Abstract
Background
In vitro maturation (IVM) of oocytes has been widely used in the field of assisted reproductive technology. However, oocytes can be injured by oxidative stress during the process of IVM.
Methods
The present study was designed to evaluate the influences of rosmarinic acid (RA) on the IVM of porcine oocytes and the subsequent development of early-stage embryos as well as its underlying mechanisms. Various concentrations of RA (5 µM, 10 µM, and 25 µM) were treated with porcine oocyte maturation medium during the period of IVM.
Results and Discussion
The results showed that 5 µM RA treatment during the period of porcine oocyte IVM improves blastocyst quality and hatching ability after parthenogenetic activation. Furthermore, the presence of RA during the period of IVM dramatically improved the total number of cells after somatic cell nuclear transfer compared to the number of cells in the control group. Notably, RA treatment during the period of porcine oocyte IVM decreased intracellular reactive oxygen species generation not only in oocytes but also in cumulus cells. Further analysis showed that the intracellular free thiols levels in the oocytes were enhanced by treatment with RA during the period of porcine oocyte IVM compared to the free thiols levels in the control groups. These results indicate that RA improves the developmental competence of porcine oocytes during the IVM period by attenuating oxidative stress.
Keywords: Rosmarinic acid, Porcine oocyte, In vitro maturation, Oxidative stress
Introduction
In vitro maturation (IVM) of oocytes refers to the process in which immature oocytes obtained from ovaries are cultured under the appropriate conditions in vitro so that they undergo a series of complex physiological and biochemical transformations, ultimately developing into mature oocytes that are able to be fertilized (Lonergan & Fair, 2016; Schultz & Wassarman, 1977). IVM of oocytes is a pivotal step in assisted reproductive technology (ART) and has broad applications in helping infertility patients and improving the breeding and reproductive efficiency of livestock (Chian et al., 2009; Gil et al., 2010; Hoelker et al., 2017; Jin et al., 2014). However, the quality and developmental competence of in vitro matured oocytes remains low compared with that of in vivo matured oocytes (Rizos et al., 2002; Sutton, Gilchrist & Thompson, 2003). The main reason may be that the unstable extracellular environments during IVM, resulting in the maturation of the nucleus and cytoplasm, are not synchronized (Funahashi & Day, 1993; Zhao et al., 2014). The oocytes continuously produce reactive oxygen species (ROS) during growth and development, and the IVM process is likely to produce additional ROS due to mechanical handling, air, light and other factors (Cetica et al., 2001; Kitagawa et al., 2004; Morado et al., 2013; Waiz et al., 2016). Although ROS are a normal product of cell metabolism, excessive ROS generation will cause DNA damage, mitochondrial dysfunction, lipid peroxidation and protein oxidation modification, thus deteriorating oocytes and blocking their subsequent developmental potential (Rajani et al., 2012; Tamura et al., 2008). Antioxidants can bind to oxygen free radicals, thus inhibiting the initiation of free radical chain propagation, eliminating the damage caused by ROS (Agarwal & Majzoub, 2017; Kitagawa et al., 2004). Under oocyte in vitro culture conditions, various types of antioxidants have been added as supplements either alone or in different combinations to attenuate oxidative stress, improving oocyte quality (Liang et al., 2018; Wu et al., 2011; Xu et al., 2018; Yu et al., 2018). Pigs are very important livestock animals for biomedical research and agricultural production. However, porcine oocytes are more sensitive to oxidative stress than those of other livestock animals such as cattle and sheep (Somfai et al., 2011). Therefore, reducing harmful ROS is an effective means of improving the quality of in vitro matured porcine oocytes and increasing their developmental potential.
Rosmarinic acid (RA) is a water-soluble polyphenol and a naturally occurring hydroxylated compound commonly found in various medicinal herbs of the family Lamiaceae (Pereira et al., 2005; Zhang et al., 2014). A growing body of evidence shows multiple beneficial effects of RA on different animal cells, including antioxidant, antimutagenic, anti-inflammatory and radical-scavenging properties (Huang & Zheng, 2006; Kantar Gok et al., 2018; Lee et al., 2008; Makino et al., 2002; McKay & Blumberg, 2006). Previous studies have identified RA as one of the most potential antioxidants in the hydroxycinnamic acid group of polyphenols (Ghaffari et al., 2014; Soobrattee et al., 2005). It has been indicated that supplementation with RA not only increases glutathione levels but also activates antioxidant enzyme activity in in vitro cell studies (Chkhikvishvili et al., 2013; Fallarini et al., 2009; Kantar Gok et al., 2018). Recent research has shown that RA can improve boar sperm quality during cryopreservation by protecting against oxidative stress (Luno et al., 2014).
Although the beneficial biological functions of RA are well documented, the effect of RA during oocyte maturation has not been investigated to date. Here, we hypothesized that RA treatment of porcine oocytes during the IVM period would improve the quality of the oocytes and improve their subsequent in vitro developmental potential. In the present study, we first investigated the effect of RA on porcine oocyte nuclear maturation and subsequent embryonic development competence after parthenogenetic activation (PA) and somatic cell nuclear transfer (SCNT). Subsequently, the mechanism underlying the effect of RA on porcine oocyte maturation was determined empirically.
