Abstract
KRAS mutation is a well‐known marker for poor response to targeted treatment and patient prognosis in microsatellite stable (MSS) colorectal cancer (CRC). However, variation in clinical outcomes among patients wild‐type for KRAS underlines that this is not a homogeneous population. Here, we evaluated the prognostic impact of KRAS alternative splicing in relation to mutation status in a single‐hospital series of primary MSS CRCs (N = 258). Using splicing‐sensitive microarrays and RNA sequencing, the relative expression of KRAS‐4A versus KRAS‐4B transcript variants was confirmed to be down‐regulated in CRC compared to normal colonic mucosa (N = 41; p ≤ 0.001). This was independent of mutation status, however, gene set enrichment analysis revealed that the effect of splicing on KRAS signaling was specific to the KRAS wild‐type subgroup, in which low relative KRAS‐4A expression was associated with a higher level of KRAS signaling (p = 0.005). In concordance, the prognostic value of KRAS splicing was also dependent on mutation status, and for patients with Stage I–III KRAS wild‐type MSS CRC, low relative KRAS‐4A expression was associated with inferior overall survival (HR: 2.36, 95% CI: 1.07–5.18, p = 0.033), a result not found in mutant cases (p interaction = 0.026). The prognostic association in the wild‐type subgroup was independent of clinicopathological factors, including cancer stage in multivariable analysis (HR: 2.68, 95% CI: 1.18–6.09, p = 0.018). This suggests that KRAS has prognostic value beyond mutation status in MSS CRC, and highlights the importance of molecular heterogeneity in the clinically relevant KRAS wild‐type subgroup.
Keywords: KRAS, alternative splicing, colorectal cancer, microsatellite stable, prognosis
Short abstract
What's new?
Patients with microsatellite stable (MSS) colorectal cancer (CRC) that lacks KRAS mutation benefit from targeted therapy. Nonetheless, variations in clinical outcome suggest that KRAS wild‐type CRC is a heterogeneous disease. Here, two KRAS transcript variants, KRAS‐4A and KRAS‐4B, generated through alternative splicing, were investigated in relation to KRAS mutation status and MSS CRC prognosis. Aberrant splicing resulting in low expression of the KRAS‐4A transcript variant, relative to the KRAS‐4B transcript, was associated with increased KRAS signaling and poor patient prognosis specifically in KRAS wild‐type MSS CRC. The findings suggest that KRAS splicing is of prognostic relevance in KRAS wild‐type CRC.
Colorectal cancer (CRC) is a heterogeneous disease, both at the molecular level and with respect to disease outcome and treatment response. There is a need for biomarkers to improve the stratification of patients into therapeutically relevant subgroups. Currently, microsatellite instability (MSI) and KRAS mutation status are among the few biomarkers recommended for clinical use.1 KRAS is primarily a predictive factor, however, studies indicate that mutated KRAS also confers poor prognosis in CRC, but this is limited to the microsatellite stable (MSS) subtype,2, 3, 4 and possibly also to subgroups within MSS. Recently, the negative prognostic value of KRAS mutations was suggested to be specific to the “epithelial‐like” MSS cancers of the gene expression‐based consensus molecular subtypes (CMS) 2/3,4 highlighting the clinical importance of evaluating biomarkers in the context of molecular subtypes. Overall, patients with RAS (KRAS/NRAS) wild‐type metastatic CRC benefit from anti‐epidermal growth factor receptor (EGFR) treatment,5 although resistance occurs in more than half.6 This indicates that KRAS wild‐type CRC is not a homogeneous entity, and although a few additional resistance factors have been identified,7 there is a need for detailed biological understanding of this clinically important subgroup.
