Abstract
The aim of this study is to report the combined application of extracorporeal membrane oxygenation (ECMO) with intra‐aortic balloon pumping (IABP) in postcardiotomy cardiac shock (PCS). A total of 60 consecutive patients who received both ECMO and IABP (concomitantly 24 hours) for PCS from February 2006 to March 2017 at Fuwai Hospital were included in our study. Clinical characteristics of the patients were collected retrospectively and compared between survivors and non‐survivors. Logistic regression analysis was used as predictors for survival to discharge. The study cohort had a mean age of 51.4±12.7 years with 75% males. ECMO was implanted intra‐operatively in 38 (63%) patients and post‐operatively in 22 (37%) patients. ECMO was implanted concurrently with IABP in 38 (63%) patients. Heart transplantation (38%) and coronary artery bypass graft (33%) were the main surgical procedures. ECMO was weaned successfully in 48% patients, and the rate of survival to discharge was 43%. Survivors showed less bedside ECMO implantation (12% vs. 41%, P=0.012) and more concurrent implantation of ECMO with IABP (81% vs. 50%, P=0.014). Concurrent implantation of IABP with ECMO (OR=0.177, P=0.015, 95% CI: 0.044‐0.718) was an independent predictor of survival to discharge. As for complications, the rate of renal failure (59% vs. 15%, P=0.001) and multiple organ dysfunction syndrome (29% vs. 0, P=0.003) was higher in patients who failed to survive to discharge. Patients who had heart transplantation had a better long‐term survival than others (P=0.0358). In summary, concurrent implantation of ECMO with IABP provides better short‐term outcome for PCS and combined application of ECMO with IABP for PCS after heart transplantation had a favorable long‐term outcome.
Keywords: Postcardiotomy cardiogenic shock, Intra‐aortic balloon pumping, Extracorporeal membrane oxygenation.
Among patients receiving cardiac surgery, the incidence of postcardiotomy heart dysfunction is about 3–5% 1, and nearly 1% require circulatory support for postcardiotomy cardiogenic shock (PCS) 2. Extra corporeal membrane oxygenation (ECMO) as a short‐term mechanical circulatory support has been widely used in patients with severe respiratory and/or circulatory failure 3. However, the rate of ECMO removal in PCS remains one of the lowest rates of all the indications 4 varying from 58 to 67%, and the in‐hospital mortality varies from 50 to 74% 5, 6, 7, 8, 9 Although the survival rate of ECMO support remains unsatisfactory with severe complications, such as bleeding, infection, end‐organ failures, lower limb ischemia, and thrombosis, ECMO is still a favorable technique treatment for PCS because of its easy and rapid applicability.
Although ECMO provides circulatory support with rapid application, it increases left ventricular (LV) afterload and decreases the blood flow in coronary arteries due to retrograde blood flow, which potentially deteriorates cardiac function. An intra‐aortic balloon pump (IABP) could reduce LV afterload and increase coronary artery blood flow through inflating during diastole and deflating during systole. In addition, IABP implantation showed reduced pulmonary artery occlusion pressure and hydrostatic pulmonary edema, and increased cerebral blood flow in patients with ECMO 10, 11, 12. Combined use of IABP with ECMO is common in PCS, with a rate of 25 to 74% in various centers 5, 6, 7, 8, 9, 13. In this study, we reported the combined application of IABP with ECMO support at Fuwai Hospital, and evaluated the outcome, risk factors, and complications of the therapy.
Methods
Patients
From February 1, 2006 to March 31, 2017, a total of 60 patients who received venoarterial ECMO and IABP concomitantly (>24 h) for refractory PCS at Fuwai Hospital were consecutively included in the study. ECMO was instituted intra‐operatively for circulatory instability during or immediately after weaning from cardiopulmonary bypass after the primary surgery procedure. Secondary indications included delayed PCS for progressive heart failure, refractory ventricular arrhythmia, postoperatively cardiac arrest. For all the patients, clinical variables were retrospectively collected.
