Abstract
BACKGROUND
Solar radiation is assumed to be a major factor limiting the efficacy of entomopathogenic fungi used as biocontrol agents in open field applications. We evaluated 12 natural UV‐protective co‐formulants for their effect on the survival of UV‐exposed Beauveria bassiana spores on agar plates, colza leaf discs and in the field.
RESULTS
Colony‐forming unit (CFU) counts of unformulated conidia on agar plates and leaf discs dropped to ≤ 50% after exposure to UV radiation. The highest UV protection was achieved with humic acid, which provided > 90% protection of UV‐B‐exposed conidia in laboratory experiments. In the field, 10% humic acid increased spore persistence up to 87% at 7 days after application. Sesame and colza oil also provided high UV protection in both assays (> 73% and > 70%, respectively).
CONCLUSIONS
This study shows that it is possible to increase the persistence of B. bassiana spores under exposure to UV radiation by formulation with natural UV‐protective additives. UV protectants might, therefore, increase the efficacy of entomopathogenic fungi as biocontrol agents in open field applications. © 2018 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Beauveria bassiana, UV protection, biocontrol, humic acid, formulation
1. INTRODUCTION
The entomopathogenic ascomycete Beauveria bassiana is an established biocontrol agent in greenhouses and is used as an active ingredient in over 50 plant‐protection products worldwide.1 Although the stable conditions in greenhouses facilitate use of this entomopathogenic fungus (EPF), open field applications, particularly against airborne insect pests, are often limited by adverse environmental conditions such as direct solar radiation, temperature fluctuations or drought stress.2, 3 Beauveria bassiana is not adapted to tolerate direct sunlight and its conidia are rapidly inactivated upon exposure to ultraviolet (UV) radiation.4, 5, 6 This is assumed to be one of the main factors limiting the persistence and efficacy of EPFs in the field.7, 8, 9 Several mechanisms are involved in the inactivation of conidia by UV radiation. For example, UV‐B radiation (UV radiation at wavelengths between 280 and 320 nm) causes direct damage to DNA as strand breaks and base lesions that may lead to mutations or failures of transcription.10 Radiation at wavelengths between 320 and 400 nm (UV‐A) causes indirect damage by the production of radicals, which again have the potential to damage DNA. Thus, any effective use of B. bassiana as a microbial control agent under open field conditions, e.g. against airborne insect pests in arable crops, requires the development of a formulation that allows conidia to extend their survival on the phylloplane. Such a formulation must include co‐formulants that provide UV protection.
Similar formulations including UV protection for microbial control agents, such as viruses or bacteria, have been developed in several studies. For the biological control agent Bacillus thuringiensis, increased survival and efficacy under direct sunlight conditions was achieved by formulating a biological control agent with chemical UV absorbants and starch encapsulation.11, 12 For viruses, substances that showed UV protection include natural, organic compounds suchs as lignin, black and green tea, coffee and cocoa extracts, and other plant‐derived antioxidants.13, 14, 15, 16, 17, 18 However, only a few efficient and cost‐effective formulations have been commercialized to date.19
For EPFs, a number of substances showed excellent protection of conidia from sunlight inactivation in controlled environments, but reports of enhanced persistence in field settings are less common. Promising substances tested to date include optical brighteners, such as Tinopal, and natural compounds like clay, lignin and vegetable or mineral oils.9, 20, 21, 22, 23 Various oils helped to increase spore survival after UV‐B exposure in laboratory studies, but lost part of their protective potential in field assays or when applied on plant material.20, 24 Moreover, some formulations showed a UV‐protective effect, but seemed to interfere with the virulence of the EPF against the target host, e.g. lignin‐coated B. bassiana spores for control of Lygus lineolaris.22 Significant UV protection of B. bassiana conidia under field conditions was demonstrated by Inglis et al.20 using clay, a natural sun blocker, and Tinopal, an optical brightener, achieving a photoprotection of 25% and 37%, respectively.
However, the current choice of substances available as co‐formulants for effective UV protection of EPFs remains very limited. This constitutes a major drawback in the use of EPFs against airborne pests in open field applications.
The aim of this study was to test the potential of UV‐protective substances to enhance the survival of B. bassiana conidia in open field applications. In a previous study, B. bassiana isolates showed promising results as microbial control agents of pollen beetles (Meligethes spp.) in colza (Brassica napus).25 Because application of the microbial control agent is envisaged for all types of farming systems, we limited the choice of potential co‐formulants to natural substances, which may also be acceptable as adjuvants in organic colza production. In a first step, we tested several candidate natural UV protectants for their compatibility with B. bassiana conidia. We then evaluated their potential to protect conidia from UV‐B radiation in the laboratory. Finally, we assessed survival of conidia with and without the most promising formulation in the field.
2. MATERIAL AND METHODS
2.1. Fungus strain and conidia production
Beauveria bassiana strain ART2587 originates from a mycosed pollen beetle (Meligethes sp.) collected in Zurich, Switzerland.25 Single‐spore isolates of the strain are stored at −70 °C in 10% skim milk (Difco, Becton Dickinson, Franklin Lakes, NJ, USA). For use in experiments, the strain was grown on plates with 20 mL Sabouraud agar, modified, supplemented with 1% yeast extract (SDAY; Difco, Becton Dickinson), and incubated in the dark at 22 °C and 75% relative humidity (RH) for 2 weeks. Conidia were collected by rinsing plates with 0.1% Tween 80.
2.2. Selection and formulation of UV‐protective additives
Selection of natural substances with potential UV‐protective effects was based on previous studies with a focus on water‐soluble additives.14, 15, 17, 20, 22, 26, 27, 28, 29, 30 In addition, substances were included that are already used in organic colza cultivation as plant growth stimulants, wetting agents or adhesives for insecticides (Table 1).
Table 1.
