Abstract
BACKGROUND
Biofortified yellow cassava is being cultivated in countries with high cassava consumption to improve its population's vitamin A status. The carotenoid retention in biofortified cassava when processed as boiled, fufu, and chikwangue was evaluated in this study. Commercial biofortified varieties Kindisa and Vuvu and the experimental genotypes MVZ2011B/360 and MVZ2012/044 were used. Fresh cassava roots were processed as boiled, fufu, and chikwangue. Provitamin A carotenoids (pVACs) content of fresh and processed cassava was measured by high‐performance liquid chromatography, and total carotenoids was measured by spectrophotometer.
RESULTS
pVACs content of fresh peeled cassava was 1.79–6.65 µg g−1 on a fresh weight basis, whereas in boiled cassava, fufu, and chikwangue the pVACs content was 1.71–6.91 µg g−1, 0.04–0.37 µg g−1, and 0.52–1.75 µg g−1 respectively. True retention of carotenoids after cooking was 93.2–96.8%, 0.8–3.1%, and 4.0–18.1% for boiled cassava, fufu, and chikwangue respectively. Significant total carotenoids loss was observed during storage.
CONCLUSION
The results indicated that biofortified boiled cassava could be an effective food product to improve pVACs intake in areas where vitamin A deficiency exists, and processing of chikwangue and fufu should be improved before promoting biofortified cassava in vitamin‐A‐deficient areas with high cassava consumption. © 2018 The Authors. Journal of the Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: biofortification, yellow cassava, carotenoid retention, chikwangue, fufu
INTRODUCTION
Vitamin A deficiency, which is associated with risk of infections and xerophthalmia, is a severe public health problem in countries like Nigeria, Democratic Republic of Congo (DRC) and the Republic of the Congo, where cassava is the main source of carbohydrates.1, 2 Biofortified staple crops with higher micronutrient density, including varieties of biofortified yellow cassava with provitamin A carotenoids (pVACs), have been developed to contribute reducing micronutrient deficiencies across the world.3 Biofortified cassava varieties developed by conventional breeding techniques have been released in the main cassava‐producing countries, such as the DRC and Nigeria. In 2008, the first biofortified cassava variety (Kindisa), with pVACs levels between 6 and 8 µg g−1 β‐carotene equivalents (βCE), was released in the DRC. Varieties of biofortified cassava with higher pVACs content (target level of 15 µg g−1 βCE) are being developed by the Institut National pour l'Etude et la Recherche Agronomiques (INERA) in the DRC and the Nigerian National Root Crops Research Institute in Nigeria. The pVACs target level for cassava, set to reach 50% of the estimated average requirement (EAR) for children and pregnant women in the DRC and Nigeria, assumes that up to 50% of pVACs in peeled roots is lost during processing, storage, and cooking.4, 5
Carotenoid retention higher than 50% in boiled cassava has been reported in different studies.6, 7 A study in Kenya demonstrated that feeding children 2–4 years old with boiled biofortified yellow cassava improved their vitamin A status.8 However, commonly consumed cassava‐derived food products in African countries, such as fufu, gari, chikwangue, and lafun, are produced with fermented cassava roots to eliminate most of the toxic cyanogenic compounds present in raw cassava.9 Cassava is mainly commercialized either as dry pieces of fermented cassava roots, cossettes, that are milled into cassava flour to prepare fufu or lafun, or as fermented cassava paste, kimpuka, used to prepare chikwangue. Generally, fufu is prepared by cooking fermented cassava flour in boiling water, whereas chikwangue is prepared by precooking and steaming fermented cassava paste.10, 11, 12, 13 Limited information on carotenoid retention is available for both products, making it difficult to estimate their potential impact on vitamin A status of children and women. In Nigeria, a study found that apparent carotenoid retention in fufu prepared with fermented cassava flour was 17–32%, but no information on true retention was presented.14 The same study also found that apparent retention of carotenoids was 86–90% when fufu was prepared with a wet paste without a drying step. Another study in Nigeria reported true carotenoid retention between 12 and 36% when processing biofortified cassava roots into fufu using fermented cassava paste without a drying step.15 Furthermore, cassava chips to prepare fufu and paste to prepare chikwangue are transported for long distances and stored during extended periods up to 1 month, which could affect the final pVACs of cooked products made with them.