The decision and the optimum time to initiate ECMO and IABP support was made by the surgical team and the physicians from the intensive care unit according to their own experience, which was independent of the study. The clinical criteria for ECMO institution for PCS were defined as follows: hypotension with systolic arterial pressure (SAP) <80 mm Hg and mean arterial pressure (MAP) <60 mm Hg; signs of renal failure (urinary volume <20 mL/h), anaerobic metabolism, and metabolic acidosis (pH <7.3, lactate level >3.0 mmol/L,) despite optimized supportive measures such as IABP, inotropes, nitric oxide, and phosphodiesterase inhibitors. Hemodynamic criteria were cardiac index (CI) less than 30 mL/s/m2 and pulmonary capillary wedge pressure of at least 20 mm Hg. Respiratory criteria included arterial partial pressure of oxygen (PaO2) less than 50 mm Hg or alveolar‐arterial oxygen pressure difference (PA–aO2) more than 620 mm Hg.
IABP management
The primary care surgeon judged the decision of IABP (Datascope System 98, Datascope Corporation, Fairfield, NJ, USA or AutoCAT2 wave, Teleflex Incorporated, Hilversum, The Netherlands) insertion. IABP was inserted through a femoral sheath and with the tip located near the second rib with a 30 or 40 mL IABP balloon, which was judged according to the patient’s height. The support was initiated at a 1:1 inflation‐deflation to cardiac cycle ratio, triggering by the R wave of the electrocardiogram. The weaning criterion of IABP was the systolic blood pressure above 100 mm Hg without inotropic agent after removal of ECMO. The support was decreased at a 1:3 inflation–deflation to cardiac cycle ratio when weaning program was initiated, and the patients were weaned off of IABP if the hemodynamic condition was stable.
ECMO management
In our center, the surgical team evaluated the patient to judge whether the ECMO was indicated according to inclusion criteria mentioned above. The ECMO circuit consisted of a centrifugal pump console (Bio‐Medicus BP‐550, Medtronic Inc. Minneapolis, MN, USA; or RotaFlow RF‐32, Maquet Cardiopulmonary AG, Hirrlingen, Germany.) in conjunction with a microporous membrane oxygenator (Carmeda coating Affinity NT or Maxima PRF Plus, Metrodonic) or a polymethylpentene oxygenator with a plasma‐tight diffusion membrane (Quadrox D, Maquet Cardiopulmonary AG) with integrated heat exchanger and adapted tube. All components were heparin bonded and connected by the shortest possible tubing system. All patients had peripheral ECMO via the cannulae of the femoral vein and artery. An additional 16Fr intravenous needle casing was inserted distally into the femoral artery to prevent leg ischemia.
The ECMO system was implanted under full heparinization, and activated clotting time was kept longer than 300 s. Half of the heparin was antagonized with protamine when full ECMO flow was established, aiming for an activated clotting time of 140–180 s unless there was ongoing coagulopathy with hemorrhage. During the first 24–48 h, ECMO blood flow was adequately adjusted to maintain CI of 40 mL/s/m2, with an aim to keep mixed venous oxygen saturation (SvO2) around 70%, and MAP of 60–65 mm Hg. The oxygenator was examined twice a day for early detection of thrombus formations. After 48 h, cardiopulmonary recovery was daily assessed by hemodynamic, clinical, and echocardiographic measurements to define the optimal time of weaning. Weaning was cautiously begun when SvO2 ≥70%, hematocrit of 30–35%, absence of bleeding, tamponade or left heart distension, left ventricular ejection fraction (LVEF) ≥35%, and normal blood lactate levels. Flow rate was reduced stepwise to approximately 1 L/min under continuous monitoring of hemodynamic and respiratory variables. When signs of insufficient perfusion occurred during ECMO weaning, the flow was increased again to full, allowing prolonged ECMO support. When the patients were hemodynamically stable on the minimal ECMO flow with satisfactory recovery of myocardial function evaluated by echocardiograph, patients were weaned off of ECMO support and IABP was retained for further evaluation.
Outcomes
The primary outcome was all‐cause mortality during hospitalization. Other short‐term outcomes were complications including renal failure, access‐site bleeding, gastrointestinal bleeding, limb ischemia, thrombosis, neurological complications, multiple organ dysfunction syndrome during hospitalization. Long‐term outcome was all‐cause mortality during follow‐up.
