Abstract
Microneedles are promising devices for transdermal delivery and diagnostic applications, due to their minimally invasive and painless nature of application. However, so far, applications are limited to small scale research projects. Material selection and production for larger projects remain a challenge. In vitro testing using human cell culture could bridge the gap between cost effective screening of suitable materials and concerns for safety and ethics. In this study, materials were tested for effects on viability and morphology of human endothelial cells and keratinocytes. In addition, materials were assessed for their potential to influence cellular differentiation and barrier formation. Elution‐based testing of inflammatory markers revealed no negative effects in all applied tests, whereas the assessment of differentiation markers on cells in direct contact with the material showed differences and allowed the selection of candidate materials for future medical device applications. This study illustrates that elution‐based biocompatibility testing can paint an incomplete picture. Advanced staining techniques and cell types specific for the application of the medical device improve material selection to reduce and replace animal testing at an early stage in the development process. © 2018 The Authors. journal Of Biomedical Materials Research Part A Published By Wiley Periodicals, Inc. J Biomed Mater Res Part A: 107A: 505–512, 2019.
Keywords: microneedle, biocompatibility, cyclic olefin polymer, keratinocytes, replacement
INTRODUCTION
Microneedle‐based transdermal drug delivery is highly attractive due to its needle‐free nature and potential for self‐administration, with corresponding benefits in increased patient compliance, reduced clinical time, elimination of needle‐stick injuries, and sharps waste.1, 2, 3, 4 Microneedle‐based devices have also shown significant potential in transdermal diagnostics5: electrodes may be used to detect biopotential signals such as ECG or EEG without the use of electrolytic gels or skin preparation, functionalised for applications in electrochemical diagnostics, or used to withdraw fluids for biomarker analysis.6, 7, 8
A microneedle is a short, sharp, spike‐like structure, generally less than 1 mm in length, and is often provided in arrays that may number anything up to several hundred per square centimeter and that can penetrate the epidermal layer of the skin. Although microneedles were originally fabricated using techniques borrowed from the semiconductor industry, injection molding has emerged as a viable alternative for their manufacture, especially as technical advances have facilitated the accurate reproduction of the microscale features associated with microneedles.9, 10 Furthermore, injection molding is a low‐cost, high‐throughput process that has traditionally been compliant with medical device regulations, materials and manufacturing procedures.
Cyclic olefin polymers (COPs) have low viscosity, good mechanical strength, high chemical resistance, and low water absorption. COPs have been processed in a variety of ways to create biomedical microdevices,11 and are also a candidate for injection molding of microneedle arrays.12, 13 However, the different compositions and monomers that make up commercially available medical grade polymers have raised questions about biocompatibility testing of these materials.14 Different additives have already been shown to have an effect on cytotoxicity and inflammation.15
In this article, we show that different COP materials have varying degrees of biocompatibility with human skin cell types. We have found that these differences are not obvious from standard cytotoxicity testing methods as described in USP <87>16 and EU ISO 10993‐5,17 but can be shown by the assessment of inflammation and differentiation markers in human cells in vitro. New cell culture based methods could give detailed information about human biology and could reduce and replace animal testing in rodents during early phases of product development.
MATERIALS AND METHODS
Polymer materials
A selection of COP granulate resins for medical applications was purchased from Zeon specialty materials. Tested materials were Zeonor 1020R (COP 1), Zeonex 690R (COP 2), and Zeonex 5000 (COP 3) (Zeon, San Jose, CA). All materials had been tested in the MEM‐Elution test previously and were labeled suitable for medical applications by the manufacturer.
Microneedle fabrication
Silicon microneedle templates, consisting of 100 mm diameter wafers, were produced by the Tyndall National Institute using the potassium hydroxide wet process described previously.6 These wafers were covered in microneedles measuring 500 μm tall at a pitch of 1.725 mm. As a result of the wet etching process, microneedles were shaped like octagonal pyramids with a sidewall angle of 71°. Needle templates were then copied at Profactor GmbH (Steyr, Austria) to obtain a negative shim (i.e., featuring microcavities instead of microneedles). Shims were then mounted in a mold and used for injection molding with COP materials at STRATEC Consumables GmbH (Anif, Austria).
Cell culture
Human umbilical vein endothelial cells (HUVEC) were isolated from human umbilical cords. The use of human umbilical cords for the isolation of HUVECs has been approved by the Ethics Committee of the Medical University of Vienna (EK no. 1123/2009 renewed as 009/06/2018). Umbilical veins were washed in PBS without Ca2+ and Mg2+ (Lonza, Basel, Switzerland) and incubated with dispase (Corning, Bedford, MA) for 10 min at 37°C. Endothelial cells were flushed from the cord with HBSS (Lonza, Verviers, Belgium), centrifuged and cultivated on tissue culture plastic (Corning, NY) coated with 1% gelatine (Sigma, St. Louis, MO) in medium M199 (Sigma, St. Louis, MO) containing 20% FCS (Gibco, Grand Island, NY), and endothelial cell growth supplement (Lonza, Verviers, Belgium) at 37°C and 5% CO2. Human keratinocyte cell line HaCaT was kindly provided by Michael Mildner, Medical University Vienna. Keratinocytes were cultured in serum free keratinocyte growth medium (Lonza, Verviers, Belgium) at 37°C and 5% CO2. Cells were passaged at 75%–90% confluency.