To determine the potential contribution of biofortified cassava to vitamin A EAR of populations with high consumption of cassava, this study measured the retention of carotenoids during the processing of biofortified cassava into boiled cassava, fufu, and chikwangue.
MATERIALS AND METHODS
Cassava roots and food products
Two released biofortified genotypes of cassava, Kindisa and Vuvu, and two yellow advanced experimental genotypes, MVZ2011B/360 and MVZ2012/044, were used to determine the carotenoid retention during boiling and production of fufu and chikwangue. The materials were selected based on their root color and acceptable root yield in breeding fields. Cassava was grown under conventional agronomic practices in February 2015 at the INERA station in Mvuazi, Kongo Central, and harvested in April 2016. Roots from each genotype were harvested in batches of 70 kg per processing repetition. Harvesting of each batch was done on different days and the processing of each batch started the day of harvest. From each batch, 10 kg was used to produce boiled cassava, 30 kg to produce fufu, and 30 kg to produce chikwangue. In addition, to determine carotenoid retention during storage of fermented cassava paste and chips, 420 kg of roots (Kindisa) was harvested and distributed among seven cassava processors across Kongo Central to produce fermented cassava paste and chips.
Processing of boiled cassava
A 10 kg batch of fresh cassava roots was peeled manually, and chopped into pieces of 8–10 cm length and 3–5 cm width and height. A 2 kg sample of the peeled cassava pieces was boiled for 40 min at >98 °C in a pot containing 4 L of boiling water, using an electric stove. Raw and cooked cassava were weighed and a sample of each was taken for measurement of total carotenoids (TCs), pVACs, and dry matter content.
Processing of fufu
A 30 kg batch of cassava roots was washed, manually peeled, and chipped using an AGRIMAC mechanical chipper (Kinshasa, DRC) with a cutting disc to produce small cassava chips, microcossettes, of approximately 25 mm length, 5 mm width, and 2 mm height. Chipped cassava was transferred into a polypropylene sack and fermented for 3 days inside a plastic bucket containing 27 L of water and covered with a dark plastic to prevent direct exposure to light. After fermentation (pH <4.0), cassava was pressed four times in a hydraulic press (5 min each) to remove excess water. Pressed cassava was sundried for 3 days (4 h per day) to obtain fermented cassava chips with 9.8–11.0% moisture. The fermented microcossettes were milled using a hammer mill to obtain fermented cassava flour with particle size <0.5 mm. The freshly milled fermented cassava flour was mixed for 4 min with boiling water in a 1 : 3–1 : 4 ratio with a wooden spatula until obtaining a uniform cooked paste called fufu. Samples of pressed fermented cassava (paste), milled microcossettes (flour), and cooked fufu were taken for measurement of TCs, pVACs, and dry matter content.
Processing of chikwangue
A 30 kg batch of cassava roots was washed, peeled manually, and grated using an AGRIMAC mechanical grater (Kinshasa, DRC). Grated cassava was put in a polypropylene sack and fermented for 4 days inside a plastic bucket containing 27 L of water and covered as noted earlier. After fermentation (pH 3.6–3.8), water was drained, roots were smashed by hand, and large pieces of fibers were removed, resulting in a fermented cassava paste, also named kimpuka. The kimpuka was sieved with excess water to remove small pieces of fiber residue, sedimented, decanted to remove free water, drained in polypropylene sacks for 16 h to remove excess water, and finally pounded to obtain a uniform fine paste. The fine paste was placed inside 1 kg plastic bags and partially cooked in boiling water for 20 min. The partially cooked paste was kneaded to form a dough and molded into pieces of approximately 700 g, placed inside polyethylene bags that were wrapped in leaves (Megaphrynium marostachyum), and finally steamed for 40 min to obtain the cooked chikwangues. Samples of the fine paste, cooked chikwangues, and fiber residue were taken for measurement of TCs, pVACs, and dry matter content.