Statistical analyses
The normality of the variables was tested by Shapiro‐Wilk test. Continuous variables with normal distribution were expressed as mean ± standard deviation (SD) and compared by Student’s t‐test; while those that were not normally distributed were reported as medians (first and third interquartile range; IQR) and compared by Mann–Whitney U test. Categorical variables were expressed as percentages and compared by Chi‐Square test. Logistic regressions were used to analyze the predictors of in‐hospital mortality. The multivariate logistic regression was performed with a level of significance of less than 0.10 in bivariate analysis to identify independent factors using an “Enter” method. The cumulative survival since discharge from hospital was determined by the Kaplan–Meier method, and differences in survival between groups were evaluated with the log‐rank tests. P values were two sided, and values <0.05 were considered statistically significant. SPSS statistical software (Version 22, IBM Corp., Armonk, NY, USA) was used for the analyses.
Results
Study population
The mean age of the patients was 51.4 ± 12.7 years and male accounted for 75%. Among all patients, 27 (45%) suffered coronary artery disease and 19 (32%) cardiomyopathy. The original surgery procedures mainly included heart transplantation (38%) and coronary artery bypass graft (CABG, 33%), Fig. 1. ECMO was implanted intra‐operatively in 38 (63%) patients and postoperatively in 22 (37%) patients. Among the 38 patient who received ECMO intra‐operatively, 20 (53%) had heart transplantation, 14 (37%) had coronary artery bypass graft (CABG) and 4 (11%) had other surgical procedures. While among the 22 patients who received ECMO postoperatively, 12 (55%) had CABG, 3 (14%) had heart transplantation and 7 (32%) had other surgeries. The baseline characteristics of the patients are shown in Table 1. ECMO was implanted concurrently with IABP in 38 (63%) patients, and sequentially in 22 (37%) patients. Among sequentially implanted patients, 11 patients received IABP first and then were treated with ECMO due to failure in maintaining circulation; the other 11 patients received ECMO first, and then were treated with IABP due to insufficient perfusion of coronary artery or peripheral organs. There was no difference as for baseline characteristics between patients who received ECMO and IABP concurrently and sequentially, Table 1.
Figure 1.
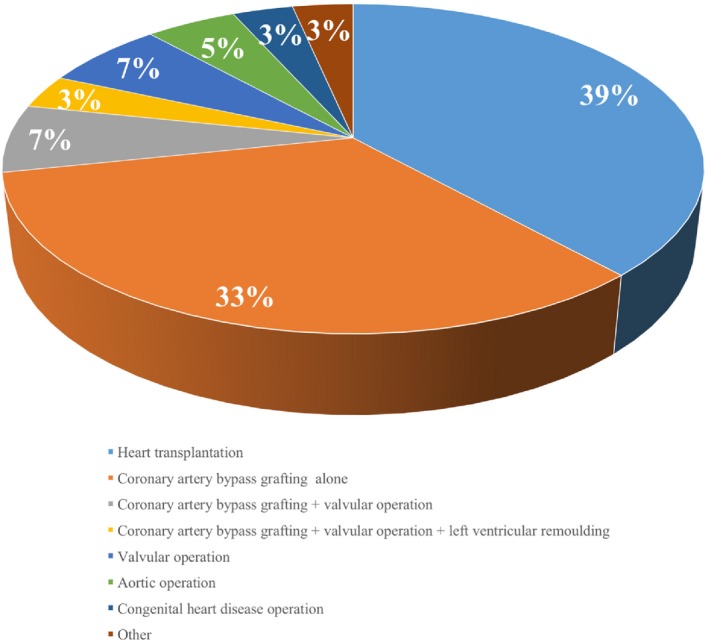
Surgical procedures of all patients. [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Baseline characteristics of the patients
| All (N = 60) | Concurrent (N = 38) | Sequential (N = 22) | P value | |
|---|---|---|---|---|