Medium eluate preparation of COPs
COP material granules or manufactured microneedle arrays were weighed and transferred to sterile 6‐well plates (Corning, NY). Materials were covered with 1 mL M199 medium or keratinocyte medium per 0.2 g material and incubated at 37°C for 24 hours. Medium eluates were recovered from the plate and used for subsequent experiments.
Morphology and viability assays
Cells were seeded to 96‐well plates and grown to confluence in endothelial growth media. Upon confluence growth medium was replaced by prepared supernatants or control medium with or without 0.1% H2O2 as control and cells were incubated for 6 hours, 24 hours, or up to 14 days. At respective time points, cell morphology was imaged with an Axiovert40 microscope (Carl Zeiss, Germany). For assessing cell viability, cells were incubated with 2 μM CalceinAM and 4 μM EthD‐1 (Life technologies, Eugene, OR) for 15 min at 37°C. Cells were imaged with the Axiovert40. For metabolic activity, cells were incubated with MTS reagent (Promega, Madison, WI) for 1 hour at 37°C. Absorbance of the supernatant was measured at 490 nm in a Tristar LB941 microplate reader (Berthold Technologies, Germany).
Flow cytometry
Primary antibodies: E‐Selectin‐FITC (BD Biosciences, NY), ICAM‐1‐PE (BD Biosciences) and HLA‐A/B/C‐PECy5 (BD Biosciences).
For testing of material eluates, cells were seeded in 24‐well plates and grown to confluence. Upon confluence the endothelial or keratinocyte medium was replaced with the respective material eluates or control medium with or without 10 ng/mL TNFα (endothelial cells) or 50 ng/mL TNFα and IFNγ (keratinocytes) as control. Cells were incubated for 6 hours, 24 hours or 48 hours. At respective time point, cells were detached with PBS without Ca2+ or Mg2+ and washed twice. Antibodies were added in PBS with 1% BSA for 30 min on ice. Cells were washed again and measured in a Cytoflex flow cytometer (Beckman Coulter, Indianapolis, IN).
Immunofluorescence
Primary antibodies: E‐cadherin‐488 (Biolegend, San Diego), Vimentin‐NL557 (R&D Systems, Minneapolis, MN).
Keratinocytes were seeded in direct contact with the microneedle materials and cultured for 7 or 14 days. Samples were fixed in 4% neutral buffered formaldehyde, permeabilized with 0.5% Triton X‐100 and stained with directly labeled E‐cadherin and vimentin antibodies. Images were taken with a LSM700 confocal microscope (Zeiss).
Statistical analysis
Data were analyzed with SPSS v. 22 software package (IBM Corp., Armonk, NY). Statistical significance was assessed by ANOVA. A p‐value below or equal to .05 was considered statistically significant.
RESULTS
No cytotoxicity of tested materials was detected with the standard elution method
Arrays of sharp microneedle structures were manufactured by injection molding of cyclic olefin polymers (COP) (Fig. 1). Standard injection molding as well as injection compression molding techniques were investigated and two different heating methods (isothermal and variothermal) were utilized for process optimization. In addition, some mold parts were modified to be able to mount the shim into an existing mold. After extensive process tests a reliable isothermal molding process without compression was found. The microneedle arrays were easily demouldable and sharp needle tips were achieved.
Figure 1.
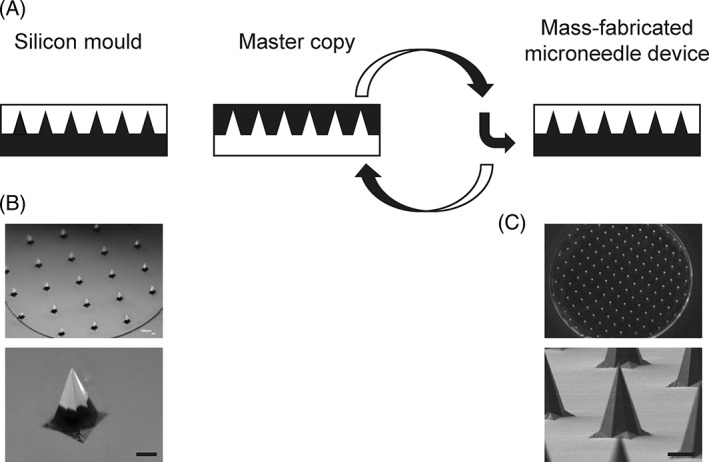
Production process and finished microneedle arrays made from COP. A schematic layout of the mass‐fabrication process used for the microneedle devices in this study can be seen in (A). Brightfield microscopic images of microneedle arrays in silicon are seen in (B). (C) Shows a brightfield microscopic image of an exemplary microneedle array in COP and a detailed electron microscopic image of an exemplary needle in COP. Scale bars equal 200 μm.