Cassava flour and paste for storage
Samples of microcossettes and kimpuka prepared with biofortified cassava variety Kindisa were collected from seven processing centers across Kongo Central province to determine retention of TCs during storage. Samples were collected immediately after the processing operations ended at each of the processing centers and transported inside an insulated foam container to a storage facility within 24 h. The fermented microcossettes and paste collected were stored at room temperature (24–28 °C, daily average) in a dark room for 20 days and 30 days respectively. The microcossettes were stored in transparent polyethylene bags and samples were taken at days 0, 10, and 20 of storage for TC analysis, whereas the paste was stored in the same bag type and samples were taken at days 0, 10, 20, and 30 of storage.
Reagents for carotenoid extraction and quantification
All reagents and chemical used were of high‐performance liquid chromatography (HPLC) and analytical grade. The all‐trans‐β‐carotene (βC), 9‐cis‐β‐carotene (9‐cis‐βC), and 13‐cis‐β‐carotene (13‐cis‐βC) standards were purchased from CaroteNature (Ostermundigen, Switzerland). Butylated hydroxytoluene, methyl‐tert‐butyl ether, hexane, ethanol, methanol, and ammonium acetate were obtained from Sigma Co. (St Louis, MO, USA).
TCs measurement by spectrophotometer
The extraction method described by Rodriguez‐Amaya with some modifications was used to measure TCs using a spectrophotometer.16 Each sample was homogenized and 10 g was mixed with 3 g of Celite and 25 mL of acetone. The mixture was pounded with a pestle for 3 min and transferred to a filter funnel. To ensure complete removal of carotenoids from the filter funnel, an additional 30 mL of acetone was added. Filtered extract was placed into a 500 mL separatory funnel containing 40 mL of petroleum ether. Acetone was separated from the petroleum ether by slowly adding 50 mL of deionized water. To further remove acetone from the petroleum ether phase, 50 mL of water (10 g kg−1 sodium chloride) was added four times. The resulting extract was filtered with 15 g of anhydrous sodium sulfate and placed into a 50 mL graduated flask. Petroleum ether was added until carotenoid extract was diluted to 50 mL. An aliquot of the extract was placed in a quartz cuvette and was read at 450 nm using a Genesys 10S UV–visible spectrophotometer. To calculate the TCs content, the following equation was used:
where TC (µg g−1) is the TCs in the sample, A is the absorbance at 450 nm; V (mL) is the final volume of extract, is the molar absorptivity coefficient of β‐carotene in petroleum ether), and P (g) is the weight of sample.