| Age, years | 51.4 ± 12.7 | 52.3 ± 13.0 | 49.8 ± 12.3 | 0.462 |
| Male, n (%) | 45 (75) | 31 (82) | 14 (64) | 0.122 |
| Body mass index, kg/m2 | 23.4 ± 4.0 | 23.5 ± 4.0 | 23.2 ± 4.1 | 0.898 |
| Etiology, n (%) | ||||
| CAD | 27 (45) | 18 (47) | 9 (41) | 0.628 |
| Cardiomoypathy | 19 (32) | 11 (29) | 8 (36) | 0.552 |
| VHD | 5 (8) | 3 (8) | 2 (9) | 1.000 |
| VHD + CAD | 2 (3) | 1 (3) | 1 (5) | 1.000 |
| Aortic disease | 3 (5) | 2 (5) | 1 (5) | 1.000 |
| CHD | 3 (5) | 2 (5) | 1 (5) | 1.000 |
| Hypertensive heart disease | 1 (2) | 1 (3) | 0 (0) | 1.000 |
| Complications, n (%) | ||||
| Hypertension | 20 (33) | 12 (32) | 8 (36) | 0.705 |
| Diabetes mellitus | 10 (17) | 7 (18) | 3 (14) | 0.732 |
| Dyslipidemia | 16 (27) | 10 (26) | 6 (27) | 0.936 |
| Atrial fibrillation | 11 (18) | 8 (21) | 3 (14) | 0.731 |
| Pulmonary arterial hypertension | 12 (20) | 7 (18) | 5 (23) | 0.744 |
| Current smoker, n (%) | 10 (17) | 6 (16) | 4 (18) | 0.811 |
| Myocardial infarction history, n (%) | 14 (23) | 6 (16) | 8 (36) | 0.112 |
| PreECMO cardiac arrest, n (%) | 15 (25) | 8 (21) | 7 (32) | 0.353 |
| LVEF, % | 45.3 ± 18.4 | 42.9 ± 19.0 | 49.5 ± 16.9 | 0.188 |
| NYHA class, n (%) | 0.102 | |||
| I | 13 (22) | 7 (18) | 6 (27) | |
| II | 15 (25) | 7 (18) | 8 (36) | |
| III | 23 (38) | 19 (50) | 4 (18) | |
| IV | 9 (15) | 5 (13) | 4 (18) | |
| Creatinine, μmol/L | 115.7 ± 108.8 | 94.0 ± 40.6 | 153.2 ± 167.4 | 0.117 |
| Total bilirubin, μmol/L | 28.7 ± 23.0 | 26.2 ± 14.2 | 33.1 ± 33.4 | 0.374 |
CAD, coronary artery disease; CHD, congenital heart disease; ECMO, extracorporeal membrane oxygenation; LVEF, left ventricle ejection fraction; NYHA, New York Heart Association classification; VHD, valvular heart disease.
Outcomes
Twenty‐nine (48%) patients were successfully weaned from mechanical life support, in which 26 (43%) survived to discharge and 3 (5%) died from reoccurred heart failure after weaning the mechanical support. Thirty‐one (52%) patients were not weaned from ECMO support, of whom 17 (28%) died due to failure of cardiac recovery, and 14 (23%) quit the treatment. Overall, the in‐hospital survival rate was 43%. Mean ECMO and IABP support time was 5.3 ± 2.8 days and 6.9 ± 4.3 days, respectively.
Characteristics of the survivors and nonsurvivors were compared as listed in Table 2. Compared to nonsurvivors, survivors had more concurrent implantation of ECMO with IABP (81 vs. 50%, P = 0.014) and less bedside implantation of ECMO (12 vs. 41%, P = 0.012). Multivariate logistic regression analysis was used to analyze relevant factors that might affect in‐hospital mortality, which indicated concurrent implantation of ECMO with IABP (OR = 0.177, P = 0.015, 95% CI: 0.044–0.718) was an independent predictor for survival of discharge, Table 3.
Table 2.