Samples of pelleted COP material and finished microneedle devices were tested for biocompatibility. In agreement with supplier data, co‐incubation of human umbilical vein endothelial cells (HUVEC) with pelleted polymer material eluates before microneedle manufacturing did not exert any cytotoxic effect as seen from morphology or viability of human cells (Fig. 2). All materials showed less than 20% difference from medium control samples as required by standard cytotoxicity test methods.16, 17 To test for cytotoxicity of the finished microneedles after the manufacturing process, eluates were prepared from the finished microneedle arrays and incubated with two different human cell types found in the skin, vascular endothelial cells, and keratinocytes. Morphology and viability [Fig. 3(A,B)] as well as metabolic activity [Fig. 3(C)] of human cells incubated with microneedle eluates did not differ from cells incubated with control cell culture medium. Likewise, the viability and metabolic activity of keratinocytes in direct contact with COPs for up to 7 days was not different from medium controls [Supporting Information Fig. S1(A,B)]. Thus, human endothelial cells and keratinocytes showed no cytotoxicity of COP materials according to standard methods commonly used for fibroblast cell lines.
Figure 2.
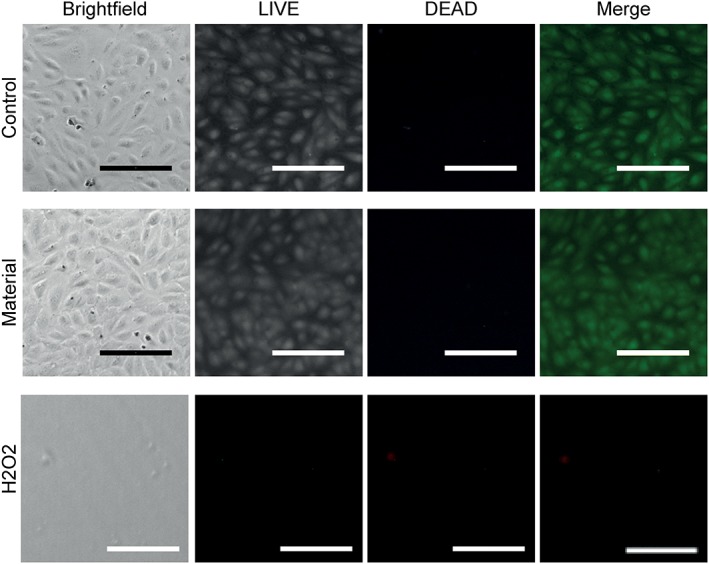
Unchanged cell morphology and viability with incubation of COP raw material eluates. Brightfield images are shown of human umbilical vein endothelial cells (HUVEC) incubated with or without pelleted COP material for 24 hours. Viable HUVEC were labeled with CalceinAM (LIVE, green), dead cells were labeled with EthD‐1 (DEAD, red). Scale bar represents 100 μm.
Figure 3.
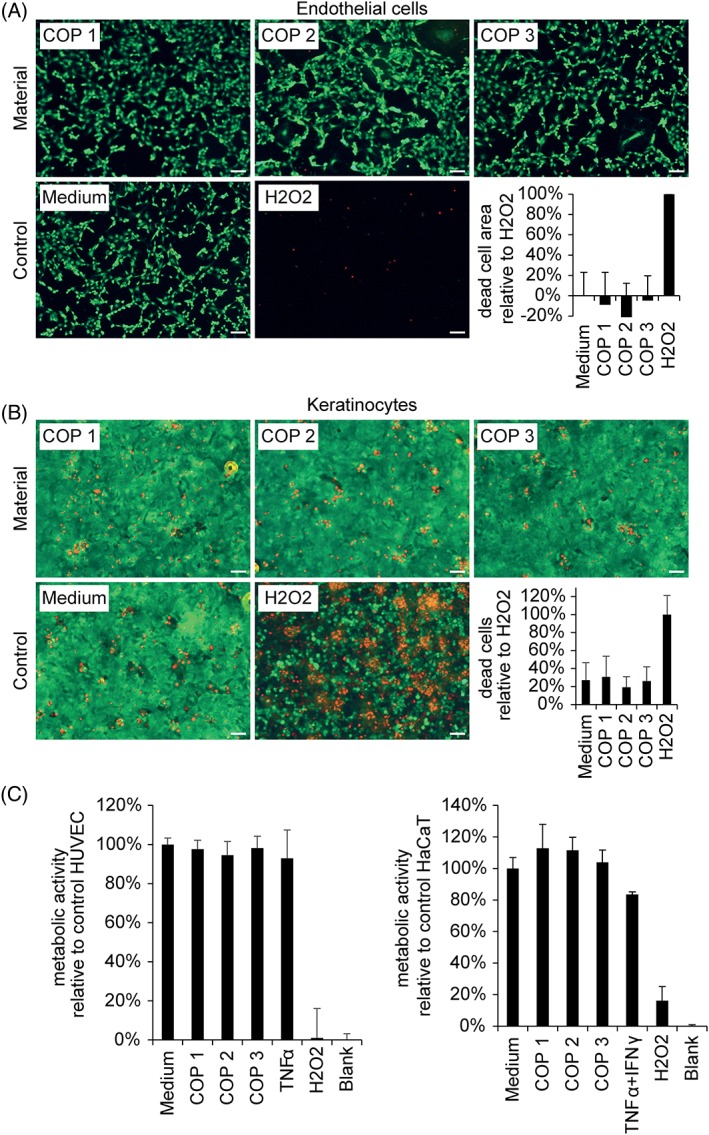
Eluates from COP materials did not elicit cytotoxicity in human endothelial or epithelial cells. Cells were incubated with eluates from indicated materials, medium control or 0.1% H2O2 for 24 hours. Viable cells were stained with CalceinAM (green), apoptotic cells were stained with EthD‐1 (red). Scale bar equals 100 μm. Apoptotic