Substances evaluated for their UV‐protective potential of Beauveria bassiana conidia
| Substance | Ingredients | Manufacturer/distributor |
|---|---|---|
| Humic acid, potassium | 78% humic acid, 0.45% potassium | WH Pharmawerk Weinboehla GmbH, Weinboehla, Germany |
| Humic acid, sodium | 65% humic acid, 7.2% sodium | WH Pharmawerk Weinboehla GmbH, Weinboehla, Germany |
| Reseda luteola extract | 62.5% polyphenols (luteolin, apigenin), 2% ash, 4% water | NIG Nahrungs‐Ingenieurtechnik GmbH, Magdeburg, Germany |
| Hippophae rhamnoides extract | 55% polyphenols (ellagitannins, flavonoids), 9.8% proteins, 3% water, 15% titratable acid, 7% ash, 0.3% fat | NIG Nahrungs‐Ingenieurtechnik GmbH, Magdeburg, Germany |
| Lignin, alkali | Lignin | Sigma‐Aldrich, St Louis, MO, USA |
| Yeast extract | Yeast extract | Merck Millipore, Darmstadt, Germany |
| Skim milk | Skim milk | Difco, Becton Dickinson, Franklin Lakes, NJ, USA |
| Surround® | 95% kaolin | Staehler Suisse SA, Zofingen, Switzerland |
| KlinoSpray (zeolite) | 70% silica, 2.8% potassium oxide, 2.5% calcium | Unipoint AG, Ossingen, Switzerland |
| Black tea | 143 000 mg kg−1 catechin, 109 000 mg kg−1 epicatechin, 179 000 mg kg−1 gallic acid, 216 000 mg kg−1 tannic acid | Tee Gschwender, Meckenheim, Germany |
| Green tea | 106 000 mg kg−1 catechin, 80 700 mg kg−1 epicatechin, 133 000 mg kg−1 gallic acid, 160 000 mg kg−1 tannic acid | Twining and Company Limited, London, UK |
| Colza oil | 274.4 ng μL−1 alpha‐tocopherol, 7.06% saturated fatty acids, 0.06% free fatty acids, 0.04% myristic acid, 64.5% oleic acid, 18.3% linoleic acid | Omya AG, Oftringen, Switzerland |
| Sesame oil | 78.7 ng μL−1 alpha‐tocopherol, 15.8% saturated fatty acids, 0.7% free fatty acids, 39.6% oleic acid, 40.8% linoleic acid | Migros‐Genossenschafts‐Bund, Zürich, Switzerland |
Liquid stock suspensions of powdery substances were freshly prepared for each assay in 0.1% Tween 80 (180 mg to 1320 μL 0.1% Tween 80). Tea was brewed in sterile distilled water at 70 °C for 30 min (2.2 g bulk tea to 17.8 mL distilled water) and the liquid portion complemented with Tween 80 to obtain a stock solution containing 0.1% Tween 80. Pure oils were mixed with 15% v/v Tween 80 as emulsifier. For the assays, stock solutions were added to spore suspensions to obtain 10% v/v of the UV protectant.
2.3. Compatibility of B. bassiana with UV‐protective additives on agar plates
The number of colony forming units (CFUs) of B. bassiana growing on plates with and without UV‐protective substances was used as a measure of the compatibility of the fungus with the tested co‐formulants. Conidia (1 × 103 conidia mL−1) suspended in 0.1% Tween 80 were complemented with 10% v/v of test substance, thoroughly mixed and 100 μL plated with sterile glass beads onto SDAY plates. Conidia suspended in 0.1% Tween 80 only served as a control. A variant with 1.7% v/v Tween 80 was included because it is an emulsifier in the tested oil variants. Agar plates were incubated in the dark at 22 °C and 75% RH and CFUs counted after 5 days. All substances were tested in three independent runs, each with three replicates.
2.4. UV‐B protection on agar plates
The detrimental effects of UV exposure on conidia seem to be mainly caused by UV‐B radiation.9, 31, 32, 33 This fraction of the solar spectrum is therefore used to evaluate UV‐protective substances and the tolerance of fungus isolates to solar exposure.6, 24, 34
SDAY plates with conidia suspensions were prepared as described above, and incubated for 30 min without light prior to UV exposure. After that, plates were transferred to a climate chamber (Climecab 1400, Kälte 3000 AG, Switzerland) onto shelves at three levels. Each level was equipped with polychromatic SolarRaptor T5 UV‐B (Econlux GmbH, Cologne, Germany) strip‐lights at a distance of 25 cm from the exposed plates.
Strip‐lights emitted an average UV‐B irradiance of 2792 mW m−2 to agar plates at this distance, which was measured with a manual solarmeter model 6 (Solar Light Co., Glenside, PA, USA). Plates were incubated for 4 h at 22 °C and 75% RH, resulting in a total UV‐B dose of 40.3 kJ m−2. This corresponds to the cumulative UV‐B dose during 2 days in lowland Switzerland in mid‐April, the point when treatments against pollen beetles in colza are usually carried out. To get this UV‐B dose, UV erythema measurements of the weather station in Payerne, Switzerland, provided by Meteo Swiss (Zurich, Switzerland), were used. Using a tropospheric ultraviolet and visible radiation model (TUV) calculator (http://cprm.acom.ucar.edu/Models/TUV/Interactive_TUV) the average ratios of UV erythema and UV‐B during the course of a day were calculated and applied to the real UV erythema measurements.
Four plates for each treatment were exposed to UV‐B irradiance at the same time. To ensure equal irradiance of test samples, agar plates were placed on a rotating disc 25 cm in diameter, mounted on shelves beneath the strip‐lights. Another three plates with the same treatments were placed in a separate part of the climate chamber, which was protected from UV radiation by aluminum foil shields. After incubation, agar plates were covered with lids, transferred to another climate chamber and kept in the dark at 22 °C and 75% RH for 5 days. Percentage survival of UV‐B radiated conidia for each variant was calculated as proportion of CFUs of irradiated and non‐irradiated plates of the same treatment within each experiment. To avoid potential influence of plate border reflection on CFU formation, only CFUs from the central circular surface of each plate with a diameter of 5 cm were included in the analysis. Each substance was tested in three independent runs, once on each shelf level of the climate chamber.