Comparison of the characteristics of survivors and nonsurvivors
| Survivors (N = 26) | Nonsurvivors (N = 34) | P value | |
|---|---|---|---|
| Age, years | 48.2 ± 13.0 | 53.9 ± 12.1 | 0.085 |
| Men, n (%) | 20 (77) | 25 (74) | 0.764 |
| Body mass index, kg/m2 | 22.5 ± 3.2 | 24.1 ± 4.5 | 0.109 |
| Concomitant disease, n (%) | |||
| Atrail fibrillation | 6 (23) | 5 (15) | 0.406 |
| Pulmonary arterial hypertension | 8 (31) | 4 (12) | 0.068 |
| Renal failure | 0 (0) | 2 (6) | 0.501 |
| History of cardiac surgery, n (%) | 4 (15) | 4 (12) | 0.717 |
| Surgery procedure, n (%) | |||
| Emergency surgery | 7 (27) | 12 (35) | 0.490 |
| Off‐pump surgery | 2 (8) | 5 (15) | 0.402 |
| Secondary exploratory thoracotomy | 6 (23) | 9 (26) | 0.764 |
| Heart transplantation | 14 (54) | 9 (26) | 0.037 |
| CABG | 9 (35) | 17 (50) | 0.233 |
| Preoperative test | |||
| Albumin, g/L | 40.9 ± 4.3 | 42.3 ± 4.9 | 0.246 |
| Total bilirubin, μmol/L | 27.2 ± 11.9 | 29.9 ± 28.9 | 0.669 |
| BUN, mmol/L | 9.4 ± 6.2 | 14.5 ± 33.2 | 0.443 |
| Creatinine, μmol/L | 87.1 ± 23.8 | 88.9 ± 22.5 | 0.759 |
| PTT, s | 13.9 ± 1.6 | 13.8 ± 1.1 | 0.677 |
| LDH, IU/L | 205.4 ± 106.1 | 193.1 ± 48.5 | 0.586 |
| HSCRP, mg/L | 2.8 ± 3.0 | 2.7 ± 2.6 | 0.934 |
| ECMO implantation | |||
| Bedside implantation, n (%) | 3 (12) | 14 (41) | 0.012 |
| Concurrently with IABP, n (%) | 21 (81) | 17 (50) | 0.014 |
| ECMO supporting time, days | 5.6 ± 2.0 | 5.0 ± 3.2 | 0.373 |
| IABP supporting time, days | 7.8 ± 2.8 | 6.3 ± 5.1 | 0.140 |
BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; ECMO, extracorporeal membrane oxygenation; HSCRP, high sensitivity C‐reactive protein; IABP, intra‐aortic balloon pump; LDH, lactic dehydrogenase; PTT, prothrombin time.
Table 3.
Univariate and multivariate logistic regression
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 0.964 | 0.923–1.006 | 0.091 | 0.970 | 0.918–1.024 | 0.265 |
| Pulmonary hypertension | 0.300 | 0.079–1.140 | 0.077 | 0.316 | 0.060–1.660 | 0.174 |
| Heart transplantation | 0.309 | 0.104–0.912 | 0.034 | 0.709 | 0.163–3.087 | 0.646 |
| Bedside implantation | 5.367 | 1.345–21.406 | 0.017 | 3.831 | 0.858–17.111 | 0.079 |
| Concurrent implantation | 0.238 | 0.073–0.778 | 0.018 | 0.177 | 0.044–0.718 | 0.015 |
ECMO, extracorporeal membrane oxygenation; CI, confidence interval; OR, odds ratio.
Complications
Complications of all the patients are shown in Table 4. Renal failure was the most common complication and was detected in 24 (40%) patients, followed by lower limb ischemia in 13 (22%) patients, bleeding at the access‐site in 11 (18%) patients, multiple organ dysfunction syndrome (MODS) in 10 (17%) patients, thrombosis in 9 (15%) patients, neurological complications in 9 (15%) patients, and bleeding in gastrointestinal tract in 4 (7%) patients. Survivors had less renal failure (15 vs. 59%, P = 0.001) and MODS (0 vs. 29%, P = 0.003) was much lower than that other patients.
Table 4.
Complications
| Complications, n (%) | All (N = 60) | Survivors (N = 26) | Nonsurvivors (N = 34) | P value |
|---|---|---|---|---|
| Renal failure | 24 (40) | 4 (15) | 20 (59) | 0.001 |
| Access‐site bleeding | 11 (18) | 5 (19) | 6 (18) | 1.000 |
| Gastrointestinal bleeding | 4 (7) | 3 (12) | 1 (3) | 0.307 |
| Limb ischemia | 13 (22) | 5 (19) | 8 (24) | 0.689 |
| Thrombosis | 9 (15) | 5 (19) | 4 (12) | 0.482 |
| Neurological complications | 9 (15) | 1 (4) | 8 (24) | 0.064 |
| MODS | 10 (17) | 0 (0) | 10 (29) | 0.003 |
MODS, multiple organ dysfunction syndrome.
Long‐term survival
The average follow‐up time was 3.9 years (median 3.7 years), Fig. 2A. Among the 26 patients who survived to discharge, 3 patients died within the first year. The 1–3 year survival rate was 85, 65, and 58%. All patients who had heart transplantation were alive until last follow up. The survival rate of patients who had heart transplantations was better than those who had other surgical procedures (P = 0.0358), Fig. 2B.
Figure 2.