KRAS is expressed as two transcript variants caused by alternative splicing,8 KRAS‐4A and KRAS‐4B, both of which will encode oncogenic proteins when KRAS is mutated. Although the evidence for differential functions is limited, mutated KRAS‐4A and KRAS‐4B differ in their ability to induce anchorage‐independent growth and cell migration.9 Furthermore, studies in mice indicate that wild‐type KRAS‐4A has tumor suppressive and pro‐apoptotic activity, while wild‐type KRAS‐4B is anti‐apoptotic.10, 11 KRAS splicing has been shown to be altered in CRC, by reduced relative expression of the KRAS‐4A transcript,12 indicating that the balance of KRAS splice variants may have a role in CRC tumorigenesis. In contrast to KRAS mutations, few studies have evaluated the clinical relevance of KRAS splice variants. Although the expression level of the KRAS‐4A protein has been shown to be prognostic in CRC,13 another study comparing the expression of KRAS‐4A relative to KRAS‐4B at the transcript level failed to show a prognostic association in Stage I–II CRC.14
Here, we analyzed the mutation status‐dependent prognostic impact of KRAS splicing in the MSS subtype in a single‐hospital series of primary CRCs.
Material and Methods
Patient samples
Altogether 258 fresh frozen primary MSS CRCs from a population‐representative series of patients who underwent surgical resection at Oslo University Hospital, Oslo, Norway, in the time period 2005–2014 were included. All samples were analyzed for gene‐expression using splicing‐sensitive platforms, either by microarrays (N = 168), RNA sequencing (N = 128) or both (N = 38 overlapping samples). The microarray dataset represented a consecutive series of Stage I–IV MSS CRCs and has previously been published15 (Gene Expression Omnibus accession number GSE96528), while the RNA sequencing dataset was selected to include mostly Stage II or III MSS cancers (Supporting Information Table 1). Additionally, normal mucosa samples taken from disease‐free areas of the colon of 41 CRC patients were analyzed either by microarrays (N = 29) or RNA sequencing (N = 12), 30 of which also had their corresponding primary tumor analyzed. The study is part of a project approved by the Regional Committee for Medical and Health Research Ethics, South Eastern Norway (REC number 1.2005.1629). Written informed consent was obtained from all patients.
Total RNA was isolated using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen GmBH, Hilden, Germany). Genomic DNA was isolated using either a standard phenol/chloroform extraction method or metallic beads (Maxwell 16 DNA Purification Kit; Promega, Madison, WI). All procedures were performed according to the manufacturer's recommendations.
MSI status, determined by analysis of the Bethesda marker panel, and mutation hotspots in KRAS (exon 2: codons 12 and 13, exon 3: codons 59 and 61), assessed using Sanger sequencing, have previously been published.15 Additionally, mutations in KRAS codons 117 and 146 were analyzed by amplification of exon 4 using the primers: sense 5′‐TGACAAAAGTTGTGGACAGGT‐3′ and antisense 5′‐AAGAAGCAATGCCCTCTCA‐3′. When referring to KRAS at the transcript level, the 5′‐untranslated exon number 1 at the DNA level is denoted Exon 0, and exon number 5 at the DNA level is Exon 4A, according to previous reports on KRAS splice variants (Fig. 1 a).8
Figure 1.
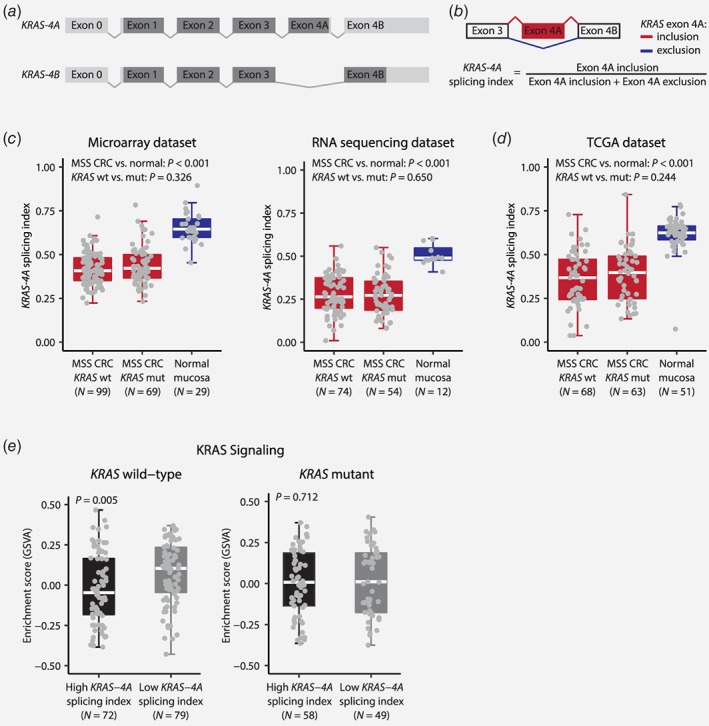
Expression of KRAS‐4A and KRAS‐4B splice variants in MSS CRC. (a) Schematic of the two splice variants of KRAS with protein coding regions in dark gray and non‐protein coding regions in light gray. To follow convention when referring to KRAS at the transcript level, the 5′‐untranslated exon number 1 at the DNA level is denoted Exon 0, and exon number 5 at the DNA level is Exon 4A. (b) KRAS splicing (denoted as the KRAS‐4A splicing index) was estimated using the signal of exonic and junction probes or RNA sequencing reads mapping to the exon 4A inclusion (red) and exclusion (blue) splice variants of KRAS. The KRAS‐4A splicing index was significantly decreased in MSS CRCs compared to normal colonic mucosa (c) in the microarray and RNA sequencing datasets, as well as (d) in an independent cohort of TCGA MSS CRC patients. No association was observed between the KRAS‐4A splicing index and KRAS mutation status. (e) KRAS wild‐type CRCs with low KRAS‐4A splicing indices had a higher level of KRAS signaling compared to tumors with high KRAS‐4A splicing indices. KRAS mutant cases showed no association between KRAS splicing and KRAS signaling.