2.5. UV‐B protection on colza leaf discs
Leaf discs of greenhouse‐grown colza were surface‐sterilized in 70% ethanol for 30 s, followed by 1 min in 1% chloramine‐T trihydrate and subsequently rinsed in sterile distilled water (adapted from Vidal et al.35). One replicate consisted of three leaf discs of 1 cm diameter placed in the center of a 2% water agar plate, 55 mm in diameter. Three 1‐μL droplets of conidia suspension (3 × 105 conidia mL−1 suspended in 0.1% Tween 80 and mixed with 10% v/v test substance) were pipetted onto the upper surface of each leaf disc. Conidia suspended in 0.1% Tween 80 served as a negative control. A treatment with 1.7% v/v Tween 80 as a test substance was included because this amount is used as an emulsifier in the tested oil variants. Droplets were left to air‐dry for 30 min. Afterwards, two plates of each treatment were exposed to UV‐B radiation as described above. UV radiation time was 5 h with a total UV‐B dose of 49.3 kJ m−2. This is the average UV‐B irradiance measured over 2.5 days in lowland Switzerland mid of April. Another two plates of each treatment were incubated in a separate, aluminum foil‐shielded part of the same climate chamber. Only one shelf level of the climate chamber was used in leaf disc experiments.
After UV‐B radiation, all three leaf discs of one plate were placed in 1 mL of 10 mm phosphate‐buffered saline (PBS) with 0.05% Tween 20 (pH 7.4) and homogenized with tungsten carbide beads in a TissueLyser (Qiagen, Hilden, Germany). One hundred microliters of the homogenate was spread onto each of three SDAY plates and incubated in the dark at 22 °C and 75% RH for 7 days before CFUs were counted. Percentage survival of UV‐B radiated conidia for each variant was calculated as proportion of CFUs found on irradiated and non‐irradiated plates of the same treatment. Each substance was tested in three independent runs, each with two replicates.
2.6. Field experiment
Based on the results of the above‐described laboratory trials, humic acid sodium was tested as a UV‐protective co‐formulant in a field trial in 2017, consisting of three treatments: (i) 1 × 1013 ha−1 B. bassiana conidia in 0.1% Tween 80, (ii) 1 × 1013 ha−1 B. bassiana conidia in 0.1% Tween 80 and 10% humic acid sodium, and (iii) an untreated control. B. bassiana conidia were produced on sterile barley kernels by Mycelia GmbH (Nevele, Belgium), and harvested with a MycoHarvester (MH5, VBS Agriculture Ltd, Beaconsfield, UK). The unformulated powder contained 1.3 × 1011 conidia/g with >70% viability. The colza field (variety Avatar) was situated in eastern Switzerland (Taenikon, 47°28′46″N, 8°54′26″E). It was divided into plots of 285 m2 (15 × 19 m) with six plots per treatment arranged randomly in a rectangle. Test substances were applied at a rate of 600 L ha−1 (17.1 L plot−1) using a machine mounted 15 m spray bar (Fischer AG, Schenkon, Switzerland) equipped with 30 nozzles AIC 11005 (TeeJet, Wheaton, IL, USA) on 6 April 2017. Meteorological parameters including total solar radiation, temperature and precipitation were recorded by a local weather station.
Survival of fungus spores on colza was assessed on days 3, 7 and 14 after application. At each sampling date, the main inflorescences of five randomly selected colza plants were collected in the center of each plot, brought to the laboratory and stored at 6 °C for a maximum of 7 days. Buds were separated from stems with sterilized scissors and transferred to an extraction bag (Bioreba, Reinach, Switzerland). Bags were filled with 15 mL of 0.01 m PBS containing 0.05% Tween 20 and the plant material was homogenized using an electronic homogenizer (Bioreba). One hundred microliters of the homogenate was plated in triplicate on Sabouraud 2% glucose agar (SDA) containing antibiotics (cycloheximide, 0.05 g L−1; streptomycinsulfate, 0.6 g L−1; tetracycline, 0.05 g L−1) and the fungicide dodine (50 mg L−1).36 CFUs were counted after incubation at 22 °C and 75% RH for 14 days and storage at 6 °C until evaluation. To get the average number of CFUs per main inflorescence, the CFUs per total plant homogenate was calculated and divided by five.
2.7. Statistical analyses
Linear mixed effect models were used for statistical evaluation. They were fitted by REML using the package ‘nlme’ (version 3.1‐128; Pinheiro et al. 2017)37 in the statistical software R (version 3.2.3; R Development Core Team, 2015).
For compatibility tests of B. bassiana spores with UV‐protective substances, the number of CFUs was chosen as the response variable, which was log‐transformed to meet the assumption of normally distributed residuals. The UV‐protective substance tested was the explanatory variable. The replicate numbers nested within the single runs were included as random factors.
To analyze B. bassiana conidia survival after UV‐B exposure on agar plates and on colza leaf discs, the CFU count per plate was the response variable, which was log‐transformed to meet the assumption of normally distributed residuals. To determine whether a UV‐protective substance had a positive effect on spore survival upon UV‐B exposure, relative to the spore survival of the untreated variant, the interactions of the UV‐protective substances tested and the treatment (UV exposure yes/no) were included as explanatory variables. The replicate number nested within the experiment was included as a random factor. For the agar plate assay, the shelf level in the climate chamber nested within each single run was also included as random factor.
The effect of humic acid sodium on the survival of B. bassiana conidia in the field experiment was analyzed with the CFU counts per main inflorescence as the response variable, which was log‐transformed to meet the assumption of normally distributed residuals. The tested treatment was the explanatory variable and the plot number was included as a random factor.