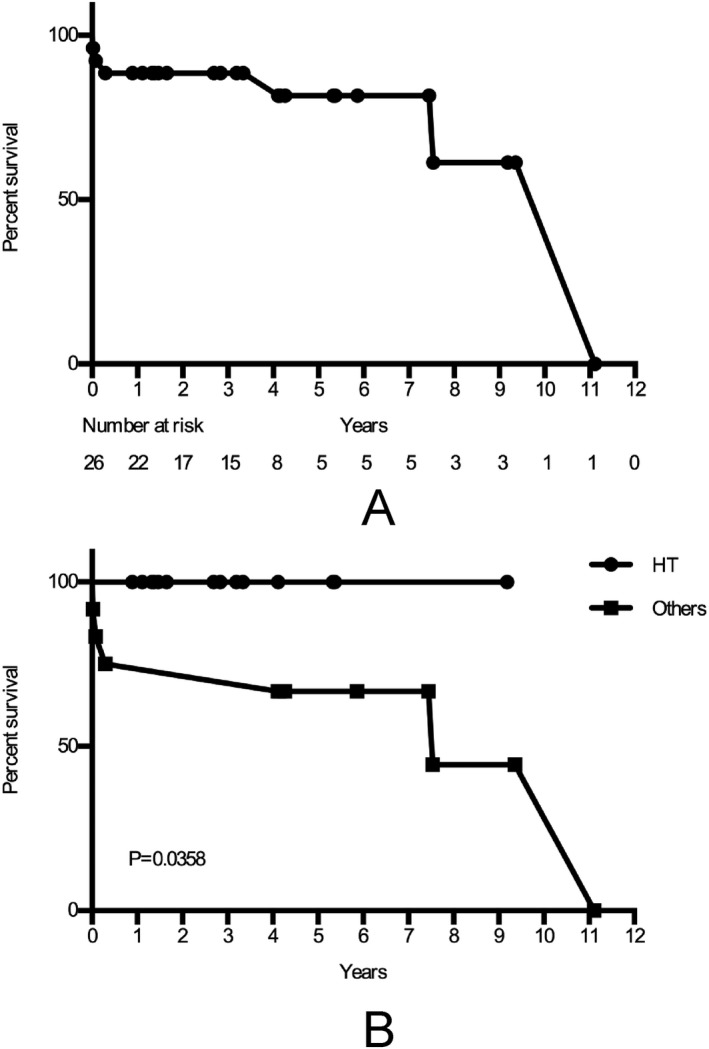
Kaplan–Meier analysis for long‐term survival. A. Kaplan–Meier curve for all patients who discharged from the hospital. B. Kaplan–Meier curve for discharged patients who had heart transplantation and other surgical procedures.
Discussion
To the best of our knowledge, this is the largest cohort of patients who received combined support with ECMO and IABP in mainland China up to now. A total of 60 patients from our 10‐years practice were analyzed retrospectively. The overall rate of weaning and survival to discharge was 48 and 43%. Survivors showed higher rate of concurrent implantation of ECMO and IABP, which was an independent predictor for survival to discharge.
With a high mortality rate, PCS is a horrible complication of cardiac surgery. Short‐term circulatory assist therapy provides a chance for myocardial recovery by off‐loading the heart. ECMO is an alternative treatment for these patients to support ventricular and pulmonary function, which allows rapid restoration of circulation. With an acceptable cost and convenient cannulation, ECMO should be the optimal choice for short‐term assist in China. Despite the advantages of ECMO support, severe complications due to anticoagulation and ECMO components remain an unsolved problem. Hence, there is no guideline for ECMO support for PCS. In practice, the ECMO initiation is determined according to the patient’s specific condition and the surgeon’s experience.
As ECMO increases afterload of LV and reduces coronary artery blood flow, combining with IABP could make up for the shortages of ECMO support. Previous studies showed that additional application of IABP reduced pulmonary artery occlusion pressure and hydrostatic pulmonary edema, and increased cerebral blood flow in patients with ECMO 10, 11, 12. However, it is still controversial whether combining application of IABP improves survival of patients with ECMO support. Gass et al. found that prior IABP use and axillary cannulation were independent predictors of reduced in‐hospital mortality, stroke or vascular injury 14. Similarly, Aso et al. reported that 28‐day mortality and in‐hospital mortality were significantly lower in the combined group 15. However, Lin et al. found the mortality rates at two weeks after ECMO implantation were not different between the ECMO group and combined group 16, which was consistent with a systematic review stating that there was a lack of survival benefit when using combined IABP and ECMO 17. Previous study included patients with all indications for short‐term mechanical circulatory support, which would induce confounding factors when interpreting the yield of combined use. Cardiac surgery with extracorporeal circulation is associated with myocardial ischemia‐reperfusion injury 18. As ECMO provides sufficient circulatory support with an impact on coronary artery perfusion, and IABP reduces afterload of LV and increases coronary blood flow, combined use of ECMO and IABP might have a potential advantage for PCS. Thus, we focused on patients who had PCS in this study.