endothelial cells detach and leave a cell free area. Cell free area per high power field was quantified and summarized in (A). Apoptotic epithelial cells adhere to the material surface. Dead cell count per high power field was quantified and summarized in (B); mean+/‐SD n = 6. Metabolic activity of human endothelial and epithelial cells in response to cyclic olefin polymer eluates is quantified in (C). Cells were incubated with eluates from indicated materials, medium control, TNFα, IFNγ or 0.1% H2O2 for 24 hours. Metabolic activity was determined by the conversion of the tetrazolium compound MTS and quantification of absorbance at 490 nm. Mean +/‐SD n = 10.
Absence of inflammatory marker upregulation in human endothelial and epithelial cells
To get additional information about the status of endothelial and epithelial cells the inflammation markers E‐Selectin, ICAM‐1 and HLA‐A/B/C were analyzed by flow cytometry. Endothelial and epithelial cells displayed no upregulation of inflammation‐specific markers after incubation with eluates from COPs [Fig. 4(A,B), respectively] or after direct contact (Supporting Information Fig. S1(C)]. Furthermore, prolonged incubation of endothelial cells with COP materials for 7 days did not lead to upregulation of inflammation markers (data not shown). Thus, no negative effects of additives leaching from manufactured microneedles were seen in culture with human cells.
Figure 4.
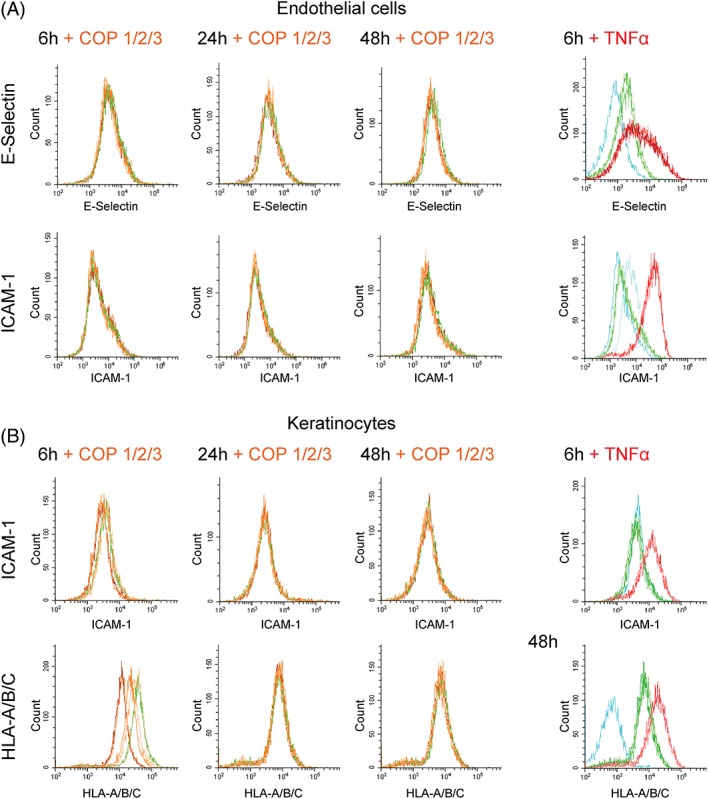
Eluates from COP materials did not upregulate inflammatory adhesion molecules in human endothelial or epithelial cells. Plots show surface expression of inflammatory adhesion molecules E‐Selectin and ICAM‐1 as well as MHC class I molecules HLA‐A/B/C. Endothelial cells (A) and epithelial cells (B) were incubated with eluates from material, medium control or TNFα for 6 hours, 24 hours, or 48 hours. Cells were gated to aquire 5000 viable cells per condition. Results are shown for COP 1 (light orange), COP 2 (orange), COP 3 (dark orange), Medium (green), and TNF (red). Unstained cells and IgG controls are shown in blue.