Carotenoid extraction and measurement by HPLC
Samples were analyzed at the Food and Nutritional Evaluation Laboratory at the International Potato Center (CIP), BecA ILRI Research Hub, Nairobi, Kenya. Carotenoid extraction and analysis was performed as described by Kurilich and Juvik, with some modifications.17 Samples were equilibrated to room conditions and 1 g was weighed in duplicate in 25 mL glass tubes; 6 mL of ethanol with 1 g kg−1 butylated hydroxytoluene was added to the tube, vortexed for 1 min, and incubated in a hot‐water bath at 85 °C for 10 min. Samples were saponified by adding 120 µL of 14 mol L–1 potassium hydroxide into the tubes, vortexing for 1 min and incubating in a water bath at 85 °C for 5 min. After saponification, the tubes were immediately placed in an ice bath and 3 mL of cold deionized distilled water was added. Hexane (5 mL) was added to each tube and vortexed for 1 min and centrifuged at 800×g for 10 min. The upper phase of hexane was pipetted into a 25 mL test tube. The hexane extraction was repeated three more times on the residue. The combined hexane fractions were washed by adding 4 mL of deionized water, vortexing, centrifuging at 800×g for 5 min and the upper layer containing the hexane fractions transferred into separate test tubes. The hexane fractions were dried to complete dryness under a gentle stream of nitrogen using an Organomation N‐Evap System (Organomation, Berlin, MA, USA). The residue was reconstituted in 5 mL of ethanol and immediately vortexed and sonicated for 30 s. A 1.5 mL aliquot was transferred into a 2 mL HPLC vial from which 50 µL was taken for HPLC analysis. The carotenoids were separated using a YMC C30 column, 3 µm, 150 mm × 4.6 mm column (YMC Europe GMBH, Dinslaken, Germany) in an Alliance 2695 HPLC system (Waters, Milford, MA, USA). The flow rate was 1 mL min−1 by a gradient elution with two mixtures of methanol, methyl‐tert‐butyl ether, and water (mixture A: 83/15/2 (v/v/v); mixture B: 8/90/2 (v/v/v); both solvents with 15 g kg−1 ammonium acetate). The gradient time program was 0–1 min 100% A, 1–8 min linear gradient to 70% A, 8–13 min 70% A, 13–22 min linear gradient to 45% A, 22–24 min 45% A, 24–34 min linear gradient to 5% A, 34–38 min 5% A, 38–40 min linear gradient to 100% A, and 40–50 min 100% A. A Waters 2996 photodiode array detector at 450 nm and Waters Empower 2 software were used to identify and quantify the carotenoids from extracts by determining the peak area of extracts and standard dilutions.
Apparent and true carotenoid retention
For all samples, percentage of true retention (TR) and apparent retention (AR) were calculated as follows:
TR percentage values were used to determine the total proportion of carotenoids lost during processing, storage, and cooking. AR percentage values were used to determine the carotenoid concentration in intermediate or final (cooked) products without considering losses of solids during processing, storage, or cooking. AR is useful to understand the contribution of a processed product to vitamin A intake based on the intake of such product. TR is useful to understand the contribution of a crop to vitamin A intake based on the expected amount of crop available per person.
Data analysis
Data were expressed as mean plus/minus standard deviation of three processing repetitions. The effect of processing step and genotype was evaluated using a two‐factor analysis of variance model for each cassava food product. Each repetition was represented by a different processing day. Analysis of variance was analyzed using the mixed procedure in SAS 9.4 (SAS Institute, Cary, NC, USA). Means separation was calculated using Tukey's method. Differences between means were considered significant at p < 0.05. The total pVACs was expressed as βCE and was calculated as follows: βCE = βC + (0.5)(9‐cis‐βC) + (0.5)(9‐cis‐βC). The conversion factor of βCE to retinol equivalent to calculate contribution to vitamin A EAR was 5 : 1.18
Rate of carotenoid degradation k was calculated as follows:
where x is the concentration of carotenoid at time t (number of days in storage), x 0 is the concentration of carotenoid at the beginning of storage, and k (day−1) is the reaction rate constant.
RESULTS AND DISCUSSION
Carotenoid profile of cassava roots
Changes in the TCs and pVACs ranking among the four genotypes were found when expressed as fresh weight (FW) basis compared with dry weight (DW) basis (Table 1). For this reason, the carotenoid concentration was expressed in FW and DW basis for peeled cassava and final cooked cassava products; for all other intermediate steps, the concentration values were presented in DW only. TCs content of peeled cassava roots measured by UV–visible spectrophotometer was 2.35–6.86 µg g−1 FW and 5.21–25.80 µg g−1 DW. Total pVACs content quantified by HPLC was 1.79–6.65 µg g−1 FW and 3.96–25.02 µg g−1 DW, where MVZ2011B/360 had the highest pVACs content and Vuvu the lowest. In all genotypes, the main carotenoid found in peeled cassava roots was βC, whereas 9‐cis‐βC and 13‐cis‐βC were found in lower concentrations (Table 1). The proportions of βC, 9‐cis‐βC, and 13‐cis‐βC isomers were respectively 74.8%, 9.1% and 16.0% of the total β‐carotene identified in fresh cassava roots. These values are within the range reported by Thakkar et al.19 and Ceballos et al.20 in which βC represented 55–85% of the total β‐carotene isomers and 13‐cis‐βC and 9‐cis‐βC represented 15–45%. The pVACs in fresh roots represented 11.9–44.3% of the 15 µg g−1 βCE target value for biofortified cassava.