Materials and Methods
All chemicals used in this study were purchased from Sigma-Aldrich (St Louis, MO, USA) unless noted otherwise.
Collection and IVM of porcine oocytes
Ovaries were obtained from slaughtered pre-pubertal gilts at a local slaughterhouse and transported to the laboratory in sterile saline in a vacuum flask. Cumulus-oocyte complexes (COCs) were aspirated from 3–6 mm antral follicles, and those showing a multilayer of compact cumulus cells and homogeneous ooplasm were specifically collected using Tyrode’s lactate–hydroxyethylpiperazine ethane sulfonic acid (HEPES) medium with 0.1% polyvinyl alcohol (PVA, w/v) and 0.05 g/L gentamycin under a stereomicroscope. The COCs were cultured in tissue culture medium 199 (TCM-199, Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) porcine follicular fluid, 0.91 mM Na pyruvate, 1% penicillin G-streptomycin sulfate, 0.5 µg/mL follicle stimulating hormone, and 0.5 µg/mL luteinizing hormone. The maturation medium was covered with mineral oil and incubated at 38.5 °C in an atmosphere containing 5% CO2 at 100% humidity for 42 h. The RA stock solution was diluted with TCM-199 (2.5 mM) and stored in the dark at −20 °C.
PA, SCNT and in vitro culture
PA and SCNT were performed according to our previously described procedures (Liang et al., 2018; Liang et al., 2017b). Porcine ear fibroblasts were isolated and used as nuclear donors. After PA and SCNT, the activated oocytes or reconstructed embryos were cultured in bicarbonate-buffered PZM-5 containing 4 mg/mL BSA and 7.5 µg/mL cytochalasin B for 3 h to suppress extrusion of the pseudo-second polar body. Next, the activated oocytes or reconstructed embryos were thoroughly washed and cultured in bicarbonate-buffered PZM-5 supplemented with 4 mg/mL BSA for 7 days at 38.5 °C in an atmosphere containing 5% CO2 at 100% humidity. The cleavage rate was examined on day 2 after activation. Blastocyst diameter, formation and hatching rates were examined on day 7 after activation. The total number of cells in blastocysts was determined by staining the cells with 10 µg/mL Hoechst 33342 for 15 min and counting the cells that exhibited blue fluorescence under a fluorescence microscope (IX70, Olympus, Tokyo, Japan).
Measurement of intracellular ROS and free thiols levels in oocytes
Oocyte intracellular ROS and free thiols levels were measured by incubating the cells with 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (Invitrogen, NY, USA) and 10 µM 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (Invitrogen) for 15 min and 30 min, respectively. The fluorescence signal was captured as TIFF files using a digital camera (DP72; Olympus) connected to a fluorescence microscope (IX70, Olympus). NIH ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to analyse the fluorescence intensities of the oocytes.
Measurement of intracellular ROS levels in cumulus cells via flow cytometry
At the end of IVM, cumulus cells were removed from the oocyte by treatment with 1 mg/mL hyaluronidase and collected in a 1.5-ml centrifugal tube. At least 50 COCs were collected from each group. The intracellular ROS content of the cumulus cells was measured with 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (Invitrogen, NY, USA) and analysed by flow cytometry.
Statistical analyses
Statistical analyses were performed using analysis of variance (ANOVA), and data obtained from two groups were compared using the Student’s t-test embedded in GraphPad Prism version 6.01(GraphPad Software, La Jolla, CA, USA). The number of independent replicates (Re) of each experiment is shown in the figure legends. The data were expressed as the mean ± standard error of the mean (SEM). A p value of <0.05 was considered significant.