Splicing‐sensitive gene expression microarrays
Microarray analyses were done using GeneChip Human Transcriptome Arrays 2.0 (Thermo Fisher Scientific, Waltham, MA), which have probes interrogating both individual exons and known splicing junctions. Fragmented and labeled single‐stranded cDNA was prepared from 100 ng total RNA and hybridized to arrays as recommended by the manufacturer. For alternative splicing analysis, CEL files were background corrected, quantile normalized and summarized according to a custom CDF file using the aroma.affymetrix R package and the robust multi‐array average (RMA) algorithm. The CDF file was generated using the CDFfromGTF function in the R package EventPointer,16 which grouped the probes of the array into probesets according to splice graphs, representing all annotated splicing events of each gene, built by mapping the probes to the Ensembl transcriptome (GRCh37 version 90). After pre‐processing, a percent spliced in (PSI) index for each identified splicing event in each sample was estimated using the getPSI command from the R package EventPointer. The relative abundance of the two splice variants of KRAS was estimated as the PSI for KRAS‐4A, defined as the expression signal of exonic and junction probes supporting exon 4A inclusion, relative to the total expression signal at the splicing event (i.e., for probes supporting inclusion and exclusion of exon 4A), and hereafter referred to as the KRAS‐4A splicing index. For gene level analysis, CEL files were preprocessed using the modified Signal Space Transformation algorithm of RMA implemented in the Affymetrix Expression Console Software (version 1.4.1.46). For genes annotated by more than one transcript cluster, the entry from RefSeq was used.
RNA sequencing
RNA sequencing libraries were generated from 500 ng total RNA using the TruSeq Stranded Total RNA Library Prep Gold kit (Illumina, San Diego, CA), and submitted to 2×101 base‐pair paired‐end sequencing using the Illumina HiSeq 2500 system at the Oslo University Hospital Genomics Core Facility (The Norwegian Radium Hospital, Oslo, Norway). The demultiplex software package CASAVA (version 1.8.2) was applied to generate raw FASTQ files. For alternative splicing analysis, RNA sequencing reads were processed using the SpliceSeq software17 (version 2.1, default parameters), with a reference splice graph database built using the GRCh38 reference genome downloaded from the NCBI FTP server and feature annotation from Ensembl (GRCh38 version 85) with all transcript support levels. The sample‐wise PSI indices of KRAS‐4A estimated by SpliceSeq corresponded with the microarray analysis and are similarly referred to as the KRAS‐4A splicing index. For gene level analysis, sequencing reads were aligned to the GRCh38 reference genome (downloaded from https://ccb.jhu.edu/software/hisat2/index.shtml) using HISAT218 (version 2.0.4), and the resulting BAM files were preprocessed with the standard GATK/Picard19 pipeline. Read counts were calculated using the htseq‐count tool from the python package HTSeq20 (version 0.9.1) with feature annotation from GENCODE21 (GRCh38; gencode.v27.annotation.gtf). Normalized gene expression levels were calculated as fragments per kilobase of transcripts per million mapped reads (FPKM). The FPKM values were log2 transformed after a constant of 0.005 was added to FPKM values of 0 to avoid infinite values.
