Abstract
The focus of this literature review is on the three interacting brain areas that participate in decision‐making: basal ganglia, ventral motor thalamic nuclei, and medial prefrontal cortex, with an emphasis on the participation of the ventromedial and ventral anterior motor thalamic nuclei in prefrontal cortical function. Apart from a defining input from the mediodorsal thalamus, the prefrontal cortex receives inputs from ventral motor thalamic nuclei that combine to mediate typical prefrontal functions such as associative learning, action selection, and decision‐making. Motor, somatosensory and medial prefrontal cortices are mainly contacted in layer 1 by the ventral motor thalamic nuclei and in layer 3 by thalamocortical input from mediodorsal thalamus. We will review anatomical, electrophysiological, and behavioral evidence for the proposed participation of ventral motor thalamic nuclei and medial prefrontal cortex in rat and mouse motor decision‐making.
Keywords: action selection, basal ganglia, cognitive functions, rodents
Introduction
Prompted by observations following frontal lobotomies in humans, Karl Pribram studied in non‐human primates decision‐making and other prefrontal‐related functions such as memory, attention, and hyperactivity (Pribram, 1975). Initially, most studies used monkeys and cats, followed by the use of other species later. Considering the difficulties in comparing brain areas between species and the enduring confusion in terminology and inevitable generalizations, we circumscribe our review to research using mice and rats performing decision‐making tasks. Nonetheless, we must also point out that the validity of research in rats and mice regarding decision‐making continues to give examples of misconceptions mainly when comparing terminology and assignations of functions to certain areas. Moreover, it should not be assumed that results from rodents can be directly extrapolated to primates and vice versa (Hardman et al., 2002; Smith et al., 2014b).
Projections of ventral motor thalamic nuclei, in particular the ventromedial (VM) and ventral anterior (VA) nuclei to prefrontal cortical layer 1, provide an important link between the basal ganglia, especially striatum, and the prefrontal cortex to mediate processes like motor decision‐making. We will briefly describe the structures involved (i.e., basal ganglia, striatum, prefrontal cortex, and ventral motor thalamic nuclei), before reviewing evidence of the association between ventral motor thalamic nuclei and medial prefrontal cortex. We will highlight the participation of layer 1 and review evidence of ventral motor thalamic nuclei involvement in the modulation of movement. Since only recently, the study of decision‐making in mice and rats has become prominent, we will describe some of the tasks used to determine motor decision making in these animals, before describing the areas of the frontal cortex where thalamic‐prefrontal‐striatal links could be relevant and suggesting how cortical layer 1 may participate in the process.
Anatomical links
Prefrontal cortex
This area is defined as the region rostral to motor and premotor areas. To determine cortical homologies between species, the pattern of specific connections, functional properties, and the presence and distribution of neurotransmitters are taken into consideration. In 1948, Rose and Woolsey proposed that the extent of the prefrontal cortex can be defined by the reciprocal connections with mediodorsal thalamus (MD) (Uylings et al., 2003). Using retrograde tracing, three neuronal populations originating in the MD were observed in monkeys and rats (Preuss & Goldman‐Rakic, 1987; Groenewegen, 1988). Recently, the projection from MD to the prefrontal cortex has been confirmed as three separate afferent pathways with little overlay: to the ventromedial (cingulate and infralimbic), dorsomedial (prelimbic), and orbitofrontal cortex (Alcaraz et al., 2016). Outputs from MD to prefrontal cortex, primarily innervate the dendrites of prefrontal pyramidal neurons located in layer 3, with fewer contacts in layers 5 and 6 (Kuroda et al., 1998; Delevich et al., 2015). Recent evidence also indicates that MD contacts cortico‐cortical neurons in layer 2/3 (Collins et al., 2018). Moreover, sparse MD projection to layer 1 has been observed together with those of intralaminar nuclei (Kuroda et al., 1998; Collins et al., 2018).
Outputs of the medial and central subdivisions of MD project to the dorsomedial and ventromedial prefrontal cortex and those from the lateral subdivision project to the orbitofrontal cortex (Cavdar et al., 2001). Although the participation of MD afferents to prefrontal cortex is an essential and an integral part of its function, our focus is on the ventral motor thalamic nuclei. See Fig. 1 for a cartoon of the prefrontal‐defining afferents to layers 2/3 from MD thalamus and inputs to those same prefrontal areas from ventral motor thalamic nuclei to layer 1 described below.
Figure 1.

Cartoon of the rodent brain illustrating the prefrontal cortex (shaded light pink), and its sensory and motor inputs from mediodorsal (layer 2/3, red) and ventral motor thalamic nuclei (layer 1, blue) that mediate associative learning, action selection, and decision‐making in rats and mice. a‐ prefrontal cortex, b‐ motor cortical areas, c‐somatosensory cortical areas.
Basal ganglia and striatum
The basal ganglia are a collection of subcortical nuclei that include striatum, substantia nigra‐ pars reticulata (SNr), and pars compacta‐, subthalamic nucleus, globus pallidus (globus pallidus ‐external segment‐ in other species), and entopeduncular nucleus that is embedded in the fibers of the corticofugal tracts (equivalent to the primate medial globus pallidus ‐internal segment‐ GPi) (Albin et al., 1989; Kha et al., 2000). In recent literature involving mice and rats, the term endopeduncular nucleus is being replaced by the term globus pallidus internus (GPi), thus, to maintain nomenclatures consistent, we will keep both terms: entopeduncular/GPi.
In rats, entopeduncular/GPi and SNr form the output of the basal ganglia (Deniau & Chevalier, 1984; Bolam & Smith, 1992; Kha et al., 2000; Kolomiets et al., 2003; Aceves et al., 2011). This output contacts ventral motor thalamic nuclei ‐particularly the ventromedial nucleus (Beckstead et al., 1979; Di Chiara et al., 1979; Herkenham, 1979; Gerfen et al., 1982; Williams & Faull, 1988; Kha et al., 2000, 2001; Gulcebi et al., 2012). SNr further sends contacts to the lateral part of MD thalamus, that also receives inputs from the dentate deep cerebellar nucleus (Cavdar et al., 2014). Other thalamic nuclei targeted by basal ganglia outputs are the external‐parallamenar MD, parafascicular, centromedial, paracentral, and reticular nuclei (Gerfen et al., 1982; Tsumori et al., 2000, 2002; Gulcebi et al., 2012). Finally, SNr output to brainstem includes the mesencephalic reticular formation, superior colliculus, pedunculopontine nucleus, central gray region, dorsal raphe, and laterodorsal tegmental nucleus (Cebrian et al., 2005).
Striatal output neurons and interneurons receive glutamatergic input from the cerebral cortex and thalamus (Smith et al., 2014a). Bilateral input arrives from most cortical areas as demonstrated in species such as: rats (Webster, 1961; Wilson, 1986, 1987), mice (Hattox & Nelson, 2007; Sohur et al., 2014), rabbits (Carman et al., 1963), cats (Webster, 1965), and primates (Kemp & Powell, 1970; Goldman‐Rakic, 1983; Cavada & Goldman‐Rakic, 1991; Haber, 2016).
This large array of striatal inputs hints to its diverse and integrative functions related to movement (Feger et al., 1976; Lee, 1984). The description of the innervation from the whole prefrontal cortex into segregated striatal areas, led to the exploration of basal ganglia functions associated with prefrontal cortical functions in monkeys (Graybiel, 1991; Romo et al., 1992; Joel & Weiner, 1994; Jaeger et al., 1995; Afifi, 2003). Some recently described striatal functions related to prefrontal cortical integration in rats that are of interest to this review, include motivational drive (Kreitzer & Berke, 2011), generation of self‐paced movements (Klaus et al., 2017), and context‐dependent facilitation of appropriate movements (Kreitzer & Berke, 2011), as well as learning involved in risk‐based decision‐making (Leblond et al., 2011), solution of cognitive conflicts between actions (Laurent et al., 2017), selection of action alternatives (Canales & Graybiel, 2000), decision‐making particularly, in relation to the effort invested to obtain a reward (Carvalho Poyraz et al., 2016) and cost‐benefit decision‐making (Friedman et al., 2015).
Ventral motor thalamic nuclei
The ventromedial (VM), ventral anterior (VA), and ventrolateral (VL) nuclei form the ventral motor nuclei of the thalamus. These nuclei are linked to subcortical and cortical structures that participate in motor control and likely convey motor related information to the prefrontal cortex. The input to ventral motor thalamic nuclei from basal ganglia is through GABAergic projections from substantia nigra pars reticulata (SNr) (Di Chiara et al., 1979; Sawyer et al., 1991; Deniau & Chevalier, 1992; Kha et al., 2001; Kase et al., 2015) and entopeduncular/(GPi) (Kha et al., 2000).
Frequently the literature refer to these nuclei as the “motor thalamus” (for example, a PubMed search for the title “motor thalamus” resulted in 142 references). Among the most recent is Gaidica et al. (2018). However, as we were pointed out in the review process “this is a vague term that embraces many different thalamic nuclei with different anatomical and functional specificity”. In fact, we have proposed that motor and non‐motor information, before it diverges to prefrontal, motor, and parietal cortices, is integrated in the ventrolateral, ventromedial, ventral anterior, parafascicular, and mediodorsal nuclei. Ideally these “motor” and “oculomotor” thalamic nuclei could form part of an “extended motor thalamus”. Having these “motor/sensory”nuclei placed into a single concept, could allow the inclusion of the related parietal cortex into the basal ganglia‐cerebellar and “motor” thalamo‐cortical circuits. This inclusion could promote conceptual expansion and new theoretical propositions for greater understanding of complex functional networks and insights into the motor and non‐motor aspects of movement.
Early evidence reported that in coronal brain sections VA and VL are difficult to separate resulting in the term VA‐VL complex. From a Golgi study in rats, mice and kittens, Scheibel & Scheibel (1966) indicated that VA was better identified in sagittal sections where afferents from entopeduncular/GPi cross obliquely through the internal capsule, run posterior to the nucleus reticularis and delimit VA from its ventrolateral aspect. These studies also indicated that efferents from VA are observed in premotor, motor and some frontal areas such as the orbitofrontal cortex. Early tracing studies also reported that cerebellum and basal ganglia contacted separate areas of the ventral motor thalamic nuclei, since their pattern of innervation did not overlap (Deniau et al., 1992). Kuramoto et al. (2009) divided VA‐VL nuclei into rostromedial and caudolateral portions designated as inhibitory (IZ) or excitatory (EZ) afferent‐dominant zones respectively based on a differential immunoreactivity for the calbindin and the GABA marker (GAD67), more intense in the rostromedial than the caudolateral portions. When VA‐VL and VM were described as inhibitory associated with basal ganglia and excitatory with cerebellum, large and small gabaergic GAD67‐immunopositive as well as glutamatergic vGLUT2‐immunopositive were observed in VA‐VL with a predominant gabaergic input in the rostroventral VA‐VL and VM, and a predominant glutamatergic input in the caudodorsal VA‐VL (Kuramoto et al., 2011). Later Nakamura et al. (2014) delineated the ventral motor thalamic nuclei into a functional parcellation called the basal ganglia‐recipient zone or BZ that includes VM and VA and a cerebellar recipient zone or CZ that includes VL. Particularly interesting for those working on cortical layers, is that BZ is defined as terminating in layer 1 whereas CZ as terminating in layers 3 to 5. Comparison of spontaneous rat neuronal activity recorded in VM‐VA and VL indicates that the innervation of layer 1 from VM‐VA is probably responsible for the large coincident excitation of cortical neurons and the temporal coupling to motor cortex oscillatory frequency (Nakamura et al., 2014). See Hintzen et al. (2018) for a discussion on the functional implications of the overlaps between cerebellar and basal ganglia outputs.