Results
Effects of RA treatment during IVM on porcine oocyte maturation and subsequent in vitro embryo development after PA
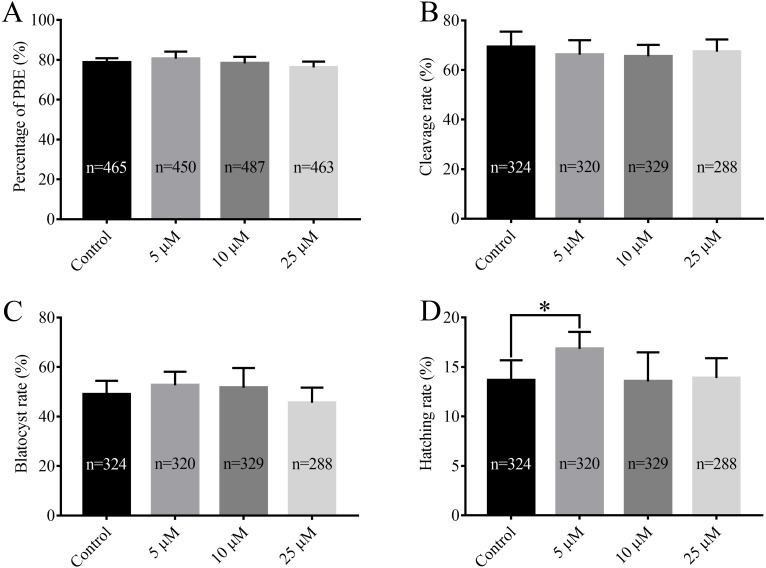
After being collected, immature porcine oocytes were cultured with various concentrations of RA. The polar body extrusion (PBE) rates were examined after 42 h of IVM. Figure 1A shows that PBE rates were not affected by treatment with 5 µM, 10 µM, or 25 µM RA (78.69 ± 2.13%, 80.54 ± 3.61%, 78.22 ± 3.24%, and 76.18 ± 2.95%, respectively). After PA, the ability of embryos to develop in vitro was analysed. As shown in Figs. 1B and 1C, we did not observe significant differences in the cleavage rate (69.34 ± 6.20%, 66.20 ± 5.86%, 66.53 ± 4.61%, and 67.40 ± 5.00%, respectively) or blastocyst formation rate (48.94 ± 5.23%, 52.64 ± 5.46%, 51.67 ± 7.95%, and 45.61 ± 6.09%, respectively) between the control and RA-treated groups during the IVM period. We further investigated the ability of the blastocysts to hatch; the results are shown in Fig. 1D. The results revealed that treatment with 5 µM RA during the IVM period significantly increased the rate of blastocyst hatching compared to that of the control group (16.83 ± 1.72% vs. 13.67 ± 2.01%; p < 0.05). Further analysis showed that treatment with 5 µM RA during the IVM period sharply increased the diameter of blastocysts (205.4 ± 4.39 vs. 180.4 ± 4.29; p < 0.05; Figs. 2A and 2B) and the total number of cells in blastocysts (43.04 ± 1.34 vs. 38.77 ± 1.37; p < 0.05; Figs. 2C–2I).
Figure 1. Effects of RA at various concentrations during IVM on porcine oocyte PBE and subsequent in vitro development of parthenogenetic embryos.
(A) PBE rate. Re = 8. (B) Cleavage rate, (C) blastocyst rate and (D) hatching rate after parthenogenetic activation. Re = 7. *p < 0.05.
Figure 2. Effects of RA treatment during IVM on the of quality blastocysts.
(A) and (B) Average diameter of blastocysts on day 6 in the control and RA treatment groups. Scale bar = 500 µm; Re = 4. (C-H) and (I) Average total cell count of blastocysts on day 7 in the control and RA treatment groups. Scale bar = 100 µm; Re = 3. *p < 0.05.
Effects of RA treatment during IVM on in vitro development of SCNT embryos
We next assayed whether RA treatment during the IVM period improved the developmental competence of porcine SCNT embryos. Consistent with the above results, RA treatment during the IVM period did not significantly increase the cleavage rate of SCNT embryos (72.30 ± 2.59% vs. 68.38 ± 2.22%; Fig. 3A). We further examined the blastocyst formation rate of SCNT embryos on days 6 and 7. Although the blastocyst formation rate was increased on day 6 (22.35 ± 1.02% vs. 20.74 ± 1.11%) or day 7 (24.20 ± 1.03% vs. 22.16 ± 0.73%), the results showed that there was no significant difference between the RA treatment and control groups (Fig. 3B). Compared with the control group, the treated group showed obvious effects of RA on SCNT quality: RA treatment during the IVM period remarkably increased the total number of cells in blastocysts (40.33 ± 1.00 vs. 38.26 ± 0.66; p <0.05; Figs. 3C and 3D).
Figure 3. Effects of RA treatment during IVM on the developmental competence of porcine SCNT embryos.
(A) Cleavage rate of SCNT embryos in the control and RA treatment groups. (B) Blastocyst formation rate in the control and RA treatment groups on days 6 and 7. (C) and (D) Average total cell count of blastocysts on day 7 in the control and RA treatment groups. Re = 3. *p < 0.05.
Effects of RA treatment during the IVM period on intracellular ROS levels in porcine oocytes and cumulus cells
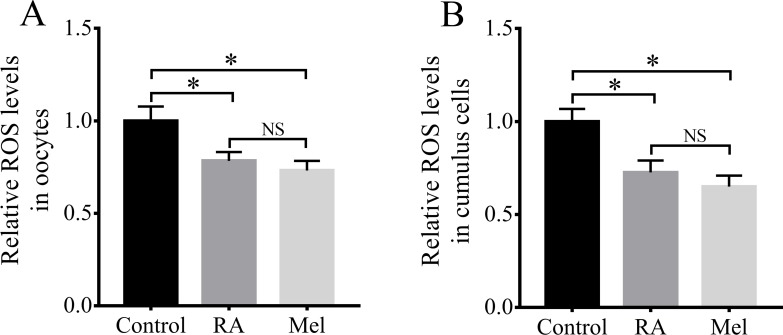
Because RA has antioxidant properties, we examined whether RA treatment during the IVM period would improve the resistance of porcine oocytes to oxidative stress. The results revealed that there were significantly lower intracellular ROS levels in the oocytes (Fig. 4A) and cumulus cells (Fig. 4B) of the RA treatment group than in those of the control group that was not treated with RA during the IVM period. When compared with the control group, the levels of ROS in the oocytes were much lower in the melatonin (Mel, 1 µM) and RA treatment group, whereas it showed no significant changes between RA and Mel treatment groups. In addition, to further determine the effect of RA against oxidative stress, the oocytes were pre-incubated with RA for 3 h. After that, H2O2 (50 µM) was added for 30 min. As shown in Fig. S1, the H2O2 and RA + H2O2 treatment group showed a higher intracellular ROS levels compared to the control group. However, the levels of intracellular ROS levels was no significant changes in the oocytes between RA and RA + H2O2 treatment group.
