Abstract
The study describes the patterns of concurrent wasting and stunting (WaSt) among children age 6–59 months living in the 1980s in Niakhar, a rural area of Senegal under demographic surveillance. Wasting and stunting were defined by z scores lower than −2 in weight for height and height for age. Both conditions were found to be highly prevalent, wasting more so before age 30 months, stunting more so after age 30 months. As a result, concurrent WaSt peaked around age 18 months and its prevalence (6.2%) was primarily the product of the two conditions, with an interaction term of 1.57 (p < 10−6). The interaction was due to the correlation between both conditions (more stunting if wasted, more wasting if stunted). Before age 30 months, boys were more likely to be concurrently wasted and stunted than girls (RR = 1.61), but the sex difference disappeared after 30 months of age. The excess susceptibility of younger boys could not be explained by muscle mass or fat mass measured by arm or muscle circumference, triceps, or subscapular skinfold. Concurrent WaSt was a strong risk factor for child mortality, and its effect was the product of the independent effect of each component, with no significant interaction.
Keywords: anthropometry, child survival, concurrent wasting & stunting, Senegal, stunting, wasting
Key messages.
Prevalence of concurrent wasting and stunting (WaSt) among under‐five children is the product of independent wasting and stunting, plus an interaction term of 57%.
Prevalence of WaSt peaks around age 18 months.
Prevalence of WaSt is about 50% higher among boys than among girls before age 30 months, largely because their higher susceptibility to stunting but not higher thereafter.
Excess mortality associated with WaSt is the product of that associated with wasting and that associated with stunting.
1. INTRODUCTION
Malnutrition in children under five is still widely prevalent in the world. It is the focus of numerous research works and of numerous public health interventions (Black et al., 2008; Briend, Collins, Golden, Manary, & Myatt, 2013; McDonald et al., 2013). Child malnutrition is primarily defined by anthropometric indicators, in reference to international growth standards elaborated in populations free of malnutrition. These anthropometric indicators describe the various dimensions of child growth in particular linear growth (bones), fat, and muscle. The aetiology of malnutrition is complex, as nutritional status is the product of numerous interactions between food intake, disease, metabolism, immunity, and endocrine system. Of particular importance for lower‐and‐middle‐income countries (LMIC) is the well‐documented synergistic effect of nutrition and infection (Scrimshaw, 2003; Scrimshaw & SanGiovanni, 1997). A recent review summarized current knowledge on the complex interactions between nutritional status (linear growth, fat, muscle) and body functions (metabolism, immunity, endocrine system; Briend, Khara, & Dolan, 2015). Of particular importance is the role of muscle on the production of amino acids necessary for vital organs (heart, kidney, thymus) and that of fat as an energy provider. Fat also has an influence on hormonal production, in particular leptin, which has numerous effects on the immune system as well as on linear growth. Various forms of malnutrition may result from deficits in diet (low food intake, deficits in micronutrients and vitamins, imbalance between proteins, carbohydrates, and lipids), from infections, or from dysfunctions of organs. In turn, these anomalies may have an impact on child growth measured by anthropometric indicators.
In nutritional epidemiologic studies, nutritional status is first defined by weight and height in relation to age. In the early 1970s, John Waterlow introduced a distinction between wasting (acute malnutrition) defined by low weight for height and stunting (chronic malnutrition) defined by low height for age. Both indicators were assumed to be largely independent of each other (Waterlow, 1972). The proportion of children who are wasted and the proportion of children who are stunted are the leading indicators of nutritional status used in Demographic and Health Surveys, conducted throughout LMIC (Akombi, Agho, Merom, Renzaho, & Hall, 2017). Wasting is more typical in younger children (6–29 months) and stunting in older children (30–59 months), although both could occur at any age. Children under 6 months of age, most of them fully breastfed, have been assumed to be relatively free of malnutrition, although this assumption has been challenged in the literature (Kerac, Mwangome, McGrath, Haider, & Berkley, 2015). In most countries, and in particular in sub‐Saharan Africa, sex differences in prevalence of malnutrition are small, if not negligible (Garenne, 2003). Large sex differences in nutritional status and mortality are found mostly in South‐Asia and are associated with family composition and differential food allocation by sex and birth order, with girls of birth order 2+ being at higher risk (Das Gupta, 1987; Mishra, Roy, & Retherford, 2004; Pande, 2003). No such effects have been documented so far in Africa (Garenne, 2003).
Recent research has revisited the relationships between wasting and stunting, partly because it is now recognized that many common factors could cause both conditions, that their effect on mortality is due to the same mechanisms, with similar changes in body composition and partly because prevention strategies are similar for both conditions (Briend et al., 2015; Victora, 1992; Martorell & Young, 2012). In addition, there is a renewed interest in concurrent WaSt (defined as being both wasted and stunted at the same time), a condition that has been rarely studied. This more severe pathology may necessitate special treatments or special attention because of its high mortality risk (McDonald et al., 2013). In recent studies, concurrent WaSt was found more prevalent than assumed earlier and is now seen as a specific problem requiring special attention (Angood, Khara, Dolan, & Berkley, 2016; Bergeron & Castleman, 2012; Khara & Dolan, 2014; Khara, Mwangome, Ngari, & Dolan, 2018; Myatt et al., 2017; Myatt et al., 2018; Richard et al., 2012).
