Abstract
Introduction:
Prevention of infection remains the ultimate goal for HIV vaccination, and there is compelling evidence that antibodies directed to Envelope are necessary to block infection. Generating antibodies that are sufficiently broad, potent, and sustained to block infection by the diverse HIV-1 strains circulating worldwide remains an area of intense study.
Areas Covered:
In this review, we have summarized progress from publications listed as PubMed citations in 2016–17 in the areas of passive antibody studies using human neutralizing monoclonal antibodies in nonhuman primates, HIV Envelope vaccine development and active vaccination studies to generate potent neutralizing antibodies.
Expert Commentary:
Passive transfer studies in nonhuman primates using human neutralizing monoclonal antibodies have informed the potency, specificity, and cooperativity of antibodies needed to prevent infection, leading to clinical studies now testing potent antibodies for prevention of HIV. Progress in understanding the structure of Envelope has led to novel vaccine constructs, including mimetics, scaffolds and native-like proteins. As yet, no single approach ensures protection against the circulating global HIV-1 strains, but there is progress in understanding why, and intense research continues in these and other areas for a solution. We offer perspectives on how this knowledge may shape the design of future HIV vaccines.
Keywords: HIV, vaccines, antibodies, Envelope, passive, monoclonal antibodies, protective efficacy
1. Introduction
In the last three decades, successful vaccines against hepatitis B and human papilloma viruses were developed that induce protective and long-lasting virus-specific antibodies. However, the combined efforts of the global research community to develop a vaccine for HIV-1 (HIV) have fallen short. Although disappointing, the absence of a protective vaccine for HIV is not surprising given the requirement to provide coverage for the antigenic diversity of strains worldwide. Structural features of the trimeric Envelope glycoprotein (Env), the target of protective antibodies, include highly variable regions to which carbohydrate moieties are affixed that disguise and shield critical binding sites. Protective antibodies must overcome these obstacles and recognize conserved determinants on Env that are shared among circulating strains and clades in order to block infection. In the absence of protection, HIV-1 integrates into the genome of the host, leading to a rapid establishment of widely dispersed and latently infected cells that are invisible to the immune system sometimes for many years. Yet, sooner or later, cells within these latent pools can become active continuously producing virus and adding a potentially insurmountable obstruction to viral clearance.
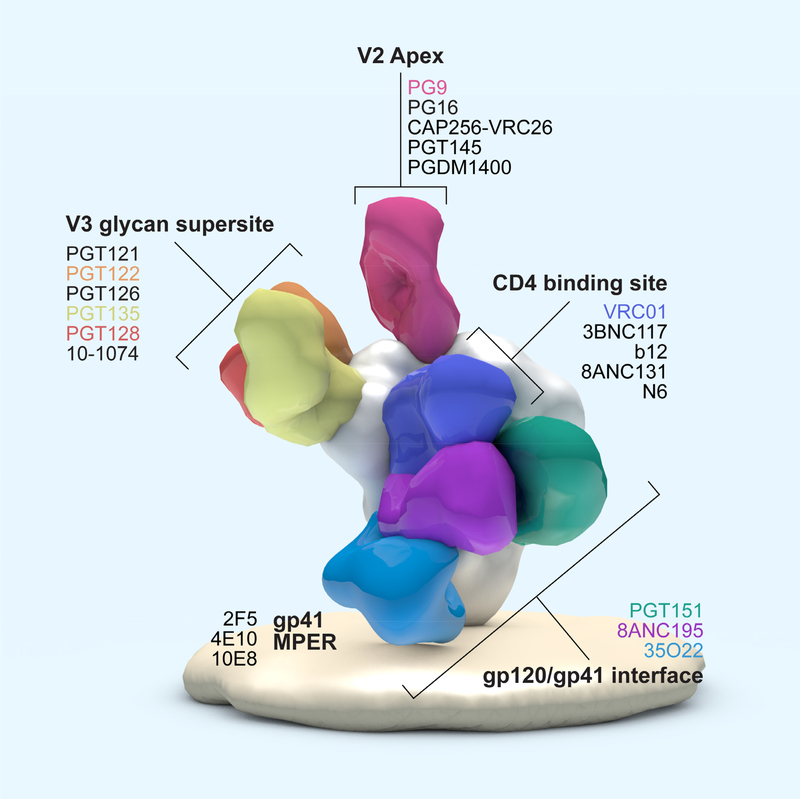
Despite these escape strategies, in most human subjects, HIV-1 infection leads to development of multiple types of antibodies, including neutralizing antibodies (NAbs), with various effector functions, recently reviewed [1]. Studies of NAb development are providing a detailed understanding of the mechanisms of antibody maturation and antigenic escape [2–5]. Technology advancement has led to the rapid discovery and characterization of highly potent human neutralizing monoclonal antibodies (NmAbs) that demonstrate extensive cross reactivity covering multiple HIV-1 clades and virus neutralization capacities [6]. These NmAbs—frequently called bNAbs to include the distinction of breadth--have become indispensable tools in revealing many aspects of the structure of Env. Co-crystals of antibody-bound Env structures have exposed intricate details of epitopes that are sites of vulnerability that may serve as candidates for vaccine targeting, recently reviewed [7] and shown in Fig. 1. At the same time, bNAbs are already in clinical testing as potential therapeutics [8], discussed in more detail below.
Figure 1. Antigenic sites on HIV-1 Envelope targeted by bNAbs.
Shown are binding locations for bNAbs to the CD4-binding site; to a high-mannose-patch referred to as the V3 glycan supersite; to the V2 apex; and to the gp41 membrane proximal external region (MPER) of HIV-1 Envelope. bNAbs associated with each of these determinants are listed. This figure is adapted from Burton and Mascola, 2015 [157].
In this review, we summarize the progress in passive antibody studies and Env-based vaccines in nonhuman primate (NHP) models of HIV infection and discuss how these studies have influenced vaccine development from the perspective of generating protective antibodies.
It is important, however, to address certain caveats of these models, which use related SIVs or laboratory chimeras of HIV and SIV—SHIVs-- due to the lack of a suitable model for HIV-1 itself. In particular, vaccine development or immunotherapy in NHP should be weighed in the context of both the advantages and limitations of these systems in order to interpret experimental outcomes for accurate translation to human studies. Here, we will offer our perspective on considerations critical to the continued and judicious implementation of NHP in HIV research. Some of the questions that will be addressed in the sections below include: (1) How has recent passive antibody research in NHP models enhanced knowledge about the impact of antibodies in blocking or controlling HIV? (2) What has this research taught us about antibody correlates of protection? (3) What are the most relevant SHIV challenge models for antibody-based vaccine studies? (4) How do we interpret findings from vaccine advances in NHPs to advance clinical studies?
2. Progress in developing primate models and lessons from passive antibody studies
2.1. Rationale for the development and use of SHIV/macaque models
NHP models for AIDS originally focused on the use of the highly adapted African-origin simian immunodeficiency virus (SIV), which causes disease in Asian macaques, the nonadapted host for SIV. SIV isolates have provided a powerful experimental advantage due to their ability to mirror most aspects of HIV-1 pathogenesis in a compressed time course of disease progression, and models have been developed that utilize various SIV strains in all three Asian macaque species (M. mulatta, M. fascicularis, and M. nemestrina). These virus-host combinations have afforded models to address many important questions in HIV research that cannot be easily addressed in clinical studies. Due to the genetic divergence of SIV and HIV, the Env proteins from these two viruses are not antigenically cross-reactive, making it impossible to study HIV Env-specific antibody responses in SIV infection. The development of chimeric simian/human immunodeficiency viruses (SHIVs) expressing the HIV env in an SIV backbone has resulted in tractable models in the same macaque species following passage in vivo in order to gain replication fitness. Since the earliest infecting virions access target cells via the coreceptor CCR5, SHIVs bearing Envs that use CCR5 were developed. However, SHIV models have come under criticism for the spontaneous and variable post-acute control of viremia in a subset of infected macaques, as well as reduced replication competence and resulting limited pathogenesis that is likely due to insufficient genetic adaptation. Nonetheless, despite limitations in these models, SHIV expression of HIV Env on the virion surface provided an avenue for human antibody-based passive protection studies in NHP models of infection, as well as a platform for testing vaccine candidates that include Env as an immunogen.
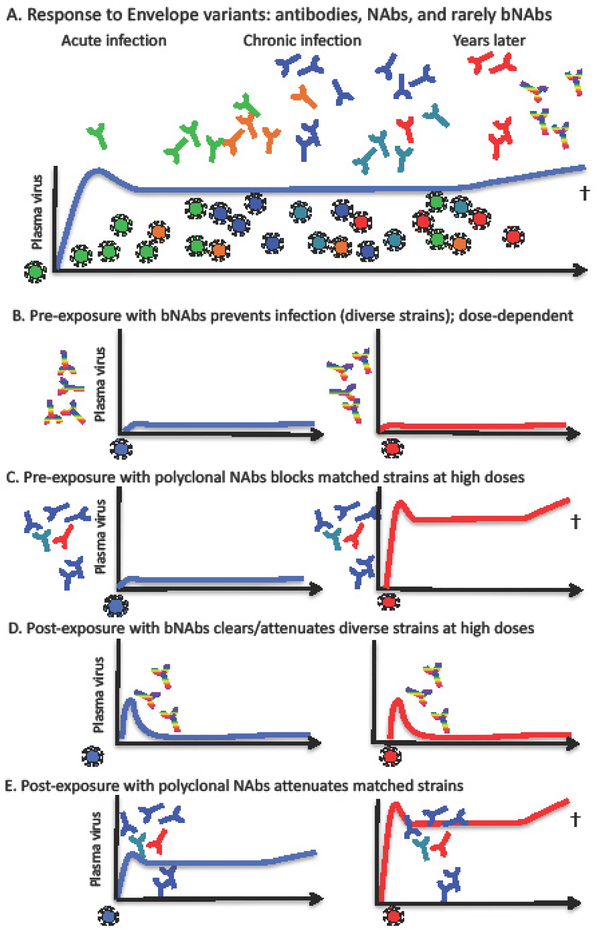
Clinical studies have shown that the development of antibodies commences shortly after infection and recognizes the Env of the infecting transmitted and early founder (T/F) virus, typically one or a few viral variants that persist for at least the first six weeks [9]. T/F strains of HIV have different phenotypic properties than chronic viruses, notably they are more infectious and contain on average more copies of Env per particle but have shorter variable loops and fewer glycan sites. Over time, as variants accrue with mutations in Env, antibodies emerge that bind to these autologous variant Envs, but their detection is delayed relative to the appearance of the antigen, depicted in Figure 2A. Autologous NAbs can be measured in a few months after infection and over years, in most subjects, there are increasing levels of heterologous NAbs that develop during chronic infection [10], despite only being exposed to variants of the infecting virus. In the figure, these new antibodies are shown to emerge after the variants that precede them in time. Their development occurs as antibodies co-evolve with the autologous virus and undergo somatic hypermutation (SHM), diverging the nucleotide sequences of variable and light chain regions (VHJH, VLJL) from away from germline. In a very small subset of people called “elite neutralizers,” this polyclonal response becomes very “broad” and potent. However, of the broad and potent bNAbs isolated from individuals, the level of SHM is high, ranging from 732%, and varies with bNmAb specificity [11–17]. Other characteristics of potent bNAbs include frequent insertions and deletions (reviewed in [18]) that have been associated with critical protein or glycan contacts [12,16]. In some cases, this exceptional breadth is due to a single clone, as shown by the isolation and characterization of bNAbs from these subjects [19–21].
Figure 2. Roles of antibodies at various stages of HIV infection.