Figure 4. RA treatment during IVM attenuated intracellular ROS levels in porcine oocytes and cumulus cells.
Relative intracellular ROS levels in oocytes (A; Re = 3) and cumulus cells (B; Re = 4). Mel: Melatonin. *p < 0.05.
Effects of RA treatment during the IVM period on intracellular free thiols levels in porcine oocytes
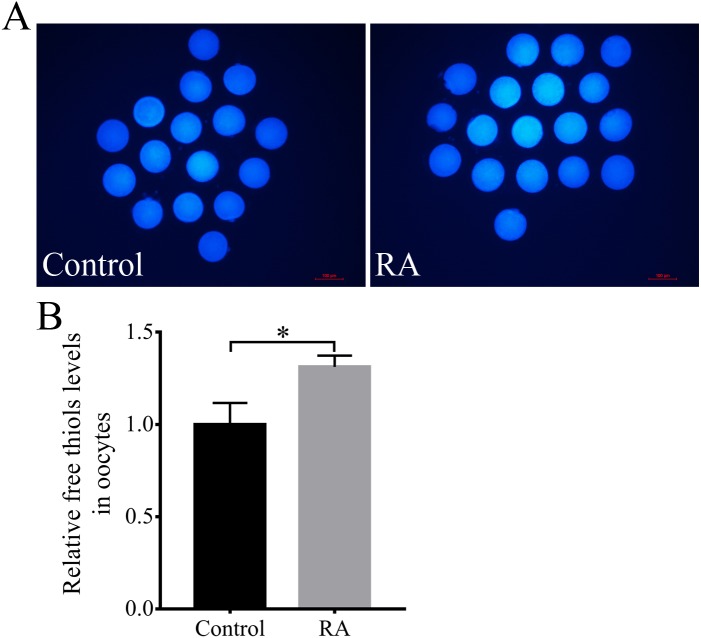
Next, the intracellular free thiols levels in the porcine oocytes were evaluated at the end of the IVM period. As shown in Figs. 5A and 5B, the intracellular free thiols levels were obviously increased in the RA treatment group compared to the control group without RA treatment during the IVM period.
Figure 5. RA treatment during IVM improves intracellular free thiols levels in porcine oocytes.
(A) Representative fluorescent images of intracellular free thiols levels in oocytes from the control and RA treatment groups. Scale bar = 100 µm. (B) Relative intracellular free thiols levels in oocytes. Re = 3. *p < 0.05.
Discussion
IVM of oocytes is closely associated with the enhancement of oxidative stress production, which induces various impairments in oocytes and causes developmental competence to decline (Liang et al., 2017a; Tamura et al., 2008). In the present study, the effects of RA supplementation on IVM of porcine oocytes were evaluated. The results of the current study indicate that RA supplementation during IVM of porcine oocytes has beneficial effects on the quality of the oocytes, leading to improved embryo developmental competence after PA and SCNT.
IVM of oocytes consists of artificially simulating the in vivo environment in which the immature oocytes grow and develop into mature ones and discharge the first polar body (Holzer et al., 2007; Sun & Nagai, 2003). Oocyte IVM is a highly significant stage of in vitro embryo production. When the quality of an in vitro matured oocyte is improved, its developmental competence is also greatly influenced. In the current study, we found that 5 µM RA treatment during the process of IVM markedly improved the quality of porcine PA and SCNT embryos, as indicated by increased blastocyst hatching and total cell counts. Unexpectedly, however, RA treatment during the process of IVM did not affect PBE by porcine oocytes. Further analyses revealed that RA treatment during the process of IVM also did not affect the cleavage rate or the blastocyst formation rate after PA or SCNT. These results partly support our hypothesis, which is that RA treatment during the process of IVM improves the in vitro developmental potential of porcine oocytes. In the future, more data will be obtained from in vitro fertilization and embryo transfer to detect the developmental potential of RA treated oocytes.