The aim of this paper is to describe the demographic patterns (prevalence, age pattern, sex differences, mortality) of concurrent WaSt, in a rural community of Senegal, using data collected in the 1980s.
2. DATA AND METHODS
The data used for this analysis were collected in the early 1980s as part of a comprehensive research project on the relationships between nutritional status and mortality among preschool children. This dataset is large and comprehensive and has been used for comparative studies. The project has been described in details elsewhere (Briend, Garenne, Maire, Fontaine, & Dieng, 1989; Briend, Maire, Fontaine, & Garenne, 2012; Garenne et al., 2009; Garenne, Maire, Fontaine, & Briend, 2006, 2012; Garenne, Maire, Fontaine, Dieng, & Briend, 1987, 2000). In brief, the study was conducted in a rural community of about 25,000 people living in 30 villages near Niakhar, Fatick region, in central Senegal. This population was living on farming, growing millet and peanuts, and raising cattle, sheep, and goats. Living standards were still low at that time, prevalence of malnutrition moderate, and under‐five mortality was very high (271 per 1,000 live births).
The Niakhar study was conducted in the framework of a comprehensive Demographic Surveillance System, with full counts of population dynamics (births, deaths, inmigration and outmigration). All under‐five children living in the study area were visited four times at 6‐month interval (May and November 1983 and 1984), with comprehensive anthropometric assessment (weight, height, head circumference, arm circumference, triceps skinfold, subscapular skinfold). For this study, children age 6–59 months were selected, who had complete measures at nutritional assessment (N = 12,638 in total). Children could be seen one, two, three or four times depending on their age and availability: 2,708 children in May 1983, 3,253 children in November 1983, 3,380 children in May 1984, 3,297 children in November 1984, for an average of 3,160 children per visit. The unit of analysis was the visit, a combination of child and age. All children were monitored for survival for 6 months after each nutritional assessment (304 deaths were recorded). More details on the study can be found elsewhere (Garenne et al., 1987, 2000, 2006, 2009, 2012). Anthropometric measures were standardized (z scores) using the CDC‐2000 growth standards: weight‐for‐age (WAZ), height‐for‐age (HAZ), and weight‐for‐height (WHZ) (CDC, 2000). The use of CDC‐2000 standard was justified here because it provided the highest sensitivity and specificity for screening the mortality risk.
2.1. Case definitions
This study focuses on wasting, stunting, and concurrent WaSt, defined as follows:
Stunted: HAZ < −2.0.
Wasted: WHZ < −2.0.
Concurrently wasted and stunted (WaSt): WHZ < −2.0 and HAZ < −2.0.
Severity of concurrent WaSt: “mild” = WHZ < −1.0 and HAZ < −1.0; “moderate” = WHZ < −2.0 and HAZ < −2.0; “severe” = WHZ < −3.0 and HAZ < −3.0 (categories are exclusive of each other, with highest severity prevailing).
For multivariate analysis, two other indicators were calculated:
Combined WaSt z score: WaStZ = (WHZ + HAZ)/2 if WHZ < 0 and HAZ < 0; = 0 otherwise.
Interaction between wasting z score and stunting z score: WaxStZ = −(WHZ × HAZ) if WHZ < 0 and HAZ < 0; = 0 otherwise.
2.2. Methods
Straightforward statistical methods were used for this study: basic cross‐tabulations and multivariate analysis, using linear models (on z scores) and Logit‐linear models (on prevalence of conditions). All calculations were done using SPSS‐17.
3. RESULTS
3.1. Prevalence of wasting, stunting, and interaction
The prevalence of wasting (16.3%), stunting (24.2%), and concurrent WaSt (6.2%) was moderate in Niakhar, with higher wasting and lower stunting than the national average for Senegal in 1992 and than the average of African countries in the 1990s (6.8% wasting and 36.4% stunting). Considered by 12‐month age groups, wasting was highest at 12–23 months, stunting at 24–35 months, and concurrent WaSt at 12–23 months. The prevalence of concurrent WaSt was 1.57 times the product of the prevalence of wasting by that of stunting, indicating an interaction between the two conditions (under the hypothesis of independence it should be equal to the product). This interaction was highest for children age 6–11 months (2.16, p = 0.038) and lowest (1.21) for children age 48–59 months (Table 1).
Table 1.
Prevalence of wasting, stunting, and concurrent wasting and stunting, by age group, Niakhar 1983–1984 (N = 12,638 measures)
| Prevalence (%) | Interaction | ||||
|---|---|---|---|---|---|
| Age group (months) | Wasted (WHZ < −2) | Stunted (HAZ < −2) | Wasted and stunted | Value | 95% CI |
| (Wa) | (st) | (wast) | (wast/Wa × st) | Min‐max | |
| 6–11 | 19.9% | 10.6% | 4.5% | 2.16* | 1.62–2.87 |
| 12–23 | 29.5% | 26.0% | 11.0% | 1.44* | 1.25–1.66 |
| 24–35 | 18.2% | 32.4% | 9.1% | 1.54* | 1.29–1.82 |
| 36–47 | 7.2% | 26.3% | 3.6% | 1.91* | 1.49–2.45 |
| 48–59 | 6.9% | 20.7% | 1.7% | 1.21 | 0.86–1.69 |
| Total | 16.3% | 24.2% | 6.2% | 1.57* | 1.43–1.72 |
Note. Interaction terms were statistically significant (RR <> 1), with p values <10−6, except for the 48–59 age group (not significant). p values and confidence intervals calculated with standard formulae for risk ratios. (*) p < 0.05.