Table 1.
Carotenoid content in fresh roots of four yellow cassava genotypes
| Fresh weight basis (µg g−1 ± SD) | Dry weight basis (µg g−1 ± SD) | ||||||
|---|---|---|---|---|---|---|---|
| Genotype | TCs | 13‐cis‐βC | βC | 9‐cis‐βC | pVACs | TCs | pVACs |
| MVZ2011B/360 | 6.86 ± 0.09a | 1.12 ± 0.05a | 5.87 ± 0.20a | 0.43 ± 0.02b | 6.65 ± 0.23a | 25.80 ± 0.94a | 25.02 ± 1.75a |
| MVZ2012/044 | 4.69 ± 0.07c | 0.81 ± 0.04c | 3.54 ± 0.03c | 0.39 ± 0.05b | 4.15 ± 0.07c | 22.42 ± 0.60b | 19.84 ± 0.83b |
| Kindisa | 5.58 ± 0.33b | 0.99 ± 0.01b | 4.95 ± 0.27b | 0.63 ± 0.03a | 5.76 ± 0.29b | 15.38 ± 1.00c | 15.87 ± 0.95c |
| Vuvu | 2.35 ± 0.01d | 0.45 ± 0.01d | 1.33 ± 0.06d | 0.46 ± 0.02b | 1.79 ± 0.06d | 5.21 ± 0.11d | 3.96 ± 0.14d |
Different lowercase letters indicate significant differences within columns (p < 0.05).
Values are average plus/minus standard deviation (SD) of three processing replicates. 13‐cis‐βC = 13‐cis‐β‐carotene; 9‐cis‐βC = 9‐cis‐β‐carotene; βC = all‐trans‐β‐carotene; pVACs, provitamin A carotenoids expressed as β‐carotene equivalents; TCs, total carotenoids measured using UV–visible spectrophotometer.
Carotenoid retention in boiled cassava
The pVACs TR values after boiling cassava roots were 93.2–96.8%, whereas AR values were 97.0–102.6% (Table 2). No significant differences were found in TR and AR between genotypes (p > 0.05). These retention values were similar to AR >100.0% found by Thakkar et al.19 for three varieties of biofortified yellow cassava, and within the TR (76.0–96.7%) reported by Ceballos et al.20 for six biofortified yellow cassava genotypes. Total pVACs after boiling was 1.71–6.91 µg g−1 FW and 3.90–25.63 µg g−1 DW (Table 2). As in fresh roots, boiled roots of MVZ2011B/360 had the highest pVACs content and Vuvu had the lowest. The main carotenoid found after boiling cassava was βC, representing 72.1% of total quantified carotenoids, whereas 13‐cis‐βC and 9‐cis‐βC represented 8.1% and 19.7% respectively. The proportions of β‐carotene isomers after boiling were similar to those in fresh roots, suggesting that no significant isomerization of βC to 13‐cis‐βC or 9‐cis‐βC occurred during boiling for these genotypes. Significant isomerization of βC to 13‐cis‐βC was observed by Thakkar et al.19 when boiling cassava previously soaked in water for at least 10 h. No isomerization of βC to 9‐cis‐βC or isomerization to 13‐cis‐βC instead of 9‐cis β‐carotene could be positive considering that 13‐cis‐βC has shown better bioconversion to retinol than 9‐cis‐βC has.21 The very high carotenoid retention during boiling yellow cassava makes it a potential alternative to deliver pVACs to consumers. Before promoting cassava consumption as boiled, the cyanogenic content should be evaluated because it is known that cassava could contain high levels of cyanide if not cooked properly.22
Table 2.