Compared to in vivo matured oocytes, in vitro matured oocytes are faced with increased oxidative pressure and are susceptible to oxidative stress (Combelles, Gupta & Agarwal, 2009). Under normal physiological conditions, the ROS and antioxidants in the body are in a state of dynamic balance (Valko et al., 2007). If there are more ROS than antioxidants, oxidative stress will result. During the process of IVM, COCs are at an elevated risk of being stimulated by exogenous factors that break the dynamic balance of redox homeostasis in oocytes (Han et al., 2006; Hu et al., 2001). This imbalance of homeostasis severely restricts the ability of oocytes to mature in vitro and affects their subsequent embryo developmental competency (Ali, Bilodeau & Sirard, 2003; Prasad et al., 2016). Porcine oocytes contain a large amount of cytoplasmic lipids, which are particularly sensitive to ROS (Dunning, Russell & Robker, 2014; McKeegan & Sturmey, 2011). Thus, antioxidant supplementation could control cascades of uncontrolled oxidation and protect oocytes from oxidative damage by scavenging ROS during IVM (Ali, Bilodeau & Sirard, 2003; Liang et al., 2018). RA, as powerful natural antioxidant, has powerful ROS scavenging activities and is an auxiliary factor in several important antioxidant enzyme systems (Rong, Liang & Niu, 2018). The antioxidant mechanism of RA is related to its chemical structure, in which the carboxylic acid group and the catechol structure in the aromatic ring work together to neutralize free radicals (Benavente-García et al., 1997; Luno et al., 2014). A previous study showed that RA could suppress H2O2-induced cytotoxicity in N2A cells by reducing intracellular ROS levels (Ghaffari et al., 2014). Moreover, RA also effectively attenuated amyloid- β-induced lipid hydroperoxide and ROS accumulation in PC12 cells (Iuvone et al., 2006). Luno et al. (2014) have shown that RA can effectively promote the function and fertilizing ability of sperm in cattle by preventing lipid peroxidation and DNA oxidation. A recent study has shown that RA supplementation improves sperm DNA integrity during a freeze-drying procedure (Olaciregui et al., 2017). Consistently, in the present study, RA treatment during the process of IVM effectively reduced intracellular ROS levels in porcine oocytes and cumulus cells at the end of IVM. This result is consistent with our hypothesis that RA can improve porcine oocyte quality by mitigating impairments caused by oxidative stress.
To further evaluate the underlying process and mechanism through which RA improves the quality and development potential of porcine oocytes, we examined intracellular free thiols levels. One of the markers of cytoplasmic maturation of oocytes at the end of IVM is intracellular free thiols levels (De Matos & Furnus, 2000; Funahashi et al., 1994). Several studies have shown that oocytes with higher intracellular ROS levels have lower intracellular free thiols levels, and their subsequent embryonic development potential is also insufficient (Liang et al., 2018; Liang, Nie & Zhao, 2017c; Wu et al., 2011). Previous studies have suggested that RA, which is one of the most effective antioxidants in the hydroxycinnamic acid group of polyphenols (Soobrattee et al., 2005), can protect Jurkat T cells and human neuroblastoma cells against oxidative stress and cause intracellular free thiols levels to increase (Chkhikvishvili et al., 2013; Fallarini et al., 2009). In the present study, RA treatment during the process of IVM promoted the accumulation of free thiols in the cytoplasm. This finding further supports our hypothesis that RA treatment during the process of IVM improves the quality and development potential of porcine oocytes after PA and SCNT.
Conclusion
The present study demonstrates that RA during the process of IVM protects porcine oocytes against oxidative stress and improves their subsequent in vitro developmental competence. Therefore, RA is a potential candidate antioxidant agent for oocytes during the process of IVM and will be helpful for improving the efficiency of in vitro embryo production in pigs.
Supplemental Information
Relative intracellular ROS levels in oocytes. Re = 3. *p < 0.05; **p < 0.01.
Raw data applied for data analyses and preparation for Figure 1A, 1B, 1C and 1D; Figure 2B and 2I; Figure 3A, 3B and 3D; Figure 4A and 4B; Figure 5B and Figure S1.
Funding Statement
This work was supported by the project of the Science and Technology, Education Department of Jilin Province (Grant No. JJKH20180690KJ), the Jilin Scientific and Technological Development Program (20190103149JH), and the National Natural Science Foundation of China (81774357). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Shuang Liang, Email: liangshuang85@jlu.edu.cn.
Suo Li, Email: wonderful8899@163.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yan Zhang conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper.
Jing Guo performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper.
Xiao Wei Nie analyzed the data, authored or reviewed drafts of the paper.
Zi Yue Li and Yu Meng Wang contributed reagents/materials/analysis tools.
Shuang Liang and Suo Li conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental File.