Schematic representation of the development of neutralizing antibodies (NAbs) and broadly neutralizing antibodies (bNAbs) during HIV-1 infection. Shown in panel (2A) is the depiction of the co-evolution of autologous antibodies and viral variants over time during infection. The first autologous NAbs are shown in green, the same as the infecting virus. As Envelope variants develop during years of chronic infection, antibodies that match those viruses appear, lagging behind the appearance of the variants as depicted by the colors of the NAbs. Antibodies emerge following each iteration of quasispecies development causing a delay in effective response to autologous variant Envs. In (2B), potent bNAbs (shown as rainbow colors to represent breadth against multiple clades and tiers of virus) have been shown to block infection when given before SHIV exposure to macaques. A comparison with polyclonal NAbs is shown in (2C) where it is critical that the majority of the neutralizing activity is matched (or derived from) the challenge SHIV. In (2D), bNAb cocktails, if given very soon after virus exposure can ablate early sites of infection. In contrast (2E), even if closely matched, polyclonal Nabs can, at best, attenuate infection.
In a very similar manner, adult and juvenile macaques infected with SHIV show Env divergence from the initial infecting clone or strain. In the last several years, it was shown that SHIV adaptation occurs in vivo to better access macaque CD4 (mCD4) thus, increasing virus replication in macaques with only a few and as little as a single but critical point mutation [22,23]. As seen with HIV infection, SHIV-induced antibody responses directed to the HIV Env become more complex and more neutralization-potent over time, as the quasispecies of variants develops. There are strong similarities between SIV and SHIV infection in the dynamics of innate cells, B- and T-cell subsets, cytokine expression, and the development and maturation of T cells and antibodies with various functional activities. Importantly for antibody studies, macaque B cell receptors are capable of recognizing complex epitopes similar to those recognized by certain bNmAbs isolated from HIV-1- infected humans, and, importantly, SHIV- infected macaques are able to develop NAbs directed to glycan-specific epitopes recognized by human bNAbs [24].
Important progress in the last few years has led to the development of SHIV strains that encode newly transmitted HIV Env proteins and that incorporate modifications to interact more efficiently with rhesus CD4, enhancing virus entry and replication making them pathogenic in vivo [25,26]. These strains can be transmitted by mucosal routes in NHP and are promising for future work. Other SHIV isolates that may be critical for vaccine work include an A/E chimera recently titered in vivo that matches strains prevalent in Bangkok, Thailand, identified in the RV144 trial [27]. The T/F viruses are more difficult to neutralize in vivo, and their plasma viremia persists longer than many current SHIVs, providing hope that they may be more relevant models for HIV in humans. As with all animal models, a successful clinical trial with clear correlates of protection is necessary to validate one or more of them. If the ongoing human trials of poxvirus plus protein (e.g. HVTN106, www.hvtn.org) in South Africa are successful, then we may be able to test vaccines or test vaccine-elicited antibodies in NHP models to understand and validate them, as described below. Their use is further discussed as it applies to vaccine development, in Section 3.
2.2. Early infection events: viral dissemination and latency
Early innate immune events that occur in response to mucosal HIV infection are not fully characterized, although there has been some significant progress in the last few years. By using fluorescently labeled virus, it has been possible to track migration into vaginal tissues [28] in efforts to visualize the earliest infection stages, where there is evidence that CD4-positive Th17 cells are preferentially targeted by SIV [29]. The HIV-1 viral eclipse phase was described as the period just after virus exposure during which virus replicates in mucosal and proximal lymphoid tissues but is not yet detectable in the circulating plasma [30]. It has been estimated that the eclipse phase in SIV-infected macaques lasts for approximately 4–7 days, and for HIV-1 infected humans it is about 7–21 days in duration. This predicted eclipse phase either differs in primate models, or may be a result of assay sensitivity, as there are now recent data from several NHP models showing that SIV and SHIV RNA and DNA are detectable in the blood by 3 days after infection in newborn macaques. Moreover, high dose oral inoculation of either SIV or SHIV results in rapid dissemination to lymph nodes and gut within 1–3 days with tissue reservoirs greatly expanded by day 14 [31,32]. The kinetics of SIV distribution to lymphoid tissues following vaginal challenge in adult macaques was observed in 2005 [33], and recent studies with SHIV confirmed these observations [34].
Understanding the kinetics of virus dissemination is important for successful post-exposure prophylaxis by antiretroviral therapy (ART), and this treatment is very effective to prevent infection after needlestick incidents when initiated within 72 hours of exposure. Treatment with ART in individuals with established infection can drive the virus to undetectable levels but it does not eliminate the infected cells, and viral rebound is observed even after extended treatment [35]. Defining the time frame of intervention is important, because once latently infected cells exist, the window of opportunity to eradicate virus appears to be closed, leaving the host vulnerable to breakthrough infections and continued viral replication. In primates, initiating ART during the eclipse phase before detectable viremia, as early as 3 days post inoculation, does not prevent establishment of infection [36]. Elegant clinical studies published in the last year indicate that the source of the continued virus in the blood may arise more commonly from distal sites rather than circulating PBMCs [37]. The application of tissue virus quantification to NHP models has begun to reveal the full impact of SIV and SHIV viral reservoirs in blood and in tissues [32,38]. Likewise, refinements to immunological assays in primates have allowed us to examine the direct effects of the virus on specific subsets of immune cell populations. SIV is found in high levels in lymphoid tissues [39,40], and one major sanctuary is in the B cell follicle in T follicular helper cells [41,42]. This area of research has emerged as the field begins to consider whether it is possible to reduce or eliminate the viral reservoirs [35]. Given these kinetics, how have studies in primates informed the role of antibodies in preventing infection?
2.3. Passive antibody for pre-exposure prophylaxis,and modulation of immunity
Combinations of human mAbs (and in some cases humanized murine mAbs) are being used effectively in cancer therapy to ablate certain populations of cells, such as B cells, or more recently to modulate immunity. Their use in infectious diseases has been slower to develop, although a number of influenza-specific mAbs are in clinical testing [43]. Recently, two different combinations of human mAbs were shown to protect nonhuman primates against all signs of Ebola [44,45], and most recently, Zika virus [46]. Despite these successes, antibodies as therapeutics for HIV infection have been dismissed, until very recently, as “too little, too late” for reasons of expense and practicality. During the last decade of HIV-1 antibody discovery, the use of passively transferred antibodies for prevention and cure strategies has gained in relevance because the recent discoveries of extremely potent bNAbs have made a clinically relevant dose more achievable. It is anticipated that the ongoing trials of human bNAbs as PrEP (pre-exposure prophylaxis), if successful, could not only prevent the establishment of infection but could also provide insight into what may constitute a protective antibody response.
Historically, SHIV/macaque models have shown that even when a single type of bNAb is present before virus exposure, infection by mucosal routes can be blocked and apparent sterilizing immunity can be achieved, summarized in a recent review [1]. This activity is depicted in Figure 2B, where bNAbs can block infection by diverse isolates in NHP models. It is hoped that eventually vaccine-induced pre-existing NAbs at the site of entry will either block infection or provide significant viral clearance before subpopulations of CD4+ T cells become productively infected.
2.4. Interpreting passive antibody results in NHP models
Data from SHIV challenges consistently shows that the concentration of antibodies required to block infection in vivo is dose-dependent and tracks directly with the in vitro neutralizing activity of either bNAbs or polyclonal serum IgG preparations, making viral neutralization the primary antiviral function associated with in vivo protection [47–49]. Historically, peripheral blood mononuclear cell (PBMC)-based HIV-1 and SHIV neutralization assays with human and/or macaque cells were used to determine the in vitro 50% and 90% inhibitory concentrations (IC50, IC90) of NAbs. These assays, and later, neutralization assays using pseudoviruses in TZM-bl cells, were used to determine NAb titers in plasma or serum at the time of challenge during passive transfer studies (plasma dilution resulting in 50% inhibition, [ID50]. Importantly, a determination of the potency of a transferred mAb using the concentration measured in plasma at the time of challenge is also essential to understanding the protective outcome of any particular study. This value has been termed the “plasma IC50 concentration” and is determined based on the in vitro neutralization potency of the transferred mAb against the challenge virus as follows: plasma concentration of the transferred mAb divided by the in vitro mAb IC50 measured (reviewed in Pegu et al. [1]). The resulting value incorporates both the amount of mAb in circulation after passive transfer (pg/ml) and the in vitro potency of the mAb (pg/ml) and represents the “fold-over the in vitro IC50.” Both of these values have been used to determine protective efficacy in passive transfer studies, but efficacy in these models has been shown to vary, even among potent and broadly neutralizing mAbs (bNAbs) depending upon the challenge virus, targeted epitope, mAb half-life, and Env glycosylation [50].
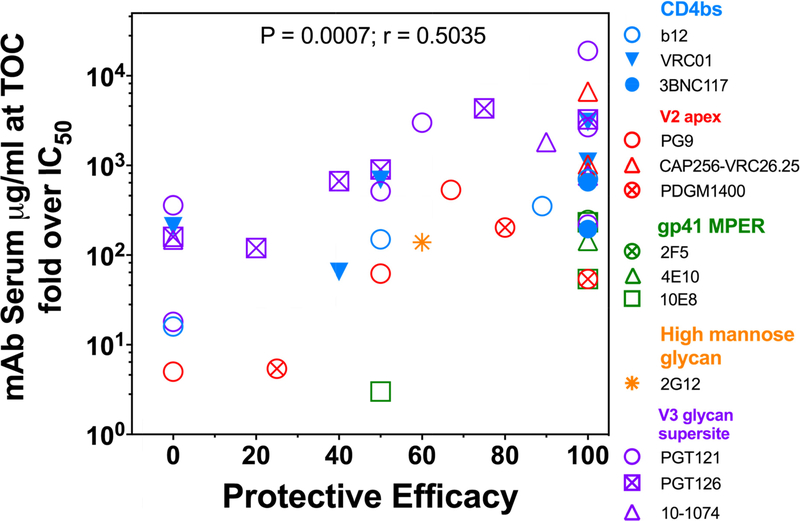
Antibody in vivo titration studies have shown that complete protection in SHIV models with macaques is seen when serum concentrations constituting neutralizing titers are at least tenfold and more often a few hundred-fold higher than the in vitro IC50 [1,51,52]. However, this range is dependent upon the neutralization assay used to assess the IC50, and even considering this caveat, protection efficacy varies among bNAbs and Env targeting specificities. The great potency of the bNAbs discovered since 2010 has effectively translated to requiring much less antibody in macaque serum at the time of challenge for protection. These findings have extremely important implications for prevention and immunotherapy applications, allowing clinically relevant doses to be achieved at reasonable costs. For vaccine research, the potential for a much lower titer of antibody induced by immunization also bolsters the search for immunogen designs and strategies to elicit potent NAbs, if a reduced risk of infection is expected to be predominatly mediated by neutralization. The bar for achieving this goal may still be out-of-reach unless bNAbs of the greatest potency are elicited. To envision how bNAbs used in passive protection studies compare, we have graphed the protective efficacy observed as a function of concentration recorded as the “fold over IC50” (Fig. 3). See Table 1 for specifics associated with each passive protection study depicted in this figure. It is important to note that apparent sterilizing immunity provided by even the most potent bNAbs indicates a requirement for serum concentrations many times the in vitro IC50 against the challenge virus, as shown in the 100% efficacy column. However, because the in vitro IC50s of potent bNAbs are extremely low (usually <0.01 pg/ml), the serum concentrations attributable to protection are also very low, thus making potent bNAbs an attractive therapeutic [1]. The recent report of protection with two broad and potent V2-specific bNAbs, PGDM1400 and CAP256-VRC.25 against a clade C SHIV designed precisely for the study [53], is consistent with the findings of the protection studies shown here because when the bNAbs and challenge virus are carefully matched, the protection results in very low serum concentrations needed for protection. However, even with the most potent bNAbs known today, there is a realization in the field that targeting multiple Env determinants will be needed for expectations of broadening protection coverage either with multi-specific Ab-like constructs or cocktails. This will be especially important for vaccine designers.
Figure 3. Correlation of neutralilzation potency and protection from SHIV challenge in macaque passive studies.
Data from multiple published passive antibody protection studies in macaques have been graphed showing the strong association between the potency of the antibody present in the sera at the time of challenge and the resulting protective efficacy. The fold over IC50 was calculated as the passive antibody serum concentration at time of challenge (TOC) divided by the antibody neutralization potency (50% inhibitory concentration, [ID50] in vitro), in pg/ml. Details of each study depicted here are detailed in Table 1.
Table 1.