Association between ventral motor thalamic nuclei and prefrontal cortex
Reciprocal connectivity
Recently, Collins et al. (2018) using optogenetic stimulation of prefrontal corticothalamic neurons of layer 6 and some of layer 5, evoked monosynaptic excitatory potentials, with similar synaptic properties, in MD and VM. Moreover, colocalization of thalamic retrograde markers from MD and VM in corticothalamic neurons is observed with bifurcated axons contacting both MD and VM. Furthermore, the authors confirmed as reported by others (see above; Anatomical links: prefrontal cortex), that both MD and VM project to the prefrontal cortex: MD to corticocortical more than corticothalamic neurons located in layer 5, and with a preference for corticocortical neurons in layer 2/3 over layer 5. VM in contrast, was observed to concentrate its axons in layer 1 and induce subthreshold, possibly modulatory, responses in corticocortical neurons in layers 5 and 2/3. In summary, although they use different layers, thalamic sensory MD, VM, and prefrontal cortex establish reciprocal connectivity.
Thalamic axonal terminal fields in layer 1
VM and VA motor thalamic nuclei project to layer 1 (Fig. 2). VM neurons form an axon terminal field characterized by swellings that travel along cortical layer 1 (Herkenham, 1979; Arbuthnott et al., 1990; Rubio‐Garrido et al., 2009; Cruikshank et al., 2012). Reports of some terminal swellings have also been reported in layers 2/3 of motor cortex (Arbuthnott et al., 1990) and layers 3 and 5 in the dorsal and lateral sectors of the cortex rostral to the genu (Herkenham, 1979). Cortical pyramidal neurons located in layers 2/3, 4, 5, and 7 (now known as layer 6b) whose apical dendrites reach layer 1, were detected using fluorescent retrograde markers placed on the cortical surface (Mitchell & Cauller, 2001; Rubio‐Garrido et al., 2007). The extent of the cortical arborization of VM axon terminal field has been described by several authors. Following tracing studies in rats, Herkenham (1979) concluded that “the bulk of the projection is distributed to pregenual frontal cortical areas while a smaller number of fibers innervates parietal and occipital areas…”. Similarly, by recording cortical antidromic activity driven by cortical stimulation, Arbuthnott et al. (1990) described that [a] “network of terminal axons is seen in layer 1 as far back as the injection site [AP −5.5], as far anterior as the frontal pole and as far lateral as the rhinal sulcus in which areas the caudal‐most fibers are regularly found”. These areas in rats (Neafsey et al., 1986) and mice (Tennant et al., 2011; Deffeyes et al., 2015) include the typically extensive motor associated cortical areas including forelimb and hindlimb regions and prefrontal cortical regions. Rubio‐Garrido et al. (2009) report that a small deposit of biotinylated dextran amine on the cortical surface backfilled approximately 150 neurons in VM whose axon arbors can be observed in layer 1 in the frontal pole anterior to the middle cerebral artery. Cortical areas that receive fibers from VM are the primary somatosensory and associated sensory orbital and cingulate areas, including the agranular insular area, cingulate cortex, dorsolateral orbital area, frontal cortex, frontal association area, medial orbital area, prelimbic area, and ventrolateral orbital area (Kuramoto et al., 2009, 2015). Figure 3 illustrates immunohistochemical evidence of cortical projections from VM that include the cingulate, prelimbic, and infralimbic medial prefrontal cortex of the rat brain.
Figure 2.
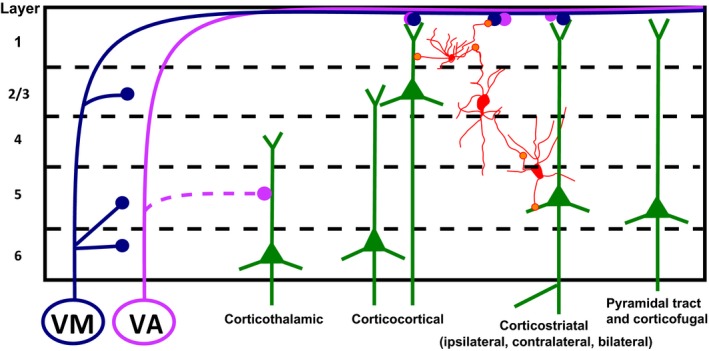
Innervation of cortex by ventromedial (VM) and ventral anterior nuclei (VA) of the ventral motor thalamic nuclei or basal ganglia zone (BZ) as defined by (Nakamura et al., 2014). Afferents of VM (blue) mainly terminate in cortical layer 1, with some ramifications in layers 3 and 5, 6. Afferents of VA innervate cortical layers 1 and 5. Afferents of ventral motor thalamic nuclei to superior cortical layers have been demonstrated to drive cortical pyramidal neurons and may drive layer 1 inhibitory interneurons that can, in turn, induce feedforward inhibition in lower level pyramidal neurons. Here, we propose that VA and VM afferents to layer 1 of the medial prefrontal cortex can provide an important modulation on corticostriatal, corticocortical, and corticofugal pyramidal neurons.
Figure 3.
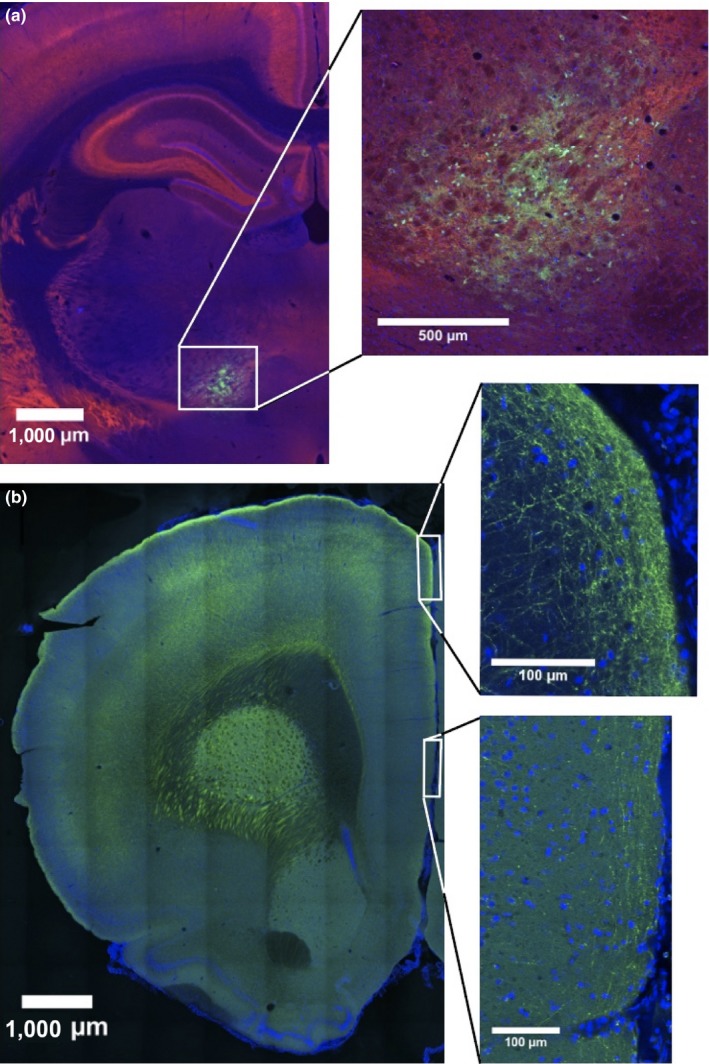
Immunohistochemistry of cortical projections from the ventromedial motor thalamic nucleus (VM) to cortex. (A) Section at the level of the injection site in ventral motor thalamus (GAD67 immunostaining), magnified on the right‐hand side insert. (B) Frontal section of the brain to illustrate the presence of VM axon terminals in the medial prefrontal cortex (expressing GFP). Axon terminal in layer 1 decrease in magnitude from a marked anterior cingulate projection (top insert) to a noticeable prelimbic and a less prominent infralimbic projection (lower insert). Projections to the orbitofrontal cortex have not been reported from VM, see text. Method: Briefly, an anesthetized Sprague‐Dawley rat received a stereotaxic unilateral injection of 90 nL of AAV5‐CAG‐ArchT‐GFP (UNC Vector Core) in VM (interaural AP +7.0 mm, ML +1.2 mm and DV −6.56 mm from Bregma). After a survival time of three weeks, the rat was sacrificed with an overdose of anesthetic and perfused with 4% paraformaldehyde. Tissue slices (60 μm) were counterstained for GAD67 (red). Cell nuclei were stained with with 4′,6‐Diamidino‐2‐Phenylindole, dihydrochloride DAPI (blue). Expression of VM axon terminals labeled by AAV5‐CAG‐ArchT‐GFP (green) can be observed in the prefrontal cortex and infected cells in VM (the GABA rich‐area in thalamus).
Doing experiments in cats and dogs Jinnai et al. (1987) and Tanaka et al. (1986), respectively, confirmed the anatomical limits of VA previously reported (Scheibel & Scheibel, 1966) and also reported VA neurons projecting to cortical layer 1 (Fig. 2). In rats, VL innervation of layer 1 is much weaker than VM or VA, only 2–15% of cortical VL terminals are found in layer 1, while the majority is found in deeper cortical layers (Kuramoto et al., 2009, 2015). For an illustration of VM projections to prefrontal cortex see Figs 2 and 3.
Layer 1 modulation of cortical pyramidal neurons: electrophysiological results
Although most recordings to date are from sensorimotor and motor cortical areas, data suggests a role of afferents to layer 1 in other cortical areas including medial prefrontal cortex. Layer 1 modulates cortical pyramidal and intratelencephalic cortical efferents. An example of modulation of cortical activity initiated in layer 1 are local field potentials in layers 2, 3, and 5 recorded in the somatosensory cortex to a whisker deflection, following brief electrical stimulation of layer 1. In this study, it is observed that layer 1 imposes a time‐dependent regulation on cortical field potentials. An amplified response is seen if the electrical stimulation in layer 1 is administered within 10 ms of whisker deflection, but inhibition occurs if stimulation is administered between 20 and 40 ms of the whisker deflection. Therefore, activity in layer 1 can adjust the magnitude of cortical responses in other layers (Shlosberg et al., 2006).