References
- Agarwal & Majzoub (2017).Agarwal A, Majzoub A. Role of antioxidants in assisted reproductive techniques. The World Journal of Men’s Health. 2017;35:77–93. doi: 10.5534/wjmh.2017.35.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, Bilodeau & Sirard (2003).Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology. 2003;59:939–949. doi: 10.1016/S0093-691X(02)01125-1. [DOI] [PubMed] [Google Scholar]
- Benavente-García et al. (1997).Benavente-García O, Castillo J, Marin FR, Ortuño A, Del Río JA. Uses and properties of citrus flavonoids. Journal of Agricultural and Food Chemistry. 1997;45:4505–4515. doi: 10.1021/jf970373s. [DOI] [PubMed] [Google Scholar]
- Cetica et al. (2001).Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001;51:57–64. doi: 10.1080/15216540119253. [DOI] [PubMed] [Google Scholar]
- Chian et al. (2009).Chian RC, Gilbert L, Huang JY, Demirtas E, Holzer H, Benjamin A, Buckett WM, Tulandi T, Tan SL. Live birth after vitrification of in vitro matured human oocytes. Fertility and Sterility. 2009;91:372–376. doi: 10.1016/j.fertnstert.2007.11.088. [DOI] [PubMed] [Google Scholar]
- Chkhikvishvili et al. (2013).Chkhikvishvili I, Sanikidze T, Gogia N, McHedlishvili T, Enukidze M, Machavariani M, Vinokur Y, Rodov V. Rosmarinic acid-rich extracts of summer savory (Satureja hortensis L.) protect Jurkat T cells against oxidative stress. Oxidative Medicine and Cellular Longevity. 2013;2013 doi: 10.1155/2013/456253. Article 456253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combelles, Gupta & Agarwal (2009).Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18:864–880. doi: 10.1016/S1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matos & Furnus (2000).De Matos DG, Furnus CC. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development effect of beta-mercaptoethanol, cysteine and cystine. Theriogenology. 2000;53:761–771. doi: 10.1016/s0093-691x(99)00278-2. [DOI] [PubMed] [Google Scholar]
- Dunning, Russell & Robker (2014).Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and beta-oxidation. Reproduction. 2014;148:R15–R27. doi: 10.1530/rep-13-0251. [DOI] [PubMed] [Google Scholar]
- Fallarini et al. (2009).Fallarini S, Miglio G, Paoletti T, Minassi A, Amoruso A, Bardelli C, Brunelleschi S, Lombardi G. Clovamide and rosmarinic acid induce neuroprotective effects in in vitro models of neuronal death. British Journal of Pharmacology. 2009;157:1072–1084. doi: 10.1111/j.1476-5381.2009.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi et al. (1994).Funahashi H, Cantley TC, Stumpf TT, Terlouw SL, Day BN. Use of low-salt culture medium for in vitro maturation of porcine oocytes is associated with elevated oocyte glutathione levels and enhanced male pronuclear formation after in vitro fertilization. Biology of Reproduction. 1994;51:633–639. doi: 10.1095/biolreprod51.4.633. [DOI] [PubMed] [Google Scholar]
- Funahashi & Day (1993).Funahashi H, Day BN. Effects of the duration of exposure to hormone supplements on cytoplasmic maturation of pig oocytes in vitro. Journal of Reproduction and Fertility. 1993;98:179–185. doi: 10.1530/jrf.0.0980179. [DOI] [PubMed] [Google Scholar]
- Ghaffari et al. (2014).Ghaffari H, Venkataramana M, Jalali Ghassam B, Chandra Nayaka S, Nataraju A, Geetha NP, Prakash HS. Rosmarinic acid mediated neuroprotective effects against H2O2-induced neuronal cell damage in N2A cells. Life Sciences. 2014;113:7–13. doi: 10.1016/j.lfs.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Gil et al. (2010).Gil MA, Cuello C, Parrilla I, Vazquez JM, Roca J, Martinez EA. Advances in swine in vitro embryo production technologies. Reproduction in Domestic Animals. 2010;45(Supp 2):40–48. doi: 10.1111/j.1439-0531.2010.01623.x. [DOI] [PubMed] [Google Scholar]
- Han et al. (2006).Han D, Lan GC, Wu YG, Han ZB, Wang HL, Tan JH. Factors affecting the efficiency and reversibility of roscovitine (ROS) block on the meiotic resumption of goat oocytes. Molecular Reproduction and Development. 2006;73:238–246. doi: 10.1002/mrd.20398. [DOI] [PubMed] [Google Scholar]
- Hoelker et al. (2017).Hoelker M, Kassens A, Salilew-Wondim D, Sieme H, Wrenzycki C, Tesfaye D, Neuhoff C, Schellander K, Held-Hoelker E. Birth of healthy calves after intra-follicular transfer (IFOT) of slaughterhouse derived immature bovine oocytes. Theriogenology. 2017;97:41–49. doi: 10.1016/j.theriogenology.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Holzer et al. (2007).Holzer H, Scharf E, Chian RC, Demirtas E, Buckett W, Tan SL. In vitro maturation of oocytes collected from unstimulated ovaries for oocyte donation. Fertility and Sterility. 2007;88:62–67. doi: 10.1016/j.fertnstert.2006.11.087. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2001).Hu Y, Betzendahl I, Cortvrindt R, Smitz J, Eichenlaub-Ritter U. Effects of low O2 and ageing on spindles and chromosomes in mouse oocytes from pre-antral follicle culture. Human Reproduction. 2001;16:737–748. doi: 10.1093/humrep/16.4.737. [DOI] [PubMed] [Google Scholar]