Summary of SHIV bNAb Passive Protection Studies in Macaques
| Region of Env | Antibody | SHIV(Route) Tier(Clade) | Challenge dose (TCID50) | mAb dose (mg/kg) | IC50 (Mg/ml)/ Neutralization Assay | TOC serum concentration (Mg/ml) | Fold >IC50 | No. Protected /Total | Protective Efficacy (percent) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4bs | b12 | SF162P4(IVAG) Tier 1(B) | 300 | 25 | 1.0/rhPBMC | 705 | 705 | 4/4 | 100 | [51] | |
| 5 | 149 | 149 | 2/4 | 50 | |||||||
| 1 | 16 | 16 | 0/4 | 0 | |||||||
| SF162P3(IVAG) Tier 2(B) | 300 | 25 | 1.6/rhPBMC | 563 | 352 | 8/9 | 89 | [152] | |||
| VRC01 | SF162P3(IVAG) Tier 2(B) | 300 | 20 | 1.86/TZM-bl | 65 | 35 | 4/4 | 100 | [50] | ||
| SF162P3(IR) Tier 2(B) | 20 | 79 | 42 | 4/4 | 100 | ||||||
| BAL(IR) Tier 1(B) | 12,800 | 20 | 0.02 | 61 | 3,050 | 6/6 | 100 | [153] | |||
| 5 | 22 | 1,100 | 6/6 | 100 | |||||||
| 0.3 | 1.3 | 65 | 4/10 | 40 | |||||||
| AD8EO(IR) Tier 2(B) | 1,000 | 50 | 0.94/TZM-bl | 649 | 690 | 1/2 | 50 | [153] | |||
| 20 | 197.3 | 210 | 0/2 | 0 | |||||||
| DH12-V3AD8 Tier 2(B) | 30 | 2.35 | 350.9 | 149 | 1/2 | 50 | |||||
| 3BNC117 | AD8EO(IR) Tier 2(B) | 1,000 | 5 | 0.14/TZM-bl | 91 | 650 | 2/2 | 100 | [153] | ||
| 1 | 27.35 | 195 | |||||||||
| DH12-V3AD8 Tier 2(B) | 20 | 0.14/TZM-bl | 283.8 | 2,027 | 2/2 | 100 | |||||
| 5 | 123.85 | 885 | 1/2 | 50 | |||||||
| 1 | 26.45 | 650 | 1/2 | 50 | |||||||
| 0.2 | 4.8 | 195 | 0/2 | 0 | |||||||
| V2 | CAP256-VRC26.25 | 325c(IVAG) Tier2(C ) | 500 | 2 | 0.003/TZM-bl | 20 | 6,667 | 4/4 | 100 | [53] | |
| 0.4 | 2.5 | 833 | |||||||||
| 0.08 | 0.75 | 250 | |||||||||
| PGDM1400 | 500 | 2 | 0.037/TZM-bl | 7.5 | 203 | 4/5 | 80 | ||||
| 0.4 | 2 | 54 | 5/5 | 100 | |||||||
| 0.08 | 0.2 | 5.4 | 1/4 | 25 | |||||||
| V1V2 | PG9 | BAL.P4(IR) Tier 1(B) | 12,800 | 20 | 0.06/TZM-bl | 32 | 533 | 4/6 | 67 | [50] | |
| 5 | 3.7 | 62 | 3/6 | 50 | |||||||
| 0.3 | 0.28 | 5 | 0/6 | 0 | |||||||
| V3 glycan | PGT121 | SF162P3(IVAG) Tier 2(B) | 300 | 5 | 0.005/TZM-bl | 95 | 19,000 | 5/5 | 100 | [49] | |
| 1 | 15 | 3,000 | 3/5 | 60 | |||||||
| 0.2 | 1.8 | 360 | 0/5 | 0 | |||||||
| AD8EO(IR) Tier 2(B) | 1,000 | 20 | 0.1/TZM-bl | 260.75 | 2,608 | 2/2 | 100 | [153] | |||
| 5 | 51.45 | 515 | 1/2 | 50 | |||||||
| 1 | 21.85 | 219 | 2/2 | 100 | |||||||
| 0.2 | L8 | 18 | 0/2 | 0 | |||||||
| DH12-V3AD8(B) | 1,000 | 20 | 0.01/TZM-bl | 207.9 | 20,790 | 4/4 | 100 | ||||
| 1 | 22.2 | 2,220 | 1/2 | 50 | |||||||
| 0.2 | 2.35 | 235 | 2/2 | 100 | |||||||
| 0.05 | 1.15 | 115 | 0/2 | 0 | |||||||
| PGT126 | SF162P3(IVAG) Tier 2(B) | 300 | 10 | 0.03/TZM-bl | 98 | 3,267 | 5/5 | 100 | [154] | ||
| 2 | 20 | 667 | 2/5 | 40 | |||||||
| 0.4 | 3.6 | 120 | 1/5 | 20 | |||||||
| SF162P3(IR) Tier 2(B) | 10 | 130 | 4,333 | 3/4 | 75 | ||||||
| 2 | 27 | 900 | 2/4 | 50 | |||||||
| 0.4 | 4.8 | 160 | 0/4 | 0 | |||||||
| 10–1074 | AD8EO(IR) Tier 2(B) | 1,000 | 20 | 0.15/TZM-bl | 273.75 | 1,825 | 2/2 | 100 | [153] | ||
| 5 | 768 | 768 | 2/2 | 100 | |||||||
| 1 | 149 | 149 | 0/2 | 0 | |||||||
| 20 | 0.06/TZM-bl | 231.8 | 3,863 | 2/2 | 100 | ||||||
| 5 | 95.95 | 1,599 | 2/2 | 100 | |||||||
| 1 | 23.5 | 392 | 1/2 | 50 | |||||||
| 0.2 | 19.55 | 326 | 0/2 | 0 | |||||||
| Man-9glycans | 2G12 | SF162P3(IR) Tier 2(B) | 500 | 40 | 7.6/TZM-bl | 1053 | 139 | 3/5 | 60 | [155] | |
| 200/rhPBMC | 53 | ||||||||||
| MPER | 10E8 | BAL.P4(IR) Tier 1(B) | 12,800 | 20 | 0.57/TZM-bl | 133 | 233 | 6/6 | 100 | [50] | |
| 5 | 31 | 54 | 6/6 | 100 | |||||||
| 0.3 | 1.8 | 3 | 3/6 | 50 | |||||||
| 4E10 | BAL(IR) Tier 1(B) | 2,000 | 50 | 0.8/TZM-bl | 866 | 1,125 | 6/6 | 100 | [156] | ||
| 6.0/rhPBMC | 144 | ||||||||||
| 2F5 | BAL(IR) Tier 1(B) | 2,000 | 50 | 0.4/TZM-bl | 742 | 1,855 | 6/6 | 100 | |||
| 3.0/rhPBMC | 247 | ||||||||||
Studies in NHP models have shown the dose-dependence of bNAb protection by repeated viral challenge during the decay phase of the passively transferred antibody cocktail [54]. However, the case for neutralizing potency in antibody protection is still strong, even in light of the modest efficacy of RV144 and correlation with high titer binding IgG directed to variable regions [27,55]. When compared for protection against a topical SHIVSF162P4 (Tier 1) vaginal challenge, no protection was achieved with the weakly-neutralizing mAb, b6, a CD4bs mAb that recognizes a similar and overlapping epitope to bNmAb b12. Surprisingly, a non-neutralizing mAb, F240, that targets the immunodominant region of gp41, protected 2 of 5 macaques [56]. The partial protection by F240 is intriguing and complicates interpreting results of the study. One possibility based on in vitro analysis, was the greater ability of F240 compared to b6 and b12 to capture infectious virions although recently this characteristic has not held up as advantageous [57]. The mechanisms behind F240 protection are unknown, but it was hypothesized that even though functionally inactive, the gp41 “stump” epitope recognized by F240 on otherwise infectious virions provided a footing for Fc receptor mediated mechanisms that decreased infectivity of the virus inoculum. This passive study has implications for vaccine-elicited Abs targeting many of the exposed regions of Env during various stages of infection. Thus, unraveling the F240 protection results in the context of RV144 correlates pointing to high titers of binding Abs directed to the V2 region of Env, is extremely important today for parsing the roles of neutralizing, binding, and effector function contributions toward protection.
Polyclonal antibody preparations are also capable of protection against SIV or SHIV infection in NHP models, but only high doses of HIV immune globlulin (purified from HIV-positive human or chimpanzee serum) have shown this activity [58]. Similarly, SIVIG and SHIVIG, polyclonal preparations purified from SIV- or SHIV-infected macaques, can protect only at high concentrations (100–200 mg/kg) due to the relatively small proportion of NAbs in the IgG preparation [59] [60] (earliest work reviewed in [61]). Furthermore, since the development of bNAbs takes years to develop in SHIV-infected NHP, SHIVIG is typically only capable of neutralizing a homologous virus, and heterologous SHIV challenge typically cannot be defended against, as depicted in Figure 2C. Even at high doses, if the potency of SHIVIG against the challenge virus is too low, there is no permanent effect on viremia [62].
More studies are needed, but if cocktails of NmAbs or therapies with bi-modal, or multispecific Ig-based variants (discussed in 2.8 below) are shown to be effective at preventing or limiting viral seeding or preventing viral latency in clinical trials, then logically it follows that if achievable, a ready and present NAb titer of significant breadth will be a critical element of a vaccine-elicited response in order to expect efficacy of a global population. V2-directed autologous Abs were identified as one of two immune correlates of the RV144 clinical vaccine trial [55], but none of the published passive studies to date have fully evaluated the potential of V2-targeted mAb cocktails of the type identified in the vaccine trial analysis for protective efficacy.
2.5. Combinations of bNAbs as post-exposure immunotherapy
In primate models, experiments published in the last two years have shown that bNAbs can significantly impact SHIV infection if present early after exposure in blocking infection and/or to modulate disease. As mentioned above, combining different specificities in NmAb cocktails may reduce viral escape and may be a logical choice for effective immunotherapy applications, as these combinations show improvement of neutralization potency in vitro [63]. Cocktails that incorporate PGT121 along with VRC01 and its clonal relatives suppressed viral replication and inhibited entry of HIV induced from latent reservoirs into CD4+ T cells [64]. Similar cocktails were given to SHIV-infected macaques on day 10 of infection and resulted in significant reductions in peak viremia and viral replication [65]. The findings in these studies of substantial impact by bNAb cocktails on established viremia spurred our interest in testing bNAb intervention in our infant macaque model developed to study mother-to-child-transmission of HIV-1 (MTCT) at birth. Despite evidence for virus in the blood and tissues by day 1, passive bNAb treatment with the cocktail of PGT121 and VRC07–523 completely protected all ten infants with no evidence of viremia in blood or tissues after six months when given subcutaneously one day after oral SHIV infection [32]. Another recent study also reported the benefits of bNAb therapy applied three days post infection in SHIV-exposed adult macaques infected with SHIV-AD8 [66]. Thus, bNAbs can either attenuate or eradicate different virus strains, as long as they are present near the time of viral acquisition, as shown in Figure 2D.
In addition to the effects seen by bNAbs, polyclonal IgG given early can contribute to viral control and promote beneficial immunomodulatory effects in infected animals, originally shown in SIV infection and more recently with SHIV infection [67,68], depicted in Figure 2E. In this setting, polyclonal IgG is typically not potent enough to block infection, but can control viremia [62] against matched SHIV but not mismatched SHIV, as well as viral seeding in PBMC [69].
We recently reported that polyclonal NAbs given to adult macaques before intrarectal (IR) SHIV infection, but at doses too low to block infection, mitigated B cell dysregulation; this preservation of B cells was correlated with the ability of the animals to develop Env-specific Abs [69].