Recordings of dendritic tufts of layer 5 pyramidal neurons in layer 1, have shown attenuating and non‐propagating action potentials initiated by focal stimulation of distal and proximal tufts (Larkum et al., 2009) and calcium‐dependent regenerative action potentials in the dendritic tuft, produced by spontaneous synaptic input or by local stimulation in layer 1 (Larkum & Zhu, 2002).
Optical stimulation of VM fibers in layer 1 of prefrontal cortex, induces synaptic depolarizing potentials in interneurons of the same layer. Nonetheless, when high frequency stimulation (10‐Hz) is applied, the postsynaptic depolarizing potentials are depressed (Cruikshank et al., 2012). As expected from the presence of GABAergic interneurons in layer 1, paired whole‐cell recordings revealed postsynaptic inhibition between two layer 1 interneurons and between a layer 1 interneuron and a layer 2 pyramidal neuron (Cruikshank et al., 2012). These results indicate that VM afferents to layer 1 can provide excitatory drive to local inhibitory interneurons, with a subsequent inhibitory action over dendritic tufts of pyramidal neurons in layers 2/3 (Cruikshank et al., 2012; Yamawaki & Shepherd, 2015). In addition, layer 1 afferents may provide inhibitory control over pyramidal neurons in lower layers via feedforward inhibition induced in cortical inhibitory interneurons (Jiang et al., 2013; Lee et al., 2015) or modulation of pyramidal tufts reaching layer 1 (Mitchell & Cauller, 2001; Rubio‐Garrido et al., 2007).
Comparison of spontaneous rat neuronal activity recorded in VM‐VA and VL indicates that the innervation of layer 1 from VM and VA is probably responsible for the large coincident excitation of cortical neurons and the temporal coupling to motor cortex oscillatory frequency (Nakamura et al., 2014).
In summary, these electrophysiological experiments have dissected in great detail areas where layer 1 modulation takes place, but more electrophysiological evidence is needed about thalamic VM and VA afferents to the prefrontal cortex.
Modulation of movement by the ventral motor thalamic nuclei
Since ventral motor thalamic nuclei projects to motor, sensorimotor and prefrontal cortex its effects on movement are expected. For example, the output of deep cerebellar nuclei converge on VL to then project to motor cortex. Recordings of VL neurons to stimulation of entopeduncular/GPi show decrease in spontaneous activity with rebound firing at the end of the stimulation, accompanied by increased activity of local field potentials in primary motor cortex and increased electromyographic activity of neck and forelimb (Kim et al., 2017). Moreover, optogenetic stimulation of VA induced transient improvement of a drug‐induced parkinsonian akinesia (Seeger‐Armbruster et al., 2015) and photoinhibition of the entopeduncular/GPi that increases VM neuronal activity, improves parkinsonian akinesia (Moon et al., 2018). Unilateral chemical stimulation of VM induces a hypermotility that results in turning behavior (Starr & Summerhayes, 1983). Bilateral VM and VL lesions impair motor performance in the rotarod and acquisition of sensorimotor and spatial learning in the Morris maze (Jeljeli et al., 2003).
All this evidence clearly underlines the important role of ventral motor thalamic nuclei in conveying and perhaps also integrating information from basal ganglia and cerebellum, before sending it to specific layers of motor, sensorimotor and prefrontal cortex. In spite of decision‐making being related to several cortical areas including prefrontal, premotor, motor, and sensory areas, direct evidence of thalamic modulation of prefrontal cortical activity and function by ventral motor thalamic nuclei is still missing. Studies such as those mentioned above involving afferents from VA and VM will be welcome.
Decision‐making in mice and rats
Considering their low rank in the mammalian evolutionary scale, decision‐making in mice or rats may sound preposterous; nonetheless, adaptation of tasks used in human and non‐human primates to study cognitive processes, has helped define in a more operational way what can be considered decision‐making. For comparative studies in social and adaptive decision‐making see Tremblay et al. (2017) and Izquierdo & Belcher (2012).
Associated to sensory and motor decision‐making, processes such as incentive motivation, attention, and prediction are important. Tasks to study decision‐making in rats and mice at least involve, detection of signals from the environment, working memory if a delay is considered, and a plan of action based on the animal's choice or decision. The following paragraph contains brief descriptions of some examples of basic tasks used in mice and rats to study decision‐making. Summaries do not include prior habituation, basic training or motivational procedures, for more references on general procedures see Floresco et al. (2008a), Heilbronner (2017) and Winstanley & Floresco (2016). Since our interest is ventral motor thalamic nuclei, we concentrate on motor decision‐making. For perceptual decision‐making see section below and Carandini & Churchland (2013).
Effort‐based decision‐making usually includes a choice performed before entering an arm of a T‐maze. One arm offers some reinforcement (e.g., 2 food pellets) whereas the other offers a higher reinforcement (e.g., 4 food pellets) that becomes available only after climbing an obstacle (Floresco & Ghods‐Sharifi, 2007). Apart from climbing, another cost can involve for example, maintaining an upright position and poking a hole many times, to obtain a larger reward. In these tasks, control animals usually prefer the larger effort for a larger reward. For variations of this paradigm see Hauber & Sommer (2009) and Hillman & Bilkey (2010). Cost‐benefit decision‐making is derived from the paradigm described above, but instead of a physical effort, animals rank options in order to receive a higher reinforcement. For example, the animal is exposed to i‐ a high cost‐high benefit choice: between pure chocolate reward paired with the presence of an aversive strong light or ii‐ a low cost‐low benefit choice: between diluted chocolate milk paired with dim light (Friedman et al., 2017). In risk‐based decision‐making the animal has to choose between two chambers, each associated with different reinforcement delivery rates. For example, one pellet every 10 lever presses (low risk), or three pellets in 3 of 10 presses (high risk) (Pais‐Vieira et al., 2007). In risk‐discounting decision‐making, also called probabilistic discounting task, the testing chamber has two retractable levers on each side of the food tray. Sessions begin with the chamber light turning on and presentation of both levers. One lever delivers one food pellet every time it is pressed (low risk or “small/certain” choice) and the other delivers four pellets with an attached probability of 1, 0.5, 0.25, or 0.125 (high risk or “larger/risky” choice) (Floresco & Whelan, 2009; Jenni et al., 2017). For variations of this paradigm see for example Ghods‐Sharifi et al. (2009). Effort‐discounting decision‐making takes place in an environmental situation as above, but each trial lasts 40 s and starts with light illumination. One lever is designed to deliver a high reward (e.g., four food pellets) and the other a low reward (e.g., two food pellets). If the low reward lever is pressed, delivery follows immediately and lever is retracted. The high reward lever requires several presses within 25 s, and the number of necessary presses increases with training. The effort‐discounting consists in more presses for the same amount of food (Floresco et al., 2008b). For variations of this paradigm see Ghods‐Sharifi et al. (2009). A delay‐based decision‐making is often used to determine impulsivity in the decision‐making process. When performing this task, the animal has the choice of two levers, one provides one food pellet and the other five food pellets delivered after varying delays, that increment during the session (Evenden & Ryan, 1996). The use of a radial maze in delay‐based decision‐making, allows determination of the ability of the animal to track its own actions and environmental conditions. In this case, the location of the rewards changes between and within trials (Lapish et al., 2008). For variations of this paradigm see Khani et al. (2015). Sensory decision‐making requires the acquisition of some sensory information prior to making a choice or decision. This paradigm is frequently used when the animal touches objects placed in front of their whiskers. For example, to discriminate between different textures (Chen et al., 2013) or distance between objects (Celikel & Sakmann, 2007). For variations of this paradigm see Gire et al. (2013) and Hanks & Summerfield (2017). For references on the level of performance in decision‐making tasks between mice and rats see Jaramillo & Zador (2014).
Prefrontal cortex in decision‐making
The prefrontal cortex has been associated with higher cognitive functions including attention, decision‐making, working memory, and inhibition of previously established responses; for references in rats see Dalley et al. (2004). Although research in human and non‐human primates has contributed to the field of decision‐making by clarifying cognitive processes and participation of subjective evaluations among many others, research in rats and mice, has contributed to clarify neurobiological processes involved in spite of the vast inter‐ and intra‐species differences (Ellenbroek & Youn, 2016). Here, we use the nomenclature used in the brain atlas for mice (Franklin & Paxinos, 2008) but not without underlining that the connectivity and function between species of areas with the same name is seriously different. For a short comprehensive comparative anatomo‐functional description of the medial prefrontal cortex in the rat see Sesack et al. (1989).
Heidbreder & Groenewegen (2003) divided the territories of the rat prefrontal cortex in dorsal and ventral compartments. The dorsal compartment consisting of the medial precentral area and the anterior cingulate cortex (AC) and the ventral consisting of prelimbic (PrL), infralimbic (IL), and orbitofrontal (OFC) cortical areas. AC and OFC in rats and primates correspond to similar areas and PrL and IL in the rat, correspond to the ventromedial prefrontal cortex in primates (Chiba et al., 2001).
The prefrontal cortical areas AC, PrL, IL and OFC are interconnected, receive afferents from somatosensory and motor cortical areas (Bedwell et al., 2014) and send direct inputs to striatum (Sesack et al., 1989; Takagishi & Chiba, 1991; Hoover & Vertes, 2011).
Consistent with previous observations, for example, using antidromic activation (Thierry et al., 1983), Sesack et al. (1989) confirmed prefrontal cortical projections to other cortical areas, the forebrain, diencephalic, and brainstem structures. Projections from the prefrontal cortex include: other cortical areas [motor, sensorimotor and visual cortical fields]; the forebrain [striatum and amygdala], diencephalic areas [lateral hypothalamus and thalamus [reticular, anteromedial, anteroventral, ventrolateral nuclei], and brainstem [superior colliculus, ventral tegmengtum, periaqueductal gray, mesencephalic reticular formation, raphe nuclei, locus coeruleus].
