Abstract
Aim
Recommendations for maximum blood draw in children range from 1 to 5% despite limited evidence. The aim of the study was to assess the safety of blood draws in children aged six months to 12 years targeting volumes of 3% of total blood volume.
Methods
Children who experienced three‐monthly blood draws during participation in one of three investigators initiated clinical trials conducted in our institution were examined. In total, 629 venous blood draws were performed in 141 children. Adverse events and blood counts were assessed.
Results
Overall, 608 adverse events were reported. None of these included symptoms that reflected concerns on blood draw volumes or frequency. Anaemia and red cell or haemoglobin measurements outside the normal age range were not observed. A reduction in haemoglobin, haematocrit, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration and mean corpuscular volume was noted in children participating in one of the three trials analysed.
Conclusion
Regular blood draws of up to 3% of total blood volume were not associated with signs of anaemia or hypovolaemia in young children. We suggest that the European recommendations be revised for clinical studies in which children are not exposed to treatments that are associated with anaemia risk.
Keywords: Blood collection, Blood draw safety in children, Paediatric research, Safe phlebotomy, Single blood draw
Abbreviations
- AEs
Adverse events
- BL
Baseline
- EOT
End of treatment
- MCHC
Mean corpuscular haemoglobin concentration
- MCH
Mean corpuscular haemoglobin
- MCV
Mean corpuscular volume
- RBC
Red blood cells
- TBV
Total blood volume
Key notes.
There is controversy in the proposed recommendations of blood volumes taken in children varying between 1 and 5% of total blood volume.
Our study demonstrates that repeated blood draws of up to 3% of total blood volume were not associated with signs of anaemia or hypovolaemia in young children.
We suggest that the European recommendations should be revised for clinical studies in which children are not exposed to treatments that are associated with anaemia risk.
Introduction
Safety and efficacy data on many medicines used in children are surprisingly scarce. To improve clinical care of children, the European Union, World Health Organisation and US National Institutes of Health have made commitments to support more studies focusing on children's health with the goal to develop treatments, drugs and devices specific to children. Paediatric trials are challenging to conduct because of participants’ developmental and physiological stage and particular ethical concerns. One aspect of controversy in conducting clinical trials in children is the currently proposed guidelines and recommendations of blood volumes taken at a single time point. Such blood volumes can be critical for assessing safety and factors that predict response to treatment.
While the European Union recommends a blood draw limit of 0.8–0.9 mL/kg body weight corresponding to 1% of total blood volume at a single time point and 2.4 mL blood/kg body weight (3% of total blood volume) during a period of four weeks 1, other institutional review boards, national health authorities or institutional committees have a larger blood draw allowance 2, 3, 4. The US National Institutes of Health guideline proposes to collect no more than 5 mL/kg body weight (5% of total blood volume) in children for research purposes in a single day and no more than 9.5 mL/kg (11% of total blood volume) over any eight‐week period 5. The World Health Organisation concluded from a review of guidelines and available data from studies in which single blood draws ranged from 1% to 5% of total blood volume that there was no indication of risk or adverse event to the study participants for any of the reviewed blood volumes, but that the majority of guidelines lacked direct evidence on which to base their recommendations 4.
In order to assess whether current European Union blood draw guidelines in children could be safely extended, we examined safety data that included blood counts and haemoglobin concentration from three randomised controlled trials in 141 children at risk of type 1 diabetes in which repeated blood draws of 2.4 mL/kg body weight (3% of total blood volume) were performed.