3. RESULTS
3.1. Compatibility of B. bassiana conidia with UV‐protective additives
All substances tested, except for the H. rhamnoides extract, showed good compatibility with no significant inhibition of B. bassiana conidia. The H. rhamnoides extract significantly reduced (P < 0.001) B. bassiana CFU formation by 97.2 ± 5.8% (mean ± SD). Compatibilities of the remaining substances ranged from 82% to 109%, with black tea and sesame oil showing the lowest compatibilities of 82.1 ± 21.8% and 83.7 ± 35.7%, respectively. Humic acid sodium and the zeolite showed best compatibilities with 109 ± 38.1% and 106 ± 37.7%, respectively. All substances except for the H. rhamnoides extract were further evaluated for their UV‐protective potential.
3.2. UV‐B protection on agar plates
Four hours of exposure of UV‐B radiation led to a clear reduction in viable conidia in the unformulated control, with a relative CFU count (CFU of irradiated plates/CFU of non‐irradiated plates) of 48.1 ± 23.3% (Fig. 1). Of the 12 additives tested, six showed a clear UV‐protective effect with significantly higher conidia counts after irradiation compared with unformulated conidia (P < 0.05; Fig. 1 and Table 2). The highest UV‐B protection was achieved by the addition of humic acid sodium, resulting in a relative conidia count of 112.6 ± 14.0%. Treatments containing black tea, colza oil, sesame oil, luteolin and humic acid potassium also showed significantly increased CFU counts compared with controls, resulting in relative CFU counts of 53.8–94.7%. Conidia formulated with green tea exhibited significantly lower CFU counts after UV‐B exposure compared with the control. An adverse effect of green tea on conidia survival was, however, not detected in non‐irradiated plates. The same was true for all the other substances tested, confirming the compatibility results.
Figure 1.

Relative colony‐forming unit (CFU) count of Beauveria bassiana conidia on agar plates after exposure to UV‐B radiation for 4 h. Test substances were used at a 10% concentration. Polysorbate 80 was tested at a 1.77% concentration because it was used as an emulsifier for oil additives. Percentage survival was calculated in relation to non‐irradiated plates within each experiment. hum. ac. K, humic acid potassium; hum. ac. Na, humic acid sodium; ps‐80, polysorbate 80; R. luteola, Reseda luteola.
Table 2.
UV‐protective effect of tested substances on Beauveria bassiana conidia after UV‐B exposure
| Agar assay | Leaf disc assay | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substance | Estimate | SE | df | t‐value | P‐value | Estimate | SE | df | t‐value | P‐value |
| Polysorbate 80 | 0.021 | 0.08 | 52 | 0.263 | 0.790 | 0.325 | 0.09 | 148 | 3.535 | 0.001 |
| Green tea | −0.277 | 0.08 | 52 | −3.342 | 0.002 | 0.210 | 0.09 | 148 | 2.423 | 0.017 |
| Black tea | 0.294 | 0.08 | 52 | 3.597 | 0.001 | 0.070 | 0.09 | 148 | 0.771 | 0.441 |
| Humic acid K | 0.221 | 0.08 | 52 | 2.751 | 0.008 | 0.732 | 0.09 | 148 | 7.834 | 0.000 |
| Humic acid Na | 0.301 | 0.08 | 52 | 3.850 | 0.000 | 0.689 | 0.09 | 148 | 7.581 | 0.000 |
| Lignin | 0.145 | 0.08 | 52 | 1.799 | 0.078 | 0.645 | 0.09 | 148 | 7.118 | 0.000 |
| R. luteola | 0.305 | 0.08 | 52 | 3.742 | 0.001 | −0.198 | 0.09 | 148 | −2.187 | 0.030 |
| Colza oil | 0.177 | 0.08 | 52 | 2.198 | 0.032 | 0.580 | 0.09 | 148 | 6.371 | 0.000 |
| Sesame oil | 0.173 | 0.08 | 52 | 2.197 | 0.033 | 0.567 | 0.09 | 148 | 6.281 | 0.000 |
| Skim milk | −0.045 | 0.08 | 52 | −0.574 | 0.569 | 0.596 | 0.09 | 148 | 6.618 | 0.000 |
| Yeast extract | −0.018 | 0.08 | 52 | −0.225 | 0.823 | 0.435 | 0.09 | 148 | 4.825 | 0.000 |
| Kaolin | 0.068 | 0.08 | 52 | 0.857 | 0.400 | 0.497 | 0.09 | 148 | 5.500 | 0.000 |
| Zeolite | −0.112 | 0.08 | 52 | −1.381 | 0.172 | 0.270 | 0.09 | 148 | 2.981 | 0.003 |
A linear mixed effect model was built using logarithmic colony‐forming unit (CFU) counts as the response variable and the interactions of the UV‐protective substances tested and the treatment (UV exposure yes/no) as explanatory variables. Only the statistics of the interaction terms (compared with the unprotected control) are displayed. The UV‐protective effect of a substance is indicated by a significant interaction term.
3.3. UV‐B protection on colza leaf discs
After 5 h of UV‐B radiation, the relative CFU count of unformulated conidia was reduced to 21.7 ± 9.2% (Fig. 2). All compounds tested, except for black tea and R. luteola extract, caused a significant reduction in conidial mortality from UV‐B exposure (P > 0.05, Fig. 2, Table 2). The best UV‐B protection was provided by humic acid potassium with a mean relative CFU count of 111.9 ± 46.6%, followed by humic acid sodium and lignin (Fig. 2). The addition of R. luteola extract caused a significant decrease in the survival of conidia exposed to UV‐B (Fig. 2, Table 2). CFU counts in non‐irradiated plates were similar for all substances tested, with the exception of green tea, which exhibited a significantly lower CFU formation relative to the control plates (P = 0.049).
Figure 2.