The interactions between wasting and stunting are further illustrated in Figure 1: The proportion of wasted children increased markedly with the degree of stunting, in a dose–response (log linear) relationship. Similarly, the proportion of stunted children increased with the degree of wasting, although the relationship was less linear (Figure 1).
Figure 1.
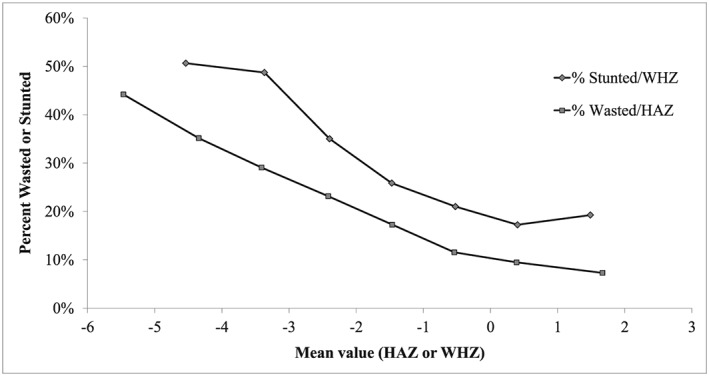
Interactions between wasting and stunting, Niakhar 1983–1984. (a) Effect of wasting on stunting, (b) Effect of stunting on wasting
The interaction between wasting and stunting (defined by −2 z score) was further investigated by linear‐logistic regression of the proportion wasted (dependent variable) on stunting (independent variable), after controlling for age and sex. The effect of stunting on wasting was strong and highly significant and similar for younger children age 6–29 months and for older children age 30–59 months. An increase in stunting of −2 units in z score increased the probability of being wasted 2.43 times below age 30 months and 2.11 times above 30 months. Surprisingly, male sex had a positive effect below age 30 months (+12% more wasting among boys at the same level of stunting) but negative above age 30 months (−20% less wasting among boys at the same level of stunting) (Table 2).
Table 2.
Net effect of stunting, age, and sex on wasting, Niakhar 1983–1984
| Variable | Children 6–29 months | Children 30–59 months | Difference between age groups | ||
|---|---|---|---|---|---|
| B coefficient | p value | B coefficient | p value | p value | |
| HAZ | −0.4435 | <10–6* | −0.3725 | <10–6* | 0.114 |
| Sex (male) | +0.1170 | 0.057 | −0.2240 | 0.015* | 0.002* |
| Age | +0.2094 | <10–6* | −0.3559 | <10–6* | <10–6* |
| Age2 | −0.0063 | <10–6* | +0.0039 | <10–6* | <10–6* |
| Constant | −3.1984 | <10–6* | +5.0068 | <10–6* | <10–6* |
Note. Dependent variable = proportion wasted (WHZ < −2); (*) p < 0.05. The net effect of each variable can be computed as = exp (bX). Age and age2 were used because the relationship with age was curvilinear. Sex = 1 for males, 0 for females.
3.2. Age patterns
The age patterns of wasting, stunting, and interaction are displayed in Figure 2. After the age of 6 months, the proportion of wasted children increased rapidly with age, peaked around 16 months (31%), and declined first slowly, then rapidly to reach low levels (7%) after 42 months. The proportion of stunted children increased less rapidly with age, reached a later peak around 28 months (33%), before declining slowly and remaining at high level (20%) by age 59 months. The proportion of children concurrently wasted and stunted followed the product wasted × stunted, with a fast increase from 6 to 18 months, a peak around 19 months (12%), followed by a decline after 24 months and becoming negligible after 48 months (1.7%). Age is therefore a fundamental aspect of the dynamics of the patterns of malnutrition among these children.
Figure 2.
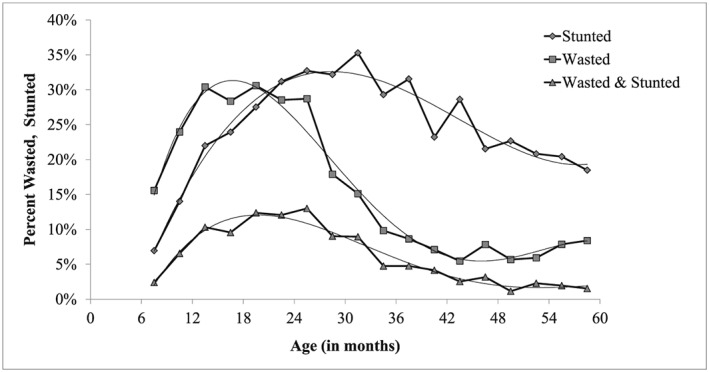
Prevalence by age of wasting, stunting, and concurrent wasting and stunting, Niakhar 1983–1984
3.3. Sex differences
As already noticed (see Table 2), sex differences in susceptibility to concurrent WaSt were very marked, atypical, and changing with age. The issue of sex differences was further investigated by calculating the sex ratios of prevalence, defined as the ratio of male prevalence/female prevalence. Wasting was more prevalent among young boys, (before age 24 months) and similar in older boys and girls. Stunting was always more prevalent among boys and more so before 36 months; interactions were roughly equivalent between boys and girls, with a small (not significant) trend towards being more pronounced for younger boys (age 6–23 months) and less pronounced for older boys (36–59 months) (Table 3).
