Abstract
Toll‐like receptors (TLRs) are germline‐encoded receptors that are central to innate and adaptive immune responses. Owing to their vital role in inflammation, TLRs are rational targets in clinics; thus, many ligands and biologics have been reported to overcome the progression of various inflammatory and malignant conditions and support the immune system. For each TLR, at least one, and often many, drug formulations are being evaluated. Ligands reported as stand‐alone drugs may also be reported based on their use in combinatorial therapeutics as adjuvants. Despite their profound efficacy in TLR‐modulation in preclinical studies, multiple drugs have been terminated at different stages of clinical trials. Here, TLR modulating drugs that have been evaluated in clinical trials are discussed, along with their mode of action, suggestive failure reasons, and ways to improve the clinical outcomes. This review presents recent advances in TLR‐targeting drugs and provides directions for more successful immune system manipulation.
Keywords: adjuvant, clinical trial, drug, innate immunity, Toll‐like receptor
1. INTRODUCTION
Toll‐like receptors (TLRs) are integral membrane bound receptors that are vital for innate immunity and help to shape the adaptive immune response. These receptors are triggered by a variety of pathogen‐associated molecular patterns (PAMPs), and danger‐associated molecular patterns (DAMPs). PAMPs are parts of pathogens such as lipoproteins, lipopeptides, and flagella, as well as nucleic acids (either single‐stranded or double‐stranded DNA or RNA),1 while DAMPs are self‐molecules that include multiple heat shock proteins, S100, and high mobility group box 1 (HMGB1) that are released in response to injury or any other anomaly in the cells.2, 3 DAMPs or PAMPs can engage a variety of TLRs; those situated on the cell surface primarily engage TLRs that function on the cell membrane, whereas intracellularly localized TLRs are activated by nucleic acid components, which are made available after pathogen endocytosis or replication. Given their functions, TLRs are considered as the first line in immune defense.1, 4
The number of functional TLRs can vary in mammals; however, they all have conserved functions, namely the activation of inflammatory mediators. Humans have 10 TLRs; TLR2 (can heterodimerize with TLR1 or TLR6), TLR4, TLR5, and TLR10 are present at the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are functionally localized to endosomes (Table 1).1, 5, 6 These receptors invariably work as homodimers or heterodimers, and several studies have suggested that they exhibit unusual dimer characteristics.7, 8 All TLRs form homo‐ and heterodimers, except for TLR3 and TLR5, which are currently considered strictly homodimeric, in the absence of empirical evidence to the contrary.
Table 1.
Natural and synthetic ligands for TLRs
| TLR | Expression | PAMPs | DAMPs | Nature of molecules investigated in clinical trials |
|---|---|---|---|---|
| TLR2 (1/6) | B, Mo, Mac, DCs, Plt N, MyDCs, Mc | Lipoproteins, zymosan, peptidoglycan | HSPs, HMGB1, hyaluronan, HDL (modified) | Lipopeptide, recombinant protein, antibody |
| TLR3 | DC, B, Plt | Viral dsRNA | Self dsRNA | Polyinosinic‐polycytidylic acid (polyIC, poly‐ICLC, polyIC12U), anti‐TLR3 antibody |
| TLR4 | Mo, Mac, N, MyDCs, Mc, B, IE, Plt | Lipopolysaccharide | HSPs, fibrinogen, heparin sulfate, fibronectin, HA, HMGB1, hyaluronan, oxidized LDL, ANG II | Lipid A derivates (glycolipids), anti‐TLR4 antibody, polysaccharide |
| TLR5 | Mo, Mac, DC, IE | Flagellin | HMGB1 | Flagella and flagella based molecules |
| TLR7 | Mo, Mac, pDC, B, Plt | Viral ssRNA | Self ssRNA | SM |
| TLR8 | Mo, Mac, DC, Mc | Viral ssRNA | Self ssRNA | SM |
| TLR9 | Mo, Mac, pDC, B, Plt | Bacterial and viral CpG DNA | Self DNA | DNA based, synthetic ssDNA molecules |
| TLR10 | LN, Mo, S, B, L | NA | NA | NA |
Abbreviations: ANG II, angiotensin II; B, B cell; DAPM, danger‐associated molecular pattern; DC, dendritic cell; dsRNA, double‐stranded RNA; HA, hyaluronic acid; HDL, high‐density lipoprotein; HMGB1, high mobility group box 1; HSPs, heat shock proteins; IE, intestinal epithelium; L, lung; LC, liver cell; LDL, low‐density lipoprotein; LN, lymph node; Mac, macrophage; Mc, mast cell; Mo, monocyt; MyDC, myeloid dendritic cell; N, neutrophil; NA, not available; PAMP, pathogen‐associated molecular pattern; pDC, plasmacytoid DC; Plt, platelet; S, spleen; SM, small molecule; ssRNA, single‐stranded RNA; TLR, Toll‐like receptor.
TLRs are composed of three distinct domains, an extracellular domain (ECD) that senses the ligand, a transmembrane domain (TM) that anchors the TLR within membranes, and Toll/interleukin‐1 (IL‐1) receptor (TIR) domain that interacts with other TIR‐containing adapters to initiate signaling (Figure 1A).9, 10, 11 TLRs signaling depends on its dimer assembly, and in the absence of any ligand, TLRs exist either in monomeric forms or weakly dimeric forms that are unable to initiate the signaling. While ligand binding confers dimer stability and induces a conformational change that reorients the TIR domain and initiates signaling.12, 13 TLRs are ideal targets for immune modulation strategies, since they have both known modulators and proven therapeutic potential (Table 1).
Figure 1.
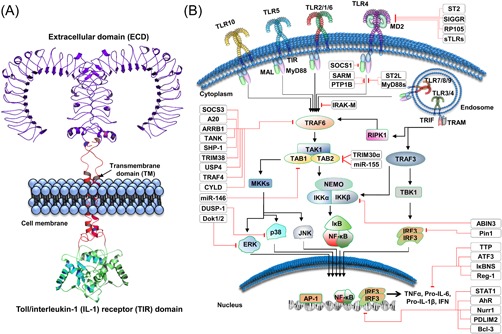
Generalized structure and signaling mechanisms of Toll‐like receptors. A, A typical TLR is composed of three distinct domains, an ECD, a TM domain, and a TIR domain. B, Conventionally, TLRs are divided into two categories; cell surface functional, and endosomally functional TLRs. Endosomal TLRs are mainly activated through nucleic acids, while cell surface–expressed TLRs are activated by a variety of ligands, including proteins and lipoproteins. Upon sensing PAMPs or DAMPs, TLRs dimerize and reorient their TIR domains, allowing the docking of intracellularly localized TIR‐containing proteins, including MAL, MyD88, TRIF, and TRAM. The majority of TLRs convey downstream signals through MyD88; however, TLR3 can signal only through TRIF. Exceptionally, TLR4 can transmit signals through both the MyD88 and TRIF adapter proteins. Therapeutics targeting immune‐related diseases mediated by TLRs are reported to modulate these signaling mechanisms. There are many internal mechanisms that come into action to regulate TLR‐mediated inflammation. These act at all levels starting from cell surface interaction to dent the TLR dimerization, and cytoplasmic interactions to block adapter molecules, alter the posttranslational modification state, and finally in the nucleus to counter overexpression of various interleukins and cytokines. There are many microRNAs that reduce the mRNA stability of different cytokines. All these mechanisms ensure a balanced response toward the invading pathogen or DAMP that unbalance the homeostasis. ABIN3, A20 binding and inhibitor of NFκB‐3; AhR, aryl hydrocarbon receptor; AP1, activated protein 1; ATF3, activating transcription factor 3; Bcl‐3, B‐cell lymphoma 3‐encoded protein; CYLD, cylindromatosis; DOK, downstream of tyrosine kinases; DUSP, dual specificity phosphatases; ECD, extracellular domain; ERK, extracellular‐regulated kinase; IFN, interferon; IkB, inhibitor of κB; IKK, inhibitor of κB kinase; IL, interleukin; IRF, interferon response factor; IRAK‐M, interleukin receptor‐associated kinase M; JNK, c‐Jun N‐terminal kinase; MAL, MyD88 adapter like; MD2, myeloid differentiation factor 2; MIR, microRNA; MKK, mitogen‐activated protein kinase kinase; mRNA, messenger RNA; MyD88, myeloid differentiation primary response 88; MyD88s, myeloid differentiation primary response 88 short; NEMO, NF‐κB essential modulator; NFKBID, NF‐κB inhibitor δ; NF‐κB, nuclear factor κB; Nurr1, nuclear receptor related 1 protein; p38, protein 38; PDLIM2, PDZ and LIM domain 2; PTP1B, protein tyrosine phosphatase‐1B; Reg‐1, regnase‐1; RIPK‐1, receptor interacting protein kinase 1; RP105, radioprotective 105 kDa protein; SARM, sterile α and armadillo‐motif containing protein; SHP‐1, Src homology region 2 domain‐containing phosphatase‐1; SIGGR, single immunoglobulin IL1R‐related molecule; SOCS, suppressor of cytokine signaling; ST2, suppression of tumorigenity 2; ST2L, membrane bound ST2; STAT, signal transducers and activators of transcription; sTLRs, soluble Toll‐like receptor; TAB, TAK‐1‐binding protein; TAK1, transforming growth factor β‐activated kinase 1; TANK, TRAF‐associated NF‐κB activator; TBK1, TANK‐binding kinase 1; TIR, Toll/interleukin‐1 receptor; TLR, Toll‐like receptor; TM, transmembrane domain; TNF‐α, tumor necrosis factor α; TRAF, tumor necrosis factor receptor (TNF‐R)‐associated factor; TRAM, TRIF‐related adapter molecule; TRIF, TIR‐domain‐containing adapter‐inducing interferon‐β; TRIM38, tripartite motif 38; TTP, tristetraprolin; USP4, ubiquitin‐specific protease 4 [Color figure can be viewed at wileyonlinelibrary.com]
Therefore, it is rational to harness their potential for improving vaccines efficacy, to treat cancers (breast and bladder cancers14, 15), to inhibit their activity in inflammatory diseases (for instance; rheumatoid arthritis (RA)16 and multiple sclerosis17), to modulate them in autoimmune diseases such as systemic lupus erythematosus (SLE),18 to fine tune them to generate specific responses (humoral vs cell‐mediated immune responses) and to curb the menopausal osteoporosis.19, 20, 21 Other than these, there are numerous diseases where TLRs play important roles, for that, the interested reviewers are encouraged to consult recent reviews.22, 23, 24 Given their extensive potential benefits, they are the target‐of‐choice for many therapeutic endeavors, and these efforts are bearing fruit, with many compounds that target TLRs are currently at various stages of evaluation in clinical trials.19
We have therefore revised and gathered relevant data and wish to present it to the scientific community to guide them in their future investigations. The data in this paper has been collected from ongoing clinical trials that either target TLRs or use them to induce improved responses. Data have been organized in a reader‐friendly manner, focusing on the clinical condition, the type of TLR being targeted, the failures and successes of drugs in different phases of clinical trials, and the synergistic efficacy of TLR ligands as adjuvants.
2. TLR SIGNALING
In induction of inflammatory responses, TLRs primarily act via the myeloid differentiation response protein 88 (MyD88) and TIR‐domain‐containing adapter‐inducing interferon‐β (TRIF)–mediated pathways.25, 26, 27, 28 On sensing PAMPs or DAMPs, TLRs dimerize and reorient their TIR domains, which allow docking of the TIR containing proteins, MyD88 and MyD88 adapter‐like (MAL). MAL is a bridging adapter frequently involved in TLR4, and to a lesser extent in TLR2, signaling pathways, and it interacts with MyD88 through TIR‐TIR interaction. In addition to the TIR domain, MyD88 contains a death domain that facilitates its interaction with interleukin‐1 receptor (IL1R)‐associated kinase 4 (IRAK4), which can both auto‐phosphorylate and trans‐phosphorylate IRAK2/1.29 This inter‐domain interaction results in a large multimeric molecule, referred to as myddosome, the phosphorylation of which leads to the activation and dimerization of tumor necrosis factor (TNF) receptor–associated factor 6 (TRAF6).30 TRAF6, an E3 ligase that is activated via autoubiquitination in an sequestosome 1 (SQSTM1/p62)‐dependent manner, mediates the ubiquitination of transforming growth factor‐β–activated kinase 1 (TAK1).31, 32 TAK1 belongs to the mitogen‐activated protein kinase kinase kinase (MAPKKK) family and forms a complex with the TAK1 binding proteins, TAB1–3. TAK1 deficiency reduces inflammatory signaling across TLRs; however, no such difference has been observed in response to a deficiency of TAB proteins.33, 34 TAK1/TABs signaling then branches into two arms: activation of nuclear factor κB (NF‐κB) and MAPK. NF‐κB is held inactive in the cytoplasm by inhibitor of κB (IκB), which is phosphorylated by IκB kinase α (IKKα) and IKKβ, and degraded via ubiquitin mediated‐proteasomal degradation, exposing a nuclear localization signal in NF‐κB, and subsequently translocating to the nucleus as reviewed by Kawai and Akira.35 NF‐κB is a hub molecule for inflammatory signals and it induces the expression of a wide array of molecules that cause inflammation, alteration in cell surface receptors, expression of pro‐ and anti‐cancerous molecules, and perturbation in cell motility, among other responses. TAK1 also activates MAPK family members, including MKK7 and/or MKK6/3, resulting in the phosphorylation of p38 and JNK, and culminating in the activation of activated protein 1 (AP1) family transcription factors and messenger RNA (mRNA) stabilization of various genes involved in the regulation of inflammation (Figure 1B).4, 36
TRIF–dependent signaling is a separate arm of TLR signaling perpetuated only by TLR3 and TLR4, where TRIF interacts with TRAF3 and TRAF6. TRAF6 interacts with receptor interacting protein kinase 1 (RIPK‐1), which transduces the signal by activating TAK1, a crucial branch point in the TLR signaling pathway. TRAF3 activates IKK‐related kinases, such as TANK‐binding kinase 1 (TBK1) and IKKi, along with NEMO, and the transduced signals culminate in interferon (IFN)‐regulatory factor 3 (IRF3) phosphorylation, which translocates into the nucleus after dimerization, inducing expression of type I IFN genes.4, 36 The production of IFNs is the prominent outcome of TLR3 and TLR4 pathways to counter viral infections, which in turn regulated by IRF3. Recently, it has been shown that phosphatidylinositol 5‐phosphate (PtdIns5P) can regulate IRF3 activation. This inositol lipid can bind to and facilitate complex formation between IRF3 and TBK1, leading to the IRF3 phosphorylation by TBK1, situated proximally.37 Furthermore, during viral infection, production of the inositol lipid, PtdIns5P, could be observed by evaluation of PIKfyve activity.38
3. ENDOGENOUS REGULATION OF TLR SIGNALING
Regulation of TLR signaling is achieved through various molecules that restrict it to an appropriate level to avoid any detrimental consequences in the form of autoimmune or inflammatory diseases. These regulatory molecules bind to key components of TLR signaling and quench their activities as reviewed elsewhere.39 The MyD88‐dependent pathway can be suppressed by spleen tyrosine kinase, Cbl‐b, and suppressor of cellular signaling 1 (SOCS1), while the TRIF arm is negatively regulated by sterile α‐ and armadillo‐motif‐containing protein (SARM) and TRAM adapter with Golgi dynamics domain (TAG).40, 41 The inhibition mechanisms of molecules can be unique or may overlap. Similarly, SOCS3 and deubiquitinating enzyme A (DUBA) negatively regulate TRAF342 while A20, cylindromatosis, TANK, tripartite motif 38 (TRIM38), ubiquitin‐specific protease 4, and small heterodimer partner can negatively influence TRAF6 (Figure 1B).39, 43, 44 TAK1 activation is regulated by A20 and TRIM30α.45 NF‐κB is pivotal in TLR signaling; therefore, it is regulated by numerous molecules, including NF‐κB inhibitor δ (NFKBID), B‐cell lymphoma 3‐encoded protein (BCL‐3), activating transcription factor 3 (ATF3), Nurr1, and PDZ and LIM Domain 2 (PDLIM2).46 IRF3 is an important player in TRIF–dependent pathways that is suppressed by Pin1 and replication and transcription activator‐associated ubiquitin ligase (RAUL).47 Various microRNAs (miRNAs) have been implicated in mRNA level regulation of TLR signaling molecules, including miR‐21, ‐29, ‐126, ‐146a, ‐155, ‐199a, ‐148/152, and ‐466l.39 Moreover, cytokine mRNA stability can also be regulated by regulatory Regnase‐1 and tristetraprolin.4, 48 Collectively, TLR signaling homeostasis is established and maintained by these endogenous modulators (Figure 1B).