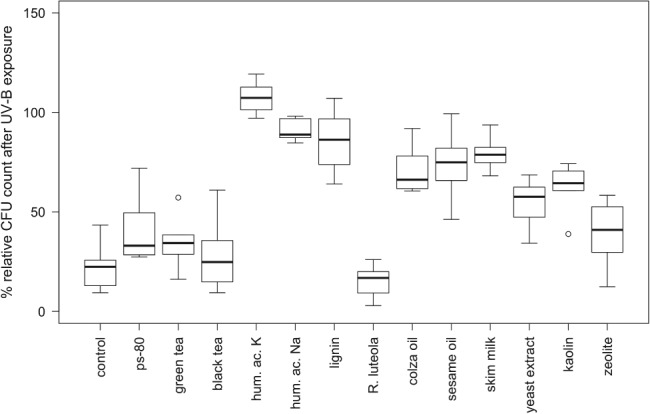
Relative colony‐forming unit (CFU) count of Beauveria bassiana conidia on colza leaf discs after exposure to UV‐B radiation for 5 h. Test substances were used at a 10% concentration. Polysorbate 80 was tested at a 1.77% concentration because it was used as an emulsifier for oil additives. Percentage survival was calculated in relation to non‐irradiated plates within each experiment. hum. ac. K, humic acid potassium; hum. ac. Na, humic acid sodium; ps‐80, polysorbate 80; R. luteola, Reseda luteola.
3.4. Field experiment
The first 3 days after application were cloudless with a total solar radiation of 7 × 104 kJ m−2. Based on the estimation that 5% of the total solar radiation is UV radiation and 5% of that is UV‐B radiation38, the UV‐B dose in the field trial exceeded the dose applied in laboratory trials after 1 day.
Subsequent days were more variable with total solar radiation of 7.9 × 104 and 8.1 104 kJ m−2 during the second and third periods between sampling dates, respectively. Whereas the first 8 days were rainless, precipitation was recorded for 5 days from day 9 after application (27 mm in total).
CFU counts in fungus treatments were significantly higher on day 3 than in the untreated control. However, they were not significantly different between fungus treatments (Fig. 3). CFU counts dropped substantially between day 3 and day 7 in the fungus treatment without UV protectant, whereas they remained almost unchanged in the treatment with the UV protectant included. Significantly more conidia survived until day 7 when formulated with the UV protectant humic acid sodium with 7.8 times higher CFU counts on colza main inflorescences than in the unformulated variant. Comparison at day 14 revealed a further loss in viable conidia in both formulations. However, the difference in conidia survival was still significant between treatments, with three times more CFUs growing in samples from the treatment including the UV protectant. By contrast, CFU counts of the fungus treatment without UV protectant reached the same low levels as the untreated control.
Figure 3.
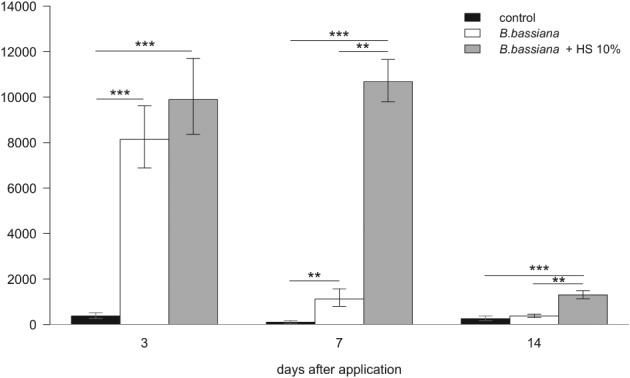
Number of Beauveria bassiana colony‐forming units (CFUs) per main colza inflorescence at days 3, 7 and 14 after application. Treated variants include B. bassiana conidia with and without 10% humic acid sodium. B. bassiana CFUs from main inflorescences of untreated plots served as the control. Standard errors are based on back‐transformed logarithmic values of six replicates. Asterisks designate significant differences between treatments: *P < 0.05, **P < 0.01, and ***P < 0.001.
4. DISCUSSION
UV‐B radiation caused a significant drop in survival of conidia in our laboratory and field trials if exposed without UV‐protective additives. This is in line with previous studies showing the susceptibility of B. bassiana conidia to UV radiation.5, 6 Our study has shown that it is possible to increase the persistence of B. bassiana spores under exposure to UV radiation by formulation with natural UV‐protective additives. Among our tested substances we identified humic acid as the most promising additive with > 90% UV‐B protection in laboratory experiments, in contrast to a significant reduction in CFU counts of unformulated conidia of 50% or more. Moreover, under realistic field conditions, we achieved a significant increase in spore survival in the field, 7 and 14 days after application. To the best of our knowledge, this is the first time that humic acid has been tested as a UV protectant in a formulation with an entomopathogenic fungus, although its potential has been indicated in previous studies. Bitton et al.26 and Muela et al.27 tested humic acid successfully as a UV protectant of bacteria, and John39 was able to protect a human cell line from UV deactivation using humic acid. Its UV‐protective effect seems to be based mainly on the specific absorption of UV radiation by organic molecules.
A similar UV‐absorbing capacity of the organic molecule may also explain the efficacy of lignin as a UV‐protective co‐formulant, as shown in our experiments. In a previous study, a formulation with cross‐linked lignin‐coated B. bassiana conidia also showed high UV protection under simulated sunlight.22 However, this formulation also showed reduced insecticidal effects of the fungal spores against its target insect, the bug Lygus lineolaris.
Some vegetable and mineral oils have shown excellent UV‐absorbing properties as well. Significant UV protection has been shown when conidia of B. bassiana, suspended in oils were spread on glass slides under controlled conditions. The same oils, however, did not enhance spore survival in field trials. One reason for this loss of UV protection of oils on plant foliage is their possible absorption into the mesophyll cells of plant leaves.20, 24 In our experiments, however, we observed high UV protection with vegetable oils such as sesame and colza oil spread on agar plates, and similar levels of protection on colza leaf discs. The waxy upper leave surface of colza (and brassicas in general) may have prevented absorption of the oils into the leaf mesophyll. However, it remains questionable whether the waxy, and therefore lipophilic, layer on the colza leaf surface is able to prevent absorption of other lipophilic substances.