Different COP materials showed an effect on keratinocyte differentiation markers
To further characterize the interaction of COP material with human skin cells, keratinocytes were seeded in direct contact with the finished microneedle arrays. Differences in keratinocyte morphology were noted in direct contact with one of the COP materials (Fig. 5). Materials 1 and 2 showed good adherence and growth of human keratinocytes [Fig. 5(A)] and unchanged morphology compared to controls on standard tissue culture plastic (data not shown). Differentiation and barrier forming properties were assessed by staining keratinocyte monolayers for E‐cadherin, an important cell–cell‐adhesion protein and a marker for intact epithelial barrier formation. Materials 1 and 2 supported keratinocyte growth and E‐cadherin expression, whereas material 3 did not [Fig. 5(B) and Supporting Information movies S2 and S3]. Therefore COP 3 should be tested further before using it as a material for the fabrication of long‐term medical devices applied in direct contact with the human epidermis. Notably, COP eluates did not lead to changes in E‐cadherin expression [Supporting Information Fig. S1(D)].
Figure 5.
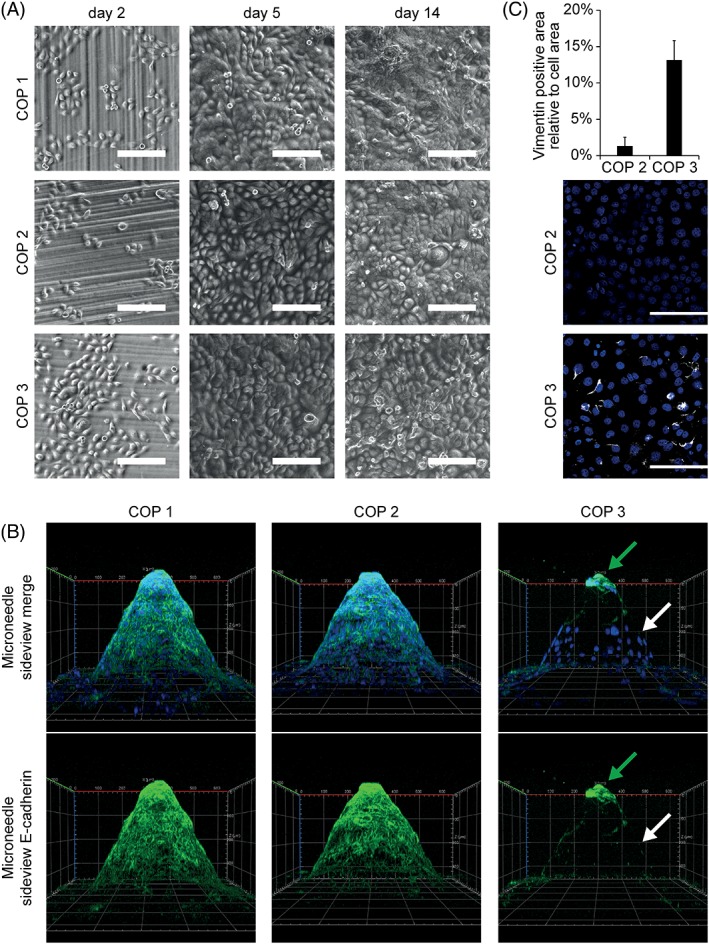
Differences in cell–cell barrier and EMT markers in human keratinocytes after incubation in direct contact with COP materials. (A) Cells were seeded on top of microneedle arrays in direct contact with the material and observed by brightfield microscopy at indicated time points. (B) After 14 days cells were stained with Alexa‐488 labeled E‐cadherin Antibody (cell–cell contacts—green) and DAPI (cell nuclei—blue). HaCaT cells seeded on top of material three showed less E‐cadherin expression after 14 days in culture. White arrow marks keratinocytes without E‐cadherin expression. Green arrow marks E‐cadherin positive keratinocytes on needle tip with limited contact to COP 3. Representative 3D images of three independent experiments are shown. X‐axis and Y‐axis equal 600 μm, Z‐axis equals 350 μm. (C) HaCaT cells seeded on top of COP 3 showed increased expression of EMT marker vimentin after 14 days in culture. Cells were seeded on top of microneedles in direct contact with the material. After 14 days cells were stained with directly labeled vimentin antibody and vimentin positive area per total cell area was quantified for three high power fields per condition. Mean +/‐SD n = 3.
In keratinocytes loss of E‐cadherin can be a marker for epithelial to mesenchymal transition (EMT), a hallmark of many adverse events, for example, during inflammation, wound healing or cancer progression. Therefore, we also assessed the expression of vimentin, another marker for EMT. COP eluates did not lead to increased vimentin expression after 7 days in culture [Supporting Information Fig. S1(E)]. Likewise, material 2 which was established as a suitable material in all previous tests showed low to no expression of the EMT marker vimentin in direct contact and was thus confirmed to be suitable for the fabrication of microneedle devices. In contrast, material 3 which already tested inferior in E‐cadherin expression, also showed increased levels of vimentin expression, which implies greater caution for the use of this material for medical device production [Fig. 5(C)].