4. TLRS AND DISEASES
TLRs are involved in a wide spectrum of diseases that either directly or indirectly exacerbate the conditions. In recent years, many endeavors have been dedicated to delineate this relationship and compile data regarding the TLR involvement in various diseases.4, 20, 49, 50, 51 Here, we would like to present a brief overview of how TLRs influence the pathobiology of inflammatory, autoimmune, and cancerous diseases.22, 23, 24, 52
Sepsis is the worst outcome of host‐pathogen interaction and is the leading cause of death in United States.53, 54 The infection by Gram‐positive and Gram‐negative bacteria equally contribute to the development of sepsis where exaggerated immune response lead to multiorgan failure and septic shock.55 These bacteria harbor ligands that trigger TLR2 and TLR4; particularly, the presence of LPS significantly contributes into sepsis development. The septic shock is due to the body immune response rather than infection itself.56 For sepsis management, various TLR inhibitors are evaluated clinically, and new modalities are being devised recently.57
Chronic pulmonary obstructive disease (COPD) is characterized by the poor reversible air flow and bronchial inflammation.52, 58 This condition can also be worsened when TLRs react in response to viral infections. It has been observed that the COPD patients exhibit higher inflammatory cytokines, TNF‐α and CCL5 in infections.59 Among various treatments, the inhibition of TLRs can also be an approach to curb the COPD.49
The involvement of TLRs in RA, an inflammatory disease, is well known. The exact mechanism of RA initiation is yet debatable; however, it is believed that the PAMPs from commensal flora is crucial for RA initiation.60 After initial insult, an autocrine loop perpetuates that increases matrix metalloproteinases (MMP) and worsen the damage. Moreover, the DNA and peptidoglycan from intestinal bacteria have also been observed in RA joints.61 This result in damaged cells that will release DAMPs such as RNAs, HMGB1, S100‐A8; the presence of such molecules activate TLRs that over‐inflame the situation.
SLE is an autoimmune disease that featured autoantibodies against double‐stranded DNA and nucleic acid‐bound proteins that served both as diagnostic and prognostic markers; however, the initial events are still a mystery.62 SLE patients manifest deficiency in clearing apoptotic cells that promote the formation of the immune complex (IC), and these ICs can trigger the endosomal TLRs. The role of TLR7 (inflammatory) and TLR9 (protective) in SLE can be different due to variation in study samples among different studies; nevertheless, TLR9−/− murine models displayed higher TLR7‐mediated inflammation concluding a regulatory role of TLR9.63, 64, 65
An autoimmune disease where the immune system destroys the fluid secreting glands, for instance, the salivary gland, has a potential TLR involvement and is known as Sjogren's syndrome (SS). The patients with SS exhibit higher TLR expression, with increased expression of inflammatory cytokines in response to TLR7 and TLR9 activation.66, 67
TLRs participation in cancers act as double‐edge swords; their activation can regress the tumor growth or conversely promote the tumor cells.20, 67 The accumulating data strongly advocate both aspects. Furthermore, it is now well‐acknowledged that the inflammation and cancer are strongly correlated in various diseases.68 Similarly, organs with higher PAMPs density such as gastrointestinal tract and skin are prone to TLR‐mediated oncogenesis along with the organs that expose to indirect TLR agonist such as the liver. The dual role of TLRs in cancers has a significant correlation with the length and amplitude of receptor activation. TLR4 has been reported to promote colon cancer, and its deficiency can alleviate the inflammation as well as tumor burden.69, 70 The liver cancer has also been related to TLR4 activation71; however, its role may be context dependent in skin cancer.72, 73 Similarly, TLRs are also critical for the cellular transformation in breast cancers, as reviewed before,14 can critically modulate the metabolism in the tumor microenvironment,74 and can regulate other signaling networks to favor pro‐ or anti‐tumor outcomes.75, 76, 77
5. TLR LIGANDS: ADJUVANTS VS DRUGS
TLR signaling activates innate immunity and assists in shaping adaptive immunity. Hence, TLR ligands are attractive for use in immunotherapy and are primarily exploited as adjuvants to specifically trigger humoral and/or cell‐mediated responses as reviewed elsewhere.78, 79 They can also magnify the immune response toward certain poorly antigenic targets. Therefore, in the majority of clinical trials, TLR ligands are evaluated as adjuvants.
The number of trials that involve TLR ligands as adjuvants (64%) are double than those considering TLR ligands as drugs (35%). This highlights the immune‐therapy role of TLRs in various diseases and their potential utilization for further exploration for immunomodulation therapy. Additionally, TLR activation can also alter other signaling pathways and it is desirable to cotarget multiple pathways with the aim of achieving improved treatment efficacy. Apart from many ongoing trials, Food and Drug Administration approved TLR ligands, MPLA80 (TLR4 agonist), and imiquimod81 (TLR7 agonist) could be highlighted to address adjuvant or drug roles. MPLA has been used in various vaccine formulations, for instance, Fendrix (Hepatitis B vaccine, GSK), as an adjuvant and imiquimod is famously used to cure viral diseases as a drug.82, 83 The majority of TLRs produce redundant responses (inflammatory vs antiviral); however, there are slight, but distinct, differences in outcomes.84 These differences are largely attributable to the relative roles of ligands and tissue‐dependent TLRs expression.85, 86
6. THERAPEUTIC INTERVENTIONS TARGETING TLRS
Given their vital roles in pathogen clearance, inflammation induction, and cancer pathogenesis, TLRs are attractive targets for manipulation of the immune system in favor of the patients. Therefore, many research centers and pharmaceutical companies are attempting to develop TLRs modulators (Figure 2A). Scaffolds of naturally occurring modulators are ideal candidates for targeting these receptors; thus, these have been heavily investigated in clinical studies and are emerging as a fruitful approach in clinical trials. An exhaustive search of the literature also supports this notion.
Figure 2.
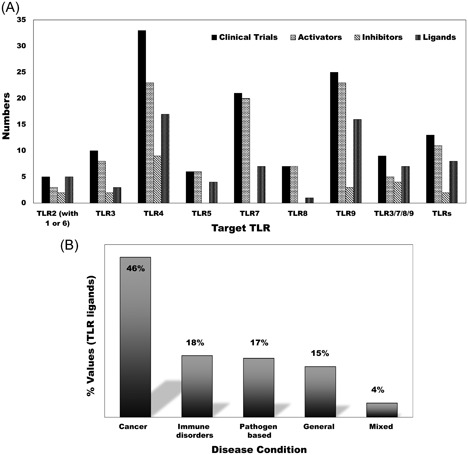
TLRs targeting ligands with respect to their relative clinical trials and disease conditions. A, The total number of clinical trials, activators (including agonists) and inhibitors (including antagonists), and the diversity of ligands are presented. The majority of ligands have been extensively pursued in different diseases, making it difficult to determine their exact numbers. The data indicate that total number of clinical trials exceeds the number of active drugs, suggesting the use of single drugs in multiple clinical trials. B, Clinical trial data showing the current status of drugs targeting TLRs from the disease perspectives. TLR ligands have been evaluated in multiple diseases including cancers, immune disorders, and viral and bacterial diseases. The largest proportion of clinical trials focuses on cancers, followed by immune disorders. “Mixed” indicates those cases where cancer and immune disease have been targeted simultaneously. The category “general” covers vaccination, clinical trials involving healthy volunteers, and those that are not covered by prior instances. This data was gathered from the clinical trials website (clinicaltrials.gov) using various keywords (cancers, immune disorders, TLR, TLRs, TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, and adjuvant) from June 2017 to Jan 2018. TLR, Toll‐like receptor
6.1. TLR2 (TLR1/TLR6)
TLR2, in combination with TLR1 or TLR6, detects the lipoproteins, diacyl lipid, or triacyl lipid, respectively, which makes it unique in forming functional heterodimers with other TLRs. Further, TLR2 interacts with modified proteins such as glyco‐ and lipoproteins, peptidoglycan, and zymosan, allowing it to detect a variety of PAMPs.87 This heterogeneity in TLR2 PAMP detection ranges across all types of pathogens, including viruses, bacteria, fungi, and parasites. The TLR2 expression has been detected in immune, endothelial, and epithelial cells,88 indicating that it is a functionally ubiquitous molecule. The homodimerization of TLR2 has been reported; however, further studies are required to confirm these findings.8, 12, 89 The ubiquitous nature and pivotal role of TLR2 make it an attractive drug‐target for various diseases; consequently, many clinical trials have been initiated to evaluate the efficacy of various lipopeptide derivates. Compounds being evaluated in clinical trials include lipopeptides, lipoproteins, oxidized low‐density lipoproteins, and TLR2 specific humanized IgG4 antibody, either alone or in various combinations (Table 2).
Table 2.
TLR2 (with TLR1 or TLR6) targeting ligands in clinical trials
| Ligand | Phase | Application | Target TLR adjuvant/drug | Sponsor/collaborators | NCT number | Type | Purpose |
|---|---|---|---|---|---|---|---|
| CBLB612 | Phase 2 | Breast cancer | TLR2 agonist/drug | Cleveland BioLabs, Inc | NCT02778763 | Synthetic lipopeptide | Neutropenia/recover blood cells count |
| SV‐283 (NY‐ESO‐1) | Phase 1 | Cancer | TLR2 agonist/adjuvant | SapVax LLC | NA | Combination of peptide and SM | Immune stimulation |
| ISA‐201 | Phase 2 | Head and neck tumor | TLR2/1 agonist/adjuvant | ISA Pharmaceuticals BV | NCT02821494 | Combination of peptide and SM, peptide | DC maturation |
| OPN‐305‐110 | Phases 1 and 2 | Second‐line and first line lower risk myelodysplastic syndrome | TLR2 antagonist/drug | Opsona Therapeutics Ltd | NCT03337451 | Monoclonal antibody | Anti‐inflammation |
| OPN‐305 | Phase 2 | Myelodysplastic syndrome, inflammatory disease, pancreas tumor, kidney transplant rejection | TLR2 antagonist/drug | Opsona Therapeutics Ltd | NCT02363491 | Monoclonal antibody | Anti‐inflammation |
Abbreviations: DC, dendritic cells; NA, not available; NCT, national clinical trial; SM, small molecule; TLR, Toll‐like receptor.
The most recent ligands, such as CBLB612 (synthetic lipopeptide TLR2 agonist), ISA‐201 (peptide agonist for TLR2), OPN‐305 (TLR2 antagonizing IgG4 monoclonal antibody), are in phase 2 in clinical trials primarily for oncogenic therapy, and act both as drugs and as adjuvants (Figure 2B).90, 91 The chemical constituents of these molecules are either lipoprotein or protein derivates, indicating that TLR2 can be targeted using mimetics of its natural ligands. This is not an absolute requirement; however, it is useful to note the existing therapeutic trend while targeting TLR2. Moreover, other than OPN‐305, the majority of molecules in phase 2 trials are agonists of TLR2, highlighting the importance of TLR2 activation in the context of malignancies. Small molecule‐based therapeutics have potential side effects that can be overcome by the application of biologics, including monoclonal antibodies (OPN‐305).90 The inhibition of TLR2 overactivation using OPN‐305 has potential applications in the treatment of inflammatory diseases.
TLR2 in TLR2/1 or TLR2/6 complexes exhibits a cavity on the binding junction of its convex side that allows the docking of Pam3CSK4 and other TLR2‐modulating ligands.12, 92 Pam3CSK4 has two esters and one amide bound lipid chains. The ester chains interact with TLR2, while the amide bound lipid chain can be accommodated into the hydrophobic cavity provided by TLR1 (Figure 3).12, 93 The TLR2/1 complex can further be stabilized by interprotein hydrogen bonding and hydrophobic interactions.12 The hydrophobic cavity in TLR1 has been mutated with bulky amino acids (Met338 and Leu360 to Phe338/360) in TLR6 to make binding of any lipid chain unfavorable, explaining the diacyl requirement for TLR2/6 complex formation.
Figure 3.
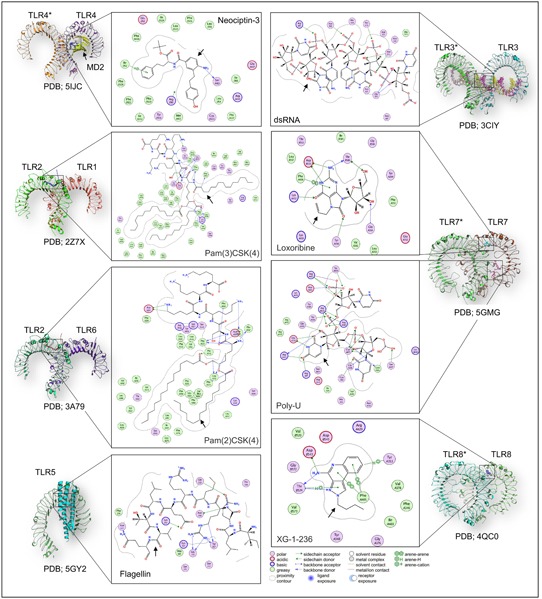
TLRs with bound ligands. The ligand binding mechanism of the extracellular TLRs (left, TLR1, 2, 4, 5, 6) and endosomal TLRs (right, TLR3, 7, 8) has been presented. Each monomer has been labeled; however, for the homodimers, the other monomer has been labeled with asterisk (*). In the case of TLR5, flagellin‐bound single monomer has been given. The respective protein databank (PDB) ID has also been given at the bottom of each structure. TLRs recognize several molecules, including protein, lipopeptide, small molecules and nucleic acids, and the bound ligand with individual TLR has been shown in 2D interaction diagram. The color code for the 2D interaction is given at the bottom of the figure. Black arrows indicate the bound TLR‐ligand. dsRNA, double‐stranded RNA; TLR, Toll‐like receptor; 2D, two‐dimensional [Color figure can be viewed at wileyonlinelibrary.com]
6.2. TLR3
TLR3 forms homodimer and signals in an exclusively TRIF–dependent manner in response to viral infections (double‐stranded RNA [dsRNA]) and stimulates the production of IFNs. The only known agonist for this TLR is poly‐ICLC (and its derivatives), which is being investigated in various clinical trials.94, 95 The success and ubiquitous nature of poly‐ICLC led to the belief that this was the only realistic possibility for targeting this TLR; however, recent studies have identified other small molecules that can either inhibit or activate TLR3.96, 97 These alternatives will not be available for clinical trials for a considerable period of time. There are a few clinical trials that involve anti‐TLR3 antibody to evaluate its efficacy in healthy individuals and asthmatic patients.98 The success of these proof‐of‐concept studies will lay the foundation of antibody‐based endosomal TLRs targeting in various diseases. However, in rhinoviral infection, the antibody could not demonstrate any improvement in asthmatic condition.99 Targeting of TLR3 is currently used as adjuvant therapy, along with other drugs or vaccines, against a variety of cancers; nonetheless, the sole activation of TLR3 to curb any disease has yet to be successfully explored (Table 3).