Anterior cingulate cortex (AC)
In contrast with the demonstrated participation of this area in human and non‐human primates in tasks requiring attention and response selection (Fan et al., 2005), motor preparation and execution (Isomura et al., 2003), risk decision‐making (Heilbronner, 2017) and evaluation of expected value of “payoff” according to physical or mental effort (Shenhav et al., 2013), research in rats and mice was inconclusive at the beginning producing produced mainly negative results. No significant differences were observed between rats with bilateral AC excitotoxic lesions and control sham lesions. No differences were observed in tasks requiring stimulus‐reinforcer associations, responses to conditioned stimuli in the absence of reward, acquisition of discriminative stimuli, in a delayed‐discounting task or response of amphetamine infusion to nose‐poking in the food alcove or general locomotor activity (Cardinal et al., 2003). Moreover, Schweimer & Hauber (2005) concluded that AC did not interfere with motivation and it was not necessary for all decisions in a cost‐benefit task. Later on, using recordings of AC electrical activity in behaving rats, it was determined that AC is associated with decision‐making (Lapish et al., 2008; Hillman & Bilkey, 2010); briefly, neuronal firing in assemblies change with variations in task choices (task entries), outcomes (rewards) or context (progression of environmental conditions) (Lapish et al., 2008) and ninety‐three percent of recorded AC neurons, significantly increased their firing rate during performance in a spatial‐decision task associated with the high physical effort, high reward option (Hillman & Bilkey, 2010). Based on experiments in primates that report increased AC activity following an omission of an earned reinforcement or the delivery of an unexpected reinforcement (Ito et al., 2003), several studies in rats report that neurons of the AC increase their activity in association with errors in performance (Totah et al., 2009; Bryden et al., 2011), after an unexpected change in reinforcement value (Bryden et al., 2011) or task demands for a change in an ongoing response (Bryden et al., 2018).
Prelimbic (PrL) and infralimbic (IL) prefrontal cortices
Initial studies observed participation of both areas in working memory processes necessary to perform decision‐making tasks. Bilateral neurotoxic lesions cause impairment in working memory as lesions increase the number of wrong responses following the delays between presentation of cues (e.g. tone‐light) and a response (e.g., lever press) compared with controls (Delatour & Gisquet‐Verrier, 1999; Ragozzino et al., 2002). It was also proposed that PrL uses memory of particular locations or visual objects to generate plans of action (Seamans et al., 1995; Delatour & Gisquet‐Verrier, 1999). Further research has involved PrL in retrieval of previously encoded action‐outcome contingencies rather than in the encoding processes itself, as impaired performance in a delayed spatial win‐shift task only occurs when the PrL lesion is performed between the training and the testing phase (Seamans et al., 1995).
Studies dissociating PrL and IL functions, conclude that the function of PrL and IL may heavily rely on their connections with amygdala. Both areas participate in the association between aversive events and environmental cues and both seem involved in behavior assessment of possible risks and evaluation of the outcome of a motor plan. The function of IL is important for the expression of auditory‐triggered conditioned fear in situations of latent inhibition. If IL is inhibited (e.g., infusion of GABAergic agonist, or activation of inhibitory M4 DREADD) the ‘extinction’ or outcome‐specific inhibition disappears and the extinct behavior is reinstated (Do‐Monte et al., 2015; Laurent et al., 2016) and if IL is stimulated (e.g., ChR2 or GABAergic antagonist) the extinction is strengthen (Do‐Monte et al., 2015; Lingawi et al., 2018) and attenuation of responses to the conditioned stimulus is observed (Villaruel et al., 2018). Following a slightly different approach Halladay & Blair (2017) demonstrated that bilateral IL inactivation (GABAergic agonist) increases freezing responses and conditioned motor inhibition, whereas bilateral activation (GABAergic antagonist) enhances conditioned motor activation (turning away from an anticipated eyelid shock). Based on these results, the authors suggest that since they tested IL inhibition or excitation in exactly the same conditions it is possible that IL is playing a role in risk assessment in a contextually driven action selection.
PrL inactivation, impairs place avoidance conditioning (Jiang et al., 2014), moreover during extinction of a tone‐electric shock association, PrL neurons increase their activity (Milad & Quirk, 2002) and amplitude of local field potentials (Mears et al., 2009). For the participation of PrL and IL in fear expression and extinction see Sierra‐Mercado et al. (2011). More recent evidence indicates that these areas are involved in regulating reward seeking and fear behaviors. Optical intrastriatal inactivation of PrL axon terminals of rats performing a cost‐based decision‐making conflict‐task, increases selection of a high‐cost, high‐reward choice (Friedman et al., 2015). Reversible inactivation of PrL cortex in rats performing a risk‐based decision‐making task, also affects decision‐making by increasing the proportion of large and risky choices confirming its participation in the evaluation of changing reward probabilities (St Onge & Floresco, 2010). A recent study indicates that PrL and striatal activity is associated with responses to reward predicting cues, in particular if failures to respond occur. The authors propose that cue‐related experience‐dependent changes in PrL activity are induced by striatal feedback to cortex (Stubbendorff et al., 2018), that is by the thalamo‐cortical input to layer 1.
Although prefrontal neuronal populations expressing different receptor subtypes and the many effects of different neurotransmitters and modulators is a topic for an extensive review, here we mention a recent study of decision‐making as an example of a risk‐discounting task. With an elaborate design that involved local infusions of dopamine D1 or D2 antagonists into PrL, and inactivation of basolateral amygdala or nucleus accumbens, it was observed that rats under control conditions show: a‐ preference for a large/risky option if the probability of obtaining a larger reward increases or b‐ the preference for a larger reward gradually shifts the choice if the reward probabilities decrease. In experimental animals D1 or D2 dopaminergic modulation of PrL output neurons to nucleus accumbens, impairs risk/reward decision‐making, whereas only D2 receptors in cortical output neurons to basolateral amygdala, facilitate changes from an initial unfavorable selection, toward a more profitable choice (Jenni et al., 2017).
Orbitofrontal cortex (OFC)
The OFC is subdivided into five regions (medial, ventral, ventral lateral, lateral, and dorsolateral) and is considered as a part of the prefrontal cortex as it has have reciprocal connections with MD and the medial prefrontal cortex (Ongur & Price, 2000). Each of the subdivisions of the OFC have rich cortico‐cortical connections with limbic, sensory, and viceromotor structures (Ongur & Price, 2000; Schilman et al., 2008). In the rat, apart from the MD input (Kuramoto et al., 2017), the lateral orbitofrontal area interconnects with VM, and other ventral and central thalamic nuclei (Reep et al., 1996; Hoover & Vertes, 2011). The rich cortical and thalamic connections of the OFC contribute to its large repertoire of behavioral contributions, for a review see Izquierdo (2017).
A lesion of the OFC in primates, causes the impulsivity typical of the frontal lobe syndrome. In rats, the OFC is involved in the cognitive flexibility necessary to reverse the order of a previously learned task. For instance, OFC inactivation with local anesthetics or lesions, induces response perseverance and difficulty reversing a previously learned task (Boulougouris et al., 2007; Ghods‐Sharifi et al., 2008). Typically, rats with lesions in the OFC in a delay or risk‐based decision‐making task, show preference for a smaller reinforcement if it is either more immediate or more certain (Mobini et al., 2002). However, using a risk‐based decision‐making task that addresses free‐choice under uncertainty Pais‐Vieira et al. (2007) reported that, as observed in humans, OFC lesions result in a preference bias for a large risk, if large rewards can be obtained. Interestingly, OFC and striatal circuits further encode shifts between goal‐directed and habitual actions. Learning of novel tasks requires a shift of behavior toward goal‐directed actions. Activity of OFC and dorsomedial striatum increases during goal‐directed activity, while activity of dorsolateral striatum decreases (Gremel & Costa, 2013). For comparison of lesions in different subareas of the OFC see Izquierdo (2017).
Anterior lateral motor cortex (ALM)
The mouse prefrontal cortex has been mapped for its involvement in task specific activity of whisking and its participation in a delay‐based decision‐making task. Two areas of the mouse motor cortex, ALM and the medial motor cortex were mapped by studying behavior‐related cortical activity with a location for ALM, anterior 2.5 mm, 1.5 mm lateral and medial motor cortex, anterior 1.5 mm, lateral 1 mm; both corresponding to the dorsal and medial part of the frontal association area according to (Franklin & Paxinos, 2008). ALM makes reciprocal connections with the ipsilateral primary motor and somatosensory cortex and with the contralateral ALM. Moreover, it receives connections from ipsilateral ventral motor thalamic nuclei, MD, intralaminar and posterior nuclei‐ called thalALM (Guo et al., 2017). Using a whisker‐based object location, where mice detect the position of an object with their whiskers and after a delay report it by directional licking, combined with wide‐field calcium imaging, it was observed that the medial motor cortex is involved in persistent activity related to the whisker‐based object location, whereas ALM is related to voluntary delayed licking (Chen et al., 2017). Moreover, ALM shows a persistent preparatory activity that predicts licking direction. Task performance is reduced or enhanced following either contralateral or ipsilateral ALM photoinhibition, respectively. In naïve animals, the ALM persistent preparatory activity is driven by thalALM. Guo et al. (2017) conclude that ALM persistent preparatory activity necessarily requires the input activity from thalALM, and that ALM in turn, is the major drive of thalALM excitatory activity, a reciprocal positive feedback loop.
To conclude, the rodent medial prefrontal (AC, PrL, and IL) and the OFC participate in slightly different but complementary aspects involved in decision‐making. The AC responds to surprising situations that demand attention and redirection of a plan of action; PrL evaluates any retribution obtained for performed behaviors and IL plays a role in reinforcement devaluation and latent inhibition. Furthermore, proper function of OFC controls the desire for immediate reinforcement or certainty and allows reversal of previously well‐learned motor sequences. Although research in ALM is producing interesting results it remains to be determined if they are unique to the mouse brain.
Ventral motor thalamic nuclei and cortical activity in action selection and decision‐making
Evidence in this section is not limited to the medial prefrontal cortex, as ventral motor thalamic nuclei projects to motor, somatosensory and prefrontal cortices. Although most likely, prefrontal cortical activity is most involved in the sensory and motor elements of sensory and motor decision‐making, activity in other cortical areas is not irrelevant.
When humans and non‐human primates perform a delay‐based decision‐making task, neuronal activity slowly increases (ramps up), during the delay prior to responding. This type of activity has been related to decision‐making or preparation to respond, but not to the movement itself. Under these conditions, responses associated with motor planning have been observed in many areas. For example, in the dorsolateral prefrontal cortex (Kim & Shadlen, 1999), supplementary motor cortex (Okano & Tanji, 1987) dentate deep cerebellar nucleus (Ohmae et al., 2017) and VL nucleus of the ventral motor thalamic nuclei (Tanaka, 2007). In rats, ramp‐up responses have been observed in a few cases; for example, in the prefrontal cortex (Bregma anterior 2 mm, lateral 1.3 mm) in a memory guided orienting task (Erlich et al., 2011) and in the secondary motor cortex, in a delay‐based decision‐making task (Barthas & Kwan, 2017). In mice, reports have been more frequent than in rat studies thanks to head‐fixed recording procedures in awake animals performing whisker‐tactile behaviors designed by Guo et al. (2014a). With this method responses associated to different parts of the task can be isolated, including those related to preparatory or anticipatory movements (Guo et al., 2014b; Chen et al., 2017), and ramp‐up activity has been reported for neurons in ALM (Li et al., 2015, 2016).