Methods
Safety data from 141 children who were at increased risk of type 1 diabetes and participated in one of three investigator‐initiated randomised control clinical trials were analysed to assess the potential risk of the applied blood draw volumes of up to 2.4 mL/kg body weight corresponding to 3% of total blood volume. The three studies were conducted at our institution. The Pre‐POINT Study (EudraCT‐No.: 2005‐001621‐29) was sponsored by the Faculty of Medicine, Technical University Dresden, and led by E. Bonifacio (principal investigator and sponsor‐delegated person). It was a multinational study with study centres in Munich (Technical University of Munich), Denver, USA (Barbara Davis Center), Vienna, Austria (University of Vienna), and Bristol, UK (University of Bristol). The Pre‐POINT‐Early Study (EudraCT‐no.: 2014‐005287‐15) and the Fr1da‐Insulin‐Intervention Study (EudraCT‐no.: 2015‐003028‐30) were sponsored by the Faculty of Medicine, Technical University of Munich, and led by A.‐G. Ziegler (principal investigator and sponsor‐delegated person). The trials were mono‐centre studies and were conducted at the study centre in Munich. Participants of all three studies provided written informed consent. All three studies were approved by local ethics committees as well as the respective regulatory authorities.
All three studies aimed to investigate whether daily administered oral insulin can induce a potentially protective immune response to the autoantigen insulin without causing adverse effects. The collected blood volumes were required to obtain the outcome measure which included antibody and T‐cell responses to insulin.
The Pre‐POINT Study included 25 islet autoantibody‐negative children aged two to seven years with a family history of type 1 diabetes and susceptible human leucocyte antigen class II genotypes. Children were followed for at least three months and a maximum of 18 months. Blood draws were taken at different study visits for at least three times and a maximum of seven times. Blood counts were analysed at baseline and at the end of treatment (Fig. S1a).
The Pre‐POINT‐Early Study included 44 islet autoantibody‐negative children aged six months to two years with a family history of type 1 diabetes and susceptible human leucocyte antigen class II genotypes. Of these, 25 had completed the study at the time of this analysis. Children were followed for a maximum of 12 months. Blood draws were taken three monthly with a maximum of five blood draws during a 12‐month treatment phase. Blood counts were analysed at the baseline visit and after 12 months of treatment (Fig. S1a).
The Fr1da‐Insulin‐Intervention Study aims to recruit 220 normoglycaemic children aged 2–12 years who have multiple islet autoantibodies. There were 84 children randomised into the study of which 38 had completed the study treatment phase at the time of this analysis. Blood draws were taken three monthly with a maximum of five blood draws during a 12‐month treatment phase. Blood counts were analysed at the baseline visit and after 12 months of treatment (Fig. S1a).
Table 1 summarises the number of participants in each trial, blood volumes, blood draw frequencies and the total number of visits.
Table 1.
Participant description and overview of blood draw frequency as well as amount in healthy children at risk of type 1 diabetes as of July 5, 2017
| Pre‐POINT | Pre‐POINT‐Early | Fr1da‐insulin‐intervention | |
|---|---|---|---|
| Number of children included | 25 | 44 | 72 |
| Inclusion age range | Two to seven years | Five months to two years | Two to 12 years |
| Median age of included childrena | 5.09 (2.09–7.70) years | 1.03 (0.50–2.96) years | 5.22 (2.47–10.95) years |
| Median body weight [kg]a: | |||
| At baseline: | 19.0 (15.65–21.90) | 9.90 (9.00–12.00) | 20.20 (16.40–24.40) |
| At end of treatment: | 20.7 (17.00–24.00) | 12.50 (11.90–15.10) | 23.00 (18.38–27.88) |
| Blood sample volume per single blood draw, as per protocol | 2.0 mL/kg body weight | 2.4 mL/kg body weight | 2.4 mL/kg body weight |
| Equivalent to a total blood volume (TBV) | 2.5% | 3.0% | 3.0% |
| Total volume of blood samples (mL) per visit | 16–25 mL | 18–29.5 mL | 29.5–96 mL |
| Frequency of blood samples | First two visits within 15 days, then 3 monthly | 3‐monthly visits | 3‐monthly visits |
| Number of blood draws total | 152 | 198 | 279 |
| Number of blood counts total | 46 | 53 | 112 |
Data are shown as median (+interquartile range).
Adverse events were reported from the beginning of study participation (baseline visit). All children were prospectively screened for any signs of risks or side effects regarding the blood draw volumes. In this regard, they were screened for symptoms such as pale skin, dizziness, fainting, easy fatigue and loss of energy, unusually rapid heartbeat, shortness of breath, headache, difficulty concentrating, leg cramps and insomnia.