Table 3.
Sex ratios of prevalence of wasting, stunting, and concurrent wasting and stunting, by age group, Niakhar 1983–1984
| Age group (months) | Sex ratio of prevalence (boys/girls) | Interaction | |||
|---|---|---|---|---|---|
| Wasted (WHZ < −2) | Stunted (HAZ < −2) | Wasted and stunted | Value | ||
| Sex ratio | p value | ||||
| (Wa) | (st) | (wast) | (wast) | (wast/Wa × st) | |
| 6–12 | 1.23* | 1.82* | 2.34* | 7.5E‐04* | 1.04 |
| 12–24 | 1.20* | 1.35* | 1.69* | 5.3E‐06* | 1.04 |
| 24–36 | 1.01 | 1.24* | 1.17 | 0.230 | 0.93 |
| 36–48 | 0.77 | 1.12 | 0.82 | 0.329 | 0.96 |
| 48–60 | 0.84 | 1.14 | 0.88 | 0.677 | 0.91 |
| Total | 1.08 | 1.24 | 1.36* | 2.4E‐05* | 1.02 |
Note. (*) p < 0.05. p values test the sex ratio for wasting and stunting (SR <> 1). None of the sex ratio of the interactions was significant.
Figure 3 displays the pattern of sex differences in concurrent WaSt into more details. Prevalence of WaSt was much higher for males at 6–23 months (RR = 1.77) and no difference after 24 months, with a minor tendency to higher prevalence among females. A multivariate analysis conducted after controlling for age showed that the net effect of sex on WaSt changed sign between age 6–29 months (b = +0.534, p < 10−6) and age 30–59 months (b = −0.095, p = 0.495), the change being highly significant (p < 10−6).
Figure 3.
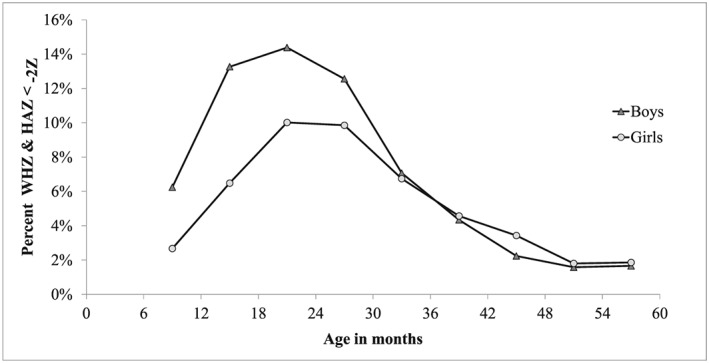
Sex differences in prevalence of concurrent wasting and stunting, Niakhar 1983–1984
3.4. Impact of severity of concurrent WaSt on sex differences
The severity of concurrent WaSt was calculated from the composite index defined above: (WHZ + HAZ)/2 if both negative, 0 otherwise. Severity categories were: “mild” for Z < −1, “moderate” for Z < −2, and “severe” for Z < −3. The pattern of sex differences was proportionate to the degree of severity of concurrent WaSt below age 30 months but not above age 30 months. (Figure 4).
Figure 4.
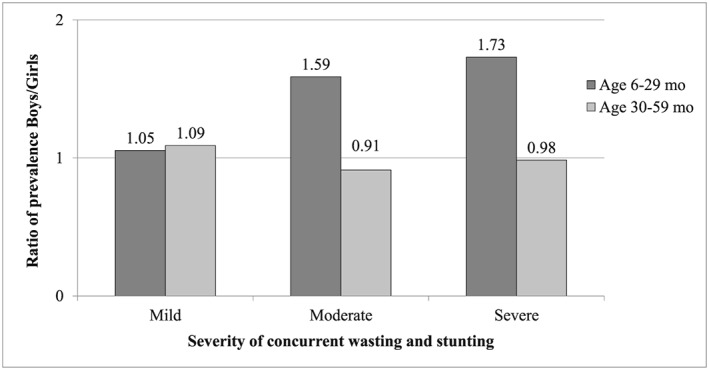
Effect of severity of concurrent wasting and stunting on sex differences in prevalence, Niakhar 1983–1984
3.5. Relationships with other anthropometric indicators
Anthropometric indicators are good proxies for body composition, although fat mass and muscle mass measured by dual‐energy X‐ray absorptiometry can only be approximated by anthropometric measures (Jensen et al., 2015). Body composition differs between sexes: Boys have a higher fat‐free mass and a lower fat mass than girls. In Niakhar (as elsewhere), boys had a larger arm circumference (143.5 mm) than girls (142.7 mm), the difference being significant (p = 0.014), and this was true at all ages. This difference did not correlate with their higher susceptibility to concurrent WaSt at younger ages. When standardized by age, boys had a lower MUAC‐for‐age z score (−1.485) than girls (−1.324), the difference being highly significant (p < 10−6). This difference was consistent at all ages, although the difference was more pronounced before age 30 months (−0.25 z score), than after age 30 months (−0.08 z score). This is the only difference that goes in the direction of observed differences in prevalence of concurrent WaSt between boys and girls. However, it does not seem to explain these observed differences. In fact, adding MUAC‐for‐age z score in a multivariate analysis reduced the net effect of sex for younger and older children but did not modify the relative risks between younger (1.88) and older children (1.68).