In order for rats to learn any decision‐making tasks, objects or object location have to become significant. This process relies on the formation of a long‐lasting trace to sustain the reference memory. The strength of input from ventral motor thalamic nuclei to motor, somatosensory and prefrontal cortices has been reported to change after memory formation, for example, Biane et al. (2016) recorded in vitro monosynaptic responses of the rat primary motor cortex to optical stimulation of ventral motor thalamic nuclei VA/VL nuclei. Cortical neurons that innervate spinal cord segments and control grasp related muscles of distal forelimb, were compared with those controlling proximal forelimb muscles. After skilled grasp training, the response amplitude of neurons that innervate grasp related muscles was significantly larger compared with those controlling proximal forelimb muscles. A clear biased response in the behaviorally relevant neurons controlling distal forelimb was observed. This evidence supports the hypothesis that afferents of ventral motor thalamic nuclei to prefrontal cortex may be involved in regulating short and longer term processes involved in decision‐making and action selection.
Other studies in mice have provided direct evidence for the involvement of thalamic afferents to prefrontal cortex in decision‐making. Usually in these experiments, neuronal activity of a wide range of areas, i.e., ALM, somatosensory and motor (primary and secondary) cortical areas, is recorded while animals decide between characteristics or locations of objects. Helmchen et al. (2018) reviews experiments in the so called somatosensory triangle, formed by the somatosensory primary and secondary cortex and the primary motor cortex, that contributes to sensory discrimination, and highlights the role of the primary sensory cortical layer 2/3 in vibrissae touch events. Moreover, they report that active sensing involves signals traveling from sensory to motor primary cortical areas. They indicate that neurons in the primary motor cortex signal object location, movement direction or the correctness of each decision, and that neuronal activity during the delay period predicts the movement direction (Chen et al., 2013). When mice are trained to withhold their response for several seconds, persistent activity in ALM during waiting for movement was dependent on neurons of ventral motor thalamic nuclei. The role of the thalamocortical loop is seen with photoinhibition, if ALM is inhibited directly, or if thalALM is inhibited and ALM activity thus reduced to 6% of control, the activity of the animals in the task is disrupted underlining the importance of the thalamocortical loop. These results underline the importance of reciprocal connections between ventral motor thalamic nuclei and cortex in the persistent cortical activity related to motor preparation before execution of a motor plan (Guo et al., 2017).
Conclusions
In examining possible influences impacting the decision to make a choice we have used the idea that the output of the basal ganglia is probably involved in the complex pathway from information to action. The major output from the basal ganglia is processed via the output nuclei to the thalamic ventromedial nucleus in rodents, and the influence of the consequent thalamic inhibition is likely, in our view, to be an important route for decisions about actions to be made. Since disease of the basal ganglia has devastating effects on intentional movements an output from the subcortical network should influence motor ability. The usual way to think of this is via subcortical (descending) output pathways, but in addition the basal ganglia also connect back to cortex indirectly via the thalamus. What can this input, with its wide distribution and small influences on individual cortical cells, achieve? It seems it does not have the power to induce actions by itself, but perhaps by transferring information from striatal assemblies through several other networks back to cortex, it might select particular combinations of cells across several cortical areas to guide decisions or choices. Thalamic input might be an important ‘voice’ in making the decision to promulgate an action, although the details of the performance resides elsewhere.
In our review of the basic literature and more modern studies we support this idea with anatomical, electrophysiological, and some behavioral data that all suggest that thalamic fibers are in an important and potentially powerful situation to influence prefrontal cortical areas and their role in decision making. Of course, in some experiments the actions seen after thalamic manipulation may derive from the corticothalamic reciprocal connections alone but the influence of the basal ganglia on motor behavior is implicated directly from the evidence of their disruption in diseases. Although for the sake of clarity we have restricted our major arguments to rodents, there are clear homologies with the thalamocortical systems in ‘higher’ animals like ourselves. We hope we have introduced a future discussion of the role of basal ganglia return route to cortex in expressing the importance of remembered and ongoing information and its relevance for decisions about action. The emphasis in prefrontal areas seems suitable for such an influence though such a speculation needs experimental support and we hope this review will incite some relevant studies.
Conflict of interest
The authors declare no conflict of interest, financial, or otherwise.
Author contributions
B.S. wrote the manuscript; M.G.M. wrote some sections and edited the manuscript; G.W.A. reviewed the manuscript and provided formulation of comprehensive research goals, mentorship, and leadership.
Abbreviations
- AC
anterior cingulate cortex
- ALM
anterior lateral motor cortex
- ChR2
channelrhodopsin‐2
- DA
dopamine
- DREADD
designer receptors exclusively activated by designer drugs
- GAD67
glutamic acid decarboxylase (67kda)
- GPh
habenula‐projecting globus pallidus
- GPi
globus pallidus ‐internal segment‐
- IL
infralimbic
- OFC
orbitofrontal
- PrL
prelimbic
- SNr
substantia nigra pars reticulata
- thalAML
thalamic connections to ALM
- VA
ventral anterior
- vGluT2
vesicular glutamate transporter 2
- VL
ventrolateral
- VM
ventromedial
Supporting information
Acknowledgments
Funding for this work was provided by the Government of Japan.
Edited by Yoland Smith. Reviewed by Robert Turner.
All peer review communications can be found with the online version of the article.
References
- Aceves, J.J. , Rueda‐Orozco, P.E. , Hernandez‐Martinez, R. , Galarraga, E. & Bargas, J. (2011) Bidirectional plasticity in striatonigral synapses: a switch to balance direct and indirect basal ganglia pathways. Learn. Mem., 18, 764–773. [DOI] [PubMed] [Google Scholar]
- Afifi, A.K. (2003) The basal ganglia: a neural network with more than motor function. Semin. Peadiatr. Neurol., 10, 3–10. [DOI] [PubMed] [Google Scholar]
- Albin, R.L. , Young, A.B. & Penney, J.B. (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci., 12, 366–375. [DOI] [PubMed] [Google Scholar]
- Alcaraz, F. , Marchand, A.R. , Courtand, G. , Coutureau, E. & Wolff, M. (2016) Parallel inputs from the mediodorsal thalamus to the prefrontal cortex in the rat. Eur. J. Neuorsci., 44, 1972–1986. [DOI] [PubMed] [Google Scholar]
- Arbuthnott, G.W. , MacLeod, N.K. , Maxwell, D.J. & Wright, A.K. (1990) Distribution and synaptic contacts of the cortical terminals arising from neurons in the rat ventromedial thalamic nucleus. Neuroscience, 38, 47–60. [DOI] [PubMed] [Google Scholar]
- Barthas, F. & Kwan, A.C. (2017) Secondary motor cortex: where ‘sensory’ meets ‘motor’ in the rodent frontal cortex. Trends Neurosci., 40, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead, R.M. , Domesick, V.B. & Nauta, W.J. (1979) Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res., 175, 191–217. [DOI] [PubMed] [Google Scholar]
- Bedwell, S.A. , Billett, E.E. , Crofts, J.J. & Tinsley, C.J. (2014) The topology of connections between rat prefrontal, motor and sensory cortices. Front. Syst. Neurosci., 8, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biane, J.S. , Takashima, Y. , Scanziani, M. , Conner, J.M. & Tuszynski, M.H. (2016) Thalamocortical projections onto behaviorally relevant neurons exhibit plasticity during adult motor learning. Neuron, 89, 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam, J.P. & Smith, Y. (1992) The striatum and the globus pallidus send convergent synaptic inputs onto single cells in the entopeduncular nucleus of the rat: a double anterograde labelling study combined with postembedding immunocytochemistry for GABA. J. Comp. Neurol., 321, 456–476. [DOI] [PubMed] [Google Scholar]
- Boulougouris, V. , Dalley, J.W. & Robbins, T.W. (2007) Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav. Brain Res., 179, 219–228. [DOI] [PubMed] [Google Scholar]
- Bryden, D.W. , Johnson, E.E. , Tobia, S.C. , Kashtelyan, V. & Roesch, M.R. (2011) Attention for learning signals in anterior cingulate cortex. J. Neurosci., 31, 18266–18274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden, D.W. , Brockett, A.T. , Blume, E. , Heatley, K. , Zhao, A. & Roesch, M.R. (2018) Single neurons in anterior cingulate cortex signal the need to change action during performance of a stop‐change task that induces response competition. Cereb. Cortex, 10.1093/cercor/bhy008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales, J.J. & Graybiel, A.M. (2000) A measure of striatal function predicts motor stereotypy. Nat. Neurosci., 3, 377–383. [DOI] [PubMed] [Google Scholar]
- Carandini, M. & Churchland, A.K. (2013) Probing perceptual decisions in rodents. Nat. Neurosci., 16, 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal, R.N. , Parkinson, J.A. , Marbini, H.D. , Toner, A.J. , Bussey, T.J. , Robbins, T.W. & Everitt, B.J. (2003) Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav. Neurosci., 117, 566–587. [DOI] [PubMed] [Google Scholar]
- Carman, J.B. , Cowan, W.M. & Powell, T.P. (1963) The organization of cortico‐striate connexionsin the rabbit. Brain, 86, 525–562. [DOI] [PubMed] [Google Scholar]
- Carvalho Poyraz, F. , Holzner, E. , Bailey, M.R. , Meszaros, J. , Kenney, L. , Kheirbek, M.A. , Balsam, P.D. & Kellendonk, C. (2016) Decreasing striatopallidal pathway function enhances motivation by energizing the initiation of goal‐directed action. J. Neurosci., 36, 5988–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada, C. & Goldman‐Rakic, P.S. (1991) Topographic segregation of corticostriatal projections from posterior parietal subdivisions in the macaque monkey. Neuroscience, 42, 683–696. [DOI] [PubMed] [Google Scholar]