In our center, intra‐operative uses of ECMO and IABP were mainly during heart transplantation and CABG. The donor hearts and the hearts suffering from coronary artery disease experience a long period of ischemia, thus it may be difficult to quickly recover from the surgery procedure. Herein, combined use of ECMO and IABP support facilitated cardiac function restoration. Postoperative uses were mainly on patients after CABG or other cardiac surgeries who were in poor condition. In such cases, ECMO and IABP allowed bridging for recovery, further evaluation or decision making.
For all patients in our study, the primary intention was bridging to recovery. The rate of successful weaning from ECMO was 48%, and only 5% of all patients with successful weaning from ECMO died in the hospital. Overall, the survival discharge rate was 43%, which is comparable to the results of previous studies for overall survival from 25 to 49% 7, 19, 20, 21, 22. When comparing to the characteristics of survivors and nonsurvivors, bedside implantation rate was higher in nonsurvivors. ECMO is implanted only when an extreme emergency event occurs as sudden cardiac arrest after the original cardiac surgery procedure. As a result, these patients might have a worse prognosis. The rate of concurrent implantation of IABP with ECMO is higher in survivors and logistic regression analysis showed that it was an independent predictor of survival to discharge. Previous studies seldom focused on the timing for ECMO and IABP implantation. Our results showed that concurrent implantation of ECMO and IABP in patients with PCS would improve the outcome.
As the support time of ECMO and IABP was similar in survivor and nonsurvivor groups, this might explain the mild difference of complications between groups. Almost all nonsurvivors in our study failed to wean from ECMO, indicating patients in nonsurvival group died of inability to restore cardiac function. As a result, the rate of renal failure and multiple organ dysfunction syndrome was higher in nonsurvivors.
Although high mortality during hospitalization was observed, long‐term outcome of the patients undergoing ECMO for PCS was favorable 21, 22. Our results also showed a favorable long‐term outcome, especially for the patients who had heart transplantation. Though the donor hearts experienced a period of cold ischemia time and ischemia‐reperfusion injury, which might induce PCS after heart transplantation, the donor heart with a normal morphology would recover with a better function than a repaired heart after mechanical support. Therefore, patients who underwent heart transplantation had a better long‐term survival than others in our study. The decision for initiation of combined support by ECMO and IABP should be considered positively in patients who undergo heart transplantation.
Limitations
As the data collection was performed prospectively and the sample size is limited, this study is subject to all limitations of a nonrandomized study. A randomized study is needed for validating the findings.
Author Contributions
S.S. Hu and J.F. Hou designed the study, revised, and approved the article. K. Chen contributed to data analysis and interpretation, statistics, and drafted the article. Funding was secured by S.S. Hu. K. Chen and H.W. Tang collected the data.
Conflict of Interest
The authors have declared no conflicts of interest for this article.
Acknowledgments
We were grateful to Information Center of Fuwai Hospital for the assistance to collecting clinical data. This work was supported by the 13th Five‐year National Science and Technology Major Project of China (grant number 2016YFC1300900).