Overall, girls had thicker triceps skinfolds (8.57 mm) and thicker subscapular skinfolds (6.31 mm) than boys (8.15 mm and 5.98 mm respectively), the differences being highly significant (p < 10−6). The difference in triceps and subscapular skinfolds between boys and girls was very small at age 6–11 months (−0.052 and − 0.048 mm) and tended to increase with age up to age 59 months (−0.522 and − 0.564 mm). Therefore, this difference did not correlate with sex differences in concurrent WaSt, and in fact, it goes in the opposite direction: The increasing advantage of girls in fat stores is associated with their decreasing advantage compared with boys in the risk of being simultaneously wasted and stunted. Overall, boys had a larger muscle circumference (117.9 mm) than girls (116.8 mm), and the difference between both (2.1 mm) was consistent over age from 6 to 59 months. Muscle mass was therefore not associated with the changing sex difference in concurrent WaSt.
Prevalence of concurrent WaSt was further analysed in multivariate analysis with respect to anthropometric indicators other than weight and height: head circumference, triceps skinfold, subscapular skinfold, and muscle arm circumference, plus age and sex. In both age groups, the net effects of anthropometric indicators were all statistically significant, in the same direction (positive or negative) and of about the same magnitude. The net effect of sex was in the same direction but not of the same magnitude. The effect of age changed markedly (as seen earlier on Figure 2). The effect of muscle circumference increased with increasing age. The changing sex ratio was conserved, with higher values before age 30 months (SR = 4.24) than after (SR = 1.75), the difference being significant (p < 10−4). In this case however, boys appeared to be always at higher risk of being concurrently wasted and stunted, after controlling for the other variables (Table 4).
Table 4.
Effect of age, sex, and anthropometric indicators on prevalence of concurrent wasting and stunting, Niakhar 1983–1984
| Variable | Children age 6–29 | Children age 30–59 | Difference in coefficients | ||
|---|---|---|---|---|---|
| Coefficient | p value | Coefficient | p value | p value | |
| Age | +0.716 | <10–6* | −0.036 | 0.746 | <10–6* |
| Age2 | −0.014 | <10–6* | +0.001 | 0.628 | <10–6* |
| Sex (male) | +1.446 | <10–6* | +0.562 | 0.0020* | <10–4* |
| Head circumference | −0.055 | <10–6* | −0.053 | <10–6* | 0.814 |
| Tricipital | −0.038 | <10–6* | −0.041 | <10–6* | 0.755 |
| Subscapular | −0.018 | 0.0054* | −0.018 | 0.060 | 0.974 |
| Muscle circumference | −0.160 | <10–6* | −0.193 | <10–6* | 0.028 |
| Constant | 34.764 | <10–6* | 47.961 | <10–6* | |
Note. (*) p < 0.05. Dependent variable: prevalence of concurrent wasting and stunting (WHZ and HAZ < −2 z score). Head circumference and muscle arm circumference in mm. Triceps and subscapular skinfold in 10 × mm. Sex = 1 for males, 0 for females.
3.6. Mortality risk
Numerous studies have documented the relationships between nutritional status and under‐five mortality (McDonald et al., 2013; Pelletier, 1994). An earlier analysis of the same data showed the independent effects of anthropometric indicators on child survival and in particular those of wasting and stunting (Garenne et al., 2006). The current analysis provides further evidence by age and sex. Wasting, stunting, and their interaction had a positive effect on mortality (lower z scores increased the risk of death), the effect being significant only for wasting at age 6–29 months and the effect being only significant for stunting at age 30–59 months. The interaction term added only little (1.08 and 1.15 respectively) and was not statistically significant in any of the two age groups. Sex differences in mortality were not significant after controlling for stunting and wasting (Table 5).
Table 5.
Effect of age, sex, stunting, wasting, and interaction on mortality, Niakhar 1983–1984
| Variable | Children age 6–29 months | Children age 30–59 months | ||||
|---|---|---|---|---|---|---|
| B coefficient | p value | Net effect | B coefficient | p value | Net effect | |
| Age | −0.0004 | 0.969 | −0.0339 | 0.027* | ||
| Sex | −0.0154 | 0.909 | −0.3785 | 0.126 | ||
| Stunting | −0.1378 | 0.150 | 1.31 | −0.4751 | 0.00007* | 2.57 |
| Wasting | −0.2608 | 0.004* | 1.66 | −0.0552 | 0.752 | 1.12 |
| Interaction | −0.0412 | 0.326 | 1.08 | −0.0714 | 0.288 | 1.15 |
| Constant | −3.8629 | <10−6* | −3.9151 | <10−6* | ||
Note. Net effect = relative risk of mortality associated with −2 z score; sex = 1 for males; stunting and wasting defined by dummy variables; (*) p < 0.05.
Altogether, the combined effect of wasting and stunting appears to have a huge effect on child survival: The mortality of children who are below −4 z score, computed as (WHZ + HAZ)/2, is 11.1 times that of children who are above the median in weight for height and height for age. In terms of attributable risk, the combined effect of WaSt explained some 51% of the total mortality (Figure 5). However, this effect remains still lower than that produced by MUAC (Garenne et al., 2006).