- Cavdar, S. , San, T. , Aker, R. , Sehirli, U. & Onat, F. (2001) Cerebellar connections to the dorsomedial and posterior nuclei of the hypothalamus in the rat. J. Anat., 198, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavdar, S. , Ozgur, M. , Uysal, S.P. & Amuk, O.C. (2014) Motor afferents from the cerebellum, zona incerta and substantia nigra to the mediodorsal thalamic nucleus in the rat. J. Int. Neurosci., 13, 565–578. [DOI] [PubMed] [Google Scholar]
- Cebrian, C. , Parent, A. & Prensa, L. (2005) Patterns of axonal branching of neurons of the substantia nigra pars reticulata and pars lateralis in the rat. J. Comp. Neurol., 492, 349–369. [DOI] [PubMed] [Google Scholar]
- Celikel, T. & Sakmann, B. (2007) Sensory integration across space and in time for decision making in the somatosensory system of rodents. Proc. Natl. Acad. Sci. USA, 104, 1395–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J.L. , Carta, S. , Soldado‐Magraner, J. , Schneider, B.L. & Helmchen, F. (2013) Behaviour‐dependent recruitment of long‐range projection neurons in somatosensory cortex. Nature, 499, 336–340. [DOI] [PubMed] [Google Scholar]
- Chen, T.W. , Li, N. , Daie, K. & Svoboda, K. (2017) A map of anticipatory activity in mouse motor cortex. Neuron, 94, 866–879 e864. [DOI] [PubMed] [Google Scholar]
- Chiba, T. , Kayahara, T. & Nakano, K. (2001) Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata . Brain Res., 888, 83–101. [DOI] [PubMed] [Google Scholar]
- Collins, D.P. , Anastasiades, P.G. , Marlin, J.J. & Carter, A.G. (2018) Reciprocal circuits linking the prefrontal cortex with dorsal and ventral thalamic nuclei. Neuron, 98, 366–379 e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank, S.J. , Ahmed, O.J. , Stevens, T.R. , Patrick, S.L. , Gonzalez, A.N. , Elmaleh, M. & Connors, B.W. (2012) Thalamic control of layer 1 circuits in prefrontal cortex. J. Neurosci., 32, 17813–17823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley, J.W. , Cardinal, R.N. & Robbins, T.W. (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev., 28, 771–784. [DOI] [PubMed] [Google Scholar]
- Deffeyes, J.E. , Touvykine, B. , Quessy, S. , & Dancause, N. (2015). Interactions between rostral and caudal cortical motor areas in the rat. J. Neurophys., 113, 3893–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatour, B. & Gisquet‐Verrier, P. (1999) Lesions of the prelimbic‐infralimbic cortices in rats do not disrupt response selection processes but induce delay‐dependent deficits: evidence for a role in working memory? Behav. Neurosci., 113, 941–955. [DOI] [PubMed] [Google Scholar]
- Delevich, K. , Tucciarone, J. , Huang, Z.J. & Li, B. (2015) The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J. Neurosci., 35, 5743–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniau, J.M. & Chevalier, G. (1984) Synaptic organization of the basal ganglia: an electroanatomical approach in the rat. Ciba Found. Symp., 107, 48–63. [DOI] [PubMed] [Google Scholar]
- Deniau, J.M. & Chevalier, G. (1992) The lamellar organization of the rat substantia nigra pars reticulata: distribution of projection neurons. Neuroscience, 46, 361–377. [DOI] [PubMed] [Google Scholar]
- Deniau, J.M. , Kita, H. & Kitai, S.T. (1992) Patterns of termination of cerebellar and basal ganglia efferents in the rat thalamus. Strictly segregated and partly overlapping projections. Neurosci. Lett., 144, 202–206. [DOI] [PubMed] [Google Scholar]
- Di Chiara, G. , Porceddu, M.L. , Morelli, M. , Mulas, M.L. & Gessa, G.L. (1979) Evidence for a GABAergic projection from the substantia nigra to the ventromedial thalamus and to the superior colliculus of the rat. Brain Res., 176, 273–284. [DOI] [PubMed] [Google Scholar]
- Do‐Monte, F.H. , Manzano‐Nieves, G. , Quinones‐Laracuente, K. , Ramos‐Medina, L. & Quirk, G.J. (2015) Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J. Neurosci., 35, 3607–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek, B. & Youn, J. (2016) Rodent models in neuroscience research: is it a rat race? Dis. Model. Mech., 9, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich, J.C. , Bialek, M. & Brody, C.D. (2011) A cortical substrate for memory‐guided orienting in the rat. Neuron, 72, 330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden, J.L. & Ryan, C.N. (1996) The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology, 128, 161–170. [DOI] [PubMed] [Google Scholar]
- Fan, J. , McCandliss, B.D. , Fossella, J. , Flombaum, J.I. & Posner, M.I. (2005) The activation of attentional networks. NeuroImage, 26, 471–479. [DOI] [PubMed] [Google Scholar]
- Feger, J. , Deniau, J.M. , Hammond‐Le Guyader, C. & Ohye, C. (1976) Connections from the basal ganglia to the thalamus. Appl. Neurophysiol., 39, 272–284. [DOI] [PubMed] [Google Scholar]
- Floresco, S.B. & Ghods‐Sharifi, S. (2007) Amygdala‐prefrontal cortical circuitry regulates effort‐based decision making. Cereb. Cortex, 17, 251–260. [DOI] [PubMed] [Google Scholar]
- Floresco, S.B. & Whelan, J.M. (2009) Perturbations in different forms of cost/benefit decision making induced by repeated amphetamine exposure. Psychopharmacology, 205, 189–201. [DOI] [PubMed] [Google Scholar]
- Floresco, S.B. , St Onge, J.R. , Ghods‐Sharifi, S. & Winstanley, C.A. (2008a) Cortico‐limbic‐striatal circuits subserving different forms of cost‐benefit decision making. Cogn. Affect. Behav. Neurosci., 8, 375–389. [DOI] [PubMed] [Google Scholar]
- Floresco, S.B. , Tse, M.T. & Ghods‐Sharifi, S. (2008b) Dopaminergic and glutamatergic regulation of effort‐ and delay‐based decision making. Neuropsychopharmacology, 33, 1966–1979. [DOI] [PubMed] [Google Scholar]
- Franklin, K.B.J. & Paxinos, G. (2008). The Mouse Brain in Stereotaxic Coordinates. Elsevier, New York. [Google Scholar]
- Friedman, A. , Homma, D. , Gibb, L.G. , Amemori, K. , Rubin, S.J. , Hood, A.S. , Riad, M.H. & Graybiel, A.M. (2015) A corticostriatal path targeting striosomes controls decision‐making under conflict. Cell, 161, 1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, A. , Homma, D. , Bloem, B. , Gibb, L.G. , Amemori, K.I. , Hu, D. , Delcasso, S. , Truong, T.F. et al (2017) Chronic stress alters striosome‐circuit dynamics, leading to aberrant decision‐making. Cell, 171, 1191–1205 e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidica, M. , Hurst, A. , Cyr, C. & Leventhal, D.K. (2018) Distinct populations of motor thalamic neurons encode action initiation, action selection, and movement vigor. J. Neurosci., 38, 6563‐6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen, C.R. , Staines, W.A. , Arbuthnott, G.W. & Fibiger, H.C. (1982) Crossed connections of the substantia nigra in the rat. J. Comp. Neurol., 207, 283–303. [DOI] [PubMed] [Google Scholar]
- Ghods‐Sharifi, S. , Haluk, D.M. & Floresco, S.B. (2008) Differential effects of inactivation of the orbitofrontal cortex on strategy set‐shifting and reversal learning. Neurobiol. Learn. Mem., 89, 567–573. [DOI] [PubMed] [Google Scholar]
- Ghods‐Sharifi, S. , St Onge, J.R. & Floresco, S.B. (2009) Fundamental contribution by the basolateral amygdala to different forms of decision making. J. Neurosci., 29, 5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire, D.H. , Whitesell, J.D. , Doucette, W. & Restrepo, D. (2013) Information for decision‐making and stimulus identification is multiplexed in sensory cortex. Nat. Neurosci., 16, 991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman‐Rakic, P.S. (1983) The corticostriatal fiber system in the rhesus monkey: organization and development. Prog. Brain Res., 58, 405–418. [DOI] [PubMed] [Google Scholar]
- Graybiel, A.M. (1991) Basal ganglia–input, neural activity, and relation to the cortex. Curr. Opin. Neurobiol., 1, 644–651. [DOI] [PubMed] [Google Scholar]
- Gremel, C.M. & Costa, R.M. (2013) Orbitofrontal and striatal circuits dynamically encode the shift between goal‐directed and habitual actions. Nat. Commun., 4, 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen, H.J. (1988) Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal prefrontal topography. Neuroscience, 24, 379–431. [DOI] [PubMed] [Google Scholar]
- Gulcebi, M.I. , Ketenci, S. , Linke, R. , Hacioglu, H. , Yanali, H. , Veliskova, J. , Moshe, S.L. , Onat, F. et al (2012) Topographical connections of the substantia nigra pars reticulata to higher‐order thalamic nuclei in the rat. Brain Res. Bull., 87, 312–318. [DOI] [PubMed] [Google Scholar]
- Guo, Z.V. , Hires, S.A. , Li, N. , O'Connor, D.H. , Komiyama, T. , Ophir, E. , Huber, D. , Bonardi, C. et al (2014a) Procedures for behavioral experiments in head‐fixed mice. PLoS ONE, 9, e88678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Z.V. , Li, N. , Huber, D. , Ophir, E. , Gutnisky, D. , Ting, J.T. , Feng, G. & Svoboda, K. (2014b) Flow of cortical activity underlying a tactile decision in mice. Neuron, 81, 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Z.V. , Inagaki, H.K. , Daie, K. , Druckmann, S. , Gerfen, C.R. & Svoboda, K. (2017) Maintenance of persistent activity in a frontal thalamocortical loop. Nature, 545, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber, S.N. (2016) Corticostriatal circuitry. Dialogues Clin. Neurosci., 18, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay, L.R. & Blair, H.T. (2017) Prefrontal infralimbic cortex mediates competition between excitation and inhibition of body movements during pavlovian fear conditioning. J. Neurosci. Res., 95, 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks, T.D. & Summerfield, C. (2017) Perceptual decision making in rodents, monkeys, and humans. Neuron, 93, 15–31. [DOI] [PubMed] [Google Scholar]
- Hardman, C.D. , Henderson, J.M. , Finkelstein, D.I. , Horne, M.K. , Paxinos, G. & Halliday, G.M. (2002) Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. J. Comp. Neurol., 445, 238–255. [DOI] [PubMed] [Google Scholar]
- Hattox, A.M. & Nelson, S.B. (2007) Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J. Neurophysiol., 98, 3330–3340. [DOI] [PubMed] [Google Scholar]
- Hauber, W. & Sommer, S. (2009) Prefrontostriatal circuitry regulates effort‐related decision making. Cereb. Cortex, 19, 2240–2247. [DOI] [PubMed] [Google Scholar]