Gene set enrichment analysis
The microarray and RNA sequencing gene level expression datasets were matched by HGNC symbols (N = 18,069 common protein‐coding genes) and merged by batch correction using the ComBat function in the R package SVA, and for overlapping samples, the expression data from the microarray dataset was used. Single‐sample gene set enrichment analysis for a set of 200 genes up‐regulated by KRAS activation (HALLMARK_KRAS_SIGNALING_UP) obtained from the Molecular Signatures Database22 was performed with the GSVA R package as described in Sveen et al.15
TCGA COADREAD samples
PSI indices of KRAS‐4A for 131 MSS CRCs and 51 normal mucosa samples were downloaded from the TCGA SpliceSeq database.23 Information on MSI and KRAS mutation status was obtained from TCGA.24
Statistical analyses
All statistical tests were performed in the R environment (version 3.4.2), including Mann–Whitney U and Wilcoxon signed rank tests using the wilcox.exact function in the R package exactRankTests, and Spearman's rank correlation test using the function cor.test. The Cox proportional hazards regression model with Wald test was applied for univariable and multivariable survival analysis using the coxph function in the R package survival. Kaplan–Meier survival curves were generated using the R package survminer and the ggsurvplot function. Survival analysis was performed on the microarray and RNA sequencing datasets in combination, and for overlapping samples, the dichotomized splicing index estimated by microarray analysis was used. Five‐year overall survival was used as endpoint, defined as the time from surgery to death from any cause, censored at last follow‐up or 5 years after surgery. All tests were 2‐sided, and p‐values lower than 0.05 are reported as significant.
Results
The KRAS‐4A splice variant is down‐regulated in CRC
The relative abundance of the two splice variants of KRAS, KRAS‐4A and KRAS‐4B (Figs. 1 a and 1 b), was analyzed by splicing‐sensitive microarrays and/or RNA sequencing in 258 Stage I–IV MSS CRCs (Supporting Information Fig. 1). Quality control showed good correlation of the KRAS‐4A splicing index among the 38 samples analyzed on both platforms (Spearman's ρ = 0.65, p < 0.001). In comparison with normal colonic mucosa, the KRAS‐4A splicing index was significantly down‐regulated in CRC in both datasets (p < 0.001; Fig. 1 c), also in paired analysis of matching tumor–normal samples (p ≤ 0.001; Supporting Information Fig. 2 a). Stratification according to mutation status showed that KRAS mutations did not influence KRAS splicing (Fig. 1 c). These results were validated in an independent patient cohort of 131 MSS CRCs, obtained from the TCGA SpliceSeq database, where the KRAS‐4A splicing index was significantly lower in MSS CRCs compared to normal mucosa, independently of KRAS mutation status (Fig. 1 d and Supporting Information Fig. 2 b).
To evaluate the impact of KRAS splicing on downstream signaling, sample‐wise enrichment scores were calculated for a gene expression signature of KRAS activity. In general, KRAS signaling was higher in CRCs than in normal colonic mucosa (p < 0.001; Supporting Information Fig. 3). Furthermore, dichotomization of the CRCs according to the median KRAS‐4A splicing index within the two datasets revealed significantly higher KRAS signaling in samples with the lowest splicing indices (p = 0.016), indicating splicing‐associated up‐regulation of KRAS signaling. Interestingly, this was found to be significant only for KRAS wild‐type tumors (p = 0.005) and not in the KRAS mutant subgroup (p = 0.712; Fig. 1 e).