Figure 5.
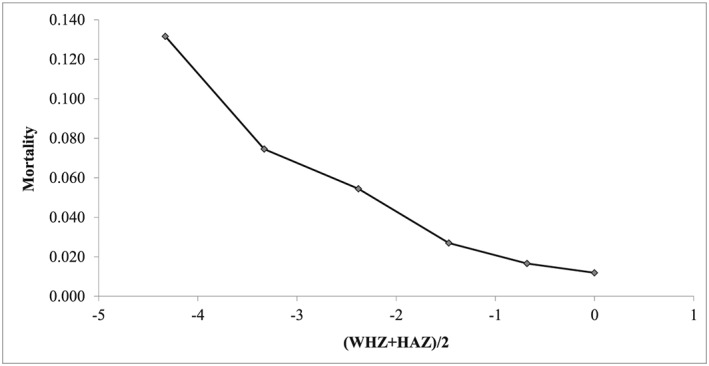
Mortality according to the combined effect of stunting and wasting, Niakhar 1983–1984
4. DISCUSSION
This study explored the interactions between wasting and stunting. Overall, wasting and stunting appear at first glance as largely independent, as they do not pertain to the same age groups and as the prevalence of concurrent WaSt by age is primarily defined by the product of both conditions. However, the interaction term (57% excess prevalence compared with the product) was significant, indicating that stunting and wasting are correlated. This interaction has implications for early detection and treatments of children at highest risk due to the multiplicative effect of both conditions on child mortality (additive effects in the logarithm of mortality risk translate into multiplicative effects).
The interactions between wasting and stunting raise a new question: Are they both produced by a common factor (nutritional, immunological, hormonal) or by increased susceptibility (stunted children being more likely to become wasted and wasted children being more likely to become stunted). This point requires further investigation.
The prevalence of concurrent WaSt can be only partly explained by linear growth, muscle mass, and fat mass, as measured by available anthropometric indicators, all playing an independent and significant role. More research is needed on the precise role of muscle mass and fat mass with more precise measures than anthropometry. This study therefore suggests the existence of other underlying factors, which cause variations by age and sex. In particular, large differences by age suggest that other strong factors are playing a role: Immunological factors, as a result of the synergistic effect of repeated infections on the immune system or hormonal factors caused by the complex production of hormones in infancy and childhood and their interactions with metabolism.
One of the most striking findings of this study was the sex difference, which changes rapidly with age. Changing sex ratios with age suggests the action of sex‐specific hormones. This has been found for a variety of infectious diseases, for auto‐immune diseases, for asthma, and other conditions. (Garenne, 2015; Garenne & Lafon, 1998). Here, the situation shows a higher susceptibility for boys below age 30 months, which disappears thereafter. This could be due to one of the sex hormones increasing susceptibility of boys at an early age and becoming silent later (Bartz et al., 2014). A potential candidate is testosterone, which is produced in early infancy and tends to disappear later, to become revived at adolescence: Testosterone could have an effect on boy's stunting in infancy, with some long‐term effects in the second year of childhood (Kuiri‐Hänninen, Sankilampi, & Dunkel, 2014). Another candidate is the difference in the dynamics of Luteinizing hormone and Follicle‐stimulating hormone (FSH) among boys and girls: In particular, FSH tends to disappear among boys after age 6 months, while it stays at high levels in girls until 3 to 4 years, which apparently matches the pattern found for concurrent WaSt (Kuiri‐Hänninen et al., 2014). Other potential candidates are Sertoli cell hormones (Müllerian inhibiting substance or the inhibin B), shown to have an effect on boys stature (Andersson et al., 1998; Morgan, Dennis, Ruffman, Bilkey, & McLennan, 2011). Further research is needed in this field to clarify these complex issues.
Whatever the aetiology of concurrent WaSt, the case deserves more attention because of its impact on child survival. Prevention, early detection, and appropriate treatments need to be identified to tackle this important public health issue, affecting millions of children in developing countries (Briend, 2015).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
MG and AB initiated the study and conducted the field work. MG conducted the statistical analysis and wrote the first draft. All authors contributed to the paper through comments and references, and all agree with the final draft.
ACKNOWLEDGMENTS
The Niakhar study was supported by the IRD (Institut de Recherche pour le Développement), formerly known as ORSTOM. Authors thank all those who participated in the field work, in particular the technical assistants, the mothers and children, and the community leaders who did welcome the study. Field work was supported by the European Union, DG‐XII, Grant number TDR‐36. The contributions of CD and TK to this paper were made possible by funding from Irish Aid (grant number HQPU/2018/ENN). The ideas, opinions and comments therein are entirely the responsibility of its authors and do not necessarily represent or reflect Irish Aid policy.