- Heidbreder, C.A. & Groenewegen, H.J. (2003) The medial prefrontal cortex in the rat: evidence for a dorso‐ventral distinction based upon functional and anatomical characteristics. Neurosci. Biobehav. Rev., 27, 555–579. [DOI] [PubMed] [Google Scholar]
- Heilbronner, S.R. (2017) Modeling risky decision‐making in nonhuman animals: shared core features. Curr. Opin. Behav. Sci., 16, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen, F. , Gilad, A. & Chen, J.L. (2018) Neocortical dynamics during whisker‐based sensory discrimination in head‐restrained mice. Neuroscience, 368, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham, M. (1979) The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J. Comp. Neurol., 183, 487–517. [DOI] [PubMed] [Google Scholar]
- Hillman, K.L. & Bilkey, D.K. (2010) Neurons in the rat anterior cingulate cortex dynamically encode cost‐benefit in a spatial decision‐making task. J. Neurosci., 30, 7705–7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintzen, A. , Pelzer, E.A. & Tittgemeyer, M. (2018) Thalamic interactions of cerebellum and basal ganglia. Brain Struct. Funct., 223, 569–587. [DOI] [PubMed] [Google Scholar]
- Hoover, W.B. & Vertes, R.P. (2011) Projections of the medial orbital and ventral orbital cortex in the rat. J. Comp. Neurol., 519, 3766–3801. [DOI] [PubMed] [Google Scholar]
- Isomura, Y. , Ito, Y. , Akazawa, T. , Nambu, A. & Takada, M. (2003) Neural coding of “attention for action” and “response selection” in primate anterior cingulate cortex. J. Neurosci., 23, 8002–8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, S. , Stuphorn, V. , Brown, J.W. & Schall, J.D. (2003) Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science, 302, 120–122. [DOI] [PubMed] [Google Scholar]
- Izquierdo, A. (2017) Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. J. Neurosci., 37, 10597–10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo, A. & Belcher, A.M. (2012) Rodent models of adaptive decision making. Methods Mol. Biol., 829, 85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger, D. , Gilman, S. & Aldridge, J.W. (1995) Neuronal activity in the striatum and pallidum of primates related to the execution of externally cued reaching movements. Brain Res., 694, 111–127. [DOI] [PubMed] [Google Scholar]
- Jaramillo, S. & Zador, A.M. (2014) Mice and rats achieve similar levels of performance in an adaptive decision‐making task. Front. Syst. Neurosci., 8, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeljeli, M. , Strazielle, C. , Caston, J. & Lalonde, R. (2003) Effects of ventrolateral‐ventromedial thalamic lesions on motor coordination and spatial orientation in rats. Neurosci. Res., 47, 309–316. [DOI] [PubMed] [Google Scholar]
- Jenni, N.L. , Larkin, J.D. & Floresco, S.B. (2017) Prefrontal dopamine D1 and D2 receptors regulate dissociable aspects of decision making via distinct ventral striatal and amygdalar circuits. J. Neurosci., 37, 6200–6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X. , Wang, G. , Lee, A.J. , Stornetta, R.L. & Zhu, J.J. (2013) The organization of two new cortical interneuronal circuits. Nat. Neurosci., 16, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Z.C. , Pan, Q. , Zheng, C. , Deng, X.F. , Wang, J.Y. & Luo, F. (2014) Inactivation of the prelimbic rather than infralimbic cortex impairs acquisition and expression of formalin‐induced conditioned place avoidance. Neurosci. Lett., 569, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnai, K. , Nambu, A. & Yoshida, S. (1987) Thalamic afferents to layer I of anterior sigmoid cortex originating from the VA‐VL neurons with entopeduncular input. Exp. Brain Res., 69, 67–76. [DOI] [PubMed] [Google Scholar]
- Joel, D. & Weiner, I. (1994) The organization of the basal ganglia‐thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience, 63, 363–379. [DOI] [PubMed] [Google Scholar]
- Kase, D. , Uta, D. , Ishihara, H. & Imoto, K. (2015) Inhibitory synaptic transmission from the substantia nigra pars reticulata to the ventral medial thalamus in mice. Neurosci. Res., 97, 26–35. [DOI] [PubMed] [Google Scholar]
- Kemp, J.M. & Powell, T.P. (1970) The cortico‐striate projection in the monkey. Brain, 93, 525–546. [DOI] [PubMed] [Google Scholar]
- Kha, H.T. , Finkelstein, D.I. , Pow, D.V. , Lawrence, A.J. & Horne, M.K. (2000) Study of projections from the entopeduncular nucleus to the thalamus of the rat. J. Comp. Neurol., 426, 366–377. [PubMed] [Google Scholar]
- Kha, H.T. , Finkelstein, D.I. , Tomas, D. , Drago, J. , Pow, D.V. & Horne, M.K. (2001) Projections from the substantia nigra pars reticulata to the motor thalamus of the rat: single axon reconstructions and immunohistochemical study. J. Comp. Neurol., 440, 20–30. [DOI] [PubMed] [Google Scholar]
- Khani, A. , Kermani, M. , Hesam, S. , Haghparast, A. , Argandona, E.G. & Rainer, G. (2015) Activation of cannabinoid system in anterior cingulate cortex and orbitofrontal cortex modulates cost‐benefit decision making. Psychopharmacology, 232, 2097–2112. [DOI] [PubMed] [Google Scholar]
- Kim, J.N. & Shadlen, M.N. (1999) Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat. Neurosci., 2, 176–185. [DOI] [PubMed] [Google Scholar]
- Kim, J. , Kim, Y. , Nakajima, R. , Shin, A. , Jeong, M. , Park, A.H. , Jeong, Y. , Jo, S. et al (2017) Inhibitory basal ganglia inputs induce excitatory motor signals in the thalamus. Neuron, 95, 1181–1196 e1188. [DOI] [PubMed] [Google Scholar]
- Klaus, A. , Martins, G.J. , Paixao, V.B. , Zhou, P. , Paninski, L. & Costa, R.M. (2017) The spatiotemporal organization of the striatum encodes action space. Neuron, 95, 1171–1180 e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomiets, B.P. , Deniau, J.M. , Glowinski, J. & Thierry, A.M. (2003) Basal ganglia and processing of cortical information: functional interactions between trans‐striatal and trans‐subthalamic circuits in the substantia nigra pars reticulata. Neuroscience, 117, 931–938. [DOI] [PubMed] [Google Scholar]
- Kreitzer, A.K. & Berke, J.D. (2011) Investigating striatal function through cell‐type‐specific manipulations. Neuroscience, 198, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto, E. , Furuta, T. , Nakamura, K.C. , Unzai, T. , Hioki, H. & Kaneko, T. (2009) Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron‐tracing study using viral vectors. Cereb. Cortex, 19, 2065–2077. [DOI] [PubMed] [Google Scholar]
- Kuramoto, E. , Fujiyama, F. , Nakamura, K.C. , Tanaka, Y. , Hioki, H. & Kaneko, T. (2011) Complementary distribution of glutamatergic cerebellar and GABAergic basal ganglia afferents to the rat motor thalamic nuclei. Eur. J. Neuorsci., 33, 95–109. [DOI] [PubMed] [Google Scholar]
- Kuramoto, E. , Ohno, S. , Furuta, T. , Unzai, T. , Tanaka, Y.R. , Hioki, H. & Kaneko, T. (2015) Ventral medial nucleus neurons send thalamocortical afferents more widely and more preferentially to layer 1 than neurons of the ventral anterior‐ventral lateral nuclear complex in the rat. Cereb. Cortex, 25, 221–235. [DOI] [PubMed] [Google Scholar]
- Kuramoto, E. , Pan, S. , Furuta, T. , Tanaka, Y.R. , Iwai, H. , Yamanaka, A. , Ohno, S. , Kaneko, T. et al (2017) Individual mediodorsal thalamic neurons project to multiple areas of the rat prefrontal cortex: A single neuron‐tracing study using virus vectors. J. Comp. Neurol., 525, 166–185. [DOI] [PubMed] [Google Scholar]
- Kuroda, M. , Yokofujita, J. & Murakami, K. (1998) An ultrastructural study of the neural circuit between the prefrontal cortex and the mediodorsal nucleus of the thalamus. Prog. Neurobiol., 54, 417–458. [DOI] [PubMed] [Google Scholar]
- Lapish, C.C. , Durstewitz, D. , Chandler, L.J. & Seamans, J.K. (2008) Successful choice behavior is associated with distinct and coherent network states in anterior cingulate cortex. Proc. Natl. Acad. Sci. USA, 105, 11963–11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum, M.E. & Zhu, J.J. (2002) Signaling of layer 1 and whisker‐evoked Ca2 + and Na+ action potentials in distal and terminal dendrites of rat neocortical pyramidal neurons in vitro and in vivo. J. Neurosci., 22, 6991–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum, M.E. , Nevian, T. , Sandler, M. , Polsky, A. & Schiller, J. (2009) Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science, 325, 756–760. [DOI] [PubMed] [Google Scholar]
- Laurent, V. , Chieng, B. & Balleine, B.W. (2016) Extinction generates outcome‐specific conditioned inhibition. Curr. Biol., 26, 3169–3175. [DOI] [PubMed] [Google Scholar]
- Laurent, M. , De Backer, J.F. , Rial, D. , Schiffmann, S.N. & de Kerchove d'Exaerde, A. (2017) Bidirectional control of reversal in a dual action task by direct and indirect pathway activation in the dorsolateral striatum in mice. Front. Behav. Neurosci., 11, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond, M. , Fan, D. , Brynildsen, J.K. & Yin, H.H. (2011) Motivational state and reward content determine choice behavior under risk in mice. PLoS ONE, 6, e25342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, R.G. (1984) Physiology of the basal ganglia: an overview. Can. J. Neurol. Sci., 11, 124–128. [DOI] [PubMed] [Google Scholar]
- Lee, A.J. , Wang, G. , Jiang, X. , Johnson, S.M. , Hoang, E.T. , Lante, F. , Stornetta, R.L. , Beenhakker, M.P. et al (2015) Canonical organization of layer 1 neuron‐led cortical inhibitory and disinhibitory interneuronal circuits. Cereb. Cortex, 25, 2114–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Chen, T.W. , Guo, Z.V. , Gerfen, C.R. & Svoboda, K. (2015) A motor cortex circuit for motor planning and movement. Nature, 519, 51–56. [DOI] [PubMed] [Google Scholar]
- Li, N. , Daie, K. , Svoboda, K. & Druckmann, S. (2016) Robust neuronal dynamics in premotor cortex during motor planning. Nature, 532, 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingawi, N.W. , Holmes, N.M. , Westbrook, R.F. & Laurent, V. (2018) The infralimbic cortex encodes inhibition irrespective of motivational significance. Neurobiol. Learn. Mem., 150, 64–74. [DOI] [PubMed] [Google Scholar]
- Mears, R.P. , Boutros, N.N. & Cromwell, H.C. (2009) Reduction of prelimbic inhibitory gating of auditory evoked potentials after fear conditioning. Behav. Neurosci., 123, 315–327. [DOI] [PubMed] [Google Scholar]
- Milad, M.R. & Quirk, G.J. (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature, 420, 70–74. [DOI] [PubMed] [Google Scholar]
- Mitchell, B.D. & Cauller, L.J. (2001) Corticocortical and thalamocortical projections to layer I of the frontal neocortex in rats. Brain Res., 921, 68–77. [DOI] [PubMed] [Google Scholar]