Table 3.
TLR3 targeting ligands in clinical trials
| Ligand | Phase | Application | Target TLR adjuvant/drug | Sponsor/collaborators | NCT number | Type | Purpose |
|---|---|---|---|---|---|---|---|
| Poly‐ICLC | Phases 1 and 2 | Low‐grade B‐cell lymphoma | TLR3 agonist/adjuvant | Joshua Brody, Icahn School of Medicine | NCT01976585 | Synthetic dsRNA | DC activation |
| Poly‐ICLC (NY‐ESO‐1) | Phases 1 and 2 | Melanoma | TLR3 agonist/adjuvant | Nina Bhardwaj, Ludwig Institute for Cancer Research, Oncovir Inc, CRI New York City, Icahn School of Medicine | NCT01079741 | Synthetic dsRNA | Activating humoral and T cell immunity |
| Poly‐ICLC | Phases 1 and 2 | Head and neck squamous cell carcinoma, sarcoma, Merkel cell carcinoma, cutaneous T‐cell lymphoma, melanoma, renal‐ bladder‐breast and prostate cancer | TLR3 agonist/adjuvant | Ludwig Institute for Cancer Research, MedImmune LLC, CRI New York City | NCT02643303 | Synthetic dsRNA | Tumor microenvironment modulation |
| Poly‐ICLC (Hiltonol) | Unknown | Primary ovarian cancer, fallopian tube cancer, primary peritoneal cancer | TLR3 agonist/adjuvant | Abramson Cancer Center of the University of Pennsylvania | NCT02452775 | Synthetic dsRNA, carboxymethylcellulose, poly‐l‐lysine | Immune stimulation |
| Poly‐ICLC (Romidepsin) | Phase 1 | Cutaneous T‐cell lymphoma | TLR3 agonist/adjuvant | New York University School of Medicine, Ludwig Institute for Cancer Research | NCT02061449 | Synthetic dsRNA | Immune stimulation |
| Poly‐ICLC (Pembrolizumab) | Phases 1 and 2 | Metastatic colon cancer, solid tumor | TLR3 agonist/adjuvant | Samir N. Khleif, Oncovir Inc, Merck Sharp and Dohme Corp, Augusta University | NCT02834052 | Synthetic dsRNA | Immune stimulation |
| Poly‐ICLC | Phases 1 and 2 | Chronic HIV‐1 infection | TLR3 agonist/adjuvant | The Campbell Foundation; Oncovir, Inc, National Institutes of Health (NIH), National Institute of Allergy & Infectious Diseases (NIAID), Nina Bhardwaj, Icahn School of Medicine at Mount Sinai | NCT02071095 | Synthetic dsRNA | Immune stimulation |
| Poly‐ICLC (DCVax‐001) | Phase 1 | HIV‐1 infection | TLR3 agonist/adjuvant | Rockefeller University | NCT01127464 | Synthetic dsRNA | Immune stimulation |
| PRV‐300 | Phase 1 | Healthy | TLR3 antagonist/drug | Janssen Research & Development, LLC | NCT02008279 | Anti‐TLR3 antibody | Anti‐inflammatory |
| PRV‐300 | Phases 1 and 2 | Asthma | TLR3 antagonist/drug | Centocor, Inc | NCT01195207 | Anti‐TLR3 antibody | Anti‐inflammatory |
Abbreviations: DC, dendritic cells; dsRNA, double‐stranded RNA; HIV, human immunodeficiency virus; NCT, national clinical trial; TLR, Toll‐like receptor.
Poly‐ICLC is a synthetic complex of polyinosinic‐polycytidylic acid (nucleic acid mimetics and pathway intermediates), carboxymethylcellulose, and poly‐l‐lysine (stabilizers). As dsRNA is a natural ligand with relatively low stability, its mimetics could be an affordable means of activating this TLR. Activation of TLR3 depends on dsRNA binding at two opposite sides of its ectodomain, which favorably relocates the C‐terminus of the ECD to facilitate further interactions and increased stabilization.100, 101 TLR3 interacts with the nucleotide backbone, rather than nucleotide bases, which explains its activation via multiple nucleotide combinations (Figure 3).100
6.3. TLR4
TLR4 is the only TLR that can function both at the cell membrane and in the endosome, and that can signal through MyD88‐ and TRIF–dependent pathways.102, 103 This has led to the evolution of additional precautions, such as an extensive ligand detection mechanism (cluster of differentiation 14, lipid binding protein, and coreceptor myeloid differentiation factor 2 [MD2]) and signal propagation mechanism (requirement of MAL for MyD88 and TRIF‐related adapter molecule [TRAM] for TRIF signaling pathways).
Among TLRs, only TLR4 has a suitable ligand binding pocket provided by MD2, rather than by the ectodomain of TLR4. This also provides an additional means of TLR4 (in)activation,104 since MD2 has a large hydrophobic cavity and lipid A derivatives are suitable binding molecules; however, other methods, such as disruption of MD2 binding with TLR4 or inhibition of interaction with the activating ligand, have also been explored.105 In case of MD2 and lipid A interaction, the lipid with six acyl chains (lipid VI‐A) can fully occupy the pocket and reorient the side chain of F126 amino acid into the binding cavity. This creates a favorable environment for the other TLR4/MD2 to dock properly (Figure 3).106 However, in case of a lower number of acyl chains, their binding is unable to reorient the side chain that, in turns, creates a steric hindrance for other heterodimer, leading to TLR4 inhibition.11, 104, 107
Prominent ligands that activate or inhibit TLR4 include lipid VI‐A and its derivatives (lipid 4A [antagonist], monophosphoryl lipid A [MPLA, a weak agonist],108 and glucopyranosyl lipid adjuvant [GLA, an agonist]),109 and recent studies have also evaluated antibody or peptide‐based drugs.105, 110 Among the TLRs, TLR4 has been extensively evaluated in the highest number of clinical trials and is of interest as a target for treatment of a variety of pathologies including cancers, viral infection, immune diseases, and inflammation. Both the agonistic and antagonistic aspects of TLR4 signaling pathways are being explored (Table 4). In addition to inhibition of MD2‐mediated TLR4 signaling, the interaction of HMGB1 with TLR4 has also been considered in recent trials to improve the efficacy of anticancer drugs (https://clinicaltrials.gov/ct2/show/NCT02995655). Besides direct modulation, the addition of a constitutively active form of TLR4 as a vaccine substitute (https://clinicaltrials.gov/ct2/show/NCT02888756) and inhibition of dipeptidyl peptidase‐4 (DPP4) that induces IL‐6 expression through TLR4 are also the subjects of therapeutic evaluations.111
Table 4.
TLR4 targeting ligands in clinical trials
| Ligand | Phase | Applications | Target TLR adjuvant/drug | Sponsor/collaborators | NCT number | Type | Purpose |
|---|---|---|---|---|---|---|---|
| GLA‐SE (MART‐1 antigen) | Phase 1 | Stage IIA–IV skin melanoma | TLR4 agonist/adjuvant | Mayo Clinic, NCI | NCT02320305 | Glycolipid | Immune stimulation |
| LPS | Phase 1 | Asthma | TLR4 agonist/adjuvant | John Sundy, Duke University | NCT00671892 | Glycolipid | Prospective inflammation |
| GLA‐SE | Phase 1 | Stage III/IV adult soft tissue sarcoma | TLR4 agonist/adjuvant | Fred Hutchinson Cancer Research Center, NCI | NCT02180698 | Glycolipid | Immune stimulation |
| GSK1795091 | Phase 1 | Cancer | TLR4 agonist/drug | GSK | NCT02798978 | Glycolipid | Immune stimulation |
| G100 | Phases 1 and 2 | Follicular lymphoma (marginal zone allowed during dose escalation only) | TLR4 agonist/adjuvant | Immune Design, Merck Sharp & Dohme Corp | NCT02501473 | Glycolipid | Tumor microenvironment alteration, DC, T and other immune cells activation |
| GLA‐AF, GLA‐SE | Phase 1 | Healthy volunteers | TLR4 agonist/adjuvant | Rockefeller University, IDRI Corporation, Immune Design | NCT01397604 | Glycolipid | Immune stimulation, DC activation |
| CCRE | Phase 1 | aHealthy individual | TLR4 agonist/adjuvant | University of North Carolina, Chapel Hill, Environmental Protection Agency (EPA) | NCT02847247 | LPS | Inflammation |
| Endotoxin | Phase 1 | Endotoxemia | TLR4 agonist/adjuvant | Radboud University | NCT00184990 | SM | Inflammation |
| GLA | Phase 1 | Hookworm infection | TLR4 agonist/adjuvant | Baylor College of Medicine, George Washington University | NCT01717950 | Glycolipid | Immune stimulation |
| Eritoran | Phase 2 | Insulin sensitivity | TLR4 antagonist/drug | The University of Texas Health Science Center at San Antonio | NCT02321111 | Glycolipid | Lipid‐induced insulin resistance |
| NI‐0101 | Phase 2 | Rheumatoid arthritis | TLR4 inhibitor/drug | NovImmune SA | NCT03241108 | Anti‐TLR4 antibody | TLR4 blocker, anti‐inflammatory |
| NI‐0101 | Phase 1 | Healthy volunteers | TLR4 inhibitor/drug | NovImmune SA | NCT01808469 | Anti‐TLR4 antibody | TLR4 blocker, anti‐inflammatory |
| CX‐01 | Phase 1 | Refractory Myelodysplastic Syndrome and Acute Myeloid Leukemia | TLR4 inhibitor/adjuvant | Washington University School of Medicine, Cantex Pharmaceuticals | NCT02995655 | Polysaccharide | Bone marrow microenvironment disruptor |
| Eritoran | Phase 2 | Insulin sensitivity | TLR4 antagonist/drug | Nicolas Musi, The University of Texas Health Science Center at San Antonio | NCT02267317 | Glycolipid | Anti‐inflammatory |
| MPL (Grass MATA) | Phase 3 | Type 1 hypersensitivity | TLR4 agonist/adjuvant | Allergy Therapeutics Plc | NCT00414141 | Glycolipid | Anti‐inflammatory |
| MPL (Grass MATA) | Phase 2 | Allergy | TLR4 agonist/adjuvant | Allergy Therapeutics Plc | NCT02582073 | Glycolipid | Anti‐inflammatory |
| GLA‐SE | Phase 1 | Merkel cell carcinoma | TLR4 agonist/adjuvant | Immune Design Corp | NCT02035657 | Glycolipid | Immune stimulation |
| GLA (H5 VLP vaccine) | Phase 2 | Influenza virus infection | TLR4 agonist/adjuvant | Medicago Inc | NCT01991561 | Combination of protein and SM | Immune stimulation |
| PEPA‐10 | Phase 2 | Cancer | TLR4 agonist/adjuvant | Immunovo BV | NA | SM | Immune stimulation |
| PET‐lipid A (ONT‐10) | Phase 1 | Advanced Breast and Ovarian Carcinoma | TLR4 agonist/adjuvant | Cascadian Therapeutics Inc | NCT02270372 | Glycolipid | Immune stimulation |
| PET‐lipid A (ONT‐10) | Phase 1 | Solid tumors | TLR4 agonist/adjuvant | Cascadian Therapeutics Inc | NCT01556789 | Glycolipid | Immune stimulation |
| PET‐lipid A (ONT‐10) | Phase 1 | Solid tumors | TLR4 agonist/adjuvant | Cascadian Therapeutics Inc | NCT01978964 | Glycolipid | Immune stimulation |
| GLA (H7N9 influenza vaccine) | Phase 1 | Influenza virus A infection | TLR4 agonist/adjuvant | Medicago Inc | NCT02022163 | Glycolipid | Enhance seroprotective antibody titers |
| G‐305 | Phase 1 | Cancer | TLR4 agonist/adjuvant | Immune Design Corp | NCT02015416 | Combination of protein and SM | Immune stimulation |
| Lipid A (SAR‐439794) | Phase 1 | Peanut hypersensitivity | TLR4 agonist/adjuvant | Sanofi, Immune Design Corp | NCT03463135 | Glycolipid | Immune stimulation |
| GLA | Phase 1 | Schistosomiasis | TLR4 agonist/adjuvant | Immune Design Corp | NCT02337855 | Glycolipid | Immune stimulation |
| JKB‐121 | Phase 2 | Nonalcoholic steatohepatitis | TLR4 antagonist/drug | TaiwanJ Pharmaceuticals | NCT02442687 | SM | Anti‐inflammatory |
| GLA‐SE (CMB‐305) | Phase 2 | Sarcoma and its various forms | TLR4 agonist/adjuvant | Immune Design Corp | NCT02609984 | Glycolipid | Immune stimulation, DC activation |
| GLA‐SE (CMB‐305) | Phase 2 | Sarcoma, melanoma, nonsmall‐lung cancer | TLR4 agonist/adjuvant | Immune Design Corp | NCT02387125 | Glycolipid | Immune stimulation, DC activation |
| JKB‐122 | Phase 2 | Chronic hepatitis C | TLR4 antagonist/drug | TaiwanJ Pharmaceuticals | NCT02293941 | SM | Anti‐inflammatory |
| JKB‐122 | Phase 2 | Autoimmune hepatitis, nonalcoholic fatty liver disease | TLR4 antagonist/drug | TaiwanJ Pharmaceuticals | NCT02556372 | SM | Anti‐inflammatory |
| Ibudilast (aka MN‐166) | Phase 2 | Alcoholism, neuropathic pain, amphetamine and opiate dependence, glioblastoma, traumatic brain injury | TLR4 antagonist/drug | MediciNova Inc | NCT01860807 | SM | Proinflammatory cytokine suppression |
| Intralipid (20%) | NA | Diabetes, obesity | TLR4 expression | The University of Texas Health Science Center at San Antonio, NIDDK | NCT01740817 | Lipid, SM | TLR4 expression |
Abbreviations: CCRE, clinical center reference endotoxin; GLA, glycopyranosyl lipid A; GSK, GlaxoSmithKline; NA, not applicable; NCI, National Cancer Institute; NCT, national clinical trial; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; SM, small molecule; TLR, Toll‐like receptor.