DISCUSSION
The biocompatibility of COPs is commonly tested by assessing cell morphology of fibroblasts after incubation with COP eluates. However, longer application of medical devices on the epidermis warrants additional testing of relevant cell types that are in contact with the device material.18, 19 Since the ban on animal testing for cosmetic products in the EU, there has been an increasing effort to develop and validate in vitro assays for testing skin sensitization potential20, 21, 22 and new procedures to replace animal testing in other fields are being evaluated.23, 24 However, to get a more complete picture of the biocompatibility of COPs and a solid basis for material selection for the mass production of medical devices, such as microneedles, further tests are needed. Therefore, in this study, human endothelial and epithelial cell types were used for biocompatibility testing with the elution method and direct contact method as described in USP <87>16 and EU ISO 10993‐517 and additional methods were applied to judge the behaviour of human skin cells in direct contact with the COP materials.
In this article, we firstly show that it is possible to use a commercial injection molding process to replicate silicon microneedles in COPs with shape and sharpness approaching that of the original master template. Secondly, microneedle arrays manufactured from a selection of COP materials were tested for biocompatibility with human endothelial cells and keratinocytes. In addition to evaluations of cell viability and morphology, the materials were assessed for their potential to influence cellular differentiation and barrier formation. Materials that penetrate the skin barrier and are in direct contact with human skin cells for long term applications should not elicit any inflammatory response due to their surface properties.25 As inflammatory markers, we have used cellular adhesion molecules E‐Selectin and ICAM‐1 to assess the potential for immune cell adhesion26, 27 and human MHC class I molecules HLA‐A/B/C to see the potential for an immunologic response.28, 29 Yet, we did not see a change in inflammatory markers, but a change in E‐cadherin and vimentin, which are markers for differentiation and epithelial to mesenchymal transition. Thus, cytotoxicity testing and monitoring of inflammatory and differentiation markers in keratinocytes enabled the selection of promising candidate materials for future medical device applications. COP materials 1 and 2 were biocompatible with human endothelial and epithelial cells and could be selected for further testing of finished microneedle devices for defined applications in vivo. The results illustrate that elution based biocompatibility testing alone can paint an incomplete picture and differentiation markers might be more suitable than markers of inflammation to assess biocompatibility of COPs and human skin cells. Thereby, more advanced staining techniques and cell types specific for the application of the medical device could improve the material selection process and replace animal testing at an early stage in the device development process.
FUNDING INFORMATION
This research was funded by the Austrian Research Promotion Agency (FFG project number 853482 Microneedle).
LIST OF ABBREVIATIONS
- COP
cyclic olefin polymer
- ECG
electrocardiography
- EEG
electroencephalography
- EMT
epithelial to mesenchymal transition
- HBSS
Hank's balanced salt solution
- HLA
human leucocyte antigen
- HUVEC
human umbilical vein endothelial cells
- ICAM
intercellular adhesion molecule
Supporting information
Supplementary Figure S1: Direct contact and eluate tests show no differences in factors other than cell–cell barrier and differentiation. (A) Direct contact of COPs with human keratinocytes does not lead to cytotoxicity. Keratinocytes were cultured in direct contact with COPs for 7 days. Viable cells were stained with CalceinAM (green), apoptotic cells were stained with EthD‐1 (red). Scale bar equals 100 μm. (B) Plots show no difference in metabolic activity of human keratinocytes in direct contact with COPs for 7 days. Metabolic activity was determined by the conversion of the tetrazolium compound MTS and quantification of absorbance at 490 nm. Mean +/‐SD n = 4. (C) No change in inflammatory markers of keratinocytes was seen after 7 days in direct contact with COPs. Cells were gated to aquire 5000 viable cells per condition. Results are shown for COP 1 (light orange), COP 2 (orange), COP 3 (dark orange), Medium (green) and TNF (red). Unstained cells and IgG controls are shown in blue. (D) No change in E‐cadherin expression of keratinocytes after 7 days in culture with COP eluates. Cells were stained with Alexa‐488 labeled E‐cadherin Antibody (cell–cell contacts ‐ green) and DAPI (cell nuclei ‐ blue). Scale bar equals 50 μm. (E) No Vimentin expression seen in keratinocytes after 7 days in culture with COP eluates. Cells were stained with directly labeled vimentin antibody and vimentin positive area per total cell area was quantified for 3 high power fields per condition. Mean +/‐SD n = 3.
Supplementary movie S2 COP1 Ecadherin DAPI
Supplementary movie S3 COP3 Ecadherin DAPI
ACKNOWLEDGMENTS
We thank Karin Neumüller for her excellent technical assistance with the cultivation of human primary cells. Images were taken at the Imaging facility of the Medical University Vienna. We thank Marion Gröger and Sabine Rauscher for their continuous support. SB is an employee of Stratec Consumables GmbH. MH and DF are employees of Functional Surfaces and Nanostructures, Profactor GmbH.
How to cite this article: Schossleitner K, O'Mahony C, Brandstätter S, Haslinger MJ, Demuth S, Fechtig D, Petzelbauer P. 2019. Differences in biocompatibility of microneedles from cyclic olefin polymers with human endothelial and epithelial skin cells. J Biomed Mater Res Part A 2019:107A:505–512.
