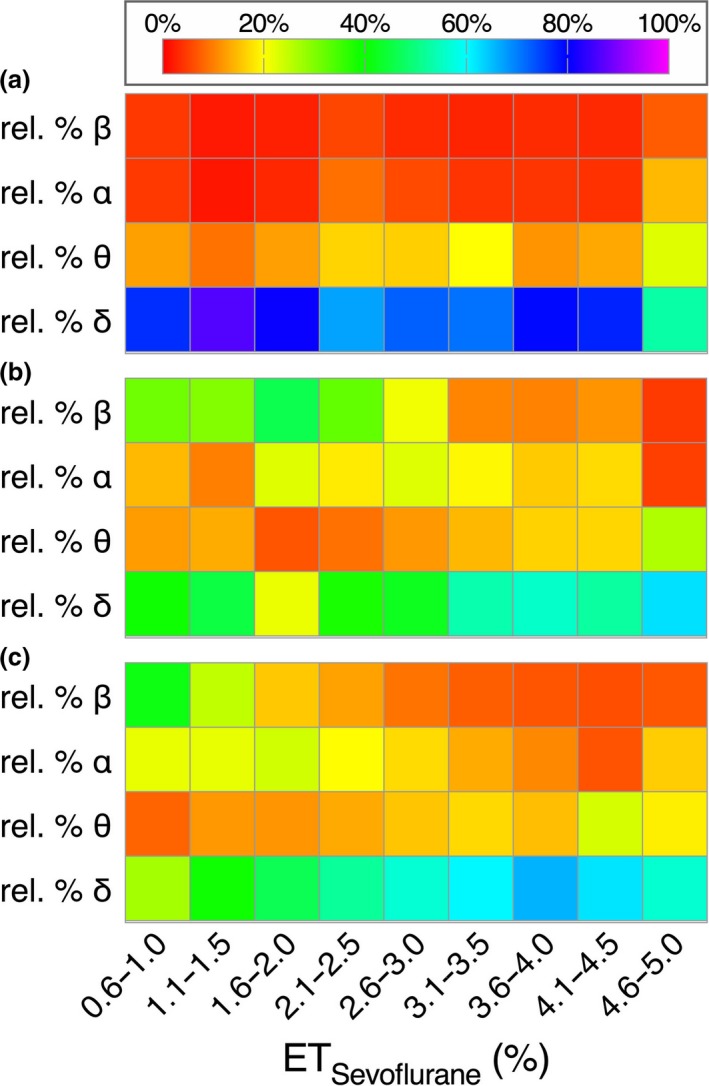
The effect of ETsevo on DSA. (a) represents infants aged 0–6 months, (b) represents infants aged 6–12 months and (c) represents children aged 1–6 years.
Figure 2 shows some typical examples of the relationship between the ETsevo concentration, the Narcotrend index and the DSA colour spectrum in each age group. Figures 2b and 2c show an example of the positive correlation of θ and δ with ETsevo in a 9‐month‐old infant and a 38‐month‐old child, respectively. The negative correlation of β and α with ETsevo is best seen in Fig. 2b. Figure 2a of a 3‐month‐old infant shows no β or α oscillations and just a little θ expression at between 0 and 30 min.
Figure 2.
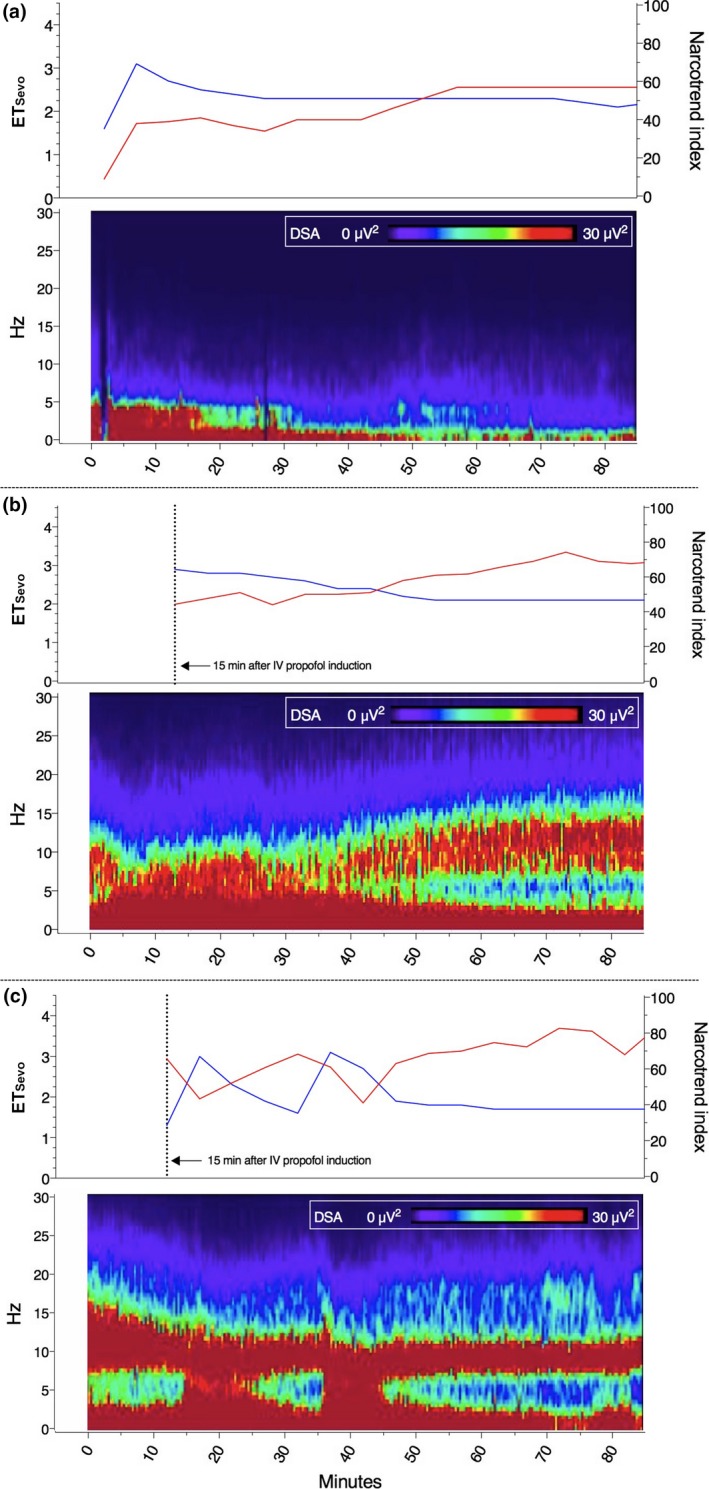
Examples of the relationship between the ETsevo concentration, the Narcotrend index and the density spectral array (DSA) colour spectrum in each age group; (a) represents a 3‐month‐old infant, a 9‐month‐old infant is represented in (b), and (c) represents a 38‐month‐old child. The red line indicates Narcotrend index and the blue line ETsevo concentration.
The Narcotrend index calculation failed in 13% of patients age < 6 months compared with 3% age 6–12 months. In 39% of patients age < 6 months Narcotrend index monitoring partially failed, compared with 7% in age 6–12 months and 6% age 1–6 years.
In three patients, propofol was administered during maintenance of anaesthesia because the surgeon reported patient movement. In two of these patients (age < 6 months) no difference in DSA colour spectrum was identified before the administration of propofol. In the third patient (age 1–6 years), due to technical issues, no EEG data could be recorded at the moment of propofol administration.
Discussion
The main finding of this clinical observational study is that different end‐tidal concentrations of sevoflurane produce changes in DSA power spectrum and appear to be age dependent.