Blood sample data at both baseline and end of treatment visits were available for 23 children in the Pre‐POINT study, 12 children in the Pre‐POINT‐Early study and 32 children in the Fr1da‐Insulin‐Intervention study. Potential changes from baseline in haematocrit, red blood cells, haemoglobin, mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and mean corpuscular volume (MCV) were compared with Wilcoxon signed‐rank tests between both visits in these children The association of each parameter with age at the respective visit was investigated in all available measurements from the baseline visits of all three studies using Spearman's correlation coefficient. To account for potential confounding by increasing age between baseline and later visits, the mean change in each parameter by one year of age was calculated from linear regression models based on merged data from the baseline visits of all three studies. This estimated change was subtracted from each 12 months visit to calculate age‐adjusted Wilcoxon signed‐rank tests. The significance level was set to 0.05 for all statistical tests, without correction for multiple testing. All calculations were carried out with SAS 9.4 (SAS Institute Inc, Cary, North Carolina) and R 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
In total, there were 629 blood withdrawals during the three trials in the 141 participating children, including 141 at baseline and 488 during the interventional part of the study (Fig. S1b). There were 608 reported adverse events of which the majority (59%) was related to infections (no local ones at the blood draw site), gastrointestinal disorders (14.6%) or fever (10.2%). None of the adverse events included dizziness, fainting, paleness or symptoms that reflected concerns on blood draw volumes or frequency (Tables 2 and S2). There were 211 blood count measurements including 76 taken after the baseline visit. None showed anaemia or were outside the normal age range for haemoglobin and red blood cells. Scatterplots of baseline values across all three studies indicated that haemoglobin, haematocrit, MCH and MCV increased by age (Fig. S2). Reduced age‐adjusted parameters were observed at the end of the treatment period as compared to baseline after repeated blood sampling in children participating in the Fr1da‐Insulin‐Intervention Study, but not in children participating in the Pre‐POINT or Pre‐POINT‐Early studies (Figs. 1, S3 and Table S1). Reductions were slight and were observed for haemoglobin p = 0.01 (Fig. 1), haematocrit p = 0.0014 (Fig. 1) MCH p = 0.01 (Fig. S3) and MCV p = 0.00003 (Fig. S3). MCHC p = 0.0009 (Fig. S3) was slightly increased in the Fr1da‐Insulin‐Intervention Study. No change in red blood cell count was observed.
Table 2.
Results of frequent blood draws in children at risk of type 1 diabetes as of July 5, 2017
| Pre‐POINT | Pre‐POINT‐Early | Fr1da‐insulin‐intervention | |
|---|---|---|---|
| Total reported adverse events (AEs) | 102 | 218 | 288 |
| Adverse events reflecting potential signs or safety concerns related to blood sampling volume, for example acquired anaemia, dizziness, fainting and paleness | 0 | 0 | 0 |
| Total number of blood count measurements after baseline | 23 | 17 | 36 |
| aNumber of haemoglobin and red blood cell concentration below reference values, that is low Hb levels | 0 | 0 | 0 |
Haemoglobin reference ranges were age‐adjusted: <one year: 12.0–14.0 g/dL; one to four years: 10.8–14.0 g/dL; four to seven years: 10.7–14.7 g/dL; seven to ten years: 11.3–14.9 g/dL; 10–11 years: 10.8–15.6 g/dL; 11–12 years: 11.5–14.5 g/dL.
Figure 1.