6.4. TLR5
TLR5 detects the bacterial monomeric flagella and mounts an immune response.112, 113 It triggers the MyD88‐dependent pathway in response to enterobacterial invasion and maintains intestinal homeostasis. TLR5 is expressed in almost all cell types with prominent expression in mucosal dendritic cells (DC); however, literature discussing TLR5 is scarce.114, 115 From a therapeutic perspective, all clinical trials targeting TLR5 include use of recombinant flagellin protein.116, 117, 118, 119 Additionally, small molecules to block the TLR5‐flagellin interaction are being tested in preclinical studies.120 In the majority of therapeutic settings, ligands for TLR5 act as adjuvants rather than as stand‐alone drugs, enhancing the efficacy and potency of vaccine candidates (Table 5). TLR5 can be an attractive target because it detects only protein. Short peptides derived from flagellin can be used as activators, while modified forms of such peptides can inhibit TLR5.118, 121 Recently, the crystal structure of zebrafish TLR5 with flagellin was determined, providing insights into its mode of activation (Figure 3).122 The leucine‐rich repeat 9 (LRR9) region in TLR5 has a critical role, and Arg89, Glu114, and Leu93 from flagellin form a hotspot with chemical and geometric complementarity.122 These regions on both proteins should be explored further to design novel therapeutics.
Table 5.
TLR5 targeting ligands in clinical trials
| Ligand | Phase | Application | Target TLR adjuvant/drug | Sponsor/collaborators | NCT number | Type | Purpose |
|---|---|---|---|---|---|---|---|
| Mobilan (M‐VM3) | Phases 1 and 2 | Prostate cancer | TLR5 agonist/adjuvant | Panacela Labs LLC | NCT02844699 | RP | Immune stimuation |
| Mobilan (M‐VM3) | Phase 1 | Prostate cancer | TLR5 agonist/adjuvant | Panacela Labs LLC | NCT02654938 | RP | Immune stimulation |
| Entolimod (aka CBLB502) | Phase 1 | Unspecified adult solid tumor | TLR5 agonist/adjuvant | Roswell Park Cancer Institute, NCI, Cleveland BioLabs Inc | NCT01527136 | RP | Immune stimulation |
| VAX125 | Phase 2 | Influenza | TLR5 agonist/adjuvant | VaxInnate Corporation | NCT00966238 | RP | Immune stimulation |
| VAX102 (flagellin.HuM2e) | Phase 1 | Influenza | TLR5 agonist/adjuvant | VaxInnate Corporation; Bill & Melinda Gates Foundation | NCT00603811 | RP | Immune stimulation |
| Entolimod (radiation therapy) | Phase 1 | Mucositis, various types of squamous cell carcinoma of various tissues | TLR5 agonist/adjuvant | Roswell Park Cancer Institute, NCI, Cleveland BioLabs Inc | NCT01728480 | RP | Immune stimulation |
Abbreviations: NCI, National Institute of Cancer; NCT, national clinical trial; RP, recombinant protein; TLR, Toll‐like receptor.
6.5. TLR7/8
TLR7 and TLR8 are functionally active in the endosomal compartment, use MyD88 adapter molecules and are activated by single‐stranded RNA (ssRNA).123, 124 Majority of ligands in clinical trials that target TLR7/8 are small molecules (eg, imiquimod [R837], resiquimod, or GSK2245035), and most are derivatives of imidazoquinoline, a tricyclic organic molecule.125, 126, 127 (Tables 6, 7, 8). There are functional differences between these two TLRs; for example, plasmacytoid DC and monocytes can be directly activated by TLR7 agonists; however, other than monocytes, TLR8 agonists can directly activate mDCs and monocyte‐derived DCs. TLR7 agonists were more potent when compared with TLR8 agonists regarding antiviral responses in the form of IFN, I‐TAC (IFN‐inducible T‐cell α chemoattractant), and IFN‐regulated cytokines from human peripheral blood mononuclear cells (PBMC).128 Proinflammatory responses, such as expression of IL‐12, TNF‐α, and macrophage inflammatory proteins‐1α (MIP‐1α) were enhanced by TLR8 agonism when compared with TLR7, leading to characteristic differential cell induction profiles.
Table 6.
TLR7 targeting ligands in clinical trials
| Ligand | Phase | Application | Target TLR adjuvant/drug | Sponsor/collaborators | NCT number | Type | Purpose/mechanism |
|---|---|---|---|---|---|---|---|
| GSK2245035 | Phase 2 | Asthma and rhinitis | TLR7 agonist/drug | GSK, PATH | NCT01607372 | SM | Type 1 IFN induction |
| Imiquimod (R837) | Phases 1 and 2 | Metastatic and recurrent breast cancer | TLR7 agonist/adjuvant | New York University School of Medicine, NCI | NCT01421017 | SM | Adaptive immune stimulator |
| Imiquimod | Phase 1 | Melanoma and metastatic cancer | TLR7 agonist/drug | University of Oklahoma, NCI | NCT00453050 | SM | Immune stimulation |
| Imiquimod | Phase 2 | Breast cancer and breast neoplasms | TLR7 agonist/drug | New York University School of Medicine | NCT00899574 | SM | Immune stimulation |
| 852A | Phase 2 | Breast, ovarian, endometrial, and cervical cancer | TLR7 agonist/drug | Masonic Cancer Center University of Minnesota, Pfizer | NCT00319748 | SM | DC activation, IFN‐α secretion |
| Imiquimod | Phase 3 | Influenza viral infection | TLR7 agonist/adjuvant | The University of Hong Kong | NCT02103023 | SM | Improve vaccine immunogenicity against influenza virus |
| Imiquimod | Phase 2 | HPV | TLR7 agonist/drug | Medical University of Vienna | NCT00941811 | SM | Immune stimulation |
| Imiquimod (intradermal HBVv) | Phases 2 and 3 | Renal failure | TLR7 agonist/adjuvant | The University of Hong Kong | NCT02621112 | SM | Improving vaccine immunogenicity |
| Imiquimod (influenza vaccine) | Unknown | Chronic illness | TLR7 agonist/adjuvant | The University of Hong Kong | NCT01508884 | SM | Activation of APC |
| GS‐9620 | Phase 2 | Chronic hepatitis B | TLR7 agonist/adjuvant | Gilead Sciences | NCT02166047 | SM | Activation of pDC |
| Single or multiple GS‐9620 | Phase 1 | Hepatitis B | TLR7 agonist/adjuvant | Gilead Sciences | NCT01590654 | SM | Activation of pDC, Immune stimulation |
| Imiquimod | Unknown | Photoaged skin and normal skin | TLR7 agonist/adjuvant | University of Michigan | NCT02889159 | SM | Immune stimulation |
| RO6864018 (aka ANA773, RO‐6864018) | Phase 1 | Healthy volunteer | TLR7 agonist/drug | Hoffmann‐La Roche | NCT02015715 | SM | Immunomodulator, Immune stimulation |
| RO7020531 | Phase 1 | Chronic Hepatitis B | TLR7 agonist/drug | Hoffmann‐La Roche | NCT02956850 | SM | Immune stimulation, B and T cell activations |
| RO6864018 | Phase 2 | Chronic Hepatitis B | TLR7 agonist/drug | Hoffmann‐La Roche | NCT02391805 | SM | Immunomodulator, Immune stimulation |
| GSK2245035 | Phase 2 | Mild asthma and allergic rhinitis | TLR7 agonist/drug | GlaxoSmithKline | NCT01788813 | SM | Immune stimulation |
| GSK2445053 | Phase 1 | Rhinitis, allergic | TLR7 agonist/drug | GlaxoSmithKline | NCT01480271 | SM | Induction of IFNα |
| GSK2245035 | Phase 2 | Allergy rhinitis, asthma, and respiratory tract allergy | TLR7 agonist/drug | GlaxoSmithKline | NCT02833974 | SM | Immune stimulation |
| Imiquimod | Phase 3 | Actinic keratosis | TLR7 agonist/drug | Graceway Pharmaceuticals, LLC | NCT00894647 | SM | Immune stimulation |
| Imiquimod | Phase 4 | Actinic keratosis | TLR7 agonist/drug | MEDA Pharma GmbH & Co. KG | NCT00777127 | SM | Immune stimulation |
| Imiquimod | Approved (China, Dec 2004) | Keratosis, mycosis fungoides, verruca vulgaris, condyloma, basal cell carcinoma, and molluscum contagiosum infection | TLR7 agonist/adjuvant | Mochida Pharmaceutical Co Ltd, 3 M Pharmaceuticals, Valeant Pharmaceuticals International Inc, iNova Pharmaceuticals Pty Ltd, Intendis GmbH, Meda AB | NCT01453179 | SM | Immune stimulation |
Abbreviations: GSK, GlaxoSmithKline; HPV, human papilloma virus; IFN, interferon; NCI, National Cancer Institute; NCT, national clinical trial; SM, small molecule; TLR, Toll‐like receptor.
Table 7.
TLR3/7/8/9 targeting ligands in clinical trials
| Ligand | Phase | Application | Target TLR adjuvant/drug | Sponsor/collaborators | NCT number | Type | Purpose/mechanism |
|---|---|---|---|---|---|---|---|
| Resiquimod, poly‐ICLC | Phase 2 | Glioma, anaplastic astrocytoma, anaplastic astro‐oligodendroglioma | TLR3/7/8 agonist/adjuvant | Jonsson Comprehensive Cancer Center | NCT01204684 | SM, synthetic dsRNA | Antitumor and antiviral immune stimulation |
| Poly‐ICLC, resiquimod (R848) | Phases 1 and 2 | Melanoma and its metastatic mucosal variants | TLR3/7/8 agonist/adjuvant | Craig L Slingluff, Jr, University of Virginia | NCT02126579 | SM, synthetic dsRNA | Antitumor immune stimulation |
| Resiquimod (R848) | Phase 1 | Influenza vaccination in seniors | TLR7/8 agonist/adjuvant | University of British Columbia | NCT01737580 | SM | Immune stimulation |
| MEDI9197 (durvalumab) | Phase 1 | Solid tumors, cutaneous T cell lymphoma | TLR7/8 agonist/adjuvant | MedImmune LLC | NCT02556463 | SM | Improving antigen presentation |
| Resiquimod (NY‐ESO‐1) | Phase 1 | Tumors | TLR7/8 agonist/adjuvant | Nina Bhardwaj, CRI New York City, Icahn School of Medicine at Mount Sinai | NCT00821652 | SM | Immune stimulation |
| IMO‐8400 | Phases 1 and 2 | Waldenstrom's macroglobulinemia | TLR7/8/9 antagonist/adjuvant | Idera Pharmaceuticals, Inc | NCT02092909 | Oligonucleotide antagonist | Immune suppression, TLR7/8/9 signaling blocker |
| IMO‐8400 | Phase 2 | Plaque psoriasis | TLR7/8/9 antagonist/drug | Idera Pharmaceuticals, Inc | NCT01899729 | Oligonucleotide antagonist | Immune suppression |
| CPG‐52364 | Phase 1 | Healthy volunteers | TLR7/8/9 antagonist/drug | Pfizer | NCT00547014 | SM | Immune suppression |
| IMO‐8400 | Phases 1 and 2 | Diffuse large B cell lymphoma | TLR7/8/9/ antagonist/drug | Idera Pharmaceuticals, Inc | NCT02252146 | Oligonucleotide antagonist | Immune suppression |
Abbreviations: CRI, Cancer Research Institute; dsRNA, double‐stranded RNA; NA, not available; NCT, national clinical trial; SLE, systematic lupus erythematosus; SM, small molecule; TLR, Toll‐like receptor.
Table 8.
TLR8 targeting ligands in clinical trials
| Ligand | Phase | Application | Target TLR adjuvant/drug | Sponsor/collaborators | NCT number | Type | Purpose/mechanism |
|---|---|---|---|---|---|---|---|
| VTX‐2337 (Motolimod) | Phase 1 | A variety and different stages of metastatic squamous neck cancer with occult primary squamous cell carcinoma | TLR8 agonist/adjuvant | University of Washington, NCI | NCT01334177 | SM | Immune stimulation |
| VTX‐2337 | Phase 1 | For various types and stages of colorectal, pancreatic, breast, melanoma, non‐small cell lung carcinoma, pancreatic, renal cell carcinoma and solid neoplasm | TLR8 agonist/adjuvant | Mayo Clinic, NCI | NCT02650635 | SM | Immune stimulation |
| VTX‐2337 | Phase 1 | Various types of ovarian cancers and fallopian tube carcinoma, recurrent ovarian carcinoma | TLR8 agonist/adjuvant | Gynecologic Oncology Group, NCI | NCT01294293 | SM | Immune stimulation |
| VTX‐2337 | Phase 2 | Epithelial ovarian cancer, fallopian tube cancer, primary peritoneal cancer | TLR8 agonist/adjuvant | VentiRx Pharmaceuticals Inc, Gynecologic Oncology Group | NCT01666444 | SM | Immune stimulation |
| VTX‐2337 (with radiotherapy) | Phases 1 and 2 | Low grade B‐Cell lymphoma | TLR8 agonist/adjuvant | VentiRx Pharmaceuticals Inc, Stanford University | NCT01289210 | SM | Immune stimulation |
| VTX‐2337 | Phase 2 | Carcinoma, squamous cell of head and neck | TLR8 agonist/adjuvant | VentiRx Pharmaceuticals Inc | NCT01836029 | SM | Immune stimulation |
| VTX‐2337 (with anti‐PD‐L1 antibody MEDI4736) | Phases 1 and 2 | Ovarian cancer | TLR8 agonist/adjuvant | Ludwig Institute for Cancer Research, MedImmune LLC, VentiRx Pharmaceuticals Inc, CRI New York City | NCT02431559 | SM | Immune stimulation |
Abbreviations: CRI, Cancer Research Institute; NCI, National Cancer Institute; NCT, national clinical trial; PD‐L1, programmed death‐ligand 1; SM, small molecule; TLR, Toll‐like recptor.
TLR7 agonists have been actively studied in phase 1 and 2 trials aiming to curb the persistent viral load in HIV‐ and HBV‐infected individuals.129 Moreover, various prodrug (pharmacologically active after metabolism) approaches have also targeted TLR7 (RO6870868 [single prodrug] or RO6864018 [double prodrug]), and use of these as TLR7 agonists was useful in treating hepatitis B infection in phase 1 clinical trials. The results of this trial were promising and paved the way for phase 2 trials (https://clinicaltrials.gov/ct2/show/NCT02015715).