- Huang & Zheng (2006).Huang SS, Zheng RL. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Letters. 2006;239:271–280. doi: 10.1016/j.canlet.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Iuvone et al. (2006).Iuvone T, De Filippis D, Esposito G, D’Amico A, Izzo AA. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. Journal of Pharmacology and Experimental Therapeutics. 2006;317:1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- Jin et al. (2014).Jin YX, Jeon Y, Lee SH, Kwon MS, Kim T, Cui XS, Hyun SH, Kim NH. Production of pigs expressing a transgene under the control of a tetracycline-inducible system. PLOS ONE. 2014;9:e86146. doi: 10.1371/journal.pone.0086146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantar Gok et al. (2018).Kantar Gok D, Hidisoglu E, Ocak GA, Er H, Acun AD, Yargicoglu P. Protective role of rosmarinic acid on amyloid beta 42-induced echoic memory decline: implication of oxidative stress and cholinergic impairment. Neurochemistry International. 2018;118:1–13. doi: 10.1016/j.neuint.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Kitagawa et al. (2004).Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004;62:1186–1197. doi: 10.1016/j.theriogenology.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2008).Lee HJ, Cho HS, Park E, Kim S, Lee SY, Kim CS, Kim DK, Kim SJ, Chun HS. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology. 2008;250:109–115. doi: 10.1016/j.tox.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Liang et al. (2017a).Liang S, Guo J, Choi JW, Kim NH, Cui XS. Effect and possible mechanisms of melatonin treatment on the quality and developmental potential of aged bovine oocytes. Reproduction, Fertility, and Development. 2017a;29:1821–1831. doi: 10.1071/rd16223. [DOI] [PubMed] [Google Scholar]
- Liang et al. (2018).Liang S, Guo J, Jin YX, Yuan B, Zhang JB, Kim NH. C-Phycocyanin supplementation during in vitro maturation enhances pre-implantation developmental competence of parthenogenetic and cloned embryos in pigs. Theriogenology. 2018;106:69–78. doi: 10.1016/j.theriogenology.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Liang et al. (2017b).Liang S, Jin YX, Yuan B, Zhang JB, Kim NH. Melatonin enhances the developmental competence of porcine somatic cell nuclear transfer embryos by preventing DNA damage induced by oxidative stress. Scientific Reports. 2017b;7 doi: 10.1038/s41598-017-11161-9. Article 11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Nie & Zhao (2017c).Liang S, Nie ZW, Zhao M. Sodium fluoride exposure exerts toxic effects on porcine oocyte maturation. 2017c;7 doi: 10.1038/s41598-017-17357-3. Article 17082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan & Fair (2016).Lonergan P, Fair T. Maturation of oocytes in vitro. Annual Review of Animal Biosciences. 2016;4:255–268. doi: 10.1146/annurev-animal-022114-110822. [DOI] [PubMed] [Google Scholar]
- Luno et al. (2014).Luno V, Gil L, Olaciregui M, Gonzalez N, Jerez RA, De Blas I. Rosmarinic acid improves function and in vitro fertilising ability of boar sperm after cryopreservation. Cryobiology. 2014;69:157–162. doi: 10.1016/j.cryobiol.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Makino et al. (2002).Makino T, Ono T, Liu N, Nakamura T, Muso E, Honda G. Suppressive effects of rosmarinic acid on mesangioproliferative glomerulonephritis in rats. Nephron. 2002;92:898–904. doi: 10.1159/000065457. [DOI] [PubMed] [Google Scholar]
- McKay & Blumberg (2006).McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytotherapy Research. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- McKeegan & Sturmey (2011).McKeegan PJ, Sturmey RG. The role of fatty acids in oocyte and early embryo development. Reproduction, Fertility, and Development. 2011;24:59–67. doi: 10.1071/rd11907. [DOI] [PubMed] [Google Scholar]
- Morado et al. (2013).Morado S, Cetica P, Beconi M, Thompson JG, Dalvit G. Reactive oxygen species production and redox state in parthenogenetic and sperm-mediated bovine oocyte activation. Reproduction. 2013;145:471–478. doi: 10.1530/rep-13-0017. [DOI] [PubMed] [Google Scholar]