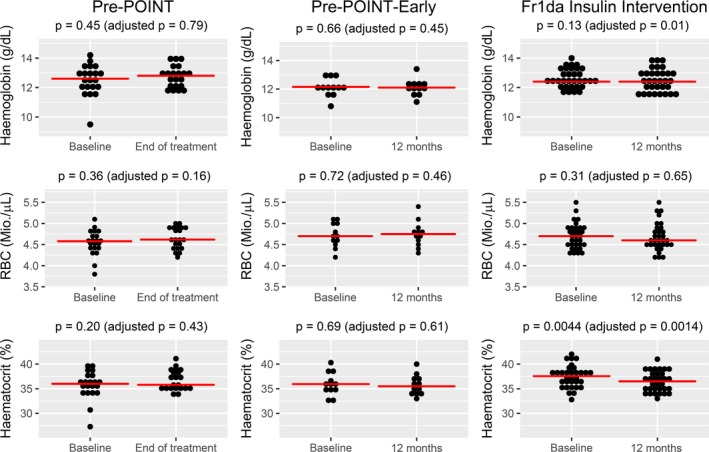
Dot plots of haemoglobin, red blood counts (RBC) and haematocrit at baseline and at end of treatment or after 12 months of treatment (as appropriate) in the Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies, respectively. Red horizontal lines indicate the groupwise medians. P‐values were derived from Wilcoxon signed‐rank tests, with and without adjustment for the estimated natural change in each parameter by increasing age between baseline and later visits. Plotting and calculations were done in all children who had both a baseline and an end of treatment/12‐month visit.
Discussion
As far as we know, this is one of the first studies providing prospectively recorded empirical data about the tolerability and safety of repeated blood draws in children up to 12 years. Data from three randomised controlled trials showed no evidence of anaemia or clinically relevant side effects after repeated blood draws of up to 2.4 mL/kg body weight (3% blood volume) in children aged six months to 12 years. These data suggest that the draft European Union blood draw guidelines can be safely extended in children who are not exposed to treatments that are associated with anaemia risk.
Our data were generated in children who had no disease‐associated increased risk of anaemia. The risk of anaemia and adverse events may increase for sick children, especially those associated with low blood haemoglobin levels or blood volume depletion and when treatment may induce anaemia. A previous study conducted in 11 children with cancer during pharmacological intervention reported that repeated blood sampling at a volume of 3–5% of total blood volume was associated with a decrease in haemoglobin concentrations but that the decrease did not correlate with the actual volume of blood taken, suggesting other factors as the cause of the reduction in haemoglobin 6. Also, our study demonstrated a slight decrease in haemoglobin, red blood cell volume and haematocrit in children participating in one of the three interventional studies but these reductions were without clinical relevance and all measurements were still in the normal range. It is difficult to explain why this slight reduction was seen in the one study which included the older children rather than in the studies performed in younger children, but one may speculate that red cell production and turnover are faster at a younger age so that blood loss can be more easily compensated.
Repeated blood draws that are over 1% of the total blood volume are documented (Table S3). The Environmental Determinants of Diabetes in the Young (TEDDY) study is an observational study in over 8000 children at increased risk of type 1 diabetes. Blood draws ranging from 1.3 to 1.7 mL/kg body weight (1.3‐2.0% of total blood volume) were taken at three‐monthly intervals from age three months to four years. TEDDY started in 2004 and was approved by institutional review boards in the United States, Germany, Finland and Sweden 7. Although not documented with actual measurements, the TEDDY study has not reported any related safety issues and has recently increased blood draw volumes to 3 mL/kg body weight (3.5% of total blood volume), with ethical approval from all participating countries.
Strengths and limitations
The strength of this analysis is that the data were collected under the rigid conditions of randomised controlled trials where data entry was quality controlled and monitored, and adverse events timely reported. A potential weakness may be that blood counts and haemoglobin measurements were taken between two and three months after blood draw so that transient anaemia soon after the blood draw may have been missed. However, since the concurrent and prospective adverse event reporting did not give any evidence for signs of anaemia or hypovolaemia in the children, we do not expect such transient anaemia to be a safety concern. It should also be noted that while the trial protocol‐based target blood draw was 2.4 mL/kg body weight, it is possible that this was not always achieved in all children.
Conclusion
We conclude that protocols that schedule regular blood draws of up to 2.4 mL/kg body weight corresponding to 3% of total blood volume at each visit are not associated with a risk of anaemia or other clinically relevant side effects in young children. We therefore suggest that the current draft European Union recommendations should be revised for studies of healthy children and where treatments are not known to induce anaemia.