In children > 6 months, heatmaps show a profound dose‐dependent sevoflurane effect on EEG power. This effect is less obvious in infants < 6 months. With increasing age, a significant increase in EEG total power was identified. This finding is in accordance with a previously performed study by Akeju et al. 4. The α coherence is defined as a prominent EEG feature associated with sevoflurane‐induced general anaesthesia 1, 6. In our study, in patients < 6 months, δ and θ coherence is present and β and α coherence is absent. Akeju et al. showed α coherence was absent in a very small group of four patients aged < 1 year 4. Cornelissen et al., who used full‐scale EEG monitoring during sevoflurane general anaesthesia, reported the emergence of α and θ oscillations in children at approximately 4 months of age 7.
Processed EEG devices such as the bispectral index monitor and the Narcotrend index monitor are more often used in paediatric anaesthesia practice than DSA. The studies designed to validate these monitors in children were mostly performed in those aged > 5 months and were mainly focused on the ability of an index value to distinguish between the awake and the unconsciousness state 8, 9.
Dennhardt et al. 10 recently investigated the effect of age on Narcotrend index monitoring during sevoflurane anaesthesia. They reported limited feasibility of the Narcotrend index monitor in infants < 4 months of age due to the lower likelihood (29.6%) of showing a Narcotrend index. In our study, the feasibility of the Narcotrend index to generate a value was also reduced in age < 6 months. McKeever et al. 11, demonstrated the relationship between age‐adjusted minimum alveolar concentration (MAC) multiples and amplitude‐integrated electroencephalography (aEEG) in young children. They found that aEEG in children < 2 years of age did not correlate with MAC. In their study, in infants aged 1–6 months, spectral edge frequency 90 (SEF90) did not correlate with MAC and the bispectral index (BIS) performed poorly. These findings are in accordance with the conclusion made by Davidson 8 that depth of anaesthesia monitors may not function in infants or young children.