TLR8 can also be activated by ssRNA as natural ligand and by VTX‐2337 (motolimod), a synthetic small molecule selective for TLR8 and is being evaluated in clinical trials.124, 130 TLR8 is a less studied receptor, as its roles overlap with those of TLR7, with which it shares multiple features. When treated with VTX‐2337, TLR8 stimulates TNF‐α and IL‐12 production at lower concentrations in human PBMCs. It also induces TNF‐α and IL‐12 secretion from monocytes and myeloid DCs through the NF‐κB pathway. IFNγ secretion was observed when NK cells were treated with VTX‐2337, which can enhance the lytic capability and antibody‐dependent cell‐mediated cytotoxicity of NK cells.130 VTX‐2337 also improves the efficacy of pegylated liposomal doxorubicin in treatment of ovarian cancer in a mouse model with humanized immune system that has been reconstituted with human CD34+ cells.131 This is the only ligand molecule that has been actively evaluated for treatment of a variety of cancers, including head and neck cancer, colorectal, pancreatic, melanoma, breast, renal cell carcinoma, nonsmall–cell lung carcinoma, and other solid neoplasms. In the majority of cases, VTX‐2337 was used in combination with other drugs; however, it is also being evaluated as a stand‐alone drug for treatment of lymphoma.130
TLR7 and TLR8 share similar activation patterns, both have z‐loops involved in ssRNA recognition, and both possess two binding sites; the first binding site binds guanosine and uridine in TLR7 and TLR8, respectively, while the second binds ssRNA in both cases (Figure 3).132 In TLR7 ssRNA binding primes the receptor for guanosine binding and subsequent dimerization, while synthetic molecules, such as R848, can activate TLR7 without the need for ssRNA.133, 134 Importantly, TLR7 remains monomeric in the absence of any ligand and dimerizes in response to ligand binding; however, its dimer conformation is similar to TLR8 and TLR9. TLR8, on the other hand, is a naturally occurring weak dimer that undergoes conformational change upon ligand binding. The Z‐loop may have an important regulatory role; when it is cleaved from TLR8, TLR8 forms a tight dimer and initiates signaling in the absence of ligand.135
6.6. TLR9
TLR9 senses CpG DNA in endosomes and induces the IFN response.136, 137 TLR9 is involved in numerous diseases and has been targeted by various therapeutic approaches. All of the ligands tested in clinical trials that exclusively target TLR9 are either nucleotides or nucleotide derivatives. There are various types of CpG DNAs that are being evaluated in different trials for treatment of diverse conditions. AZD1419 is a C‐type CpG‐based inhaled TLR9 agonist for treatment of asthma and to stimulate IFNs production in lungs. This treatment was classified as well‐tolerated and safe in phase 1 human trials with potential disease‐modifying characteristics and is a promising new therapeutic for use in various immune diseases.138 CYT003 was initially found to be effective; however, its effects were not confirmed in phase 2 clinical trials where 35 patients were treated with varying doses of CYT003.139 Another TLR9 agonist, EMD 1201081, was evaluated in phase 2, open‐label, randomized trial in patients with head and neck cancer, and was found to be ineffective in the tested dose regimen.140 GNKG168 is another CpG‐based molecule that can induce CD8+ T cell antitumor cytotoxic responses; however, it was withdrawn in clinical phase 1 because of sponsor reluctance to further support the study141 (NCT01743807) (Tables 7 and 9).
Table 9.
TLR9 targeting ligands in clinical trials
| Ligand | Phase | Application | Target TLR adjuvant/drug | Sponsor/Collaborators | NCT Number | Type | Purpose/ Mechanism |
|---|---|---|---|---|---|---|---|
| MGN1703 (Ipilimumab) | Phase 1 | Melanoma | TLR9 agonist/adjuvant | MD Anderson Cancer Center, Mologen AG | NCT02668770 | DNA‐based molecule | Antitumor immune stimulation |
| SD‐101 | Phases 1 and 2 | Lymphoma and its various forms | TLR9 agonist/adjuvant | Robert Lowsky, NCI, Stanford University | NCT02254772 | CpG‐C class oligodeoxynucleotide | Antitumor immune stimulation |
| SD‐101 | Phases 1 and 2 | Grade 1/2/3 follicular lymphoma and recurrent and refractory follicular lymphoma | TLR9 agonist/drug | Robert Lowsky, NCI, Stanford University | NCT02927964 | CpG‐C class oligodeoxynucleotide | Antitumor immune stimulation |
| CpG vaccine (autologous tumor cell) | Phase 1 | Colorectal neoplasms, anal, colon, and rectal cancers | TLR9 agonist/adjuvant | Stanford University | NCT00780988 | Oligonucleotide | Antitumor immune stimulation |
| CYT003 | Phase 2 | Moderate to severe allergic asthma | TLR9 agonist/drug | Cytos Biotechnology AG | NCT01673672 | Oligonucleotide | TH1‐mediated immune response |
| CYT003‐QbG10 | Phase 2 | Allergic Bronchial Asthma | TLR9 agonist/drug | Cytos Biotechnology AG | NCT00890734 | Oligonucleotide | TH1‐mediated immune response |
| DUK‐CpG‐001 | Phase 2 | Hodgkin lymphoma, non‐Hodgkin lymphoma | TLR9 agonist/adjuvant | David Rizzieri, MD, Duke University | NCT02115126 | Single‐stranded synthetic DNA molecules | Immune stimulation |
| MGN1703 | Phases 1 and 2 | HIV | TLR9 agonist/drug | University of Aarhus | NCT02443935 | DNA‐based molecule | Antiviral immune stimulation |
| CpG‐7909 | Phases 1 and 2 | Non‐Hodgkin lymphoma, mycosis fungoides | TLR9 agonist/adjuvant | Ronald Levy, Lymphoma Research Foundation, American Society of Clinical Oncology, Stanford University | NCT00185965 | single‐stranded synthetic DNA molecules | TH1‐like immune stimulator |
| CYT003 | Phase 2 | Asthma | TLR9 agonist/drug | Cytos Biotechnology AG | NCT02087644 | Oligonucleotide | TH1‐mediated immune response |
| CpG‐7909 (pneumococcal vaccines) | Phases 1 and 2 | HIV infections | TLR9 agonist/adjuvant | Aarhus University Hospital | NCT00562939 | Oligonucleotide | Immune stimulator |
| GNKG168 | Phase 1 | Relapsed acute lymphoblastic myelogenous leukemia | TLR9 agonist/drug | Therapeutic Advances in Childhood Leukemia Consortium | NCT01743807 | CpG‐C class oligodeoxynucleotide | Antitumor immune response |
| CpG‐7909 | Phase 1 | Malaria | TLR9 agonist/adjuvant | Oxford University, NIAID | NCT01351948 | Oligonucleotide | Immune stimulation |
| EMD 1201081 (Cetuximab) | Phase 2 | Squamous cell carcinoma of the head and neck | TLR9 agonist/adjuvant | EMD Serono | NCT01040832 | Oligonucleotide | Immune stimulation |
| IMO‐2125 | Phase 1 | Hepatitis C | TLR9 agonist/drug | Idera Pharmaceuticals, Inc | NCT00728936 | Oligonucleotide | Immune stimulation |
| CpG 10104 | Phase 1 | Hookworm infection | TLR9 agonist/adjuvant | Baylor College of Medicine, George Washington University | NCT02143518 | Oligonucleotide | Immune stimulation |
| AZD1419 | Phase 2 | Asthma | TLR9 agonist/drug | AstraZeneca | NCT02898662 | C‐type CpG Oligonucleotide | IFN induction |
| CpG‐7909 (URLC10‐177, TTK‐567) | Phases 1 and 2 | Esophageal cancer | TLR9 agonist/adjuvant | Wakayama Medical University, Human Genome Center University of Tokyo | NCT00669292 | Oligonucleotide | Immune stimulation |
| CpG‐7909 | Phases 1 and 2 | Mycosis fungoides | TLR9 agonist/adjuvant | Stanford University, NIH | NCT00226993 | Oligonucleotide | Immune stimulation |
| SD‐101 | Phase 1 | Chronic hepatitis C | TLR9 agonist/adjuvant | Dynavax Technologies Corporation, Synteract, Inc, PPD | NCT00823862 | CpG‐C class oligodeoxynucleotide | Anti0tumor immune response |
| Mycobacterium w.(Mw)‐freeze dried extract 0.5 mL | NA | Optic neuritis | TLR9 antagonist/adjuvant | Sudhalkar Eye Hospital | NCT01424735 | Bacterial mix | Immune suppression |
| Hydroxychloroquine sulfate, valsartan | Phase 4 | Primary IgA nephropathy | TLR9 inhibitor/adjuvant | Peking Union Medical College Hospital | NCT02765594 | SM | Impaired IFN‐α and TNF‐α secretion |
| Hydroxychloroquine | Phase 3 | Sjogren's syndrome | TLR9 inhibitor/drug | SNU Hospital | NCT01601028 | SM | Immune suppression |
| CpG‐ODN (K3) | Phase 1 | Lung tumor | TLR9 agonist/drug | National Institute of Biomedical Innovation, Osaka University | NA | Nucleotide based | Immune stimulation |
Abbreviations: IFN, interferon; NA, not applicable; NIAID, National Institute of Allergy & Infectious Diseases; NCI, National Cancer Institute; NCT, national clinical trial; NIH, National Institute of Health; SM, small molecule; SNU, Seoul National University; TLR, Toll‐like receptor; TNF, tumor necrosis factor.
Similar to other TLRs, TLR9 forms a symmetrical complex with CpG‐DNA; nonetheless, during inhibitory DNA interactions, it remains in a monomeric form. CpG‐DNA binding with TLR9 is symmetric and they form a stoichiometric complex of 2:2, as DNA is recognized by both TLR9 monomers, particularly via the amino‐terminal fragment (LRRNT–LRR10) from one protomer and the carboxy‐terminal fragment (LRR20–LRR22) from the other.142 CpG‐DNA‐based TLR9 inhibition is mediated by binding to the concave surface formed by LRR2–LRR10, thereby inhibiting its signaling.
6.7. TLR10–13
Other than TLR1–9, humans also have TLR10 and TLR11, whereas they lack TLR12 and TLR13.143 The expression of TLR10 has been confirmed in humans (spleen, lymph node, B cells, monocytes, and neutrophils)144; nonetheless, its function and specific ligand are yet to be determined. Recently, it was suggested that TLR10 may act as an anti‐inflammatory TLR, rather than a conventional inflammatory receptor and that it modulates TLR2‐mediated responses through the formation of heterodimers with TLR1 or TLR6.145 Humans have a pseudogene homologous to TLR11 that includes a premature stop codon, resulting in lack of protein expression.146 TLR11 and TLR12 have been studied in mouse and they have shown to detect profilin from Toxoplasma gondii and be capable of forming heterodimers.143
7. INTERDEPENDENT AND CROSS‐TALK AMONG TLR PATHWAYS
Since TLRs overlap in their structures and signaling pathways, it is rational to assume that one single ligand can activate multiple TLRs; however, this is less common among plasma membrane expressed TLRs, TLR2/1, TLR2/6, TLR4, and TLR5, and there are a few ligands that can share targets, particularly for TLR2 and TLR4. This situation is very common among endosomal TLRs, partly because they are all involved in sensing nucleic acids, and endosomes have a specific pH range that is also thought to contribute to their activation. Various ligands exert their actions on multiple endosomal TLRs (eg, TLR7/8 or TLR7/8/9), which may imply a combination of multiple pathways in their activity, a common mode of activation, and, to some extent, H+ interference of these TLRs being a common factor147, 148 (Tables 7 and 10). TLR7 and TLR8 detect ssRNA, which may explain why one ligand is equally effective against both TLRs. Some studies have also explored the independent targeting for either TLR7149 or TLR8.150 The expression patterns of various TLRs differ among tissues, and the extent to which they respond to various ligands may contribute to unexpected results of clinical trials (mostly failure and toxicity issues). TLR2/TLR4 (cell surface) can be regulated by a single ligand, and there are many examples of endosomal TLRs being regulated by single ligands, suggesting that they may have similar sensing and regulatory mechanisms that could be exploited for therapeutic purposes.
Table 10.
Ligands with either undefined or multiple target TLRs
| Ligand | Phase | Application | Mode of action adjuvant/drug | Sponsor/collaborators | NCT number | Type | Purpose/mechanism |
|---|---|---|---|---|---|---|---|
| DC‐based vaccine | Phases 1 and 2 | Melanoma | TLR agonist/adjuvant | Radboud University | NCT01530698 | Biologics, SM | DC activation |
| TLR agonist (Tuberculin nasal challenge, timothy grass pollen) | NA | Allergic rhinitis, asthma, latent tuberculosis | TLR agonist/adjuvant | Imperial College London | NCT02090374 | Bacterial mix, SM | Immune stimulation |
| Mycobacterium w | Phases 2 and 3 | Sepsis | TLR agonist/adjuvant | Postgraduate Institute of Medical Education & Research, PGIMS, Rohtak, St. John's National Academy of Health Sciences | NCT02330432 | Bacterial mix | Immune stimulation |
| DRibbles vaccine, imiquimod, HPV vaccine | Phase 2 | Carcinoma, non‐small‐cell lung cancer | TLR2/3/4/7/9 agonists/adjuvant | UbiVac | NCT01909752 | Combination of protein and SM | Immune stimulation |
| DRibbles vaccine, imiquimod, HPV vaccine | Phase 1 | Adenocarcinoma of the prostate | TLR agonist/adjuvant | UbiVac | NCT02234921 | Combination of protein and SM | Immune stimulation |
| MB‐11040 | Phase 1 | Menopause, autoimmune disease, cancer | TLR agonist/drug | KT & G Life Sciences Corp | NA | SM | Antitumor immunity |
| Mycobacterium w | Phases 2 and 3 | Severe sepsis, septic shock, immune modulation | TLR agonist/drug | Postgraduate Institute of Medical Education & Research | NCT02025660 | Bacterial mix | Immune stimulation |
| Insulin | Phase 2 | Insulin resistance | TLR inhibitor/drug | Kaleida Health, American Diabetes Association | NCT01151605 | Biomolecule | TLR downregulation |
| Autologous DC vaccination | Phases 1 and 2 | Melanoma | TLRs agonist/adjuvant | Radboud University | NCT00940004 | SM | DC activation/maturation |
| R848 (GP100, MAGE‐3) | Phase 2 | Melanoma | TLR agonist/adjuvant | MD Anderson Cancer Center | NCT00960752 | SM | Immune stimulation |
| VB‐201 | Phase 2 | Ulcerative colitis | TLR2‐4 antagonist/drug | VBL Therapeutics | NCT01839214 | SM | Immune suppression |
| PUL‐042 | Phase 1 | Healthy individuals | TLR2/6, 9 agonist/adjuvant | Pulmotect Inc | NCT02124278 | SM | Immune stimulation |
| PUL‐042 | Phase 1 | Hematologic diseases, stem cell transplants | TLR2/6, 9 agonist/adjuvant | Pulmotect Inc | NCT03097796 | SM | Immune stimulation |
Abbreviations: DC, dendritic cell; HPV, human papilloma virus; NA, not available; NCI, National Cancer Institute; NCT, national clinical trial; PGIMS, Pandit Bhagwat Dayal Sharma; SM, small molecule; TLR, Toll‐like receptor.
TLRs may antagonize one another under certain physiological conditions. For example, TLR2 and TLR9 antagonize each other in a mouse model for oral infection with Salmonella enterica.151 TLR9 deficiency is manifested as reduced survival, exaggerated cytokine responses, and salmonella hepatitis, while TLR2 deficiency produces the opposite effects. Deficiency of either TLR may disrupt NK cell cytotoxicity, and IFN‐γ and ROS production.151
Synergism is very common in TLRs. When monocyte‐derived DCs have been triggered with a TLR8 ligand, TLR3 or TLR4 are also activated, resulting in expression of IL‐6, IL‐10, IL‐12, and TNF‐α elevation. These results were also confirmed by increased binding of IRF and signal transducers and activators of transcription (STAT) transcription factors to their respective DNA binding sites, which was abolished when NF‐κB, p38, and phosphoinositide 3‐kinase (PI3K) inhibitors were used.152 These data suggest that co‐operation among TLRs is perpetuated, not only at the top level but also among different signaling pathways to ensure proper and balanced expressions of target genes.