It is our opinion that, even short of complete eradication of the virus, applications of NmAb immunotherapies during the earliest stages (days) of infection may have an impact in slowing the ravages of HIV-1 on the adaptive immune system. Once deterred, the probable effect would then be the development of strong humoral responses as well as highly functional T cell responses that could contribute to viral control after rebound [66]. Once infection is established and viral reservoirs are present, it is not known whether antibodies could be effective therapeutics, but there has been progress in testing the concept in NHP models. These studies have implications for designing therapeutic strategies to maintain virus remission in infected individuals and remove the indefinite use of ART. Studies in humanized mice and macaque models have shown that several of the known bNAbs can suppress established virus in infected animals, summarized in a recent review [70]. Animal models of viral clearance have begun to test the potential for anti-HIV bNAbs in strategies for curative approaches [8,65,68,71–73].
In summary, attaining viral clearance in macaques using cocktails of bNAbs with different specificities may offer the highest potential for successful therapies to clear infection. Several highly potent bNAbs can neutralize a large portion of known circulating isolates worldwide at extremely low IC50 concentrations. In vitro studies examining mAb combinations suggested that neutralization coverage is improved when different epitopes are targeted and more recently, mathematical models of neutralization and breadth predicted improved protection with combinations of the most potent and broadly neutralizing mAbs [63]. To date only a few in vivo studies have been published using combinations of these bNAbs [71,72] and the long term benefit of such therapies is not known.
2.6. Recent bNAb discoveries and modifications
Human bNAbs against HIV-1 that were isolated years ago did not have the potency to become a part of an effective immunotherapy in humans. Instead, studies to understand their specific interactions with Env, their neutralization, and their protective efficacies in NHP were their primary contribution to guide vaccine designers as a blueprint for immunogen design, recently reviewed [6]. New B cell technologies leading to the HIV bNAb discovery explosion, heralded in 2009 by the isolation of a panel of very potent bNAbs from an African cohort, headlined with PG9 and PG16 [11], set in motion the subsequent discoveries of many new bNAbs. Large patient cohorts and improved identification of the subjects with the most potent and broad sera continued to expand the collection of super bNAbs [74]. Structural studies of these bNAbs continue to advance understanding of Env vulnerability and requirements for immunogen design [75–78]. The five known antigenic sites on HIV-1 Envelope targeted by bNAbs are shown in Figure 1 along with bNAbs associated with those epitope regions. Since 2010 and the identification of PG9 and PG16 that targeted the Env apex epitopes, other unique determinants have been identified that are associated with discoveries of very potent bNAbs. The high mannose patch is targeted by the PGT families of bNAbs which show exceptionally potency [15]. PGT121 has shown exceptional protective efficacy at very low doses against SHIVSSF162P3, a commonly used Tier 2 virus [49]. As mentioned previously, when using a challenge virus developed to be highly susceptible to neutralization by PDGM1400 and CAP256.VRC26, protection of macaques was also achieved at very low doses and serum concentrations [53]. Together, these studies bolster the idea of targeting the V1V2 apex epitopes for vaccine discovery. Another new epitope discovery is the gp120-gp41 interfa ce harboring unique epitopes from which several bNAbs have been identified [79] [80] [81] and structural studies will no doubt benefit immunogen design concepts. Notably, the bNAb specificities of the apex, the high mannose patch and some of the gp120-gp41 are dependent upon glycans [2]. In 2016, the discovery of the CD4bs bNAb, N6, is the broadest and most potently neutralizing of any so far and covers 98% of known HIV-1 isolates [82]. However, new bNAbs are continuing to be discovered that could surpass the breadth and potency of those known today. The safety and utility of producing antibodies in plants was shown with the Ebola life-saving tri-mAb cocktail. Now, developments with many of the newest and most potent HIV-1 mAbs are also being developed utilizing Nicotians species, for testing in NHP and eventual clinical use [83–85].
2.7. Bispecific and trispecific antibodies and mimetics
Even with the recent discovery of N6, no single mAb is highly active against all viral strains. Now, innovative research with bispecific and trispecific immunoglobulins (IgGs) composed of antigen-binding fragments from mAbs of different specificities and a common Fc region are being developed as efforts to achieve pan-reactive neutralization coverage [86–89]. These bispecific and trispecific antibodies act against HIV Env with multiple antibody specificities often with improved neutralization coverage over the individual parental antibodies and, importantly, when tested in NHP, were similar in pharmacokinetics comparable to those of the parental bNAbs [86]. When tested for protection, the trispecific mAb engineered to interact with the CD4bs, MPER and the V1V2 glycan site, protected NHP against SHIVs that were resistant to single bNAbs alone [89]. Other biNAbs are being constructed with altered Fab arm flexibility by re-engineering the hinge domain variant of lgG3 [90]. Yet another approach has been to engineer the biNAbs to become positioned at the cell surface with Fab arms designed to link either an anti-CD4 or anti-CCR5 mAb to a gp41-specific bNmAb, creating an antibody-like inhibitor. Although not tested in NHP models, the 10E8v2.0/iMab protected humanized mice from HIV-1 intraperitoneal challenge and reduced virus loads when tested after established infection [82]. The approach is ingenious, providing adaptability and a multi-faceted attack for both prevention and treatment of HIV-1. Other constructs based on multiple targeting include entry inhibitors such as eCD4-Ig, a construct of the immunoadhesin form of CD4, CD4-Ig, fused to a small CCR5-mimetic peptide. Using AAV delivery to macaques, eCD4-Ig remained stable and functional for almost a year and protected macaques from repeated SHIV challenges [91]. This extremely broadly neutralizing molecule was shown to be less immunogenic and could be stably expressed in macaques with AAV for 40+ weeks and protected animals from repeated challenges with SHIV-AD8. Other groups have developed CD4-mimetics to sensitize infected cells to ADCC [92] or to redirect ADCC killing away from uninfected bystander CD4+ T cells [93,94], a surprising new discovery with therapeutic implications. The aim of using entry inhibitors is to sidestep the need for a conventional vaccine. Future developments and increased efficacy with these tools will be extremely interesting to follow, especially in NHP models of preclinical testing.
2.8. Cell-to-cell transmission
Understanding the precise mechanism of HIV-1 passage through virological synapses may become an important consideration for NmAb applications because preventing cell-to-cell transmission will be an important defining characteristic of antibody potency in therapeutic or prophylactic treatments of HIV-1. Although bNAb effectiveness against cell-to-cell transmission by neutralization of transcytosed virus has been shown [95], a recent report raises an alarm regarding the reduced potency of some bNAbs in the face of cell-to-cell infection [96]. The group studied a panel of bNAbs and compared two molecular clones and two transmitted/founder (T/F) clones and compared their sensitivies to each bNAb in cell-free and cell-to-cell infection assays. The in vitro findings revealed that bNAbs have reduced neutralization potency in cell-to- cell infections compared to cell-free infections. These results imply that a fraction of virus can escape bNAb neutralization during cell-to-cell transmission, thus compromising full blocking of infection. With new imaging technologies that are being used in animal models it may be possible to visually follow systemic virus spread following infection [39,97] [98]. These technologies applied to studies testing immunotherapy strategies in NHP will be invaluable in assessing the potential for eradication of virus and cure possibilities.
2.9. Relevance of effector function in protective efficacy
As summarized in the sections above, there is a strong correlation between the in vitro neutralization of the challenge SHIV and in vivo protection in passive antibody studies in macaques, while the weight of the contribution by other antiviral activities in the absence of neutralization is still controversial [99]. Nonetheless, there is a growing appreciation for the protective role of antibody effector function in natural infection and vaccine induced responses in NHP [100–103], and reviewed in [104]. Moreover, additional correlates analyses of the RV144 trial suggested that specific qualitative features of the humoral response may be key to protective efficacy, identifying ADCC activity [105], as well as IgG3 antibodies associated with polyfunctional effector profiles [106] as potentially contributing to efficacy.
Antibodies have the ability to modulate the recruitment of innate immune effector cells by cell type and thus the responses induced [104]. ADCC is one of the main effector functions necessary for clinical efficacy of mAbs and is mediated largely through binding of the Fc portion of the Ig molecule to a set of closely related Fcy receptors capable of activating or inhibitory activities. For HIV infection, the requirement for a particular recruitment may differ during stages of infection or modes of transmission. Also, several antibody characteristics may determine innate immune recruiting capacity including titer, affinity, epitope specificity, and polyclonality, by impacting the geometry and valency of the immune complexes formed. An important study compared bNAb ADCC activity against different scenarios of infection, including reactivation of virus from latent reservoirs. A range of Fc-mediated killing was measured in the panel of bNAbs tested, confirming that a difference in efficacies dependent on the Fab interactions with virions exposed on the surface of infected cells [107]. Beyond these variable domain features that modulate the potency of the humoral immune response, antibodies provide instructions to the innate immune system on how to clear complexed antigens via their Fc-domain, providing an additional level of regulated and bidirectional control over activity with isotype/subclass, and Fc glycan choice. A recent multi-lab study evaluated a set of 140 blinded IgG samples purified from HIV infected subjects using different FcyR-mediated assays. Surprisingly, ADCC-inducing antibodies were found to be highly enriched in elite controllers (EC) and were shown to coordinate the recruitment of ADCC, monocyte and neutrophil phagocytosis, and complement [108]. Enhanced FcRn function has been shown to not only increase in vivo half-life, but also to increase gut mucosal tissue localization, resulting in superior NmAb-mediated protection of macaques against SHIV challenge [109]. Together, the work highlights the critical nature of functionally coordinated antibodies in virus control.
A recent study with non-neutralizing mAbs containing mutations to enhance Fc effector function was performed in NHP [110]. However, even though effector function was enhanced, passive infusion of the non-neutralizing mAbs did not protect animals from infection, but did significantly reduced the number of T/F genomes. This suggests that antibodies that bind HIV-1 Env but fail to neutralize virus in traditional neutralization assays may act against infectious virions by limiting the number of T/F viruses during transmission or, as previously reported, by the ability to capture infectious virions [56].
Despite the growing body of literature that supports the importance of Fc-dependent antibody activities, the impact of FcyR genotype variability in NHP on vaccine efficacy and passive transfer studies of monoclonal or polyclonal therapeutics is still being evaluated. Only recently, studies are beginning to define key similarities and differences between NHP and humans in terms of antibody and effector cell biology. These findings are fundamental to advancing our understanding and correct translation of data from NHP passive studies. A comprehensive analysis of IgG binding characteristics to human and macaque FcyRs has been performed with variants of potent bNmAbs [111]. These data provide us with the expertise and molecular details to customize Fcy modifications of the human mAbs for optimal interactions with macaque FcyRs in vivo [112]. These discoveries should help in identifying the extent to which the NHP model accurately reflects human mechanisms of protective responses, and they are necessary to provide a biological and immunological foundation for animal cohort selection based on FcyR allotypic variations between human and macaques. These studies also underscore the importance of understanding the outcomes of NHP model studies in order to select more promising approaches for clinical trials. As noted above, the first clinical trials with human bNmAbs have begun [71,72].
3. Developing vaccines that overcome Env diversity by inducing broad humoral immunity
3.1. Understanding how HIV Env elicits antibodies to shared regions to enhance vaccine design.
Inducing immune responses that are broad and potent remains a key objective for vaccines, and the types of antibodies that are elicited will be dependent upon the choice of Env(s) in the vaccine. One of the major advances in the last few years has been to develop a framework by which we can evaluate potency and breadth of bNAbs and polyclonal responses using a standardized assay. Viral Envs can then be categorized in this system for their relative susceptibility to neutralization by various antibodies, termed “Tiers.” It is expected that a successful vaccine must induce NAbs potent enough to neutralize Tier 2 isolates, which constitute much of inoculum in human transmission events (T/F, discussed in Section 1). Our current thinking is that unless the polyclonal response raised by vaccination induces a majority of targeted and potent antibody responses, it will likely fall short of protection against virus exposure. There are a number of approaches aimed at developing an immunogen effective at eliciting these antibodies. At this writing, the polyclonal responses from vaccinated primates have primarily targeted autologous Tier 2 viral clones [113,114] and have shown limited breadth [115].