The KRAS‐4A splicing index has prognostic impact dependent on mutation status
Considering the mutation status‐dependent impact of KRAS splicing on downstream signaling, we also analyzed prognostic associations according to mutation status in Stage I–III MSS CRCs. Patients from the microarray and sequencing datasets were separately dichotomized by their median KRAS‐4A splicing index within the two datasets and combined (N = 230). Among all patients, there was no association between the KRAS‐4A splicing index and overall survival (HR: 1.29, 95% CI: 0.76–2.18, p = 0.346). However, stratification according to mutation status revealed that low KRAS‐4A splicing indices were associated with a significantly lower 5‐year overall survival rate than high splicing indices specifically in the KRAS wild‐type subgroup (HR: 2.36, 95% CI: 1.07–5.18, p = 0.033), and not in the subset of patients with mutant KRAS (HR: 0.69, 95% CI: 0.32–1.48, p = 0.337, p interaction = 0.026; Fig. 2), consistent with the corresponding impact of splicing on KRAS signaling. The negative prognostic value of low KRAS‐4A splicing indices in KRAS wild‐type cases was retained in multivariable analysis including patient age and gender, as well as tumor stage and location (HR: 2.68, 95% CI: 1.18–6.09, p = 0.018, Table 1). Similar results were also seen for KRAS wild‐type patients that had not received post‐operative chemotherapy, although statistically significant only in the multivariable analysis (multivariable HR: 2.82, 95% CI: 1.11–7.16, p = 0.029, Supporting Information Table 2 and Supporting Information Fig. 4). Furthermore, a similar prognostic association was found when analyzing the KRAS‐4A splicing index as a continuous variable among KRAS wild‐type Stage I–III MSS CRCs, although statistically significant only in the sequencing dataset (multivariable HR: 0.001, 95% CI: 0.00–0.30, p = 0.017).
Figure 2.
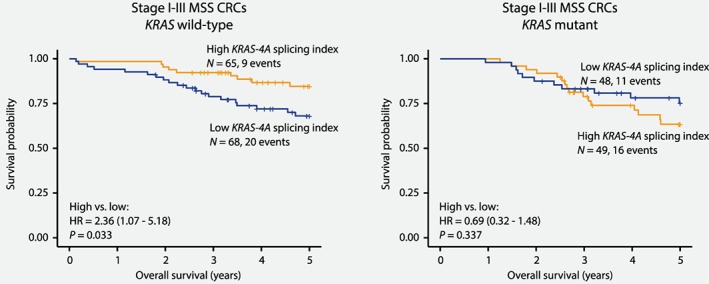
Mutation status‐dependent poor‐prognostic impact of KRAS splicing. Among patients with Stage I–III MSS CRC, a low KRAS‐4A splicing index (below median) was associated with poor survival specifically in the KRAS wild‐type subgroup (5‐year overall survival: 85% vs. 68%), but not in patients harboring tumors with KRAS mutations.
Table 1.
Univariable and multivariable analysis of the prognostic impact of the KRAS‐4A splicing index in KRAS wild‐type patients with Stage I–III MSS CRCs
| Variable | Five‐year overall survival | |||
|---|---|---|---|---|
| Univariable analysis | Multivariable analysis1 | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| KRAS‐4A splicing index | ||||
| High (above median) | 1 | 1 | ||
| Low (below median) | 2.36 (1.07–5.18) | 0.033 | 2.68 (1.18–6.09) | 0.018 |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.45 (0.69–3.03) | 0.327 | 1.14 (0.54–2.41) | 0.737 |
| Age2 | 1.04 (1.00–1.07) | 0.025 | 1.05 (1.01–1.09) | 0.012 |
| Location | ||||
| Right | 1 | 1 | ||
| Left or rectum | 1.27 (0.52–3.13) | 0.597 | 1.85 (0.70–4.86) | 0.213 |
| Stage | ||||
| Stage I and II | 1 | 1 | ||
| Stage III | 1.20 (0.57–2.51) | 0.633 | 1.26 (0.60–2.66) | 0.536 |
Included all variables in table. N = 133.
Continous variable.
Discussion
Our study shows that the clinical relevance of KRAS aberrations in MSS CRC is not limited to genetic changes, but also extends to the RNA splicing process. In a single‐hospital series of MSS CRCs investigated for KRAS splicing, relative down‐regulation of the KRAS‐4A versus KRAS‐4B splice variant was associated with high KRAS signaling and a poor patient outcome. However, both effects were specific to the KRAS wild‐type subgroup, consistent with the presumption that the effect of mutations outweighs the effect of aberrant splicing in this gene. Nonetheless, although targeting the MAP kinase pathway may be effective in KRAS wild‐type CRC, the proportion of patients that are sensitive to anti‐EGFR therapy is modest, highlighting the need for improved biological knowledge in this subgroup. Along with mutations in PIK3CA and MET amplification,7 KRAS amplification has been shown to cause resistance in a small proportion of patients.25 Based on our finding of an association with increased KRAS signaling and poor patient outcome, albeit in the primary setting, we hypothesize that consideration of KRAS splicing may also contribute to optimize the treatment of KRAS wild‐type CRC.