- Olaciregui et al. (2017).Olaciregui M, Luno V, Gonzalez N, Domingo P, De Blas I, Gil L. Chelating agents in combination with rosmarinic acid for boar sperm freeze-drying. Reproductive Biology. 2017;17:193–198. doi: 10.1016/j.repbio.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Pereira et al. (2005).Pereira P, Tysca D, Oliveira P, Da Silva Brum LF, Picada JN, Ardenghi P. Neurobehavioral and genotoxic aspects of rosmarinic acid. Pharmacological Research. 2005;52:199–203. doi: 10.1016/j.phrs.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Prasad et al. (2016).Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. Journal of Biomedical Science. 2016;23 doi: 10.1186/s12929-016-0253-4. Article 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajani et al. (2012).Rajani S, Chattopadhyay R, Goswami SK, Ghosh S, Sharma S, Chakravarty B. Assessment of oocyte quality in polycystic ovarian syndrome and endometriosis by spindle imaging and reactive oxygen species levels in follicular fluid and its relationship with IVF-ET outcome. Journal of Human Reproductive Sciences. 2012;5:187–193. doi: 10.4103/0974-1208.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizos et al. (2002).Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Molecular Reproduction and Development. 2002;61:234–248. doi: 10.1002/mrd.1153. [DOI] [PubMed] [Google Scholar]
- Rong, Liang & Niu (2018).Rong H, Liang Y, Niu Y. Rosmarinic acid attenuates beta-amyloid-induced oxidative stress via Akt/GSK-3beta/Fyn-mediated Nrf2 activation in PC12 cells. Free Radical Biology and Medicine. 2018;120:114–123. doi: 10.1016/j.freeradbiomed.2018.03.028. [DOI] [PubMed] [Google Scholar]
- Schultz & Wassarman (1977).Schultz RM, Wassarman PM. Biochemical studies of mammalian oogenesis: protein synthesis during oocyte growth and meiotic maturation in the mouse. Journal of Cell Science. 1977;24:167–194. doi: 10.1242/jcs.24.1.167. [DOI] [PubMed] [Google Scholar]
- Somfai et al. (2011).Somfai T, Kaneda M, Akagi S, Watanabe S, Haraguchi S, Mizutani E, Dang-Nguyen TQ, Geshi M, Kikuchi K, Nagai T. Enhancement of lipid metabolism with L-carnitine during in vitro maturation improves nuclear maturation and cleavage ability of follicular porcine oocytes. Reproduction, Fertility, and Development. 2011;23:912–920. doi: 10.1071/rd10339. [DOI] [PubMed] [Google Scholar]
- Soobrattee et al. (2005).Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutation Research/DNA Repair. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Sun & Nagai (2003).Sun QY, Nagai T. Molecular mechanisms underlying pig oocyte maturation and fertilization. Journal of Reproduction and Development. 2003;49:347–359. doi: 10.1262/jrd.49.347. [DOI] [PubMed] [Google Scholar]
- Sutton, Gilchrist & Thompson (2003).Sutton ML, Gilchrist RB, Thompson JG. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Human Reproduction Update. 2003;9:35–48. doi: 10.1093/humupd/dmg009. [DOI] [PubMed] [Google Scholar]
- Tamura et al. (2008).Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, Sugino N. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. Journal of Pineal Research. 2008;44:280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Valko et al. (2007).Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry and Cell Biology. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Waiz et al. (2016).Waiz SA, Raies-Ul-Haq M, Dhanda S, Kumar A, Goud TS, Chauhan MS, Upadhyay RC. Heat stress and antioxidant enzyme activity in bubaline (Bubalus bubalis) oocytes during in vitro maturation. International Journal of Biometeorology. 2016;60:1357–1366. doi: 10.1007/s00484-015-1129-0. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2011).Wu GQ, Jia BY, Li JJ, Fu XW, Zhou GB, Hou YP, Zhu SE. L-carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology. 2011;76:785–793. doi: 10.1016/j.theriogenology.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2018).Xu HY, Yang XG, Lu SS, Liang XW, Lu YQ, Zhang M, Lu KH. Treatment with acetyl-l-carnitine during in vitro maturation of buffalo oocytes improves oocyte quality and subsequent embryonic development. Theriogenology. 2018;118:80–89. doi: 10.1016/j.theriogenology.2018.05.033. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2018).Yu XX, Liu YH, Liu XM, Wang PC, Liu S, Miao JK, Du ZQ, Yang CX. Ascorbic acid induces global epigenetic reprogramming to promote meiotic maturation and developmental competence of porcine oocytes. Scientific Reports. 2018;8:6132. doi: 10.1038/s41598-018-24395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2014).Zhang S, Yan Y, Wang B, Liang Z, Liu Y, Liu F, Qi Z. Selective responses of enzymes in the two parallel pathways of rosmarinic acid biosynthetic pathway to elicitors in Salvia miltiorrhiza hairy root cultures. Journal of Bioscience and Bioengineering. 2014;117:645–651. doi: 10.1016/j.jbiosc.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2014).Zhao MH, Jin YX, Lee SK, Kim NH, Cui XS. Artificial control maturation of porcine oocyte by dibutyryl cyclicAMP. Animal Cells and Systems. 2014;18:52–58. doi: 10.1080/19768354.2014.880371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative intracellular ROS levels in oocytes. Re = 3. *p < 0.05; **p < 0.01.
Raw data applied for data analyses and preparation for Figure 1A, 1B, 1C and 1D; Figure 2B and 2I; Figure 3A, 3B and 3D; Figure 4A and 4B; Figure 5B and Figure S1.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental File.