There has been progress in identifying the ideal properties of Envs to include in vaccines, in part, through understanding the development of bNmAbs in HIV infection of elite neutralizers. One advance has been to explore in detail the co-evolution of Env on the surface of virions and the antibody response, as diagrammed in Figure 1A and described in a recent review [116]. Developing Env immunogens that engage germline B cell receptors (BCR) of potent bNmAbs has recently emerged as an attractive strategy to drive antibody maturation, and understanding the development of antibody lineages leading to neutralization breadth of the kind seen in elite neutralizers. Elegant recent work demonstrated that T/F Envs expressed on the surface of the virion can bind to germline BCR [75]. However, the mechanisms at play to drive lineages toward specific epitopes may be more complex and varied and apparently do not necessarily include T/F viruses [117].
Important advances in understanding how the glycan shield limits access to the Env protein has come from structural studies of antibodies co-crystallized with recombinant trimers, and last year was no exception with several new revealing reports [76–79,81]. From a structural perspective, it has been tempting to speculate that we can design vaccines that will present desired epitopes and avoid those that are less desirable, as described in a series of recent reviews [7,118]. One key measure is to examine the immunogenicity of these Env proteins in NHPs, compared with recombinant scaffolds that expose regions of interest to the B cells, partially or fully.
3.2. Understanding Env delivery methods, constructs and adjuvants
Further advances have been made in vector and adjuvant development that can directly influence the cell tropism, magnitude, location, and type of antibodies induced. Some of the remaining obstacles to address include the following question: Which immunogen is the most effective for generating breadth - an Env that mimics nature with relatively disordered components, or an engineered Env that presents epitopes in an ordered structure? Even if we knew which Envs were most effective in eliciting breadth, there are many other factors that influence the magnitude and longevity of the antibody response. These include: the molecular form of Env, e.g. monomer, trimer, modified trimer, or protein embedded in liposome; the Env delivery method, e.g. protein, viral vector, DNA; the amount of Env used; the regimen and the adjuvant. Each of these may contribute in different ways and may affect the ability to stimulate protective B cell memory. The following sections address these areas further.
3.2. Mimicking nature: natural Envelopes to mimic the pathway of bNAbs
A concept put forward as a strategy to “educate” the immune system of vaccinated hosts into eliciting bNAbs is to sequentially immunize with natural quasispecies sequences that have evolved during the development of breadth. The hypothesis behind this approach is that B cells target conserved, functional regions on Env by sequential exposure to viral variants and that this process can be recapitulated by chronological immunizations using specific circulating Envs isolated from these subjects. In recent years several groups, including our own, have tested this hypothesis using natural Env immunogens isolated from timepoints contemporaneous with or preceding the development of neutralization breadth. In the last year, we showed that Envs cloned from two unrelated HIV-infected subjects who developed moderate NAbs during infection could be used in co-immunization with gp160 DNA and gp140 trimeric Env protein. This Env- based vaccine using uncleaved trimeric gp140 proteins induced NAbs in vaccinated macaques that had modest breadth and the capacity to neutralize autologous Tier 2 virus. TFH activation and increasing avidity developed concomitantly during the 20 weeks of vaccination [113], which appears to be critical for optimal humoral responses [119] and was also observed for constrained Env immunogens, discussed below [120]. The commitment to sequential boosting by other groups has become increasingly innovative and has been combined with germline targeting strategies with some success in manipulating antibody evolution in a controlled manner [121–123]. A further refinement in using natural quasispecies Envs is the B cell lineage approach based on mapping the co-evolution of autologous virus and neutralizing lineages over time [116]. Using this process, it has been possible to select the rare autologous Env variants that participated in the selection of bnAb precursors by aligning their sequential engagements (including the unmutated common ancestor). Env immunogens can then be chosen with features shown to be involved in natural induction of bnAbs in vivo. Such mapping analyses also revealed the existence of cooperative lineages for the development of bNAb responses [124]. Therefore, these studies reveal how the earliest development of NAbs may inform how breadth can intensify and target conserved determinants to generate heterologous NAbs in vaccinees. Very recently this concept was tested in macaques immunized with CH505 lineage proteins delivered sequentially as gp140 trimers, where some modest neutralization of heterologous tier 2 viruses was observed in a single macaque [125].
3.3. Engineered Env immunogens that expose key epitopes
An important limitation of the HIV vaccine field is the difficulty in generating Env immunogens that can recapitulate the native Env conformation as seen on the functional viral spike. Natural Env trimers have been described as too flexible or metastable, and it has been proposed that Env on virions “breathes” by oscillating between the closed (pre-fusion), and open (post-fusion, CD4 receptor bound) states. The more open conformation allows greater access to the trimer surface by weaker, less potent NAbs and the closed conformation restricts access only to bNAbs. Therefore, several groups began to design Envs that could address this problem [126–128]. More recently, further improvements have led to modified native-like Envs that favor this closed state by increasing trimer stability and constraining its conformation to induce NAbs that exclusively interact with epitopes targeted only by bNAbs [129,130]. Thus in theory, these native-like Env immunogens will elicit NAbs that can neutralize Tier 2 virus, and would have the greatest chance for protection efficacy when tested against challenge with a heterologous Tier 2 virus.
The first generation clade B, YU-2 and JRFL gp140-Foldons were soluble trimers in which the transmembrane gp41 domain was replaced by the foldon motif (derived from T4 bacteriophage Fibritin) in order to improve trimer stability. Native flexibly linked (NFL) trimers were then developed to contain a flexible peptide covalent linkage of Env subdomains to produce soluble, homogeneous, and cleavage-independent Env mimics that elicit higher immune responses [131]. Vaccine-elicited mAbs in NHP vaccinated with the NFLs and reverted to their most likely inferred germline state could bind these gp140 immunogens, indicating progress with this strategy [132]. Env gp140 foldons were characterized as immunogens by cloning antibodies from immunized macaques using single B cell sorting [133]. Characterization of these mAbs showed that, the antibody response in NHPs is highly polyclonal and different from the bNAbs in infected human elite neutralizers due to its lower level of SHM and distinct binding specificities compared to bNAbs. This powerful technology is very important as a tool to understand how well vaccines function in primates, as the resulting mAbs can be compared with mAbs cloned from infected or vaccinated humans [134].
Another spike mimetic design is the cleaved recombinant BG505 SOSIP.664 trimer based on a T/F clade A Env sequence containing a covalent disulfide bridge between gp120 and gp41 and an I/P mutation in gp41 to improve trimerization and stability, summarized in a recent review [129]. Compared to uncleaved gp140 trimers, BG505 SOSIP best recapitulates the native Env spike on the viral surface as demonstrated by extensive structural and antigenic characterization. This SOSIP strategy has been applied to Env trimers from multiple clades (A, B, C) and significantly increased constraint was reached in the SOSIP.v4 constructs to reduce presentation of V3 and other non-neutralizing epitopes [135,136]. In immunized rabbits, these modifications significantly increased neutralization of autologous Tier 2 viral isolates but their effect on heterologous neutralization was modest [125,136]. In addition, the autologous NAb response against BG505 SOSIP.664 targets a glycan shield hole (residue 241) rarely present in Env populations, thus raising the concern of skewing the immune response towards rare epitopes [137]. NAb development best correlated with booster immunization GC B cell magnitude and Tfh characteristics of the Env-specific CD4 T cells [120]. Encouraging evidence for improvement in inducing Tier 2 NAbs was observed in macaques immunized with BG505 SOSIP.664 immunogens delivered by the subcutaneous route in an extended regimen [115].
Other engineering approaches have been developed including immunogen modifications to increase interaction with BCRs. In knock-in mouse models, the BG505 trimers were engineered to gain the ability to engage the germline BCR of PGT121 NmAb in vitro and in vivo [123]. A reengineered version of this trimer, BG505 SOSIP.v4.1-GT1, was shown to bind to multiple bNAb germline precursors and to activate B cells in two different knock-in mouse strains [138]. Iterative rounds of computational design and in vitro antigenic testing resulted in the engineering of eOD-GT6, a gp120 outer domain construct able to interact with germline and mature CD4 binding site BCRs, capable of priming VRC01-class responses in knock-in mice [139].
Additional approaches that have shown some promise include the use of synthetic liposomes for native-like trimers [130], the use of chemical cross-linking stabilized native-like trimers [140]. Other groups have made scaffolds based on natural self-folding proteins that have a multimeric or trimeric structure and can theoretically direct immune responses to the displayed regions MPER [141] or V1V2 [142] and have tested these in rabbits, but few of these have advanced to testing in NHP models.
3.4. Advances in adjuvants and vectors to enhance antibody responses
Research efforts have also focused on the development of new adjuvants to add to the very limited number licensed currently for human use. Recently, different adjuvants were compared head-to-head for their effect on Env protein vaccination in rhesus macaques. Binding and Tier 1 neutralizing antibody titers were increased by alum/TLR7 and by MF59 alone or with TLR4 while MF59, poly IC:LC, and ISCOMs (immune stimulating complexes) transiently improved Env- specific memory B cells. However, there were no changes in CDRH3 length or SHM, two antibody features associated with bNAb development [143]. MF59 was further evaluated for efficacy in NHPs vaccinated with the components of the RV144 clinical trial (ALVAC canarypox vector prime and Env gp120 modified prime-boost), since this adjuvant is included in the HVTN 100 clinical trial underway in South Africa to test the RV144 approach with a clade C Env immunogen. Surprisingly, vaccine efficacy against SIV intrarectal challenge was not associated with the MF59 adjuvanted responses despite being higher than alum-adjuvanted responses, the adjuvant used in the RV144 trial [144]. V2 specific antibodies appeared important but there were other important signals of protection, including induction of Env-dependent intestinal lymphoid cells. However, antibodies from MF59-adjuvanted ALVAC/gp120 vaccinated NHPs captured more infectious virus than the alum group, resulting in a higher number of T/F strains [57]. Thus understanding the relative importance of neutralizing and capture antibodies in mucosal acquisition models remains a key goal of adjuvant and antigen design for HIV vaccines.
The partial efficacy of the RV144 clinical trial [27] also increased interest in the development of viral vector vaccine delivery systems. In particular, the double-stranded DNA poxvirus vectors (MVA, NYVAC and ALVAC), which have an extensive safety record, have been used as priming agents to elicit T cell responses. In a recent head-to-head comparison between NYVAC and ALVAC, the vaccine regimen with NYVAC was shown to induce stronger cellular and humoral immune responses [145]. Recently, a DNA-MVA-protein vaccine was shown to elicit mucosal immunity in LNs [110]. Another type of viral vectors actively pursued is adenovirus (Ad) since it is able to target mucosal sites, an important feature for HIV vaccines. Adenoviruses can infect dividing and nondividing cells and generate humoral and cellular immune responses while also accommodating large transgenes. However, the main drawback is the anti-vector immunity in particular to highly prevalent serotypes such as human Ad5, which was included in the failed STEP trial. This issue was partially resolved by using serotypes with lower prevalence such as human Ad4, Ad26, Ad35 and A48. Ad26, Ad35 and Ad48 are non-replication competent whereas Ad4 is replication-competent which has the potential to better mimic natural HIV infection and to act as adjuvant. Ad26 used in an Ad prime/protein boost approach recently showed 50% protection against SIV intrarectal challenge [111]. Another solution was to develop Ad vectors based on Ads from other species (chimpanzees and rhesus macaques), but these vectors do not replicate in humans and may not confer immunity that is as potent. There was evidence for significantly better viral control in a recent study that combined either human Ad4 or simian Ad7 expressing Gag and Env with recombinant Env protein [146].