Synergy and tolerance of TLRs are long‐established and are critical to the innate immune response. The coadministration of LPS (TLR4 agonist) and MALP‐2 (TLR2 agonist) to mouse macrophages resulted in increased TNF‐α production.153 Repeated treatment with LPS or MALP‐2 resulted in a hyporesponse, also termed tolerance. Intriguingly, pretreatment with any ligand results in lower responses on exposure to the second ligand.153 LPS may cause downregulation of the cell surface expression of TLR4 after the second LPS treatment; however, MALP‐2‐mediated reduction in responses involve modulation of downstream signaling. The acute immune tolerance and cross‐tolerance between TLR4 and TLR9 have been studied,154 indicating that LPS selectively inhibits proinflammatory cytokines, while CpG suppresses both pro‐ and anti‐inflammatory responses. IRAK‐M is critical for the induction of this differential response, and its expression is modulated by IL‐7.154
The mycobacterium extract,155, 156 and autophagosome‐enriched cancer vaccine (DRibbles),157 which likely contain multiple biological molecules and can trigger numerous TLRs, is being evaluated in clinical trials; however, caution is required when considering the use of such substances in the clinic due to synergy and differential responsiveness of TLRs to various ligands. Moreover, DC vaccines that have been matured using TLR ligands are also therapeutically relevant, owing to the use of TLR ligands in their production.158, 159
8. FAILED CLINICAL TRIALS
The proportion of failures of clinical trials depends on the clinical stage, as well as the type of disease; particularly, failure at phase 3 is an impediment to the development of successful therapy for various diseases and TLRs are no exception. For example, eritoran, a TLR4 antagonist, that was being evaluated for treatment of sepsis could not meet its target end‐point in phase 3 when data from ~2000 patients were analyzed.160 Among the reasons of failure of eritoran, there were oversights in study design, patient population differences, improved patient care methods, and mixed bacterial infections.160 Similarly, imiquimod, a TLR7 agonist, produced a divergent result in phase 3 when evaluated for treatment of the skin disorder, molluscum contagiosum (MC) lesions, in children.161 Imiquimod was first approved by the Food and Drug Administration in 1997 for treatment of genital warts. This approval has prejudiced its subsequent off‐label use as the treatment of MC in children, since it was already shown to be effective against viral‐based diseases and its use is supported by several research and clinical investigations.82, 83 This off‐labeled use of imiquimod is still debatable.83, 161 Similarly, in a meta‐analysis, Qin et al (162) has systematically analyzed the TLR9 agonist effects as observed by other clinical investigations. It has been concluded that the safety profile of TLR9 agonists is acceptable if they are not combine with immunosuppressive drugs.162
The success rate of transition among different clinical phases (phase 1, 2, 3, and occasionally 4) is highly variable. The likelihood of a molecule passing phase 1 is 63.2%, which is the highest probability for any phase. Phase 2 has the lowest success rate (30.7%), while phase 3 has a success rate of 58.1%.163, 164 Biologics have twice the final success rate (18%), compared with that of small molecules (9%). The transition failure can comprise of drugs that have not met their specific endpoints, and there are cases where particular drugs did not show any improved effect over an existing treatment of a particular condition. The situation is exacerbated when similar molecules are evaluated in multiple interventions, causing an elevated number of failed trials.
Lack of recruitment (23%) and unstated reasons (such as unknown reasons for termination, unable to begin the study, unavailability of a drug, or protocol modification resulting in cessation of a trial; 26%) comprise the majority of reasons for failure of clinical trials targeting TLRs, followed by safety issues (18%) and financial concerns (where a sponsor withdraws the drug; 15%). Moreover, 15% of trials do not show any efficacy in subsequent clinical trials. The inadequate understanding of the biology of TLRs may also contribute to drug failure,160 underlining the need for further studies, increased understanding of the theoretical background of disease etiology and progression, modification of protocols to address problems, and trial redesign.
While many factors contribute to a failed clinical trial, a common reason underlying failure is a lack of serious focus on biomarker discovery and implementation. There is a clear trend of success among those trials including biomarker selection (25.9%) compared with those lacking selection biomarkers (8.4%).164 There are several methods that can be used to reduce failure rates, such as early identification of false drug candidates, stratification of patients, development of diagnostics, proper use of pharmacogenomics through machine learning, and other analysis tools that can provide improvements and efficiencies in patient categorization. Focusing on neglected disease areas can also help to reduce the failure burden. In recent years, the Drugs for Neglected Diseases Initiative has approved six treatments within a decade, with many more in the pipeline. This is not only dramatically reducing the cost of drug development, but also providing hope for individuals affected by neglected diseases and incentivizing the pharmaceutical industry to continue their search for new drugs.
Financial and commercial reasons are also major contributors to trial failures because sponsors are “unwilling,” or there are “failure to pursue” investigational drugs for commercially important diseases. This can be reduced if pharmaceutical companies focus on diseases that lack adequate therapeutic intervention, as drugs that show positive effects will soon be marketable. Additionally, if such a trial does fail, it has a lower cost impact on the company.
Drug development is a lengthy process that starts with lead molecule identification and progresses through optimization, animal modeling studies, pharmacokinetic and pharmacodynamic studies, and preclinical and clinical stage trials. Therefore, if a drug fails to show any effect or shows toxicity in clinical studies, there must have been a series of oversights during earlier experimental stages. It is hard to give a single reason for any failure and failures may encompass complex issues, such as the use of subjective, composite, or surrogate endpoints.165 Moreover, biases in outcome reporting and publications; underreporting of adverse events; failure to select an appropriate patient group; preference for relative outcomes, rather than absolute values; no defined core outcome sets; lack of transparency and basic science; inappropriate study population size; and lack of data integrity are among the reasons for trial failures. Finally, during clinical trials involving humans, factors that influence the drug metabolism, distribution, and secretion are diverse that predispose the pharmacokinetically and pharmacodynamically optimized drug molecules to failure.
9. PERSPECTIVES IN TLR TARGETING
Researchers are expending extensive efforts to generate appropriate solutions for various inflammatory, autoimmune, and malignant conditions; however, the process is not straightforward, rather it is littered with unexpected events and outcomes, along with unknown obstructions that severely undermine the efforts of the research community.
In the majority of studies targeting TLRs, the investigated compounds are related to or derived from natural ligands; particularly those targeting TLR3, TLR4, TLR5, and TLR9, and somewhat those for TLR2. TLR7 and TLR8 have the benefit of being targeted by small molecules rather than ssRNA. The instability of ssRNA molecules can hinder their use for TLRs activation. However, since RNAi technology is being evaluated in more than 100 clinical trials,166 stability should not be an issue, rather, tuning of small molecules is far easier than tuning biologics for therapeutic purposes.
Other than molecules derived from natural ligands, it is necessary to focus on the chemical space that can be used to target TLRs.106, 118 This broadening of the chemical space will provide more potent, specific, and less toxic molecules, resulting in fewer trial failures. Biologics are gaining popularity, as they have a higher ratio of success, and are comparatively safe and specific.167, 168, 169, 170, 171 It is estimated that the biologics will soon become the norm in therapeutics, in addition to being responsible for the majority of revenue.172 For different TLRs, the therapeutic trend can vary; however, a rise in antibody‐mediated TLR inhibition (TLR2, TLR3, and TLR4) and novel molecular backbone (independent of PAMPs) have been seen in recent therapeutics.
The evaluation of various drugs for similar or different conditions is also an optimal approach, which can facilitate the development of single drugs for multiple diseases. In this context, research laboratories can screen the outcomes of phase 2 failures that have been abandoned by their sponsors to evaluate them for other symptoms.173 Such an approach can dramatically reduce the cost, speed up the process, and will encourage pharmaceutical companies to share their data with research laboratories for application to other disease targets.
Rather than directly inhibiting TLRs, it may be more appropriate to target the transcription regulation of TLRs to suppress their expression,174 as described in a study where the authors used GST‐21 for cytokine inhibition, which could be reversed by the janus kinase 2 inhibitor, AG490. Since the majority of TLRs regulate the similar pathway, targeting of their downstream inhibitory signaling mechanisms should also be explored to further intensify the benefits of their inhibition.
Lack of clinical data is an impediment to the development of clinical research. It is estimated that approximately half of all clinical trials are not reported in either peer‐reviewed journals or clinical trial websites (clinicaltrials.gov; http://apps.who.int/trialsearch/).175, 176
It is now necessary to develop additional TLR ligands that should not mimic PAMPs, explore new biomarkers for disease progression, revise protocols, and clinical trials, target small subsets of patients, improve the understanding of the basic biology of diseases, and improve final outcomes, which must legitimately refer to the progress of the disease and the effect of the compound being applied.
10. CONCLUSIONS
TLRs are among the ideal targets for exploitation in immunotherapy; however, their biology still needs to be better understood in the context of target diseases. These receptors are capable of inhibiting disease pathophysiology, as well as exacerbating inflammatory diseases. Given this dual role, it is imperative to fine tune their activation using a multidrug approach. Cumulative evidence suggests the participation of TLRs in almost all diseases is unique and can be exploited by including their ligands as adjuvant treatment during regular immunotherapy or as part of other therapeutic regimens.
It is vital to create superior disease models that assist in early phase evaluation of drugs, improve diagnostics and evaluation of disease progression, and facilitate identification of novel biomarkers that reliably indicate disease progression and real‐time disease monitoring. Finally, the availability of clinical trial data should be ensured to guide the scientific community in their endeavors. This would also assist in the refinement of targets and lead molecules and improve the pathophysiological manifestations of diseases. Using a combination of computational power, next‐generation sequencing and proteomic data, machine learning approaches, and improved availability of results, we are hopeful that a dramatic increase in new therapeutic options for various inflammatory diseases and cancers involving TLRs and a decline in clinical trial failures will be achieved.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR'S CONTRIBUTION
MAA and SC participated in research design; MAA, MS, and JK performed data analysis; and MAA, MS, JK, and SC wrote or contributed to the writing of the manuscript.
ACKNOWLEDGMENTS
This study was supported by the National Research Foundation of Korea (grant no. NRF‐2015R1A2A2A09001059) and the Commercialization Promotion Agency for R&D Outcomes funded by the Ministry of Science and ICT (grant no. 2018K000369).
Biographies
Dr Muhammad Ayaz Anwar is a biochemist and completed his doctoral degree in computational analysis of TLRs. Later, in his postdoctoral training, he investigated the anti‐inflammatory approaches using small molecules designing and evaluation. He also participated in other projects involving mechanistic studies of TLRs, the role of TLRs in cancer propagation and peptide‐based therapeutic drugs.
Dr Masaud Shah has been trained in microbiology and pursued his doctoral degree in TLR‐based therapeutics and an interlay of TLRs in viral diseases. He has a profound experience in drug designing and evaluation, lead optimization, and manipulation of peptides for improved therapeutics. Currently, he filed several patents regarding TLR therapeutics for FDA approval.
Dr Jason Kim is currently serving as the CEO of J2H Biotech, Adjunct Professors at Cha's College of Science and Pharmacy, and Ajou University, and a board member of FDC Academic Society. He has an extensive experience in lead optimization, preclinical and clinical trials studies. Moreover, he has more than 100 national and international patents regarding protocol improvements and designing ligands for various diseases.
Prof Sangdun Choi is an accomplished scientist in TLR biology. He has dedicated his life to discovering the underlying mechanism and development of TLRs‐targeting therapeutics, that are involved in multiple diseases. He has published more than 200 papers in this area, and currently hold several FDA patents with many more being filed. He has been awarded many prizes for his contributions in immunology. He is also a leading editorial board member for several reputed research journals. Moreover, he has edited two editions of Encyclopedia of Signaling Molecules.