Garenne M, Myatt M, Khara T, Dolan C, Briend A. Concurrent wasting and stunting among under‐five children in Niakhar, Senegal. Matern Child Nutr. 2019;15:e12736 10.1111/mcn.12736
REFERENCES
- Akombi, B. J. , Agho, K. E. , Merom, D. , Renzaho, A. M. , & Hall, J. J. (2017). Child malnutrition in sub‐Saharan Africa: A meta‐analysis of demographic and health surveys (2006‐2016). PLoS One, 12(5), e0177338 10.1371/journal.pone.0177338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, A. M. , Toppari, J. , Haavisto, A. M. , Petersen, J. H. , Simell, T. , Simell, O. , & Skakkebaek, N. E. (1998). Longitudinal reproductive hormone profiles in infants: Peak of inhibin B levels in infant boys exceeds levels in adult men. Journal of Clinical Endocrinology and Metabolism, 83(2), 675–681. 10.1210/jcem.83.2.4603 [DOI] [PubMed] [Google Scholar]
- Angood, C. , Khara, T. , Dolan, C. , & Berkley, J. A. (2016). Research priorities on the relationship between wasting and stunting. PLoS One, 11(5), e0153221 10.1371/journal.pone.0153221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz, S. , Mody, A. , Hornik, C. , Bain, J. , Muehlbauer, M. , Kiyimba, T. , … Freemark, M. (2014). Severe acute malnutrition in childhood: Hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. Journal of Clinical Endocrinology and Metabolism, 99, 2128–2137. 10.1210/jc.2013-4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron, G. , & Castleman, T. (2012). Program responses to acute and chronic malnutrition: Divergences and convergences. Advances in Nutrition, 3(2), 242–249. 10.3945/an.111.001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, R. E. , Allen, L. H. , Bhutta, Z. A. , Caulfield, L. E. , de Onis, M. , Ezzati, M. , … Rivera, J. (2008). Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet, 371(9608), 243–260. 10.1016/S0140-6736(07)61690-0 [DOI] [PubMed] [Google Scholar]
- Briend, A. , Collins, S. , Golden, M. , Manary, M. , & Myatt, M. (2013). Maternal and child nutrition. Lancet, 382(9904), 1549 10.1016/S0140-6736(13)62315-6 [DOI] [PubMed] [Google Scholar]
- Briend, A. , Garenne, M. , Maire, B. , Fontaine, O. , & Dieng, K. (1989). Nutritional status, age and survival: The muscle mass hypothesis. European Journal of Clinical Nutrition, 43, 715–726. [PubMed] [Google Scholar]
- Briend, A. , Khara, T. , & Dolan, C. (2015). Wasting and stunting—similarities and differences: Policy and programmatic implications. Food and Nutrition Bulletin, 36(S1), S15–S23. 10.1177/15648265150361S103 [DOI] [PubMed] [Google Scholar]
- Briend, A. , Maire, B. , Fontaine, O. , & Garenne, M. (2012). Mid‐upper arm circumference and weight‐for‐height to identify high‐risk malnourished under‐five children. Maternal & Child Nutrition, 8(1), 130–133. 10.1111/j.1740-8709.2011.00340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) . (2000). CDC Growth charts for the United States: methods and development. CDC Series Report 11, No. 246, 201 pp.
- Das Gupta, M. (1987). Selective discrimination against female children in Rural Punjab, India. Population and Development Review, 13(1), 77–100. 10.2307/1972121 [DOI] [Google Scholar]
- Garenne, M. (2003). Sex differences in health indicators among children in African DHS surveys. Journal of Biosocial Science, 35(4), 601–614. 10.1017/S0021932003006047 [DOI] [PubMed] [Google Scholar]
- Garenne, M. (2015). Demographic evidence of sex differences in vulnerability to infectious diseases. Journal of Infectious Diseases, 211(2), 331–332. 10.1093/infdis/jiu448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garenne, M. , & Lafon, M. (1998). Sexist diseases. Perspectives in Biology and Medicine, 41(2), 176–189. 10.1353/pbm.1998.0005 [DOI] [PubMed] [Google Scholar]
- Garenne, M. , Maire, B. , Fontaine, O. , & Briend, A. (2006). Distributions of mortality risk attributable to low nutritional status in Niakhar, Senegal. Journal of Nutrition, 136, 2893–2900. 10.1093/jn/136.11.2893 [DOI] [PubMed] [Google Scholar]
- Garenne, M. , Maire, B. , Fontaine, O. , & Briend, A. (2012). Adequacy of child anthropometric indicators for measuring nutritional stress at population level: A study from Niakhar, Senegal. Public Health Nutrition, 16(9), 1533–1539. 10.1017/S136898001200448X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garenne, M. , Maire, B. , Fontaine, O. , Dieng, K. , & Briend, A. (1987). Un critère de prévalence de la malnutrition: La survie de l'enfant. Actes des 3èmes Journées Scientifiques Internationales du GERM, Saly 6–10 octobre, 1987: in D. Lemmonier et Y. Ingenbleek eds. Les carences nutritionnelles dans les pays en voie de développement. Paris, Karthala, 12–19.
- Garenne, M. , Maire, B. , Fontaine, O. , Dieng, K. , & Briend, A. (2000). Risques de décès associés à différents états nutritionnels chez l'enfant d'âge préscolaire . Etudes du CEPED n° 17, 192 p. Paris, CEPED.