4. Expert commentary
In 1984, vaccine researchers had little reason to doubt the proclamation by Health and Human Services Director Margaret Heckler and NIH researcher Dr. Robert Gallo that an HIV vaccine would be forthcoming in two years, heady from the development of the first recombinant hepatitis B vaccine. Within a few years, however, they came the realization that the road to a successful vaccine would be much longer. HIV has proven to be a formidable nemesis to vaccinology, much more challenging than predicted. Disagreements within the field developed among researchers about whether the vaccine should be designed to elicit cellular or humoral immunity delayed the testing of combination approaches. The importance of generating both types of immunity is now well appreciated, and a great deal of progress has been made in vector development as well as DNA and RNA vaccines, some of which has led to rapid clinical vaccine development for emerging viral outbreaks such as Zika [147,148]. Adjuvant development is focused on enhancing innate responses [149] and understanding how different adjuvants affect antibody specificities [150,151] . Much of the progress using Env-based approaches stems from continued refinements to NHP models that led to a better understanding of the potency and breadth of antibodies needed for protection from infection, which cannot be achieved by T cell-directed vaccines. By using the current collection of human bNAbs, it is now possible to predict which combinations of mAbs targeting different epitopes are most effective in preventing infection against strains from many different clades. This information is guiding the development of vaccines that will optimally expose these conserved regions to maximize protective immunity. There is firm evidence from NHP models supporting the inclusion of Env in HIV vaccines to elicit protective antibodies as well as to enhance the potential for viral control. Furthermore, these models have provided insights into other benefits of antibodies through analysis of passive transfer studies with antibodies that did not confer sterilizing immunity. Testing vaccine sera/IgG for protection in primates may be a next step forward as a strategy for understanding potency and breadth. Beyond measuring titers of NAbs in sera of vaccinated macaques, the HIV antibody field has moved forward with new ways to evaluate induced immunity e.g., focusing on the development of mucosal immunity, quantifying the activation of Tfh cells in germinal centers, and defining the kinetics and distribution of viremia in blood and tissues of vaccinated NHP after viral challenges. These types of studies, along with new imaging technologies [98] and advances in immunogen design and delivery are moving the field to a more comprehensive understanding of vaccinology and may lead to contributions to HIV cure research. The contributions of NHP models to HIV vaccine pursuits are both direct and indirect and argue for more investment into discovery research to understand mechanisms critical to HIV vaccine and immunotherapy success, as part of model validation, so that the current pandemic can be more rapidly contained and eliminated.
5. Five year view
The lessons learned from passive antibody studies in primate models and prophylactic vaccines in nonhuman primates and humans have provided a large body of data to serve as guidelines to inform the potency of immunity necessary for protective versus controlling HIV vaccines. However, until there is a highly successful trial that shows more than 50% protection, primate challenge models and in vitro assays for antibody activity cannot be validated. In the next five years, the outcomes from both the VRC01 PrEP trial, as well as the poxvirus plus protein HIV vaccine trial in South Africa will be available, and antibody correlates for protection from infection should be more clearly defined. It is highly likely that antibody cocktails or specifically engineered antibody-based molecules with multiple specificities will be developed that can be used as relatively infrequent PrEP, much as the long-lasting drugs in development today. We propose that antibody-based therapy may well supplant drug therapy in certain instances, where the subject requires a drug holiday for treatment of another disease, or due to disabling side effects. In the setting of mother-to-child-transmission, long lasting antibodies may provide a welcome opportunity to breastfeeding babies born to HIV-positive mothers without fear of transmission.
At a minimum, a protective vaccine-elicited polyclonal antibody response can be expected to be potently neutralizing and broadly cross-reactive, and this goal will be achieved only after immunogens are designed to engage the appropriate B-cell receptors to induce antibodies of the desired specificity. Anamnestic responses will arise too slowly requiring titers of pre-existing NAbs ready for action, preferably at mucosal sites. Neutralizing IgG or potentially IgA should be present in the gastrointestinal and genitourinary mucosal tissue, which will require establishment of long-lived plasma cells and mucosal homing factors. Avidity and persistence of the polyclonal response must be maximized. Affinity maturation of the polyclonal response in vaccinees promoted by the immunization regimen will be necessary in order to develop high affinity antibodies and autoreactive B cells must be marginalized or deleted. Many of the NmAbs isolated from HIV+ subjects after years of infection have extensive somatic mutations which may be impossible to replicate by immunizations. We also note for thoroughness in discussion of a protective vaccine response, that both arms of the adaptive immune response, cellular and humoral, will be needed to provide durable immunity. Cytotoxic T cell responses are also needed to eliminate infected cells that have slipped through a NAb blockade. Memory T cell responses are also essential, and the generation of T follicular helper (TFH) cells is clearly important for NAb development. The best hope for vaccine designers may be to aim to elicit less extensively ‘matured’ antibodies that bind to a few critical epitopes on Env and augment with potent mucosal T cell anti-HIV responses. If it is possible to generate a protective antibody response to HIV-1 by vaccination, we predict that it will be comprised of multiple epitope targeting, possibly necessitating multiple immunizations. Nonetheless, in the same time frame, we propose that Env immunogens effective at generating antibodies directed at conserved regions will be discovered, and the pathways for generating very broad antibodies in vivo will be delineated.
6. Key issues:
Despite imperfections, primate models that use SIV and SHIV have been instructive in understanding the necessity for HIV antibodies to prevent infection in models of HIV infection in macaques.
New SHIV isolates are available based on transmitted/founder viruses that are designed to interact more efficiently with rhesus CD4, thus enhancing virus entry and replication.
Neutralizing antibodies (NAbs) have multiple effects on infection in NHP models: blocking, modulating immunity, and killing infected cells; non-neutralizing antibodies have yet to demonstrate protection from infection.
Polyfunctionality of antibodies may contribute to viral control through cell killing mechanisms via the Fc portion of the molecule.
The window for antibody treatment leading to virus clearance appears to be only a few days after virus exposure in NHP models; new technologies are helping to define viral kinetics.
Achieving durable reductions in viral load in blood and tissues with passive bNAbs during established, chronic infection appears to be challenging.
Bispecific and trispecific antibodies and mimetics are promising therapeutic approaches.
Several novel strategies for Env presentation to obscure non-neutralizing regions while exposing neutralization epitopes are in testing.
Iterative testing of vaccines in NHP models, followed by cloning of mAbs from the macaques, is yielding valuable information about antigen presentation and the pathways to broadening of the immune response.
Prime-boost vaccines modeled after RV144 have provided similar levels of protection, adding confidence to the NHP models.
In NHP models, no vaccines tested to date have been shown to elicit NAbs that are capable of blocking Tier 2 virus strains, and more research is needed to break this barrier.
Acknowledgments
Funding
This manuscript was supported in part by grants from the US Dept of Health & Human services, National Institutes of Health, P51OD011092, awarded to Oregon health & Science University.
Footnotes
Declaration of interest
A J Hessell and N L Haigwood receive salary support from P51 grant noted above and are employee of Oregon health & Science University. D C Malherbe is an employee of the University of Texas Branch, Galveston, Texas.The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Pegu A, Hessell AJ, Mascola JR, Haigwood NL. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev, 275(1), 296–312 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.*.Landais E, Huang X, Havenar-Daughton C et al. Broadly Neutralizing Antibody Responses in a Large Longitudinal Sub-Saharan HIV Primary Infection Cohort. PLoS Pathog, 12(1), e1005369 (2016). Comprehensive analysis of NAb development in the largest and most diverse longitudinal cohort yet to be studied that also suggests a combination of viral and host factors as a fundamental element of the development of bNAbs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo NS, Sutton MS, Shiakolas AR et al. Multiple Antibody Lineages in One Donor Target the Glycan-V3 Supersite of the HIV-1 Envelope Glycoprotein and Display a Preference for Quaternary Binding. Journal of virology, 90(23), 10574–10586 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freund NT, Wang H, Scharf L et al. Coexistence of potent HIV-1 broadly neutralizing antibodies and antibody-sensitive viruses in a viremic controller. Sci Transl Med, 9(373) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelsoe G, Haynes BF. Host controls of HIV broadly neutralizing antibody development. Immunol Rev, 275(1), 79–88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton DR, Hangartner L. Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu Rev Immunol, 34, 635–659 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward AB, Wilson IA. The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol Rev, 275(1), 21–32 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch RM, Boritz E, Coates EE et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med, 7(319), 319ra–206. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keele BF, Giorgi EE, Salazar-Gonzalez JF et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America, 105(21), 7552–7557 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS, 28(2), 163–169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker LM, Phogat SK, Chan-Hui PY et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science, 326(5950), 285289 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou T, Georgiev I, Wu X et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science, 329(5993), 811–817 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Yang ZY, Li Y et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science, 329(5993), 856–861 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Zhou T, Zhu J et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science, 333(6049), 1593–1602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LM, Huber M, Doores KJ et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature, 477, 466–470 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pejchal R, Doores KJ, Walker LM et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science, 334(6059), 1097–1103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein F, Diskin R, Scheid JF et al. Somatic Mutations of the Immunoglobulin Framework Are Generally Required for Broad and Potent HIV-1 Neutralization. Cell, 153(1), 126–138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol, 31, 705–742 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Liao HX, Lynch R, Zhou T et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature, 496(7446), 469–476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doria-Rose NA, Schramm CA, Gorman J et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature, 509(7498), 55–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLeod DT, Choi NM, Briney B et al. Early Antibody Lineage Diversification and Independent Limb Maturation Lead to Broad HIV-1 Neutralization Targeting the Env High-Mannose Patch. Immunity, 44(5), 1215–1226 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humes D, Emery S, Laws E, Overbaugh J. A species-specific amino acid difference in the macaque CD4 receptor restricts replication by global circulating HIV-1 variants representing viruses from recent infection. Journal of virology, 86(23), 12472–12483 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd DF, Peterson D, Haggarty BS et al. Mutations in HIV-1 envelope that enhance entry with the macaque CD4 receptor alter antibody recognition by disrupting quaternary interactions within the trimer. Journal of virology, 89(2), 894–907 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker LM, Sok D, Nishimura Y et al. Rapid development of glycan-specific, broad, and potent anti-HIV-1 gp120 neutralizing antibodies in an R5 SIV/HIV chimeric virus infected macaque. Proceedings of the National Academy of Sciences of the United States of America, 108(50), 20125–20129 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Prete GQ, Ailers B, Moldt B et al. Selection of unadapted, pathogenic SHIVs encoding newly transmitted HIV-1 envelope proteins. Cell Host Microbe, 16(3), 412418 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. **.Li H, Wang S, Kong R et al. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proceedings of the National Academy of Sciences of the United States of America, 113(24), E3413–3422 (2016). An extremely important study that directly impacts the use of SHIVs in nonhuman primate models of HIV infection as an effort to mprove the infectivity of SHIVs to strengthen the validity and interpretation of passive protection and vaccine/challenge studies [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. NEnglJMed, 361(23), 2209–2220 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Carias AM, McCoombe S, McRaven M et al. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. Journal of virology, 87(21), 11388–11400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stieh DJ, Matias E, Xu H et al. Th17 Cells Are Preferentially Infected Very Early after Vaginal Transmission of SIV in Macaques. Cell Host Microbe, 19(4), 529–540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiebig EW, Wright DJ, Rawal BD et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS, 17(13), 1871–1879 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Milush JM, Kosub D, Marthas M et al. Rapid dissemination of SIV following oral inoculation. AIDS, 18(18), 2371–2380 (2004). [PubMed] [Google Scholar]