We confirm previous results showing that KRAS splicing is altered in CRC compared to normal colonic mucosa by relative down‐regulation of KRAS‐4A versus KRAS‐4B splice variants, and that this is not dependent on the presence of KRAS mutations.12 Furthermore, it has been shown also on the protein level that the expression level specifically of the KRAS‐4A variant may be prognostic in KRAS wild‐type CRC.13 However, in the latter study, the prognostic impact of KRAS‐4A and KRAS‐4B protein expression was investigated separately. Studies in preclinical models have shown that KRAS‐4A and KRAS‐4B differ in their ability to regulate apoptosis, proliferation and metastasis‐related processes,9, 10, 11 suggesting that the impact of KRAS splicing on patient outcome might ultimately be dependent on the overall balance of KRAS‐4A and KRAS‐4B expression. Furthermore, a study of carcinogen‐induced colonic adenomas in KRAS wild‐type mice unable to express KRAS‐4A revealed higher levels of KRAS‐4B expression and concomitant evidence of an increased expression of the KRAS downstream effectors ERK1/2 and AKT,11 consistent with higher KRAS signaling observed in tumors with low relative KRAS‐4A expression in our dataset. In addition, an increase of the proliferation marker Ki‐67 and a decrease of the apoptosis marker cleaved caspase‐3 in mice depleted of KRAS‐4A expression indicated that KRAS‐4A has tumor suppressor activity in the KRAS wild‐type setting,11 providing a biological rationale for the poor outcome observed for patients with low relative KRAS‐4A expression. It should be noted that the size of the patient series is limited and a search in the public domain for splicing‐sensitive expression data with corresponding clinical information identified no suitable external validation series. However, our hypothesis that KRAS splicing is a surrogate marker for activated KRAS in the wild‐type setting is intriguing and awaiting validation studies.
In conclusion, our study indicates that KRAS has prognostic value beyond mutation status in MSS CRC, and suggests relevance of investigating KRAS splicing for optimization of targeted treatment of KRAS wild‐type CRC.
Authors Contribution
Conception and design: A.S., R.A.L.; Acquisition of data: I.A.E., J.M.S., R.I.S., A.N.; Analysis and interpretation of data: I.A.E., A.S., R.A.L.; Drafting of the manuscript: I.A.E., A.S., R.A.L.; Study supervision: A.S., R.A.L. All authors were involved in revision of the manuscript and have approved the final version.
Supporting information
Supporting Information Figure S1. Consort diagram for included patients
Supporting Information Figure S2. Expression of KRAS‐4A and KRAS‐4B splice variants in paired MSS CRCs and normal colonic mucosa samples
Supporting Information Figure S3. KRAS signaling in MSS CRCs compared to normal colonic mucosa
Supporting Information Figure S4. Prognostic impact of KRAS splicing in chemotherapy naïve KRAS wild‐type patients
Supporting Information Table S1. Clinical and molecular characteristics of all in‐house MSS CRC patients included in the study, and in the microarray and RNA sequencing datasets separately
Supporting Information Table S2. Univariable and multivariable analysis of the prognostic impact of the KRAS‐4A splicing index in KRAS wild‐type patients that had not received post‐operative chemotherapy
Acknowledgement
The authors are grateful for the excellent technical assistance of Stine Aske Danielsen, Merete Hektoen and Bjarne Johannessen.
Conflict of interest: The authors declare no conflict of interests.