Contrary to what we found with vegetable oils, some of the substances tested showed inconsistent results on the two experimental systems used, agar plates and leaf discs. For example, the two stone meals tested as UV blockers, kaolin and zeolite, both showed a UV‐B protective effect only when spread on leaf discs. One explanation may be that the solid stone meal particles, which are responsible for blocking UV radiance, were absorbed by the medium in our agar plate assay and therefore no longer able to shield the conidia. This was probably not the case on leaf discs, where the wax layer on the leaf surface possibly prevented absorption and UV‐blocking particles stayed on the surface. The natural UV blocker clay has already been tested previously in field trials by Inglis et al.20 showing an average photoprotection of B. bassiana conidia on crested wheatgrass of 25–37%.40 Similarly, kaolin, a clay mineral, has been shown to provide moderate protection against UV radiation of Cydia pomonella granulosis virus in laboratory studies.16
The opposite effect, significant UV protection on agar plates and a strong decrease in efficacy on leaf discs, was found with the application of R. luteola extract as a co‐formulant. Reseda luteola plants contain high amounts of the flavonoid luteolin which is able to absorb UV‐A and UV‐B radiation. It is also known to have antimicrobial properties towards bacteria, yeast and fungi.30, 41, 42 One reason for the contradictory results achieved with R. luteola extract may therefore be a toxic side‐effect of the extract on EPF spores. We did, however, not detect any evidence of such an effect in our initial compatibility test. Evaporation of the spore suspension may, however, have led to an increase in the concentration of luteolin on treated leaf discs and, consequently, to a decrease in spore survival caused by the antimicrobial properties of the higher concentrated co‐formulant. However, the agar assay revealed more variable results than the leaf disc assay and should therefore be more carefully interpreted.
Green and black tea have shown high UV protection in entomopathogenic viruses in previous studies, whereas we did not find a similar strong effect for B. bassiana conidia in our study.14, 17, 18 Green and black tea contain high amounts of polyphenols. These act as antioxidants for radicals triggered by UV radiation but show very little UV‐A or UV‐B absorption.43 It has been shown that the UV protection of viruses and bacteria is in part attained by antioxidants and antioxidative enzymes, which are able to counteract the damage caused by radicals at the site of the DNA.44 We assume that polyphenolic compounds of green or black tea may more easily enter bacterial cells or viral bodies, whereas they may fail to get through the multilayered wall of the resting Beauveria conidia. As a consequence, they would not be able to exhibit their antioxidative activity in the latter. It seems reasonable that UV protection in B. bassiana conidia is given mainly by the UV‐blocking or absorbing capacities of additives, which act as a shield on the outside of spores.
The only substance that caused strong inhibition of B. bassiana CFU formation in our study was the H. rhamnoides extract. Its antifungal properties might be based on its high ascorbic acid content because this vitamin has been shown to have fungistatic potential towards a dermatophyte.45 Although not successful for purposes of this study, H. rhamnoides extract might be an interesting candidate for any types of treatments in which antifungal properties are desired, e.g. treatment of fungal plant pathogens, or even in human medicine.
Our results show that the formulation of EPF spores with UV‐protective substances can prolong the persistence of EPF spores exposed to UV radiation in the laboratory and in the field for several days. We assume that this is an important step towards enhancing the efficacy of EPF treatments (and treatments with any microbial control agent in general), because efficacy should increase with the length of time that target insects are exposed to viable infective spores.
Recent studies indicate that a secondary pickup of EPF spores from the foliage may indeed be as important for pest control as hitting target insects directly during spray application. Behle et al. demonstrated that B. bassiana residues on leaves may be as effective against a lepidopteran pest as direct spray treatments.46 Metarhizium anisopliae or B. bassiana residual spores on host plants of locusts or colorado potato beetles, respectively, caused a significant reduction in pest abundance, sometimes as high as in direct spray treatments.47, 48, 49, 50 However, such increase in efficacy strongly depends on the mobility and physical contact between the target insect and its host plant. Also, the feeding behaviour influences spore acquisition and thereby the route and probablity of infection. In any case, the efficacy of an EPF treatment depends on the number of infectious propagules that touch the insect cuticle, no matter if by primary or secondary pickup (see Jaronski40).
Declining pest mortalities observed in the field after application were attributed to the loss of viable spores due to adverse environmental factors, such as solar radiation.40, 46 Hence, the development of formulations to increase persistence of EPF spores under exposure to solar radiation is probably a keystone in the successful use of EPFs as biological control agents in field crops. Humic acids have proven high UV protection potential in our study, and may be an important additive to such a formulation. Field experiments in colza with a combination of B. bassiana spores and humic acid will show if the biocontrol control measure leads to a significant reduction of pollen beetle abundance. If so, the developed formulation would be a further valuable tool to foster the sustainable above‐ground control of pest insects in arable crops.
ACKNOWLEDGEMENTS
This study is funded by means of the Federation of Migros Cooperatives, Switzerland. We are grateful to Beat Kürsteiner, Christian Schweizer, Christian Schenk, Fionna Knecht and Philipp Streckeisen for technical assistance and to Suzanne Edwards for writing assistance. WH Pharmawerk Weinböhla GmbH, NIG Nahrungs‐ Ingenieurtechnik GmbH and Omya AG kindly provided test substances. We are also very grateful for many valuable comments of an anonymous reviewer on a previous version of the manuscript.