- Garenne, M. , Willie, D. , Maire, B. , Fontaine, O. , Eeckels, R. , Briend, A. , & Van den Broeck, J. (2009). Incidence and duration of severe wasting in Africa: Cases studies in Niakhar and Bwamanda. Public Health Nutrition, 12(11), 1974–1982. 10.1017/S1368980009004972 [DOI] [PubMed] [Google Scholar]
- Jensen, S. M. , Mølgaard, C. , Ejlerskov, K. T. , Christensen, L. B. , Michaelsen, K. F. , & Briend, A. (2015). Validity of anthropometric measurements to assess body composition, including muscle mass, in 3‐year‐old children from the SKOT cohort. Maternal & Child Nutrition, 11(3), 398–408. 10.1111/mcn.12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerac, M. , Mwangome, M. , McGrath, M. , Haider, R. , & Berkley, J. A. (2015). Management of acute malnutrition in infants aged under 6 months (MAMI): Current issues and future directions in policy and research. Food and Nutrition Bulletin, 36(1 Suppl), S30–S34. 10.1177/15648265150361S105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khara, T. , & Dolan, C. (2014). The relationship between wasting and stunting, policy, programming and research implications. Technical Briefing Paper, Emergency nutrition network, Oxford, UK.
- Khara, T. , Mwangome, M. , Ngari, M. , & Dolan, C. (2018). Children concurrently wasted and stunted: A meta‐analysis of prevalence data of children 6‐59 months from 84 countries. Maternal & Child Nutrition, 14(2), e12516 10.1111/mcn.12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiri‐Hänninen, T. , Sankilampi, U. , & Dunkel, L. (2014). Activation of the hypothalamic‐pituitary‐gonadal axis in infancy: Minipuberty. Hormone Research in Paediatrics, 82(2), 73–80. 10.1159/000362414 [DOI] [PubMed] [Google Scholar]
- Martorell, R. , & Young, M. F. (2012). Patterns of stunting and wasting: Potential explanatory factors. Advances in Nutrition, 3(2), 227–233. 10.3945/an.111.001107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, C. M. , Olofin, I. , Flaxman, S. , Fawzi, W. W. , Spiegelman, D. , Caulfield, L. E. , … Danaei, G. (2013). The effect of multiple anthropometric deficits on child mortality: Meta‐analysis of individual data in 10 prospective studies from developing countries. American Journal of Clinical Nutrition, 97(4), 896–901. 10.3945/ajcn.112.047639 [DOI] [PubMed] [Google Scholar]
- Mishra, V. , Roy, T. K. , & Retherford, R. D. (2004). Sex differentials in childhood feeding, health care and nutritional status. Population and Development Review, 30(2), 269–295. 10.1111/j.1728-4457.2004.013_1.x [DOI] [Google Scholar]
- Morgan, K. , Dennis, N. A. , Ruffman, T. , Bilkey, D. K. , & McLennan, I. S. (2011). The stature of boys is inversely correlated to the levels of their Sertoli cell hormones: Do the testes restrain the maturation of boys? PLoS One, 6(6), e20533 10.1371/journal.pone.0020533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt, M. , Khara, T. , Schoenbuchner, S. , Pietzsch, S. , Dolan, C. , Lelijveld, N. , & Briend, A. (2017). Children who are both wasted and stunted (WaSt) are also underweight and have a high risk of death. Action Against Hunger, Research for nutrition; Paris, Action contre la faim. [DOI] [PMC free article] [PubMed]
- Myatt, M. , Khara, T. , Schoenbuchner, S. , Pietzsch, S. , Dolan, C. , Lelijveld, N. , & Briend, A. (2018). Children who are both wasted and stunted are also underweight and have a high risk of death: A descriptive epidemiology of multiple anthropometric deficits using data from 51 countries. Archives of Public Health, 76(28), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande, R. (2003). Selective gender differences in childhood nutrition and immunization in rural India: The role of siblings. Demography, 40(3), 395–418. 10.1353/dem.2003.0029 [DOI] [PubMed] [Google Scholar]
- Pelletier, D. L. (1994). The relationship between child anthropometry and mortality in developing countries: Implications for policy, programs and future research. Journal of Nutrition, 124(10), 2047S–2208S. [DOI] [PubMed] [Google Scholar]
- Richard, S. A. , Black, R. E. , Gilman, R. H. , Guerrant, R. L. , Kang, G. , Lanata, C. F. , … Checkley, W. (2012). Childhood Infection and Malnutrition Network. Wasting is associated with stunting in early childhood. Journal of Nutrition, 142, 1291–1296. 10.3945/jn.111.154922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimshaw, N. S. (2003). Historical concepts of interactions, synergism and antagonism between nutrition and infection. Journal of Nutrition, 133, 316S–321S. 10.1093/jn/133.1.316S [DOI] [PubMed] [Google Scholar]
- Scrimshaw, N. S. , & SanGiovanni, J. P. (1997). Synergism of nutrition, infection, and immunity: An overview. The American Journal of Clinical Nutrition, 66(2), 464S–477S. 10.1093/ajcn/66.2.464S [DOI] [PubMed] [Google Scholar]
- Victora, C. G. (1992). The association between wasting and stunting: An international perspective. Journal of Nutrition, 122(5), 1105–1110. 10.1093/jn/122.5.1105 [DOI] [PubMed] [Google Scholar]
- Waterlow, J. C. (1972). Classification and definition of protein‐calorie malnutrition. British Medical Journal, 3, 566–569. 10.1136/bmj.3.5826.566 [DOI] [PMC free article] [PubMed] [Google Scholar]