References
- 1. Golding LA. Postcardiotomy mechanical support. Semin Thorac Cardiovasc Surg 1991;3:29–32. [PubMed] [Google Scholar]
- 2. Smith C, Bellomo R, Raman JS, et al. An extracorporeal membrane oxygenation‐based approach to cardiogenic shock in an older population. Ann Thorac Surg 2001;71:1421–7. [DOI] [PubMed] [Google Scholar]
- 3. Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014;63:2769–78. [DOI] [PubMed] [Google Scholar]
- 4. McCarthy FH, McDermott KM, Kini V, et al. Trends in U.S. extracorporeal membrane oxygenation use and outcomes: 2002–2012. Semin Thorac Cardiovasc Surg 2015;27:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ko WJ, Lin CY, Chen RJ, Wang SS, Lin FY, Chen YS. Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg 2002;73:538–45. [DOI] [PubMed] [Google Scholar]
- 6. Zhong Z, Jiang C, Yang F, et al. Veno‐arterial extracorporeal membrane oxygenation support in patients undergoing aortic surgery. Artif Organs 2017;41:1113–20. [DOI] [PubMed] [Google Scholar]
- 7. Rastan AJ, Dege A, Mohr M, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 2010;139:302–11, 11 e1. [DOI] [PubMed] [Google Scholar]
- 8. Doll N, Kiaii B, Borger M, et al. Five‐year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. Ann Thorac Surg 2004;77:151–7; discussion 7. [DOI] [PubMed] [Google Scholar]
- 9. Wu MY, Lin PJ, Lee MY, et al. Using extracorporeal life support to resuscitate adult postcardiotomy cardiogenic shock: treatment strategies and predictors of short‐term and midterm survival. Resuscitation 2010;81:1111–6. [DOI] [PubMed] [Google Scholar]
- 10. Petroni T, Harrois A, Amour J, et al. Intra‐aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation*. Crit Care Med 2014;42:2075–82. [DOI] [PubMed] [Google Scholar]
- 11. Brechot N, Demondion P, Santi F, et al. Intra‐aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial‐extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care 2018; 7(1):62–9. [DOI] [PubMed] [Google Scholar]
- 12. Yang F, Jia ZS, Xing JL, et al. Effects of intra‐aortic balloon pump on cerebral blood flow during peripheral venoarterial extracorporeal membrane oxygenation support. J Transl Med 2014;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Acheampong B, Johnson JN, Stulak JM, et al. Postcardiotomy ECMO support after high‐risk operations in adult congenital heart disease. Congenit Heart Dis 2016;11:751–5. [DOI] [PubMed] [Google Scholar]
- 14. Gass A, Palaniswamy C, Aronow WS, et al. Peripheral venoarterial extracorporeal membrane oxygenation in combination with intra‐aortic balloon counterpulsation in patients with cardiovascular compromise. Cardiology 2014;129:137–43. [DOI] [PubMed] [Google Scholar]
- 15. Aso S, Matsui H, Fushimi K, Yasunaga H. The effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: an analysis using a nationwide inpatient database. Crit Care Med 2016;44:1974–9. [DOI] [PubMed] [Google Scholar]
- 16. Lin LY, Liao CW, Wang CH, et al. Effects of additional intra‐aortic balloon counter‐pulsation therapy to cardiogenic shock patients supported by extra‐corporeal membranous oxygenation. Sci Rep 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng R, Hachamovitch R, Makkar R, et al. Lack of survival benefit found with use of intraaortic balloon pump in extracorporeal membrane oxygenation: a pooled experience of 1517 patients. J Invasive Cardiol 2015;27:453–8. [PubMed] [Google Scholar]
- 18. Hausenloy DJ, Garcia‐Dorado D, Botker HE, et al. Novel targets and future strategies for acute cardioprotection: position paper of the European Society of Cardiology Working Group on cellular biology of the heart. Cardiovasc Res 2017;113:564–85. [DOI] [PubMed] [Google Scholar]
- 19. Elsharkawy HA, Li L, Esa WA, Sessler DI, Bashour CA. Outcome in patients who require venoarterial extracorporeal membrane oxygenation support after cardiac surgery. J Cardiothorac Vasc Anesth 2010;24:946–51. [DOI] [PubMed] [Google Scholar]
- 20. Mikus E, Tripodi A, Calvi S, Giglio MD, Cavallucci A, Lamarra M. CentriMag venoarterial extracorporeal membrane oxygenation support as treatment for patients with refractory postcardiotomy cardiogenic shock. ASAIO J 2013;59:18–23. [DOI] [PubMed] [Google Scholar]
- 21. Unosawa S, Sezai A, Hata M, et al. Long‐term outcomes of patients undergoing extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. Surg Today 2013;43:264–70. [DOI] [PubMed] [Google Scholar]
- 22. Wang JG, Han J, Jia YX, Zeng W, Hou XT, Meng X. Outcome of veno‐arterial extracorporeal membrane oxygenation for patients undergoing valvular surgery. PLoS One 2013;8:e63924. [DOI] [PMC free article] [PubMed] [Google Scholar]