- 32. **.Hessell AJ, Jaworski JP, Epson E et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med, 22(4), 362–368 (2016). First evidence of total and durable viral clearance by passively transferred cocktails of bNAbs in a post-exposure SHIV model in infant macaques that also shows kinetics of virus and antibody distribution in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller CJ, Li Q, Abel K et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. Journal of virology, 79(14), 92179227 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Ghneim K, Sok D et al. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science, 353(6303), 1045–1049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. *.Murray AJ, Kwon KJ, Farber DL, Siliciano RF. The Latent Reservoir for HIV-1: How Immunologic Memory and Clonal Expansion Contribute to HIV-1 Persistence. J Immunol, 197(2), 407–417 (2016). An in-depth look at the earliest infection events after pre-exposure bNAb transfer that shows clearance of infection from adult macaque tissues distal from the site of challenge within two weeks of challenge, indicating systemic ongoing bNAb anti-viral activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitney JB, Hill AL, Sanisetty S et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature, 512(7512), 74–77 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. *.Boritz EA, Darko S, Swaszek L et al. Multiple Origins of Virus Persistence during Natural Control of HIV Infection. Cell, 166(4), 1004–1015 (2016). This is a comprehensive study of viral reservoirs in humans that is highly relevant to cure research with implications for antibody-based therapies to clear HIV [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen SG, Ford JC, Lewis MS et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature, 473(7348), 523–527 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deleage C, Wietgrefe SW, Del Prete G et al. Defining HIV and SIV Reservoirs in Lymphoid Tissues. Pathog Immun, 1(1), 68–106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S, Mwakalundwa G, Skinner PJ. In Situ MHC-tetramer Staining and Quantitative Analysis to Determine the Location, Abundance, and Phenotype of Antigen-specific CD8 T Cells in Tissues. J Vis Exp, (127) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukazawa Y, Lum R, Okoye AA et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med, 21(2), 132–139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mylvaganam GH, Rios D, Abdelaal HM et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proceedings of the National Academy of Sciences of the United States of America, 114(8), 1976–1981 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corti D, Cameroni E, Guarino B, Kallewaard NL, Zhu Q, Lanzavecchia A. Tackling influenza with broadly neutralizing antibodies. Curr Opin Virol, 24, 60–69 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corti D, Misasi J, Mulangu S et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science, 351(6279), 1339–1342 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Qiu X, Audet J, Lv M et al. Two-mAb cocktail protects macaques against the Makona variant of Ebola virus. Sci TranslMed, 8(329), 329ra–333. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magnani DM, Rogers TF, Beutler N et al. Neutralizing human monoclonal antibodies prevent Zika virus infection in macaques. Sci Transl Med, 9(410) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura Y, Igarashi T, Haigwood N et al. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. Journal of virology, 76(5), 2123–2130 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hessell AJ, Poignard P, Hunter M et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med, 15(8), 951–954 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moldt B, Rakasz EG, Schultz N et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proceedings of the National Academy of Sciences of the United States of America, 109(46), 18921–18925 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pegu A, Yang ZY, Boyington JC et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med, 6(243), 243ra–288. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parren PW, Marx PA, Hessell AJ et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. Journal of virology, 75(17), 8340–8347 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudicell RS, Kwon YD, Ko SY et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. Journal of virology, 88(21), 12669–12682 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Julg B, Tartaglia LJ, Keele BF et al. Broadly neutralizing antibodies targeting the HIV- 1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci Transl Med, 9(406) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. *.Gautam R, Nishimura Y, Pegu A et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature, 533(7601), 105–109 (2016). A well-designed study that demonstrates the longevity of passive bNmAbs against low-dose virus challenges and the potential for protection for an extended time after delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haynes BF, Gilbert PB, McElrath MJ et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. NEnglJMed, 366(14), 1275–1286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burton DR, Hessell AJ, Keele BF et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proceedings of the National Academy of Sciences of the United States of America, 108(27), 11181–11186 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gach JS, Venzon D, Vaccari M, Keele BF, Franchini G, Forthal DN. Relationship between Vaccine-Induced Antibody Capture of Infectious Virus and Infection Outcomes following Repeated Low-Dose Rectal Challenges with Simian Immunodeficiency Virus SIVmac251. Journal of virology, 90(19), 8487–8495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibata R, Igarashi T, Haigwood N et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nature Med, 5, 204–210 (1999). [DOI] [PubMed] [Google Scholar]
- 59.Van Rompay KK, Berardi CJ, Dillard-Telm S et al. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J Infect Dis, 177(5), 1247–1259 (1998). [DOI] [PubMed] [Google Scholar]
- 60.Sholukh AM, Byrareddy SN, Shanmuganathan V et al. Passive immunization of macaques with polyclonal anti-SHIV IgG against a heterologous tier 2 SHIV: outcome depends on IgG dose. Retrovirology, 11, 8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haigwood NL. Predictive value of primate models for AIDS. AIDS Rev, 6(4), 187–198 (2004). [PubMed] [Google Scholar]
- 62.Ng CT, Jaworski JP, Jayaraman P et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med, 16(10), 11171119 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. **.Wagh K, Bhattacharya T, Williamson C et al. Optimal Combinations of Broadly Neutralizing Antibodies for Prevention and Treatment of HIV-1 Clade C Infection. PLoS Pathog, 12(3), e1005520 (2016). The mathematical modeling used in this study is highly relevant to the HIV-1 bNAb field and provides critical predictions for the potential of combinations of bNmAbs for clinical prevention and therapeutic strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chun TW, Murray D, Justement JS et al. Broadly neutralizing antibodies suppress HIV in the persistent viral reservoir. Proceedings of the National Academy of Sciences of the United States of America, 111(36), 13151–13156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolton DL, Pegu A, Wang K et al. HIV-1 monoclonal antibodies suppress acute SHIV viremia and limit seeding of cell-associated viral reservoirs. Journal of virology,( 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. **.Nishimura Y, Gautam R, Chun TW et al. Early antibody therapy can induce long- lasting immunity to SHIV. Nature, 543(7646), 559–563 (2017). This study provides more evidence of the critical nature of delivering immunotherapy during early acute infection and emphasizes the impact of long- lasting benefits that can be directly associated with limiting progressive infection and irreversible damage to the immune system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaworski JP, Kobie J, Brower Z et al. Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques. Journal of virology, 87(19), 1044710459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. **.Schoofs T, Klein F, Braunschweig M et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science, 352(6288), 9971001 (2016). A significant and definitive report of bNmAb influence on host immune responses. Establishing immunomodulatory effects as an accessory outcome of bNmAb therapy is a critical step toward a full appreciation of clinical relevance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaworski JP, Bryk P, Brower Z et al. Pre-existing neutralizing antibody mitigates B cell dysregulation and enhances the Env-specific antibody response in SHIV-infected rhesus macaques. PLoS One, 12(2), e0172524 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Margolis DM, Koup RA, Ferrari G. HIV antibodies for treatment of HIV infection. Immunol Rev, 275(1), 313–323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bar KJ, Sneller MC, Harrison LJ et al. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. NEnglJMed, 375(21), 2037–2050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caskey M, Schoofs T, Gruell H et al. Antibody 10–1074 suppresses viremia in HIV-1- infected individuals. Nat Med, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saunders KO, Pegu A, Georgiev IS et al. Sustained Delivery of a Broadly Neutralizing Antibody in Nonhuman Primates Confers Long-Term Protection against Simian/Human Immunodeficiency Virus Infection. Journal of virology, 89(11), 58955903 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCoy LE, Burton DR. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev, 275(1), 11–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scharf L, West AP, Sievers SA et al. Structural basis for germline antibody recognition of HIV-1 immunogens. Elife, 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stewart-Jones GB, Soto C, Lemmin T et al. Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell, 165(4), 813–826 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Kerkhof TL, de Taeye SW, Boeser-Nunnink BD et al. HIV-1 escapes from N332-directed antibody neutralization in an elite neutralizer by envelope glycoprotein elongation and introduction of unusual disulfide bonds. Retrovirology, 13(1), 48 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Irimia A, Serra AM, Sarkar A et al. Lipid interactions and angle of approach to the HIV-1 viral membrane of broadly neutralizing antibody 10E8: Insights for vaccine and therapeutic design. PLoS Pathog, 13(2), e1006212 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Gils MJ, van den Kerkhof TL, Ozorowski G et al. An HIV-1 antibody from an elite neutralizer implicates the fusion peptide as a site of vulnerability. Nat Microbiol, 2, 16199 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kong R, Xu K, Zhou T et al. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science, 352(6287), 828–833 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wibmer CK, Gorman J, Ozorowski G et al. Structure and Recognition of a Novel HIV-1 gp120-gp41 Interface Antibody that Caused MPER Exposure through Viral Escape. PLoS Pathog, 13(1), e1006074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. **.Huang Y, Yu J, Lanzi A et al. Engineered Bispecific Antibodies with Exquisite HIV-1- Neutralizing Activity. Cell, 165(7), 1621–1631 (2016). The CrossMAb technology employed in this study shows the potential for exploiting bNAb characteristics by targeted engineering for next generation therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosenberg Y, Sack M, Montefiori D et al. Rapid high-level production of functional HIV broadly neutralizing monoclonal antibodies in transient plant expression systems. PLoS One, 8(3), e58724 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosenberg Y, Sack M, Montefiori D et al. Pharmacokinetics and immunogenicity of broadly neutralizing HIV monoclonal antibodies in macaques. PLoS One, 10(3), e0120451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenberg YJ, Montefiori DC, LaBranche CC et al. Protection against SHIV Challenge by Subcutaneous Administration of the Plant-Derived PGT121 Broadly Neutralizing Antibody in Macaques. PLoS One, 11(3), e0152760 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asokan M, Rudicell RS, Louder M et al. Bispecific Antibodies Targeting Different Epitopes on the HIV-1 Envelope Exhibit Broad and Potent Neutralization. Journal of virology, 89(24), 12501–12512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galimidi RP, Klein JS, Politzer MS et al. Intra-spike crosslinking overcomes antibody evasion by HIV-1. Cell, 160(3), 433–446 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gardner MR, Farzan M. Engineering antibody-like inhibitors to prevent and treat HIV-1 infection. Curr Opin HIV AIDS, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. **.Xu L, Pegu A, Rao E et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science, 358(6359), 85–90 (2017). This study is a preview of the future of preventatives against the risk of HIV-1 exposure. Innovations such as this tri-specific Ab are likely to lead the way for the future of protection by infusion of Ab-based therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bournazos S, Gazumyan A, Seaman MS, Nussenzweig MC, Ravetch JV. Bispecific Anti- HIV-1 Antibodies with Enhanced Breadth and Potency. Cell, 165(7), 1609–1620 (2016) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. *.Gardner MR, Kattenhorn LM, Kondur HR et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature, 519(7541), 87–91 (2015). One of the first demonstrations of the power of bispecific antibody-like therapeutics as gene therapy that describes elegant engineering [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ding S, Verly MM, Princiotto A et al. Small Molecule CD4-Mimetics Sensitize HIV-1- infected Cells to ADCC by Antibodies Elicited by Multiple Envelope Glycoprotein Immunogens in Non-Human Primates. AIDS Res Hum Retroviruses, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Richard J, Pacheco B, Gohain N et al. Co-receptor Binding Site Antibodies Enable CD4-Mimetics to Expose Conserved Anti-cluster A ADCC Epitopes on HIV-1 Envelope Glycoproteins. EBioMedicine, 12, 208–218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richard J, Veillette M, Ding S et al. Small CD4 Mimetics Prevent HIV-1 Uninfected Bystander CD4 + T Cell Killing Mediated by Antibody-dependent Cell-mediated Cytotoxicity. EBioMedicine, 3, 122–134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lorin V, Malbec M, Eden C et al. Broadly neutralizing antibodies suppress post- transcytosis HIV-1 infectivity. Mucosal Immunol, (2016). [DOI] [PubMed] [Google Scholar]