Anwar MA, Shah M, Kim J, Choi S. Recent clinical trends in Toll‐like receptor targeting therapeutics. Med Res Rev. 2019;39:1053–1090. 10.1002/med.21553
References
REFERENCES
- 1. Takeda K, Kaisho T, Akira S. Toll‐like receptors. Annu Rev Immunol. 2003;21:335‐376. [DOI] [PubMed] [Google Scholar]
- 2. McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC. Toll‐like receptors and damage‐associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol. 2014;306(2):H184‐H196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schaefer L. Complexity of danger: the diverse nature of damage‐associated molecular patterns. J Biol Chem. 2014;289(51):35237‐35245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kawai T, Akira S. The role of pattern‐recognition receptors in innate immunity: update on Toll‐like receptors. Nat Immunol. 2010;11(5):373‐384. [DOI] [PubMed] [Google Scholar]
- 5. Casanova JL, Abel L, Quintana‐Murci L. Human TLRs and IL‐1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol. 2011;29:447‐491. [DOI] [PubMed] [Google Scholar]
- 6. Pace E, Di Sano C, Ferraro M, et al. Budesonide increases TLR4 and TLR2 expression in Treg lymphocytes of allergic asthmatics. Pulm Pharmacol Ther. 2015;32:93‐100. [DOI] [PubMed] [Google Scholar]
- 7. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1‐m2 polarization balance. Front Immunol. 2014;5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buwitt‐Beckmann U, Heine H, Wiesmüller KH, et al. TLR1‐ and TLR6‐independent recognition of bacterial lipopeptides. J Biol Chem. 2006;281(14):9049‐9057. [DOI] [PubMed] [Google Scholar]
- 9. Manavalan B, Basith S, Choi S. Similar structures but different roles—an updated perspective on TLR structures. Front Physiol. 2011;2:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Botos I, Segal DM, Davies DR. The structural biology of Toll‐like receptors. Structure. 2011;19(4):447‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim HM, Park BS, Kim JI, et al. Crystal structure of the TLR4‐MD‐2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130(5):906‐917. [DOI] [PubMed] [Google Scholar]
- 12. Jin MS, Kim SE, Heo JY, et al. Crystal structure of the TLR1‐TLR2 heterodimer induced by binding of a tri‐acylated lipopeptide. Cell. 2007;130(6):1071‐1082. [DOI] [PubMed] [Google Scholar]
- 13. Tanji H, Ohto U, Shibata T, Miyake K, Shimizu T. Structural reorganization of the Toll‐like receptor 8 dimer induced by agonistic ligands. Science. 2013;339(6126):1426‐1429. [DOI] [PubMed] [Google Scholar]
- 14. Bhatelia K, Singh K, Singh R. TLRs: linking inflammation and breast cancer. Cell Signal. 2014;26(11):2350‐2357. [DOI] [PubMed] [Google Scholar]
- 15. Garcia PV, Seiva FRF, Carniato AP, et al. Increased Toll‐like receptors and p53 levels regulate apoptosis and angiogenesis in non‐muscle invasive bladder cancer: mechanism of action of P‐MAPA biological response modifier. BMC Cancer. 2016;16:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ospelt C, Kyburz D, Pierer M, et al. Toll‐like receptors in rheumatoid arthritis joint destruction mediated by two distinct pathways. Ann Rheum Dis. 2004;63(Suppl 2):ii90‐ii91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hossain MJ, Morandi E, Tanasescu R, et al. The soluble form of Toll‐Like Receptor 2 is elevated in serum of multiple sclerosis patients: a novel potential disease biomarker. Front Immunol. 2018;9:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rahman AH, Eisenberg RA. The role of Toll‐like receptors in systemic lupus erythematosus. Springer Semin Immunopathol. 2006;28(2):131‐143. [DOI] [PubMed] [Google Scholar]
- 19. Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR‐based immune adjuvants. Vaccine. 2011;29(17):3341‐3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pradere JP, Dapito DH, Schwabe RF. The Yin and Yang of Toll‐like receptors in cancer. Oncogene. 2014;33(27):3485‐3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ginaldi L, De Martinis M. Osteoimmunology and beyond. Curr Med Chem. 2016;23(33):3754‐3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'reilly S, Duffy L. Toll‐like receptors in the pathogenesis of autoimmune diseases: recent and emerging translational developments. Immunotargets Ther. 2016;5:69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin YT, Verma A, P. hodgkinson C. Toll‐like receptors and human disease: lessons from single nucleotide polymorphisms. Curr Genomics. 2012;13(8):633‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vijay K. Toll‐like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deguine J, Barton GM. MyD88: a central player in innate immune signaling. F1000Prime Rep. 2014;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu W, Jain A, Gao Y, et al. Differential outcome of TRIF‐mediated signaling in TLR4 and TLR3 induced DC maturation. Proc Natl Acad Sci USA. 2015;112(45):13994‐13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akira S, Takeda K, Kaisho T. Toll‐like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675‐680. [DOI] [PubMed] [Google Scholar]
- 28. Shevlin E, Miggin SM. Toll‐Like receptor adaptor protein family members In: Choi S, ed. Encyclopedia of signaling molecules. New York, NY: Springer International Publishing; 2018;9(5537‐5546). [Google Scholar]
- 29. Janssens S, Beyaert R. Functional diversity and regulation of different interleukin‐1 receptor‐associated kinase (IRAK) family members. Mol Cell. 2003;11(2):293‐302. [DOI] [PubMed] [Google Scholar]
- 30. Lin SC, Lo YC, Wu H. Helical assembly in the MyD88‐IRAK4‐IRAK2 complex in TLR/IL‐1R signalling. Nature. 2010;465(7300):885‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lomaga MA, Yeh WC, Sarosi I, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin‐1, CD40, and LPS signaling. Genes Dev. 1999;13(8):1015‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deng L, Wang C, Spencer E, et al. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin‐conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103(2):351‐361. [DOI] [PubMed] [Google Scholar]
- 33. Shim JH, Xiao C, Paschal AE, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19(22):2668‐2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin‐dependent kinase of MKK and IKK. Nature. 2001;412(6844):346‐351. [DOI] [PubMed] [Google Scholar]
- 35. Kawai T, Akira S. Signaling to NF‐kappaB by Toll‐like receptors. Trends Mol Med. 2007;13(11):460‐469. [DOI] [PubMed] [Google Scholar]
- 36. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783‐801. [DOI] [PubMed] [Google Scholar]
- 37. Shah M, Choi S. Interferon regulatory factor In: Choi S, ed. Encyclopedia of signaling molecules. New York, NY: Springer International Publishing; 2018;4(2672–2680). [Google Scholar]
- 38. Kawasaki T, Takemura N, Standley DM, Akira S, Kawai T. The second messenger phosphatidylinositol‐5‐phosphate facilitates antiviral innate immune signaling. Cell Host Microbe. 2013;14(2):148‐158. [DOI] [PubMed] [Google Scholar]
- 39. Anwar MA, Basith S, Choi S. Negative regulatory approaches to the attenuation of Toll‐like receptor signaling. Exp Mol Med. 2013;45:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Palsson‐McDermott EM, Doyle SL, McGettrick AF, et al. TAG, a splice variant of the adaptor TRAM, negatively regulates the adaptor MyD88‐independent TLR4 pathway. Nat Immunol. 2009;10(6):579‐586. [DOI] [PubMed] [Google Scholar]
- 41. Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR‐triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl‐b. Nat Immunol. 2010;11(8):734‐742. [DOI] [PubMed] [Google Scholar]
- 42. Kayagaki N, Phung Q, Chan S, et al. DUBA: a deubiquitinase that regulates type I interferon production. Science. 2007;318(5856):1628‐1632. [DOI] [PubMed] [Google Scholar]
- 43. Skaug B, Chen J, Du F, He J, Ma A, Chen ZJ. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol Cell. 2011;44(4):559‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yuk JM, Shin DM, Lee HM, et al. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll‐like receptors. Nat Immunol. 2011;12(8):742‐751. [DOI] [PubMed] [Google Scholar]
- 45. Shi M, Deng W, Bi E, et al. TRIM30 alpha negatively regulates TLR‐mediated NF‐kappa B activation by targeting TAB2 and TAB3 for degradation. Nat Immunol. 2008;9(4):369‐377. [DOI] [PubMed] [Google Scholar]
- 46. Hayden MS, Ghosh S. NF‐kappaB, the first quarter‐century: remarkable progress and outstanding questions. Genes Dev. 2012;26(3):203‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saitoh T, Tun‐Kyi A, Ryo A, et al. Negative regulation of interferon‐regulatory factor 3‐dependent innate antiviral response by the prolyl isomerase Pin1. Nat Immunol. 2006;7(6):598‐605. [DOI] [PubMed] [Google Scholar]
- 48. Cherradi N, Feige J‐J. Tristetraprolin (ZFP36) and TIS11B (ZFP36‐L1) In: Choi S, ed. Encyclopedia of Signaling Molecules. New York, NY: Springer International Publishing; 2018;9(5709–5718). [Google Scholar]
- 49. Gao W, Xiong Y, Li Q, Yang H. Inhibition of Toll‐like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front Physiol. 2017;8:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mills KHG. TLR‐dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11(12):807‐822. [DOI] [PubMed] [Google Scholar]
- 51. Joosten LAB, Abdollahi‐Roodsaz S, Dinarello CA, O'Neill L, Netea MG. Toll‐like receptors and chronic inflammation in rheumatic diseases: new developments. Nat Rev Rheumatol. 2016;12(6):344‐357. [DOI] [PubMed] [Google Scholar]
- 52. Hansbro PM, Haw TJ, Starkey MR, Miyake K. Toll‐like receptors in COPD. Eur Respir J. 2017;49(5):1700739. [DOI] [PubMed] [Google Scholar]
- 53. Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10(6):701‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief62; 2011:1‐8. [PubMed]
- 55. Friedman G, Silva E, Vincent JL. Has the mortality of septic shock changed with time. Crit Care Med. 1998;26(12):2078‐2086. [DOI] [PubMed] [Google Scholar]
- 56. Thomas L. Germs. N Engl J Med. 1972;287(11):553‐555. [DOI] [PubMed] [Google Scholar]
- 57. Savva A, Roger T. Targeting Toll‐like receptors: promising therapeutic strategies for the management of sepsis‐associated pathology and infectious diseases. Front Immunol. 2013;4:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kesimer M, Ford AA, Ceppe A, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377(10):911‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pomerenke A, Lea S, Herrick S, Lindsay M, Singh D. Characterization of TLR‐induced inflammatory responses in COPD and control lung tissue explants. Int J Chron Obstruct Pulmon Dis. 2016;11:2409‐2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schrijver IA, Melief MJ, Tak PP, Hazenberg MP, Laman JD. Antigen‐presenting cells containing bacterial peptidoglycan in synovial tissues of rheumatoid arthritis patients coexpress costimulatory molecules and cytokines. Arthritis Rheum. 2000;43(10):2160‐2168. [DOI] [PubMed] [Google Scholar]
- 61. van der Heijden IM, Wilbrink B, Tchetverikov I, et al. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000;43(3):593‐598. [DOI] [PubMed] [Google Scholar]
- 62. Barrat FJ, Meeker T, Gregorio J, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll‐like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202(8):1131‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Santiago‐Raber ML, Dunand‐Sauthier I, Wu T, et al. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9‐deficient mice. J Autoimmun. 2010;34(4):339‐348. [DOI] [PubMed] [Google Scholar]
- 64. Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll‐like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25(3):417‐428. [DOI] [PubMed] [Google Scholar]
- 65. Nickerson KM, Christensen SR, Shupe J, et al. TLR9 regulates TLR7‐ and MyD88‐dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184(4):1840‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Spachidou MP, Bourazopoulou E, Maratheftis CI, et al. Expression of functional Toll‐like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjogren's syndrome. Clin Exp Immunol. 2007;147(3):497‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Karlsen M, Jonsson R, Brun JG, Appel S, Hansen T. TLR‐7 and ‐9 stimulation of peripheral blood b cells indicate altered tlr signalling in primary Sjogren's syndrome patients by increased secretion of cytokines. Scand J Immunol. 2015;82(6):523‐531. [DOI] [PubMed] [Google Scholar]
- 68. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fukata M, Hernandez Y, Conduah D, et al. Innate immune signaling by Toll‐like receptor‐4 (TLR4) shapes the inflammatory microenvironment in colitis‐associated tumors. Inflamm Bowel Dis. 2009;15(7):997‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fukata M, Shang L, Santaolalla R, et al. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis‐associated tumorigenesis. Inflamm Bowel Dis. 2011;17(7):1464‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yusuf N, Nasti TH, Long JA, et al. Protective role of Toll‐like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis. Cancer Res. 2008;68(2):615‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cataisson C, Salcedo R, Hakim S, et al. IL‐1R‐MyD88 signaling in keratinocyte transformation and carcinogenesis. J Exp Med. 2012;209(9):1689‐1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huang L, Xu H, Peng G. TLR‐mediated metabolic reprogramming in the tumor microenvironment: potential novel strategies for cancer immunotherapy. Cell Mol Immunol. 2018;15:428‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kawai T, Akira S. Toll‐like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637‐650. [DOI] [PubMed] [Google Scholar]
- 76. Tan RST, Ho B, Leung BP, Ding JL. TLR cross‐talk confers specificity to innate immunity. Int Rev Immunol. 2014;33(6):443‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jagannathan M, Hasturk H, Liang Y, et al. TLR cross‐talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J Immunol. 2009;183(11):7461‐7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Browne EP. Regulation of B‐cell responses by Toll‐like receptors. Immunology. 2012;136(4):370‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jin B, Sun T, Yu XH, Yang YX, Yeo AET. The effects of TLR activation on T‐cell development and differentiation. Clin Dev Immunol. 2012;2012:836485‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Alving CR, Peachman KK, Rao M, Reed SG. Adjuvants for human vaccines. Curr Opin Immunol. 2012;24(3):310‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. MacReady N Short‐acting imiquimod cream approved for genital warts. In: Medscape (2011).
- 82. Arican O. Topical treatment of molluscum contagiosum with imiquimod 5% cream in Turkish children. Pediatr Int. 2006;48(4):403‐405. [DOI] [PubMed] [Google Scholar]
- 83. Badavanis G, Pasmatzi E, Monastirli A, Georgiou S, Tsambaos D. Topical imiquimod is an effective and safe drug for molluscum contagiosum in children. Acta Dermatovenerol Croat. 2017;25(2):164‐166. [PubMed] [Google Scholar]
- 84. Dowling D, Hamilton CM, O'neill SM. A comparative analysis of cytokine responses, cell surface marker expression and MAPKs in DCs matured with LPS compared with a panel of TLR ligands. Cytokine. 2008;41(3):254‐262. [DOI] [PubMed] [Google Scholar]
- 85. Deifl S, Kitzmüller C, Steinberger P, et al. Differential activation of dendritic cells by Toll‐like receptors causes diverse differentiation of naive CD4+ T cells from allergic patients. Allergy. 2014;69(12):1602‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ioannidis I, Ye F, McNally B, Willette M, Flano E. Toll‐like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J Virol. 2013;87(6):3261‐3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zähringer U, Lindner B, Inamura S, Heine H, Alexander C. TLR2—promiscuous or specific? A critical re‐evaluation of a receptor expressing apparent broad specificity. Immunobiology. 2008;213(3‐4):205‐224. [DOI] [PubMed] [Google Scholar]
- 88. Flo TH, Halaas O, Torp S, et al. Differential expression of Toll‐like receptor 2 in human cells. J Leukoc Biol. 2001;69(3):474‐481. [PubMed] [Google Scholar]
- 89. Zheng H, Tan Z, Zhou T, et al. The TLR2 is activated by sporozoites and suppresses intrahepatic rodent malaria parasite development. Sci Rep. 2015;5:18239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Reilly M, Miller RM, Thomson MH, et al. Randomized, double‐blind, placebo‐controlled, dose‐escalating phase I, healthy subjects study of intravenous OPN‐305, a humanized anti‐TLR2 antibody. Clin Pharmacol Ther. 2013;94(5):593‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dar P, Kalaivanan R, Sied N, et al. Montanide ISA 201 adjuvanted FMD vaccine induces improved immune responses and protection in cattle. Vaccine. 2013;31(33):3327‐3332. [DOI] [PubMed] [Google Scholar]
- 92. Durai P, Govindaraj RG, Choi S. Structure and dynamic behavior of Toll‐like receptor 2 subfamily triggered by malarial glycosylphosphatidylinositols of Plasmodium falciparum. FEBS J. 2013;280(23):6196‐6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kang JY, Nan X, Jin MS, et al. Recognition of lipopeptide patterns by Toll‐like receptor 2‐Toll‐like receptor 6 heterodimer. Immunity. 2009;31(6):873‐884. [DOI] [PubMed] [Google Scholar]