REFERENCES
- 1. Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng 2017. Jun 7;8(1):177–200. [DOI] [PubMed] [Google Scholar]
- 2. Ma G, Wu C. Microneedle, bio‐microneedle and bio‐inspired microneedle: A review. J Cont Rel 2017. Apr 10;251:11–23. [DOI] [PubMed] [Google Scholar]
- 3. Donnelly RF, Raj Singh TR, Woolfson AD. Microneedle‐based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv 2010. May 18;17(4):187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tuan‐Mahmood T‐M, McCrudden MTC, Torrisi BM, McAlister E, Garland MJ, Singh TRR, et al. Microneedles for intradermal and transdermal drug delivery. Eur J Pharm Sci 2013;50(5):623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chinnadayyala SR, Park KD, Cho S. Editors' choice—Review—In vivo and in vitro microneedle based enzymatic and non‐enzymatic continuous glucose monitoring biosensors. ECS J Solid State Sci Technol 2018. Apr 24;7(7):Q3159–Q3171. [Google Scholar]
- 6. O'Mahony C, Grygoryev K, Ciarlone A, Giannoni G, Kenthao A, Galvin P. Design, fabrication and skin‐electrode contact analysis of polymer microneedle‐based ECG electrodes. J Micromech Microeng 2016. Aug 1;26(8):084005. [Google Scholar]
- 7. Barrett C, Dawson K, O'Mahony C, O'Riordan A. Development of low cost rapid fabrication of sharp polymer microneedles for in vivo glucose biosensing applications. ECS J Solid State Sci Technol 2015. Aug 27;4(10):S3053–S3058. [Google Scholar]
- 8. Blicharz TM, Gong P, Bunner BM, Chu LL, Leonard KM, Wakefield JA, et al. Microneedle‐based device for the one‐step painless collection of capillary blood samples. Nat Biomed Eng 2018. Mar 19;2(3):1–7. [DOI] [PubMed] [Google Scholar]
- 9. Burton SA, Ng CY, Simmers R, Moeckly C, Brandwein D, Gilbert T, Johnson N, Brown K, Alston T, Prochnow G, Siebenaler K, Hansen K. Rapid intradermal delivery of liquid formulations using a hollow microstructured array. Pharm Res 2011. Jan;28(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nair K, Whiteside B, Grant C, Patel R, Tuinea‐Bobe C, Norris K, et al. Investigation of plasma treatment on micro‐injection moulded microneedle for drug delivery. Pharmaceutics 2015. Oct 30;7(4):471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nunes PS, Ohlsson PD, Ordeig O, Kutter JP. Cyclic olefin polymers: Emerging materials for lab‐on‐a‐chip applications. Microfluid Nanofluidics 2010. Aug 7;9(2–3):145–161. [Google Scholar]
- 12. Hamilton JD. Fabrication and analysis of injection molded plastic microneedle arrays. Atlanta, GA: M.S. thesis, Georgia Institute of Technology; 2011. [Google Scholar]
- 13. Sammoura F, Kang J, Heo YM, Jung T, Lin L. Polymeric microneedle fabrication using a microinjection molding technique. Microsyst Technol 2007. Jan 17;13(5–6):517–522. [Google Scholar]
- 14. Hiebl B, Lützow K, Lange M, Jung F, Seifert B, Klein F, Weigel T, Kratz K, Lendlein A. Cytocompatibility testing of cell culture modules fabricated from specific candidate biomaterials using injection molding. J Biotechnol 2010. Jul 1;148(1):76–82. [DOI] [PubMed] [Google Scholar]
- 15. Bernard M, Jubeli E, Bakar J, Tortolano L, Saunier J, Yagoubi N. Biocompatibility assessment of cyclic olefin copolymers: Impact of two additives on cytotoxicity, oxidative stress, inflammatory reactions, and hemocompatibility. J Biomed Mater Res Part A 2017. Dec 1;105(12):3333–3349. [DOI] [PubMed] [Google Scholar]
- 16. Pharmacopeia US . < 87> Biological reactivity tests, In Vitro. USP, Rockville, MD, USA. 2015;
- 17. European Standards . ISO 10993‐5 Biological Evaluation of Medical Devices ‐ Part 5: Tests for in vitro Cytotoxicity. 2009;
- 18. Wiegand C, Hipler U‐C. Evaluation of biocompatibility and cytotoxicity using keratinocyte and fibroblast cultures. Skin Pharmacol Physiol 2009;22(2):74–82. [DOI] [PubMed] [Google Scholar]
- 19. Ferreira AM, Mattu C, Ranzato E, Ciardelli G. Bioinspired porous membranes containing polymer nanoparticles for wound healing. J Biomed Mater Res Part A 2014. Feb;102(12):n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 20. Strickland J, Zang Q, Kleinstreuer N, Paris M, Lehmann DM, Choksi N, Matheson J, Jacobs A, Lowit A, Allen D, Casey W. Integrated decision strategies for skin sensitization hazard. J Appl Toxicol 2016. Sep;36(9):1150–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reisinger K, Hoffmann S, Alépée N, Ashikaga T, Barroso J, Elcombe C, Gellatly N, Galbiati V, Gibbs S, Groux H, Hibatallah J, Keller D, Kern P, Klaric M, Kolle S, Kuehnl J, Lambrechts N, Lindstedt M, Millet M, Martinozzi‐Teissier S, Natsch A, Petersohn D, Pike I, Sakaguchi H, Schepky A, Tailhardat M, Templier M, van Vliet E, Maxwell G. Systematic evaluation of non‐animal test methods for skin sensitisation safety assessment. Toxicol Vitr 2015. Feb 1;29(1):259–270. [DOI] [PubMed] [Google Scholar]