- 96. **.Li H, Zony C, Chen P, Chen BK. Reduced potency and incomplete neutralization of broadly neutralizing antibodies against cell-to-cell transmission of HIV-1 with transmitted founder Envs. Journal of virology, (2017). Extremely important report that should remind the field of the need to address the impact of cell-to-cell transmission of HIV by showing vulnerability of bNAbs against viral synapse transfer of virus between the host and target cell by measuring reduced neutralization potency and incomplete neutralization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kieffer C, Ladinsky MS, Ninh A, Galimidi RP, Bjorkman PJ. Longitudinal imaging of HIV-1 spread in humanized mice with parallel 3D immunofluorescence and electron tomography. Elife, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. *.Santangelo PJ, Rogers KA, Zurla C et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods, 12(5), 427–432 (2015).Breakthrough demonstration of PET applied to SIV in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewis GK, Pazgier M, Evans D et al. Beyond Viral Neutralization. AIDS Res Hum Retroviruses, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prevost J, Zoubchenok D, Richard J et al. Influence of the Envelope Gp120 Phe 43 Cavity on HIV-1 Sensitivity to ADCC Responses. Journal of virology, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Musich T, Li L, Liu L, Zolla-Pazner S, Robert-Guroff M, Gorny MK. Monoclonal Antibodies to V2, V3, the CD4-binding site and gp41 HIV-1 Mediate Phagocytosis in a Dose-dependent Manner. Journal of virology, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.von Bredow B, Arias JF, Heyer LN et al. Comparison of Antibody-Dependent Cell- Mediated Cytotoxicity and Virus Neutralization by HIV-1 Env-specific Monoclonal Antibodies. Journal of virology, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Costa MR, Pollara J, Edwards RW et al. Fc Receptor-Mediated Activities of Env- Specific Human Monoclonal Antibodies Generated from Volunteers Receiving the DNA Prime-Protein Boost HIV Vaccine DP6–001. Journal of virology, 90(22), 1036210378 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gunn BM, Alter G. Modulating Antibody Functionality in Infectious Disease and Vaccination. Trends Mol Med, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pollara J, Bonsignori M, Moody MA et al. HIV-1 vaccine-induced C1 and V2 Env- specific antibodies synergize for increased antiviral activities. Journal of virology, 88(14), 7715–7726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yates NL, Liao HX, Fong Y et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med, 6(228), 228ra–239. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruel T, Guivel-Benhassine F, Amraoui S et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. NatCommun, 7, 10844 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang Y, Ferrari G, Alter G et al. Diversity of Antiviral IgG Effector Activities Observed in HIV-Infected and Vaccinated Subjects. J Immunol, 197(12), 4603–4612 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ko SY, Pegu A, Rudicell RS et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature, 514(7524), 642–645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santra S, Tomaras GD, Warrier R et al. Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques. PLoS Pathog, 11(8), e1005042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. **.Chan YN, Boesch AW, Osei-Owusu NY et al. IgG Binding Characteristics of Rhesus Macaque FcgammaR.JImmunol, (2016). This study is highly relevant to the translatability of the macaque model of HIV infection and any potential antibody-based therapeutic regimens. The authors provide urgently needed comparisons of IgG binding characteristics of both human and macaque Fcg receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boesch AW, Osei-Owusu NY, Crowley AR et al. Biophysical and Functional Characterization of Rhesus Macaque IgG Subclasses. Front Immunol, 7, 589 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. *.Hessell AJ, Malherbe DC, Pissani F et al. Achieving Potent Autologous Neutralizing Antibody Responses against Tier 2 HIV-1 Viruses by Strategic Selection of Envelope Immunogens. J Immunol, (2016). Natural Env immunogens isolated from timepoints contemporaneous with or preceding the development of neutralization breadth elicit autologous Tier 2 Nabs and Env-specific Tfh responses in vaccinated NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bradley T, Fera D, Bhiman J et al. Structural Constraints of Vaccine-Induced Tier-2 Autologous HIV Neutralizing Antibodies Targeting the Receptor-Binding Site. Cell Rep, 14(1), 43–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. **.Pauthner M, Havenar-Daughton C, Sok D et al. Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity, 46(6), 1073–1088 e1076 (2017). Evidence for strong vaccine responses effective against ‘self virus’ and modest breadth in NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. *.Bonsignori M, Liao HX, Gao F et al. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol Rev, 275(1), 145–160 (2017). Ontogeny of rare V3-glycan bNAb is due to cooperation of two autologous neutralizing antibody lineages that selected for virus escape mutations and drove initiation and affinity maturation of specific lineage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. **.Bhiman JN, Anthony C, Doria-Rose NA et al. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat Med, 21(11), 1332–1336 (2015). This study is a new look at antibody lineage by starting with a known bNmAb and working back to identify the elements responsible during development that drove the pathway and those viral events that resulted in ‘dead-end’ antibody lineage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kwong PD, Chuang GY, DeKosky BJ et al. Antibodyomics: bioinformatics technologies for understanding B-cell immunity to HIV-1. Immunol Rev, 275(1), 108128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gonzalez-Figueroa P, Roco JA, Vinuesa CG. Germinal Center Lymphocyte Ratios and Successful HIV Vaccines. Trends Mol Med, (2017). [DOI] [PubMed] [Google Scholar]
- 120. *.Havenar-Daughton C, Carnathan DG, Torrents de la Pena A et al. Direct Probing of Germinal Center Responses Reveals Immunological Features and Bottlenecks for Neutralizing Antibody Responses to HIV Env Trimer. Cell Rep, 17(9), 2195–2209 (2016). Technical progress in longitudinally sampling germinal centers in lymph nodes by fine needle aspirates. Tier 2 autologous NAbs generated by Env protein vaccines correlated with germinal center B cell magnitude and Tfh characteristics of the Env-specific CD4 T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. *.Escolano A, Steichen JM, Dosenovic P et al. Sequential Immunization Elicits Broadly Neutralizing Anti-HIV-1 Antibodies in Ig Knockin Mice. Cell, 166(6), 1445–1458 e1412 (2016). Proof of concept study performed in knock-in mice using an ELISA-guided Env- based vaccine strategy to target the PGT121 BCR and sequential boosting immunogens to elicit SHM and Tier 2 bNAbs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. *.Briney B, Sok D, Jardine JG et al. Tailored Immunogens Direct Affinity Maturation toward HIV Neutralizing Antibodies. Cell, 166(6), 1459–1470 e1411 (2016). Design of Env-based boosting immunogens and sequential vaccine strategy in knock-in mice to guide/drive antibody maturation of VRC01-class HIV bNAb responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. *.Steichen JM, Kulp DW, Tokatlian T et al. HIV Vaccine Design to Target Germline Precursors of Glycan-Dependent Broadly Neutralizing Antibodies. Immunity, 45(3), 483–496 (2016). Vaccine strategy using stabilized native-like Env-based trimer immunogens for germline BCR priming and lineage boosting to elicit glycan-dependent PGT121- class bNAb responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. **.Bonsignori M, Zhou T, Sheng Z et al. Maturation Pathway from Germline to Broad HIV-1 Neutralizer of a CD4-Mimic Antibody. Cell, 165(2), 449–463 (2016). This study is an important guidebook for tracing the ontogeny of CD4bs bNAbs. By working through a longitudinal phylogeny of Envs from the time of infection, this group identified critical structural and mechanistic contributions to bNAb evolution [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saunders KO, Nicely NI, Wiehe K et al. Vaccine Elicitation of High Mannose- Dependent Neutralizing Antibodies against the V3-Glycan Broadly Neutralizing Epitope in Nonhuman Primates. Cell Rep, 18(9), 2175–2188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Julien JP, Cupo A, Sok D et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science, 342(6165), 1477–1483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lyumkis D, Julien JP, de Val N et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science, 342(6165), 1484–1490 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pancera M, Zhou T, Druz A et al. Structure and immune recognition of trimeric prefusion HIV-1 Env. Nature, 514(7523), 455–461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sanders RW, Moore JP. Native-like Env trimers as a platform for HIV-1 vaccine design. Immunol Rev, 275(1), 161–182 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ingale J, Stano A, Guenaga J et al. High-Density Array of Well-Ordered HIV-1 Spikes on Synthetic Liposomal Nanoparticles Efficiently Activate B Cells. Cell Rep, 15(9), 1986–1999 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Feng Y, Tran K, Bale S et al. Thermostability of Well-Ordered HIV Spikes Correlates with the Elicitation of Autologous Tier 2 Neutralizing Antibodies. PLoS Pathog, 12(8), e1005767 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dai K, Khan SN, Wang Y et al. HIV-1 Vaccine-elicited Antibodies Reverted to Their Inferred Naive Germline Reveal Associations between Binding Affinity and in vivo Activation. Sci Rep, 6, 20987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Phad GE, Vazquez Bernat N, Feng Y et al. Diverse antibody genetic and recognition properties revealed following HIV-1 envelope glycoprotein immunization. J Immunol, 194(12), 5903–5914 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karlsson Hedestam GB, Guenaga J, Corcoran M, Wyatt RT. Evolution of B cell analysis and Env trimer redesign. Immunol Rev, 275(1), 183–202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Taeye SW, Ozorowski G, Torrents de la Pena A et al. Immunogenicity of Stabilized HIV-1 Envelope Trimers with Reduced Exposure of Non-neutralizing Epitopes. Cell, 163(7), 1702–1715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Torrents de la Pena A, Julien JP, de Taeye SW et al. Improving the Immunogenicity of Native-like HIV-1 Envelope Trimers by Hyperstabilization. Cell Rep, 20(8), 18051817 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McCoy LE, van Gils MJ, Ozorowski G et al. Holes in the Glycan Shield of the Native HIV Envelope Are a Target of Trimer-Elicited Neutralizing Antibodies. Cell Rep, 16(9), 2327–2338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Medina-Ramirez M, Garces F, Escolano A et al. Design and crystal structure of a native-like HIV-1 envelope trimer that engages multiple broadly neutralizing antibody precursors in vivo. J Exp Med, 214(9), 2573–2590 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sok D, Briney B, Jardine JG et al. Priming HIV-1 broadly neutralizing antibody precursors in human Ig loci transgenic mice. Science, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schiffner T, de Val N, Russell RA et al. Chemical Cross-Linking Stabilizes Native-Like HIV-1 Envelope Glycoprotein Trimer Antigens. Journal of virology, 90(2), 813–828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Krebs SJ, McBurney SP, Kovarik DN et al. Multimeric Scaffolds Displaying the HIV-1 Envelope MPER Induce MPER-Specific Antibodies and Cross-Neutralizing Antibodies when Co-Immunized with gp160 DNA. PLoS One, 9(12), e113463 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zolla-Pazner S, Powell R, Yahyaei S et al. Rationally-Designed Vaccines Targeting the V2 Region of HIV-1 gp120 Induce a Focused, Cross Clade-Reactive, Biologically Functional Antibody Response. Journal of virology, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. *.Francica JR, Sheng Z, Zhang Z et al. Analysis of immunoglobulin transcripts and hypermutation following SHIV(AD8) infection and protein-plus-adjuvant immunization. Nat Commun, 6, 6565 (2015). Adjuvant comparison in NHPs. Adjuvants do not alter immunogen-elicited antibody SHM levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. **.Vaccari M, Gordon SN, Fourati S et al. Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat Med, 22(7), 762–770 (2016). The surprising results of this study showing qualitative differences in adjuvant- related innate and adaptive immune responses provides important insights into differences in vaccine efficacy among various immunization regimens [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garcia-Arriaza J, Perdiguero B, Heeney JL et al. HIV/AIDS vaccine candidates based on replication-competent recombinant poxvirus NYVAC-C-KC expressing trimeric gp140 and Gag-derived VLPs or lacking the viral molecule B19 that inhibits type I interferon activate relevant HIV-1-specific B and T cell immune functions in nonhuman primates. Journal of virology, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malherbe DC, Mendy J, Vang L et al. Combination Adenovirus and Protein Vaccines Prevent Infection or Reduce Viral Burden after Heterologous Clade C SHIV Mucosal Challenge. Journal of virology, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dowd KA, Ko SY, Morabito KM et al. Rapid development of a DNA vaccine for Zika virus. Science, 354(6309), 237–240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tebas P, Roberts CC, Muthumani K et al. Safety and Immunogenicity of an Anti-Zika Virus DNA Vaccine - Preliminary Report. N Engl J Med, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kasturi SP, Kozlowski PA, Nakaya HI et al. Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5alpha Restrictive Macaques. Journal of virology, 91(4) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chung AW, Kumar MP, Arnold KB et al. Dissecting Polyclonal Vaccine-Induced Humoral Immunity against HIV Using Systems Serology. Cell, 163(4), 988–998 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. *.Ackerman ME, Barouch DH, Alter G. Systems serology for evaluation of HIV vaccine trials. Immunol Rev, 275(1), 262–270 (2017). Excellent review that provides insight into all types of functional antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hessell AJ, Hangartner L, Hunter M et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature, 449(7158), 101–104 (2007). [DOI] [PubMed] [Google Scholar]
- 153.Shingai M, Donau OK, Plishka RJ et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med, 211(10), 2061–2074 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moldt B, Le K, Carnathan DG et al. Neutralizing antibody affords comparable protection against vaginal and rectal SHIV challenge in macaques. AIDS, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hessell AJ, Rakasz EG, Poignard P et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoSPathog, 5(5), e1000433 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hessell AJ, Rakasz EG, Tehrani DM et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian- human immunodeficiency virus SHIVBa-L. Journal of virology, 84(3), 1302–1313 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol, 16(6), 571–576 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]