- Mobini, S. , Body, S. , Ho, M.Y. , Bradshaw, C.M. , Szabadi, E. , Deakin, J.F. & Anderson, I.M. (2002) Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology, 160, 290–298. [DOI] [PubMed] [Google Scholar]
- Moon, H.C. , Won, S.Y. , Kim, E.G. , Kim, H.K. , Cho, C.B. & Park, Y.S. (2018) Effect of optogenetic modulation on entopeduncular input affects thalamic discharge and behavior in an AAV2‐alpha‐synuclein‐induced hemiparkinson rat model. Neurosci. Lett., 662, 129–135. [DOI] [PubMed] [Google Scholar]
- Nakamura, K.C. , Sharott, A. & Magill, P.J. (2014) Temporal coupling with cortex distinguishes spontaneous neuronal activities in identified basal ganglia‐recipient and cerebellar‐recipient zones of the motor thalamus. Cereb. Cortex, 24, 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey, E.J. , Bold, E.L. , Haas, G. , Hurley‐Gius, K.M. , Quirk, G. , Sievert, C.F. & Terreberry, R.R. (1986) The organization of the rat motor cortex: a microstimulation mapping study. Brain Res., 396, 77–96. [DOI] [PubMed] [Google Scholar]
- Ohmae, S. , Kunimatsu, J. & Tanaka, M. (2017) Cerebellar roles in self‐timing for sub‐ and supra‐second intervals. J. Neurosci., 37, 3511–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano, K. & Tanji, J. (1987) Neuronal activities in the primate motor fields of the agranular frontal cortex preceding visually triggered and self‐paced movement. Exp. Brain Res., 66, 155–166. [DOI] [PubMed] [Google Scholar]
- Ongur, D. & Price, J.L. (2000) The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex, 10, 206–219. [DOI] [PubMed] [Google Scholar]
- Pais‐Vieira, M. , Lima, D. & Galhardo, V. (2007) Orbitofrontal cortex lesions disrupt risk assessment in a novel serial decision‐making task for rats. Neuroscience, 145, 225–231. [DOI] [PubMed] [Google Scholar]
- Preuss, T.M. & Goldman‐Rakic, P.S. (1987) Crossed corticothalamic and thalamocortical connections of macaque prefrontal cortex. J. Comp. Neurol., 257, 269–281. [DOI] [PubMed] [Google Scholar]
- Pribram, K.H. (1975) The primate frontal cortex: progress report 1975. Acta Neurobiol. Exp., 35, 609–625. [PubMed] [Google Scholar]
- Ragozzino, M.E. , Detrick, S. & Kesner, R.P. (2002) The effects of prelimbic and infralimbic lesions on working memory for visual objects in rats. Neurobiol. Learn. Mem., 77, 29–43. [DOI] [PubMed] [Google Scholar]
- Reep, R.L. , Corwin, J.V. & King, V. (1996) Neuronal connections of orbital cortex in rats: topography of cortical and thalamic afferents. Exp. Brain Res., 111, 215–232. [DOI] [PubMed] [Google Scholar]
- Romo, R. , Scarnati, E. & Schultz, W. (1992) Role of primate basal ganglia and frontal cortex in the internal generation of movements. I Preparatory activity. Exp. Brain Res., 91, 385–395. [DOI] [PubMed] [Google Scholar]
- Rubio‐Garrido, P. , Perez‐de‐Manzo, F. & Clasca, F. (2007) Calcium‐binding proteins as markers of layer‐I projecting vs. deep layer‐projecting thalamocortical neurons: a double‐labeling analysis in the rat. Neuroscience, 149, 242–250. [DOI] [PubMed] [Google Scholar]
- Rubio‐Garrido, P. , Perez‐de‐Manzo, F. , Porrero, C. , Galazo, M.J. & Clasca, F. (2009) Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb. Cortex, 19, 2380–2395. [DOI] [PubMed] [Google Scholar]
- Sawyer, S.F. , Martone, M.E. & Groves, P.M. (1991) A GABA immunocytochemical study of rat motor thalamus: light and electron microscopic observations. Neuroscience, 42, 103–124. [DOI] [PubMed] [Google Scholar]
- Scheibel, M.E. & Scheibel, A.B. (1966) The organization of the ventral anterior nucleus of the thalamus. A Golgi study. Brain Res., 1, 250–268. [DOI] [PubMed] [Google Scholar]
- Schilman, E.A. , Uylings, H.B. , Galis‐de Graaf, Y. , Joel, D. & Groenewegen, H.J. (2008) The orbital cortex in rats topographically projects to central parts of the caudate‐putamen complex. Neurosci. Lett., 432, 40–45. [DOI] [PubMed] [Google Scholar]
- Schweimer, J. & Hauber, W. (2005) Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn. Mem., 12, 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans, J.K. , Floresco, S.B. & Phillips, A.G. (1995) Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav. Neurosci., 109, 1063–1073. [DOI] [PubMed] [Google Scholar]
- Seeger‐Armbruster, S. , Bosch‐Bouju, C. , Little, S.T. , Smither, R.A. , Hughes, S.M. , Hyland, B.I. & Parr‐Brownlie, L.C. (2015) Patterned, but not tonic, optogenetic stimulation in motor thalamus improves reaching in acute drug‐induced Parkinsonian rats. J. Neurosci., 35, 1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack, S.R. , Deutch, A.Y. , Roth, R.H. & Bunney, B.S. (1989) Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract‐tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol., 290, 213–242. [DOI] [PubMed] [Google Scholar]
- Shenhav, A. , Botvinick, M.M. & Cohen, J.D. (2013) The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron, 79, 217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlosberg, D. , Amitai, Y. & Azouz, R. (2006) Time‐dependent, layer‐specific modulation of sensory responses mediated by neocortical layer 1. J. Neurophysiol., 96, 3170–3182. [DOI] [PubMed] [Google Scholar]
- Sierra‐Mercado, D. , Padilla‐Coreano, N. & Quirk, G.J. (2011) Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology, 36, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, Y. , Galvan, A. , Ellender, T.J. , Doig, N. , Villalba, R.M. , Huerta‐Ocampo, I. , Wichmann, T. & Bolam, J.P. (2014a) The thalamostriatal system in normal and diseased states. Front. Syst. Neurosci., 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, Y. , Wichmann, T. & DeLong, M.R. (2014b) Corticostriatal and mesocortical dopamine systems: do species differences matter? Nat. Rev., 15, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohur, U.S. , Padmanabhan, H.K. , Kotchetkov, I.S. , Menezes, J.R. & Macklis, J.D. (2014) Anatomic and molecular development of corticostriatal projection neurons in mice. Cereb. Cortex, 24, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge, J.R. & Floresco, S.B. (2010) Prefrontal cortical contribution to risk‐based decision making. Cereb. Cortex, 20, 1816–1828. [DOI] [PubMed] [Google Scholar]
- Starr, M.S. & Summerhayes, M. (1983) Role of the ventromedial nucleus of the thalamus in motor behaviour–II. Effects of lesions. Neuroscience, 10, 1171–1183. [DOI] [PubMed] [Google Scholar]
- Stubbendorff, C. , Molano‐Mazon, M. , Young, A.M. & Gerdjikov, T.V. (2018) Synchronisation in the prefrontal‐striatal circuit tracks behavioural choice in a go no‐go task in rats. Eur. J. Neuorsci., 10.1111/ejn.13905 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Takagishi, M. & Chiba, T. (1991) Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA‐L study. Brain Res., 566, 26–39. [DOI] [PubMed] [Google Scholar]
- Tanaka, M. (2007) Cognitive signals in the primate motor thalamus predict saccade timing. J. Neurosci., 27, 12109–12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, D. Jr , Isaacson, L.G. & Trosko, B.K. (1986) Thalamostriatal projections from the ventral anterior nucleus in the dog. J. Comp. Neurol., 247, 56–68. [DOI] [PubMed] [Google Scholar]
- Tennant, K.A. , Adkins, D.L. , Donlan, N.A. , Asay, A.L. , Thomas, N. , Kleim, J.A. , & Jones, T.A. (2011). The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb Cortex, 21, 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry, A.M. , Chevalier, G. , Ferron, A. & Glowinski, J. (1983) Diencephalic and mesencephalic efferents of the medial prefrontal cortex in the rat: electrophysiological evidence for the existence of branched axons. Exp. Brain Res., 50, 275–282. [DOI] [PubMed] [Google Scholar]
- Totah, N.K. , Kim, Y.B. , Homayoun, H. & Moghaddam, B. (2009) Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. J. Neurosci., 29, 6418–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, S. , Sharika, K.M. & Platt, M.L. (2017) Social decision‐making and the brain: a comparative perspective. Trends Cogn. Sci., 21, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumori, T. , Yokota, S. , Lai, H. & Yasui, Y. (2000) Monosynaptic and disynaptic projections from the substantia nigra pars reticulata to the parafascicular thalamic nucleus in the rat. Brain Res., 858, 429–435. [DOI] [PubMed] [Google Scholar]
- Tsumori, T. , Yokota, S. , Ono, K. & Yasui, Y. (2002) Synaptic organization of GABAergic projections from the substantia nigra pars reticulata and the reticular thalamic nucleus to the parafascicular thalamic nucleus in the rat. Brain Res., 957, 231–241. [DOI] [PubMed] [Google Scholar]
- Uylings, H.B.M. , Groenewegen, H.J. & Kolb, B. (2003) Do rats have a prefrontal cortex? Behav. Brain Res., 146, 3–17. [DOI] [PubMed] [Google Scholar]
- Villaruel, F.R. , Lacroix, F. , Sanio, C. , Sparks, D.W. , Chapman, C.A. & Chaudhri, N. (2018) Optogenetic activation of the infralimbic cortex suppresses the return of appetitive pavlovian‐conditioned responding following extinction. Cereb. Cortex, 28, 4210–4221. [DOI] [PubMed] [Google Scholar]
- Webster, K.E. (1961) Cortico‐striate interrelations in the albino rat. J. Anat., 95, 532–544. [PMC free article] [PubMed] [Google Scholar]
- Webster, K.E. (1965) The cortico‐striatal projection in the cat. J. Anat., 99, 329–337. [PMC free article] [PubMed] [Google Scholar]
- Williams, M.N. & Faull, R.L. (1988) The nigrotectal projection and tectospinal neurons in the rat. A light and electron microscopic study demonstrating a monosynaptic nigral input to identified tectospinal neurons. Neuroscience, 25, 533–562. [DOI] [PubMed] [Google Scholar]
- Wilson, C.J. (1986) Postsynaptic potentials evoked in spiny neostriatal projection neurons by stimulation of ipsilateral and contralateral neocortex. Brain Res., 367, 201–213. [DOI] [PubMed] [Google Scholar]
- Wilson, C.J. (1987) Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J. Comp. Neurol., 263, 567–580. [DOI] [PubMed] [Google Scholar]
- Winstanley, C.A. & Floresco, S.B. (2016) Deciphering decision making: variation in animal models of effort‐ and uncertainty‐based choice reveals distinct neural circuitries underlying core cognitive processes. J. Neurosci., 36, 12069–12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki, N. & Shepherd, G.M. (2015) Synaptic circuit organization of motor corticothalamic neurons. J. Neurosci., 35, 2293–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