Funding
This study did not receive any specific funding.
Conflict of interest
The authors have no conflict of interest to declare.
Supporting information
Figure S1a Time points of study related blood draws and blood count measurements and number of blood draws and blood counts in the Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies.
Figure S1b Summary of blood draws and blood counts as of July 5, 2017 from Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies.
Figure S2 Scatterplots of haemoglobin, red blood cells (RBC), haematocrit, mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and mean corpuscular volume (MCV) by age at baseline visit.
Figure S3 Dot plots of mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and mean corpuscular volume (MCV) at baseline and at end of treatment or after 12 months of treatment (as appropriate) in the Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies, respectively.
Table S1 Summary statistics of haemoglobin, red blood cells (RBC), haematocrit, mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and mean corpuscular volume (MCV) at baseline (BL) and at end of treatment (EOT) or after 12 months of treatment (as appropriate) in the Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies, respectively.
Table S2 Frequency of adverse events in the Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies, respectively.
Table S3 Summary of blood volumes recommended in guideline and used in clinical studies.
Acknowledgement
We especially thank all children and parents for their participation in the Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies. In addition, we would like to thank the whole study team and laboratory technicians at the Institute of Diabetes Research for their excellent work regarding study visit conduction, blood draws and sample processing.
References
- 1. European Parliament . Ethical considerations for clinical trials on medicinal products conducted with the paediatric population: Recommendations of the ad hoc group for the development of implementing guidelines for Directive 2001/20/EC relating to good clinical practice in the conduct of clinical trials on medicinal products for human use; 2008. Available at: http://ec.europa.eu/health//sites/health/files/files/eudralex/vol-10/ethical_considerations_en.pdf.
- 2. The hospital for sick children (SickKids) Research Ethics Board. Blood Sampling Guidelines; 2003. Available at: https://www.sickkids.ca/pdfs/Research/REB/12349-Bloodguidelines.pdf.
- 3. Altamimi MI, Choonara I, Sammons H. Invasiveness of pharmacokinetic studies in children: a systematic review. BMJ Open 2016; 6(7): e010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howie SRC. Blood sample volumes in child health research: review of safe limits. Bull World Health Organ 2011; 89(1): 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Warren Grant Magnuson Clinical Center . Policy and communications Bulletin – The Clinical Center/Medical Administrative Series: Guidelines for Limits of Blood Drawn for Research Purposes in the Clinical Center; 2009. Available at: https://irb.research.chop.edu/sites/default/files/documents/g_nih_blooddraws.pdf.
- 6. Cole M, Boddy AV, Kearns P, Teh KH, Price L, Parry A, et al. Potential clinical impact of taking multiple blood samples for research studies in paediatric oncology: how much do we really know? Pediatr Blood Cancer 2006; 46(7): 723–7. [DOI] [PubMed] [Google Scholar]
- 7. The TEDDY Study Group . The environmental determinants of diabetes in the young (TEDDY) study. Ann N Y Acad Sci 2008; 1150: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1a Time points of study related blood draws and blood count measurements and number of blood draws and blood counts in the Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies.
Figure S1b Summary of blood draws and blood counts as of July 5, 2017 from Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies.
Figure S2 Scatterplots of haemoglobin, red blood cells (RBC), haematocrit, mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and mean corpuscular volume (MCV) by age at baseline visit.
Figure S3 Dot plots of mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and mean corpuscular volume (MCV) at baseline and at end of treatment or after 12 months of treatment (as appropriate) in the Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies, respectively.
Table S1 Summary statistics of haemoglobin, red blood cells (RBC), haematocrit, mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and mean corpuscular volume (MCV) at baseline (BL) and at end of treatment (EOT) or after 12 months of treatment (as appropriate) in the Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies, respectively.
Table S2 Frequency of adverse events in the Pre‐POINT, Pre‐POINT‐Early and Fr1da‐Insulin‐Intervention studies, respectively.
Table S3 Summary of blood volumes recommended in guideline and used in clinical studies.