- 22. Natsch A, Ryan CA, Foertsch L, Emter R, Jaworska J, Gerberick F, et al. A dataset on 145 chemicals tested in alternative assays for skin sensitization undergoing prevalidation. J Appl Toxicol 2013. Apr;33(11):n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 23. Hamm J, Sullivan K, Clippinger AJ, Strickland J, Bell S, Bhhatarai B, Blaauboer B, Casey W, Dorman D, Forsby A, Garcia‐Reyero N, Gehen S, Graepel R, Hotchkiss J, Lowit A, Matheson J, Reaves E, Scarano L, Sprankle C, Tunkel J, Wilson D, Xia M, Zhu H, Allen D. Alternative approaches for identifying acute systemic toxicity: Moving from research to regulatory testing. Toxicol In Vitro 2017. Jun;41:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zuang V., Barroso J., Belz S., Berggren E., Bernasconi C., Bopp S., Bouhifd M., Bowe G., Campia I., Casati S., Coecke S. CR , Dura A., Gribaldo L., Grignard E., Halder M., Holley T., Janusch Roi A., Kienzler A., Lostia A., Madia F., Milcamps A. MS , Munn S., Paini A., Pistollato F., Price A., Prieto‐Peraita P., Richarz A., Triebe J., van der Linden S., Wittwehr C., Worth A. WM . EURL ECVAM Status Report on the Development, Validation and Regulatory Acceptance of Alternative Methods and Approaches (2017) [Internet]. EUR 28823. Luxembourg: Publications Office of the European Union; 2017.
- 25. Park G, Chung H‐J, Kim K, Lim SA, Kim J, Kim Y‐S, Liu Y, Yeo WH, Kim RH, Kim SS, Kim JS, Jung YH, Kim TI, Yee C, Rogers JA, Lee KM. Immunologic and tissue biocompatibility of flexible/stretchable electronics and optoelectronics. Adv Healthc Mater 2014. Apr;3(4):515–525. [DOI] [PubMed] [Google Scholar]
- 26. Unger RE, Krump‐Konvalinkova V, Peters K, Kirkpatrick CJ. In vitro expression of the endothelial phenotype: Comparative study of primary isolated cells and cell lines, including the novel cell line HPMEC‐ST1.6R. Microvasc Res 2002;64(3):384–397. [DOI] [PubMed] [Google Scholar]
- 27. Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), and E‐selectin through nuclear factor‐kappa B activation in endothelial cells. J Biol Chem 2001. Mar 9;276(10):7614–7620. [DOI] [PubMed] [Google Scholar]
- 28. Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: Mechanisms and clinical consequences. Nat Rev Immunol 2004. Mar 1;4(3):211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson DR. Locus‐specific constitutive and cytokine‐induced HLA class I gene expression. J Immunol 2003. Feb 15;170(4):1894–1902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Direct contact and eluate tests show no differences in factors other than cell–cell barrier and differentiation. (A) Direct contact of COPs with human keratinocytes does not lead to cytotoxicity. Keratinocytes were cultured in direct contact with COPs for 7 days. Viable cells were stained with CalceinAM (green), apoptotic cells were stained with EthD‐1 (red). Scale bar equals 100 μm. (B) Plots show no difference in metabolic activity of human keratinocytes in direct contact with COPs for 7 days. Metabolic activity was determined by the conversion of the tetrazolium compound MTS and quantification of absorbance at 490 nm. Mean +/‐SD n = 4. (C) No change in inflammatory markers of keratinocytes was seen after 7 days in direct contact with COPs. Cells were gated to aquire 5000 viable cells per condition. Results are shown for COP 1 (light orange), COP 2 (orange), COP 3 (dark orange), Medium (green) and TNF (red). Unstained cells and IgG controls are shown in blue. (D) No change in E‐cadherin expression of keratinocytes after 7 days in culture with COP eluates. Cells were stained with Alexa‐488 labeled E‐cadherin Antibody (cell–cell contacts ‐ green) and DAPI (cell nuclei ‐ blue). Scale bar equals 50 μm. (E) No Vimentin expression seen in keratinocytes after 7 days in culture with COP eluates. Cells were stained with directly labeled vimentin antibody and vimentin positive area per total cell area was quantified for 3 high power fields per condition. Mean +/‐SD n = 3.
Supplementary movie S2 COP1 Ecadherin DAPI
Supplementary movie S3 COP3 Ecadherin DAPI