REFERENCES
- 1. Mascarin GM and ST J, The production and uses of Beauveria bassiana as a microbial insecticide. World J Microbiol Biotechnol 32:177 (2016). [DOI] [PubMed] [Google Scholar]
- 2. Jackson MA, Dunlap CA and Jaronski ST, Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. BioControl 55:129–145 (2010). [Google Scholar]
- 3. Wraight S, Inglis G and Goettel M, Fungi, in Field Manual of Techniques in Invertebrate Pathology, ed. by, ed. by Lacey L. and Kaya H. Springer, Dordrecht, pp. 227–228 (2007). [Google Scholar]
- 4. Inglis GD, Goettel MS and Johnson DL, Persistence of the entomopathogenic fungus, Beauveria bassiana, on phylloplanes of crested wheatgrass and alfalfa. Biol Control 3:258–270 (1993). [Google Scholar]
- 5. Fargues J, Goettel MS, Smits N, Ouedraogo A, Vidal C, Lacey LA et al, Variability in susceptibility to simulated sunlight of conidia among isolates of entomopathogenic Hyphomycetes. Mycopathologia 135:171–181 (1996). [DOI] [PubMed] [Google Scholar]
- 6. Fernandes ÉKK, Rangel DEN, Moraes ÁML, Bittencourt VREP and Roberts DW, Variability in tolerance to UV‐B radiation among Beauveria spp. isolates. J Invertebr Pathol 96:237–243 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Daoust RA and Pereirn RM, Stability of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae on beetle‐attracting tubers and cowpea foliage in Brazil. Environ Entomol 15:1237–1243 (1986). [Google Scholar]
- 8. Ignoffo CM, Environmental factors affecting persistence of entomopathogens. Florida Entomol 75:516–525 (1992). [Google Scholar]
- 9. Moore D, Bridge PD, Higgins PM, Bateman RP and Prior C, Ultra‐violet radiation damage to Metarhizium flavoviride conidia and the protection given by vegetable and mineral oils and chemical sunscreens. Ann Appl Biol 122:605–616 (1993). [Google Scholar]
- 10. Griffiths HR, Mistry P, Herbert KE and Lunec J, Molecular and cellular effects of ultraviolet light‐induced genotoxicity. Crit Rev Clin Lab Sci 35:189–237 (1998). [DOI] [PubMed] [Google Scholar]
- 11. Morris ON, Protection of Bacillus thuringiensis from inactivation by sunlight. Can Entomol 115:1215–1227 (1983). [Google Scholar]
- 12. Dunkle RL and Shasha BS, Response of starch‐encapsulated Bacillus thuringiensis containing ultraviolet screens to sunlight. Environ Entomol 18:1035–1041 (1989). [Google Scholar]
- 13. Shapiro M and Robertson JL, Enhancement of gypsy moth (Lepidoptera: Lymantriidae) baculovirus activity by optical brighteners. J Ecol Entomol 85:1120–1124 (1992). [Google Scholar]
- 14. Shapiro M, El Salamouny S and Shepard BM, Green tea extracts as ultraviolet protectants for the beet armyworm, Spodoptera exigua, nucleopolyhedrovirus. Biocontrol Sci Technol 18:591–603 (2008). [Google Scholar]
- 15. Shapiro M, El‐Salamouny S and Shepard BM, Plant extracts as ultraviolet radiation protectants for the beet armyworm (Lepidoptera: Noctuidae) nucleopolyhedrovirus: screening of extracts. JAgric Urban Entomol 26:47–61 (2009). [DOI] [PubMed] [Google Scholar]
- 16. Arthurs SP, Lacey LA and Behle RW, Evaluation of lignins and particle films as solar protectants for the granulovirus of the codling moth, Cydia pomonella . Biocontrol Sci Technol 18:829–839 (2008). [Google Scholar]
- 17. El Salamouny S, Shapiro M, Ling KS and Shepard BM, Black tea and lignin as ultraviolet protectants for the beet armyworm nucleopolyhedrovirus. J Entomol Sci 44:50–58 (2009). [DOI] [PubMed] [Google Scholar]
- 18. El‐Salamouny S, Ranwala D, Shapiro M, Shepard BM and Farrar JRR, Tea, coffee, and cocoa as ultraviolet radiation protectants for the beet armyworm nucleopolyhedrovirus. J Econ Entomol 102:1767–1773 (2009). [DOI] [PubMed] [Google Scholar]
- 19. Burges HD and Jones KA, Formulation of bacteria, viruses and protozoa to control insects, in Formulation of Microbial Biopesticides: Beneficial Microorganisms, Nematodes and Seed Treatments, ed. by, ed. by Burges HD. Springer, Dordrecht, pp. 33–127 (1998). [Google Scholar]
- 20. Inglis GD, Goettel MS and Johnson DL, Influence of ultraviolet‐light protectants on persistence of the entomopathogenic fungus, Beauveria bassiana . Biol Control 5:581–590 (1995). [Google Scholar]
- 21. Alves RT, Bateman RP, Prior C and Leather SR, Effects of simulated solar radiation on conidial germination of Metarhizium anisopliae in different formulations. Crop Prot 17:675–679 (1998). [Google Scholar]
- 22. JE L and Behle RW, Coating Beauveria bassiana with lignin for protection from solar radiation and effects of pathogenicity to Lygus lineolarsi (Heteroptera: Miridae). Biocontrol Sci Technol 15:309–320 (2005). [Google Scholar]
- 23. Posadas J, Lewylle Maricel A, Mini I and Roberto L, Natural tolerance to UV‐B and assessment of photoprotectants in conidia of six native isolates of Beauveria bassiana (Bals‐Criv) Vuillemin. World Appl Sci J 20:1024–1030 (2012). [Google Scholar]
- 24. Behle RW, Compton DL, Laszlo JA and Shapiro‐Ilan DI, Evaluation of soyscreen in an oil‐based formulation for UV protection of Beauveria bassiana conidia. J Econ Entomol 102:1759–1766 (2009). [DOI] [PubMed] [Google Scholar]
- 25. Kuske S, Pilz C and Koelliker U, Phenotypic search for promising entomopathogenic fungal isolates to control pollen beetles, in Proceedings of the 6th meeting of the IOBC‐WPRS Working Group “Integrated Control in Oilseed Crops”, ed. by, ed. by Koopmann B, Cook S, Evans N. and Ulber B. IOBC-WPRS Bulletin, Paris, France, 2008, pp. 79–81 (2013). Bulletin No. 92. [Google Scholar]