References
- 1. Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J Mol Diagn 2017;19:187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taieb J, Le Malicot K, Shi Q, et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst 2017;109:djw272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dienstmann R, Mason MJ, Sinicrope FA, et al. Prediction of overall survival in stage II and III colon cancer beyond TNM system: a retrospective, pooled biomarker study. Ann Oncol 2017;28:1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smeby J, Sveen A, Merok MA, et al. CMS‐dependent prognostic impact of KRAS and BRAFV600E mutations in primary colorectal cancer. Ann Oncol 2018;29:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. [DOI] [PubMed] [Google Scholar]
- 6. De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy‐refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010;11:753–62. [DOI] [PubMed] [Google Scholar]
- 7. Dienstmann R, Vermeulen L, Guinney J, et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer 2017;17:79–92. [DOI] [PubMed] [Google Scholar]
- 8. Capon D, Seeburg P, McGrath J, et al. Activation of Ki‐ras2 gene in human colon and lung carcinomas by two different point mutations. Nature 1983;304:507–13. [DOI] [PubMed] [Google Scholar]
- 9. Voice JK, Klemke RL, Le A, et al. Four human ras homologs differ in their abilities to activate Raf‐1, induce transformation, and stimulate cell motility. J Biol Chem 1999;274:17164–70. [DOI] [PubMed] [Google Scholar]
- 10. Plowman SJ, Arends MJ, Brownstein DG, et al. The K‐Ras 4A isoform promotes apoptosis but does not affect either lifespan or spontaneous tumor incidence in aging mice. Exp Cell Res 2006;312:16–26. [DOI] [PubMed] [Google Scholar]
- 11. Luo F, Ye H, Hamoudi R, et al. K‐ras exon 4A has a tumour suppressor effect on carcinogen‐induced murine colonic adenoma formation. J Pathol 2010;220:542–50. [DOI] [PubMed] [Google Scholar]
- 12. Plowman SJ, Berry RL, Bader SA, et al. K‐ras 4A and 4B are co‐expressed widely in human tissues, and their ratio is altered in sporadic colorectal cancer. J Exp Clin Cancer Res 2006;25:259–67. [PubMed] [Google Scholar]
- 13. Abubaker J, Bavi P, Al‐Haqawi W, et al. Prognostic significance of alterations in KRAS isoforms KRAS‐4A/4B and KRAS mutations in colorectal carcinoma. J Pathol 2009;219:435–45. [DOI] [PubMed] [Google Scholar]
- 14. Kriegl L, Jung A, Horst D, et al. Microsatellite instability, KRAS mutations and cellular distribution of TRAIL‐receptors in early stage colorectal cancer. PLoS One 2012;7:e51654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sveen A, Bruun J, Eide PW, et al. Colorectal cancer consensus molecular subtypes translated to preclinical models uncover potentially targetable cancer cell dependencies. Clin Cancer Res 2017;24:794–806. [DOI] [PubMed] [Google Scholar]
- 16. Romero JP, Muniategui A, De Miguel FJ, et al. EventPointer: an effective identification of alternative splicing events using junction arrays. BMC Genomics 2016;17:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan MC, Cleland J, Kim R, et al. SpliceSeq: a resource for analysis and visualization of RNA‐Seq data on alternative splicing and its functional impacts. Bioinformatics 2012;28:2385–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 2015;12:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next‐generation DNA sequencing data. Genome Res 2010;20:1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high‐throughput sequencing data. Bioinformatics 2015;31:166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrow J, Frankish A, Gonzalez JM, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res 2012;22:1760–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liberzon A, Birger C, Thorvaldsdottir H, et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 2015;1:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryan M, Wong WC, Brown R, et al. TCGASpliceSeq a compendium of alternative mRNA splicing in cancer. Nucleic Acids Res 2016;44:D1018–D1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. TCGA . Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valtorta E, Misale S, Sartore‐Bianchi A, et al. KRAS gene amplification in colorectal cancer and impact on response to EGFR‐targeted therapy. Int J Cancer 2013;133:1259–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1. Consort diagram for included patients
Supporting Information Figure S2. Expression of KRAS‐4A and KRAS‐4B splice variants in paired MSS CRCs and normal colonic mucosa samples
Supporting Information Figure S3. KRAS signaling in MSS CRCs compared to normal colonic mucosa
Supporting Information Figure S4. Prognostic impact of KRAS splicing in chemotherapy naïve KRAS wild‐type patients
Supporting Information Table S1. Clinical and molecular characteristics of all in‐house MSS CRC patients included in the study, and in the microarray and RNA sequencing datasets separately
Supporting Information Table S2. Univariable and multivariable analysis of the prognostic impact of the KRAS‐4A splicing index in KRAS wild‐type patients that had not received post‐operative chemotherapy