With knowledge that the processed EEG does not appear to function, and that there is no significant correlation between relative power in θ in infants < 6 months old, the question remains as to whether it makes sense at all to use EEG monitoring during anaesthesia in this age group. However, we believe there is a place for EEG monitoring in this vulnerable patient group. Although ETsevo and θ oscillations are not significantly correlated in infants < 6 months old, the oscillations are at least present. This does not appear to be the case with β and α oscillations. It is widely accepted that too deep a level of anaesthesia is almost always associated with burst suppression and disappearance of θ oscillations, leaving EEG activity in the δ range 1. Therefore, from a neurophysiological perspective, the presence or absence of θ oscillations in young infants under sevoflurane anaesthesia may be an indicator of anaesthetic depth. Future research should address this issue, aiming to clarify whether the emergence of θ oscillations in infants anaesthetised with sevoflurane helps to prevent these patients from relative sevoflurane overdose, making it a meaningful EEG signature in recording the depth of anaesthesia in infants.
A limitation of this study is that we only focused on the effect of ETsevo on DSA and did not use a clinical scale or scoring system to monitor anaesthetic depth. As a consequence, we cannot use this study as validation to investigate the ability of DSA to differentiate between different levels of consciousness in young children.
In conclusion, this observational study provides additional evidence of the applicability of DSA as a measure of anaesthetic depth in young children undergoing sevoflurane anaesthesia. We believe there is an additional value to DSA in hypnotic depth monitoring in children aged 6 months to 6 years due to a significant correlation between DSA and ETsevo. In infants < 6 months, DSA may be a useful tool in preventing sevoflurane overdosing due to the emergence of θ expression. Further research using DSA should specifically address the issue of sevoflurane overdosing in infants aged 0–6 months.
Acknowledgements
The Research Ethics Committee of the Erasmus Medical Centre approved this prospective observational study. We thank R.J. Stolker, Professor and Chair of the Department of Anaesthesia, Erasmus MC Rotterdam, The Netherlands for critical appraisal of the manuscript. No external funding and no competing interests declared.
You can respond to this article at http://www.anaesthesiacorrespondence.com
References
- 1. Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical electroencephalography for anesthesiologists: part I: background and basic signatures. Anesthesiology 2015; 123: 937–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hernandez‐Hernandez MA, Fernandez‐Torre JL. Color density spectral array of bilateral bispectral index system: electroencephalographic correlate in comatose patients with nonconvulsive status epilepticus. Seizure 2016; 34: 18–25. [DOI] [PubMed] [Google Scholar]
- 3. Zanner R, Pilge S, Kochs EF, Kreuzer M, Schneider G. Time delay of electroencephalogram index calculation: analysis of cerebral state, bispectral, and Narcotrend indices using perioperatively recorded electroencephalographic signals. British Journal of Anaesthesia 2009; 103: 394–9. [DOI] [PubMed] [Google Scholar]
- 4. Akeju O, Pavone KJ, Thum JA, et al. Age‐dependency of sevoflurane‐induced electroencephalogram dynamics in children. British Journal of Anaesthesia 2015; 115(Suppl 1): i66–i76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee JM, Akeju O, Terzakis K, et al. A prospective study of age‐dependent changes in propofol‐induced electroencephalogram oscillations in children. Anesthesiology 2017; 127: 293–306. [DOI] [PubMed] [Google Scholar]
- 6. Akeju O, Westover MB, Pavone KJ, et al. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology 2014; 121: 990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cornelissen L, Kim SE, Purdon PL, Brown EN, Berde CB. Age‐dependent electroencephalogram (EEG) patterns during sevoflurane general anesthesia in infants. eLife Journal 2015; 4: e06513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davidson AJ. Monitoring the anaesthetic depth in children – an update. Current Opinion in Anaesthesiology 2007; 20: 236–43. [DOI] [PubMed] [Google Scholar]
- 9. Palanca BJ, Mashour GA, Avidan MS. Processed electroencephalogram in depth of anesthesia monitoring. Current Opinion in Anaesthesiology 2009; 22: 553–9. [DOI] [PubMed] [Google Scholar]
- 10. Dennhardt N, Arndt S, Beck C, et al. Effect of age on Narcotrend Index monitoring during sevoflurane anesthesia in children below 2 years of age. Pediatric Anesthesia 2018; 28: 112–19. [DOI] [PubMed] [Google Scholar]
- 11. McKeever S, Johnston L, Davidson AJ. Sevoflurane‐induced changes in infants’ quantifiable electroencephalogram parameters. Pediatric Anesthesia 2014; 24: 766–73. [DOI] [PubMed] [Google Scholar]