- 26. Bitton G, Henis Y and Lahav N, Effect of several clay minerals and humic acid on the survival of Klebsiella aerogenes exposed to ultraviolet irradiation. Appl Microbiol 23:870–874 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muela A, García‐Bringas JM, Arana I and Barcina I, Humic materials offer photoprotective effect to Escherichia coli exposed to damaging luminous radiation. Microb Ecol 40:336–344 (2000). [DOI] [PubMed] [Google Scholar]
- 28. Bixby‐Brosi AJ and Potter DA, Evaluating a naturally occurring baculovirus for extended biological control of the black cutworm (Lepidoptera: Noctuidae) in golf course habitats. J Econ Entomol 103:1555–1563 (2010). [DOI] [PubMed] [Google Scholar]
- 29. Korać RR and Khambholja KM, Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn Rev 5:164–173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woelfle U, Esser PR, Simon‐Haarhaus B, Martin SF, Lademann J and Schempp CM, UVB‐induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo . Free Radical Biol Med 50:1081–1093 (2011). [DOI] [PubMed] [Google Scholar]
- 31. Fargues J, Rougier M, Goujet R, Smits N, Coustere C and Itier B, Inactivation of conidia of Paecilomyces fumosoroseus by near‐ultraviolet (UVB and UVA) and visible radiation. J Invertebr Pathol 69:70–78 (1997). [DOI] [PubMed] [Google Scholar]
- 32. Braga GUL, Flint SD, Miller CD, AJ A and Roberts DW, Both solar UVA and UVB radiation impair conidial culturability and delay germination in the entomopathogenic fungus Metarhizium anisopliae . Photochem Photobiol 74:734–739 (2001). [DOI] [PubMed] [Google Scholar]
- 33. Lee JY, Kang SW, Yoon CS, Kim JJ, Choi DR and Kim SW, Verticillium lecanii spore formulation using UV protectant and wetting agent and the biocontrol of cotton aphids. Biotechnol Lett 28:1041–1045 (2006). [DOI] [PubMed] [Google Scholar]
- 34. Férnandez‐Bravo M, Responses to abiotic environmental stresses among phylloplane and soil isolates of Beauveria bassiana from two holm oak ecosystems. J Invertebr Pathol 141:6–17 (2016). [DOI] [PubMed] [Google Scholar]
- 35. Vidal C, Lace LA and Fargues J, Pathogenicity of Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) against Bemisia argentifolii (Homoptera: Aleyrodidae) with a description of a bioassay method. J Econ Entomol 90:765–772 (1997). [Google Scholar]
- 36. Strasser H, Forer A and Schinner F, Development of media for the selective isolation and maintenance of virulence of Beauveria brongniartii, in Microbial Control of Soil Dwelling Pests, ed. by, ed. by Glare TR. and Jackson TA. AgResearch, Lincoln, pp. 125–130 (1996). [Google Scholar]
- 37. Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team, nlme: Linear and nonlinear mixed effect models, retrieved from https://CRAN.R‐project.org/package=nlme (2017).
- 38. Diffey BL, What is light? Photodermatol Photoimmunol Photomed 18:68–74 (2002). [DOI] [PubMed] [Google Scholar]
- 39. John SE, In‐vitro‐Untersuchung zur Schutzwirkung natürlicher und synthetischer Huminsäuren gegenüber UVB‐Strahlenschäden an humanen Linsenepithelzellen. Friedrich‐Wilhelms‐Universität, Bonn: (2010). [Google Scholar]
- 40. Jaronski S, Ecological factors in the inundative use of fungal entomopathogens. BioControl 55:159–185 (2010). [Google Scholar]
- 41. Zhu X, Zhang H and Lo R, Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J Agric Food Chem 52:7272–7278 (2004). [DOI] [PubMed] [Google Scholar]
- 42. Lopez‐Lazaro M, Distribution and biological activities of the flavonoid luteolin. Mini Rev in Med Chem 9:31–59 (2009). [DOI] [PubMed] [Google Scholar]
- 43. Yusuf N, Irby C, Katiyar SK and Elmets CA, Photoprotective effects of green tea polyphenols. Photodermatol, Photoimmunol Photomed 23:48–56 (2007). [DOI] [PubMed] [Google Scholar]
- 44. Ignoffo CM and Garcia C, UV‐Photoinactivation of cells and spores of Bacillus thuringiensis and effects of peroxidase on inactivation. Environ Entomol 7:270–272 (1978). [Google Scholar]
- 45. Goetz H, Die Pilzkrankheiten der Haut durch Dermatophyten, in Die Pilzkrankheiten der Haut durch Dermatophyten. Handbuch der Haut‐ und Geschlechtskrankheiten (Ergänzungswerk), Vol. 4/3 ed. by, ed. by Marchionini A. and Goetz H. Springer, Berlin: (1962). [Google Scholar]
- 46. Behle RW, Importance of direct spray and spray residue contact for infection of Trichoplusia ni larvae by field applications of Beauveria bassiana . J Econ Entomol 99:1120–1128 (2006). [DOI] [PubMed] [Google Scholar]
- 47. Bateman RP, Douro‐Kpindou OK, Kooyman C, Lomer C and Ouambama Z, Some observations on the dose transfer of mycoinsecticide sprays to desert locusts. Crop Prot 17:151–158 (1998). [Google Scholar]
- 48. Scanlan JC, Grant WE, Hunter DM and Milner RJ, Habitat and environmental factors influencing the control of migratory locusts (Locusta migratoria) with an entomopathogenic fungus (Metarhizium anisopliae). Ecol Model 136:223–236 (2001). [Google Scholar]
- 49. Fernandez S, Groden E, Vandenberg JD and Furlong MJ, The effect of mode of exposure to Beauveria bassiana on conidia acquisition and host mortality of Colorado potato beetle, Leptinotarsa decemlineata . J Invertebr Pathol 77:217–226 (2001). [DOI] [PubMed] [Google Scholar]
- 50. Van der Valk H, Review of the efficacy of Metarhizium anisopliae var. acridum against the desert locust. Desert Locust Technical Series. FAO, Rome.