- 94. Zhou Y, Guo M, Wang X, et al. TLR3 activation efficiency by high or low molecular mass poly I:C. Innate Immun. 2013;19(2):184‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Matsumoto M, Seya T. TLR3: interferon induction by double‐stranded RNA including poly(I:C). Adv Drug Deliv Rev. 2008;60(7):805‐812. [DOI] [PubMed] [Google Scholar]
- 96. Zhang L, Dewan V, Yin H. Discovery of small molecules as multi‐Toll‐like receptor agonists with proinflammatory and anticancer activities. J Med Chem. 2017;60(12):5029‐5044. [DOI] [PubMed] [Google Scholar]
- 97. Cheng K, Wang X, Yin H. Small‐molecule inhibitors of the TLR3/dsRNA complex. J Am Chem Soc. 2011;133(11):3764‐3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bunting RA, Duffy KE, Lamb RJ, et al. Novel antagonist antibody to TLR3 blocks poly(I:C)‐induced inflammation in vivo and in vitro. Cell Immunol. 2011;267(1):9‐16. [DOI] [PubMed] [Google Scholar]
- 99. Silkoff PE, Flavin S, Gordon R, et al. Toll‐like receptor 3 blockade in rhinovirus‐induced experimental asthma exacerbations: a randomized controlled study. J Allergy Clin Immunol. 2017;141:1220‐1230. [DOI] [PubMed] [Google Scholar]
- 100. Liu L, Botos I, Wang Y, et al. Structural basis of Toll‐like receptor 3 signaling with double‐stranded RNA. Science. 2008;320(5874):379‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Choe J, Kelker MS, Wilson IA. Crystal structure of human Toll‐like receptor 3 (TLR3) ectodomain. Science. 2005;309(5734):581‐585. [DOI] [PubMed] [Google Scholar]
- 102. Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145‐151. [DOI] [PubMed] [Google Scholar]
- 103. Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll‐like receptor 4 to the induction of interferon‐beta. Nat Immunol. 2008;9(4):361‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4‐MD‐2 complex. Nature. 2009;458(7242):1191‐1195. [DOI] [PubMed] [Google Scholar]
- 105. Park S, Shin HJ, Shah M, et al. TLR4/MD2 specific peptides stalled in vivo LPS‐induced immune exacerbation. Biomaterials. 2017;126:49‐60. [DOI] [PubMed] [Google Scholar]
- 106. Wang Y, Su L, Morin MD, et al. TLR4/MD‐2 activation by a synthetic agonist with no similarity to LPS. Proc Natl Acad Sci USA. 2016;113(7):E884‐E893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD‐2 and its complex with antiendotoxic lipid IVa. Science. 2007;316(5831):1632‐1634. [DOI] [PubMed] [Google Scholar]
- 108. Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, Sherwood ER. The Toll‐like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infect Immun. 2011;79(9):3576‐3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Arias MA, Van Roey GA, Tregoning JS, et al. Glucopyranosyl lipid adjuvant (GLA), a synthetic TLR4 agonist, promotes potent systemic and mucosal responses to intranasal immunization with HIVgp140. PLOS One. 2012;7(7):e41144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Monnet E, Lapeyre G, Poelgeest E, et al. Evidence of NI‐0101 pharmacological activity, an anti‐TLR4 antibody, in a randomized phase I dose escalation study in healthy volunteers receiving LPS. Clin Pharmacol Ther. 2017;101(2):200‐208. [DOI] [PubMed] [Google Scholar]
- 111. Ta NN, Li Y, Schuyler CA, Lopes‐Virella MF, Huang Y. DPP‐4 (CD26) inhibitor alogliptin inhibits TLR4‐mediated ERK activation and ERK‐dependent MMP‐1 expression by U937 histiocytes. Atherosclerosis. 2010;213(2):429‐435. [DOI] [PubMed] [Google Scholar]
- 112. Feuillet V, Medjane S, Mondor I, et al. Involvement of Toll‐like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci USA. 2006;103(33):12487‐12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Atif SM. TLR5 (Toll‐like receptor 5) In: Choi S, ed. Encyclopedia ofsignaling molecules. New York, NY: Springer International Publishing; 2018;9(5484–5491). [Google Scholar]
- 114. Parkunan SM, Astley R, Callegan MC. Role of TLR5 and flagella in bacillus intraocular infection. PLOS One. 2014;9(6):e100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Steiner TS. How flagellin and Toll‐like receptor 5 contribute to enteric infection. Infect Immun. 2007;75(2):545‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mett V, Komarova EA, Greene K, et al. Mobilan: a recombinant adenovirus carrying Toll‐like receptor 5 self‐activating cassette for cancer immunotherapy. Oncogene. 2017;37:439‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Taylor DN, Treanor JJ, Strout C, et al. Induction of a potent immune response in the elderly using the TLR‐5 agonist, flagellin, with a recombinant hemagglutinin influenza‐flagellin fusion vaccine (VAX125, STF2.HA1 SI). Vaccine. 2011;29(31):4897‐4902. [DOI] [PubMed] [Google Scholar]
- 118. Yang H, Brackett CM, Morales‐Tirado VM, et al. The Toll‐like receptor 5 agonist entolimod suppresses hepatic metastases in a murine model of ocular melanoma via an NK cell‐dependent mechanism. Oncotarget. 2016;7(3):2936‐2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Toshkov IA, Gleiberman AS, Mett VL, et al. Mitigation of radiation‐induced epithelial damage by the TLR5 agonist entolimod in a mouse model of fractionated head and neck irradiation. Radiat Res. 2017;187(5):570‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol. 2010;185(10):5677‐5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Blohmke CJ, Victor RE, Hirschfeld AF, et al. Innate immunity mediated by TLR5 as a novel antiinflammatory target for cystic fibrosis lung disease. J Immunol. 2008;180(11):7764‐7773. [DOI] [PubMed] [Google Scholar]
- 122. Song WS, Jeon YJ, Namgung B, Hong M, Yoon S. A conserved TLR5 binding and activation hot spot on flagellin. Sci Rep. 2017;7:40878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lund JM, Alexopoulou L, Sato A, et al. Recognition of single‐stranded RNA viruses by Toll‐like receptor 7. Proc Natl Acad Sci USA. 2004;101(15):5598‐5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Heil F, Hemmi H, Hochrein H, et al. Species‐specific recognition of single‐stranded RNA via Toll‐like receptor 7 and 8. Science. 2004;303(5663):1526‐1529. [DOI] [PubMed] [Google Scholar]
- 125. Wang Y, Abel K, Lantz K, Krieg AM, McChesney MB, Miller CJ. The Toll‐like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J Virol. 2005;79(22):14355‐14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ellis AK, Tsitoura DC, Quint D, Powley W, Lee LA. Safety and pharmacodynamics of intranasal GSK2245035, a TLR7 agonist for allergic rhinitis: A randomized trial. Clin Exp Allergy. 2017;47(9):1193‐1203. [DOI] [PubMed] [Google Scholar]
- 127. Smits ELJM, Ponsaerts P, Berneman ZN, Van Tendeloo VFI. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist. 2008;13(8):859‐875. [DOI] [PubMed] [Google Scholar]
- 128. Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174(3):1259‐1268. [DOI] [PubMed] [Google Scholar]
- 129. Janssen HLA, Brunetto MR, Kim YJ, et al. Safety, efficacy and pharmacodynamics of vesatolimod (GS‐9620) in virally suppressed patients with chronic hepatitis B. J Hepatol. 2018;68(3):431‐440. [DOI] [PubMed] [Google Scholar]
- 130. Lu H, Dietsch GN, Matthews MAH, et al. VTX‐2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin Cancer Res. 2012;18(2):499‐509. [DOI] [PubMed] [Google Scholar]
- 131. Monk BJ, Facciabene A, Brady WE, et al. Integrative development of a TLR8 agonist for ovarian cancer chemoimmunotherapy. Clin Cancer Res. 2017;23(8):1955‐1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tanji H, Ohto U, Shibata T, et al. Toll‐like receptor 8 senses degradation products of single‐stranded RNA. Nat Struct Mol Biol. 2015;22(2):109‐115. [DOI] [PubMed] [Google Scholar]
- 133. Zhang Z, Ohto U, Shibata T, et al. Structural analysis reveals that Toll‐like receptor 7 is a dual receptor for guanosine and single‐stranded RNA. Immunity. 2016;45(4):737‐748. [DOI] [PubMed] [Google Scholar]
- 134. Maeda K, Akira S. TLR7 structure: cut in Z‐loop. Immunity. 2016;45(4):705‐707. [DOI] [PubMed] [Google Scholar]
- 135. Tanji H, Ohto U, Motoi Y, Shibata T, Miyake K, Shimizu T. Autoinhibition and relief mechanism by the proteolytic processing of Toll‐like receptor 8. Proc Natl Acad Sci USA. 2016;113(11):3012‐3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ramirez‐Ortiz ZG, Specht CA, Wang JP, et al. Toll‐like receptor 9‐dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect Immun. 2008;76(5):2123‐2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ashkar A, Rosenthal K. Toll‐like receptor 9, CpG DNA and innate immunity. Curr Mol Med. 2002;2(6):545‐556. [DOI] [PubMed] [Google Scholar]
- 138. Jackson S, Candia AF, Delaney S, et al. First‐in‐human study with the inhaled TLR9 oligonucleotide agonist AZD1419 results in interferon responses in the lung, and is safe and well‐tolerated. Clin Pharmacol Ther. 2017;104:335‐345. 10.1002/cpt.938 [DOI] [PubMed] [Google Scholar]
- 139. Casale TB, Cole J, Beck E, et al. CYT003, a TLR9 agonist, in persistent allergic asthma—a randomized placebo‐controlled Phase 2b study. Allergy. 2015;70(9):1160‐1168. [DOI] [PubMed] [Google Scholar]
- 140. Ruzsa A, Sen M, Evans M, et al. Phase 2, open‐label, 1:1 randomized controlled trial exploring the efficacy of EMD 1201081 in combination with cetuximab in second‐line cetuximab‐naive patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). Invest New Drugs. 2014;32(6):1278‐1284. [DOI] [PubMed] [Google Scholar]
- 141. Muthusamy N, Breidenbach H, Andritsos L, et al. Enhanced detection of chromosomal abnormalities in chronic lymphocytic leukemia by conventional cytogenetics using CpG oligonucleotide in combination with pokeweed mitogen and phorbol myristate acetate. Cancer Genet. 2011;204(2):77‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ohto U, Shibata T, Tanji H, et al. Structural basis of CpG and inhibitory DNA recognition by Toll‐like receptor 9. Nature. 2015;520(7549):702‐705. [DOI] [PubMed] [Google Scholar]
- 143. Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol. 2014;14(2):109‐121. [DOI] [PubMed] [Google Scholar]
- 144. Chuang TH, Ulevitch RJ. Identification of hTLR10: a novel human Toll‐like receptor preferentially expressed in immune cells. Biochim Biophys Acta. 2001;1518(1‐2):157‐161. [DOI] [PubMed] [Google Scholar]
- 145. Oosting M, Cheng SC, Bolscher JM, et al. Human TLR10 is an anti‐inflammatory pattern‐recognition receptor. Proc Natl Acad Sci USA. 2014;111(42):E4478‐E4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang D, Zhang G, Hayden MS, et al. A Toll‐like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303(5663):1522‐1526. [DOI] [PubMed] [Google Scholar]
- 147. Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186(8):4794‐4804. [DOI] [PubMed] [Google Scholar]
- 148. Bryant CE, Gay NJ, Heymans S, Sacre S, Schaefer L, Midwood KS. Advances in Toll‐like receptor biology: Modes of activation by diverse stimuli. Crit Rev Biochem Mol Biol. 2015;50(5):359‐379. [DOI] [PubMed] [Google Scholar]
- 149. Biggadike K, Ahmed M, Ball DI, et al. Discovery of 6‐amino‐2‐{[(1S)‐1‐methylbutyl]oxy}‐9‐[5‐(1‐piperidinyl)pentyl]‐7,9‐dihydro‐8H‐pu rin‐8‐one (GSK2245035), a highly potent and selective intranasal Toll‐like receptor 7 agonist for the treatment of asthma. J Med Chem. 2016;59(5):1711‐1726. [DOI] [PubMed] [Google Scholar]
- 150. Zhang S, Hu Z, Tanji H, et al. Small‐molecule inhibition of TLR8 through stabilization of its resting state. Nat Chem Biol. 2018;14(1):58‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhan R, Han Q, Zhang C, Tian Z, Zhang J. Toll‐Like receptor 2 (TLR2) and TLR9 play opposing roles in host innate immunity against Salmonella enterica serovar Typhimurium infection. Infect Immun. 2015;83(4):1641‐1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Makela SM, Strengell M, Pietila TE, Osterlund P, Julkunen I. Multiple signaling pathways contribute to synergistic TLR ligand‐dependent cytokine gene expression in human monocyte‐derived macrophages and dendritic cells. J Leukoc Biol. 2009;85(4):664‐672. [DOI] [PubMed] [Google Scholar]
- 153. Sato S, Nomura F, Kawai T, et al. Synergy and cross‐tolerance between Toll‐like receptor (TLR) 2‐ and TLR4‐mediated signaling pathways. J Immunol. 2000;165(12):7096‐7101. [DOI] [PubMed] [Google Scholar]
- 154. Julian MW, Strange HR, Ballinger MN, Hotchkiss RS, Papenfuss TL, Crouser ED. Tolerance and cross‐tolerance following Toll‐like receptor (TLR)‐4 and ‐9 activation are mediated by IRAK‐M and modulated by IL‐7 in murine splenocytes. PLOS One. 2015;10(7):e0132921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kumar P, Tyagi R, Das G, Bhaskar S. Mycobacterium indicus pranii and Mycobacterium bovis BCG lead to differential macrophage activation in Toll‐like receptor‐dependent manner. Immunology. 2014;143(2):258‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll‐like receptors. Cell Microbiol. 2007;9(5):1087‐1098. [DOI] [PubMed] [Google Scholar]
- 157. Hilton TL, Hulett TW, Dubay C, et al. DPV‐001 an autophagosome‐enriched cancer vaccine in phase II clinical trials contains 25 putative cancer antigens, DAMPS, HSPS and agonists for TLR 2, 3, 4, 7 and 9. J Immunother Cancer. 2013;1(1):P260. [Google Scholar]
- 158. Vo MC, Lee HJ, Kim JS, et al. Dendritic cell vaccination with a Toll‐like receptor agonist derived from mycobacteria enhances anti‐tumor immunity. Oncotarget. 2015;6(32):33781‐33790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chen P, Liu X, Sun Y, Zhou P, Wang Y, Zhang Y. Dendritic cell targeted vaccines: recent progresses and challenges. Hum Vaccin Immunother. 2016;12(3):612‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Opal SM, Laterre PF, Francois B, et al. Effect of eritoran, an antagonist of MD2‐TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309(11):1154‐1162. [DOI] [PubMed] [Google Scholar]
- 161. Katz KA. Imiquimod is not an effective drug for molluscum contagiosum. Lancet Infect Dis. 2014;14(5):372‐373. [DOI] [PubMed] [Google Scholar]
- 162. Qin M, Li Y, Yang X, Wu H. Safety of Toll‐like receptor 9 agonists: a systematic review and meta‐analysis. Immunopharmacol Immunotoxicol. 2014;36(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 163. Mullard A. Parsing clinical success rates. Nat Rev Drug Discov. 2016;15(7):447‐447. [DOI] [PubMed] [Google Scholar]
- 164.Thomas DW, Burns J, Audette J, Carroll A, Dow‐Hygelund C, Hay M. Clinical development success rates 2006‐2015. In BIO Industry Analysis; 2016:1‐26.
- 165. Heneghan C, Goldacre B, Mahtani KR. Why clinical trial outcomes fail to translate into benefits for patients. Trials. 2017;18(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Barata P, Sood AK, Hong DS. RNA‐targeted therapeutics in cancer clinical trials: current status and future directions. Cancer Treat Rev. 2016;50:35‐47. [DOI] [PubMed] [Google Scholar]
- 167. Lima CX, Souza DG, Amaral FA, et al. Therapeutic effects of treatment with anti‐TLR2 and anti‐TLR4 monoclonal antibodies in polymicrobial sepsis. PLOS One. 2015;10(7):e0132336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Gross P, Brandl K, Dierkes C, et al. Lipopolysaccharide‐trap‐Fc, a multifunctional agent to battle gram‐negative bacteria. Infect Immun. 2009;77(7):2925‐2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Toshchakov VY, Fenton MJ, Vogel SN. Cutting edge: differential inhibition of TLR signaling pathways by cell‐permeable peptides representing BB loops of TLRs. J Immunol. 2007;178(5):2655‐2660. [DOI] [PubMed] [Google Scholar]
- 170. Toshchakov VY, Szmacinski H, Couture LA, Lakowicz JR, Vogel SN. Targeting TLR4 signaling by TLR4 Toll/IL‐1 receptor domain‐derived decoy peptides: identification of the TLR4 Toll/IL‐1 receptor domain dimerization interface. J Immunol. 2011;186(8):4819‐4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Arslan F, Houtgraaf JH, Keogh B, et al. Treatment with OPN‐305, a humanized anti‐Toll‐Like receptor‐2 antibody, reduces myocardial ischemia/reperfusion injury in pigs. Circ Cardiovasc Interv. 2012;5(2):279‐287. [DOI] [PubMed] [Google Scholar]
- 172. Lagassé HAD, Alexaki A, Simhadri VL, et al. Recent advances in (therapeutic protein) drug development. F1000Res. 2017;6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Petsko GA. When failure should be the option. BMC Biol. 2010;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kox M, van Velzen JF, Pompe JC, Hoedemaekers CW, van der Hoeven JG, Pickkers P. GTS‐21 inhibits pro‐inflammatory cytokine release independent of the Toll‐like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochem Pharmacol. 2009;78(7):863‐872. [DOI] [PubMed] [Google Scholar]
- 175. Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS. Failure of investigational drugs in late‐stage clinical development and publication of trial results. JAMA Intern Med. 2016;176(12):1826‐1833. [DOI] [PubMed] [Google Scholar]
- 176. Powell‐Smith A, Goldacre B. The TrialsTracker: automated ongoing monitoring of failure to share clinical trial results by all major companies and research institutions. F1000Res. 2016;5:2629. [DOI] [PMC free article] [PubMed] [Google Scholar]