Provitamin A carotenoids and carotenoid retention in four yellow cassava genotypes after different processing methods
| Process type | Genotype | pVACs FW (µg g−1 ± SD)a | pVACs DW (µg g−1 ± SD)a | True retention (% ± SD)a | Apparent retention (% ± SD)a |
|---|---|---|---|---|---|
| Boiled | MVZ2011B/360 | 6.91 ± 0.21a | 25.63 ± 1.65a | 96.8 ± 4.5a | 102.6 ± 5.5a |
| MVZ2012/044 | 4.36 ± 0.18c | 19.43 ± 0.83b | 96.4 ± 5.2a | 98.0 ± 5.4a | |
| Kindisa | 5.80 ± 0.78b | 15.42 ± 2.01c | 93.2 ± 9.0a | 97.0 ± 8.4a | |
| Vuvu | 1.71 ± 0.19d | 3.90 ± 0.41d | 94.9 ± 6.6a | 98.4 ± 6.9a | |
| Chikangue | MVZ2011B/360 | 1.75 ± 0.62a | 6.73 ± 2.35a | 5.6 ± 0.9b | 26.3 ± 6.8a |
| MVZ2012/044 | 1.21 ± 0.24a | 4.28 ± 0.92a | 4.0 ± 0.4b | 21.6 ± 4.8a | |
| Kindisa | 1.50 ± 1.24a | 4.69 ± 3.90ab | 8.10 ± 6.2b | 28.8 ± 22.4a | |
| Vuvu | 0.39 ± 0.23b | 1.04 ± 0.62b | 18.1 ± 1.7a | 35.7 ± 4.7a | |
| Fufu | MVZ2011B/360 | 0.22 ± 0.17ab | 0.63 ± 0.47b | 1.8 ± 1.2ab | 2.5 ± 1.7b |
| MVZ2012/044 | 0.37 ± 0.13a | 1.11 ± 0.38a | 3.1 ± 1.4a | 5.6 ± 1.9a | |
| Kindisa | 0.08 ± 0.04b | 0.23 ± 0.11b | 0.8 ± 0.5b | 1.5 ± 0.7b | |
| Vuvu | 0.04 ± 0.03b | 0.13 ± 0.09b | 2.5 ± 1.7ab | 3.3 ± 2.3b |
Different lowercase letters indicate significant differences within columns for each process type (p < 0.05).
Values are average plus/minus standard deviation (SD) of three processing replicates. DW, dry weight basis; FW, fresh weight basis; pVACs, provitamin A carotenoids expressed as β‐carotene equivalents.
Carotenoid retention in cooked fufu
The TR of pVACs in cooked fufu prepared with freshly fermented microcossettes was 0.8–3.1%, whereas AR was 1.5–5.6% (Table 2). The pVACs in cooked fufu was 0.04–0.37 µg g−1 FW and 0.23–1.11 µg g−1 DW. Genotype MVZ2012/044 had higher TR than Kindisa did, but no significant differences were found with the other genotypes. MVZ2012/044 had the highest pVACs AR among the four genotypes. The TR values in fufu were lower than the 12–36% TR reported for fufu produced with nondried fermented paste in Nigeria.15 The AR were also lower than the AR values reported for fufu made with oven‐dried fermented cassava flour (17–32%) in Nigeria.14 The lower carotenoid retention in fufu could be due to the exposure of carotenoids to sunlight during drying, as observed by Chávez et al.,6 who reported higher carotenoid TR (71.9%) when drying cassava with an oven compared with sun‐drying (37.9%). Fermentation and pressing was the step during preparation of fufu that produced the highest pVACs loss (56.2%), followed by 40.1% loss during sun‐drying of fermented microcossettes. The cooking step caused only 1.7% of pVACs loss (Fig. 1).
Figure 1.
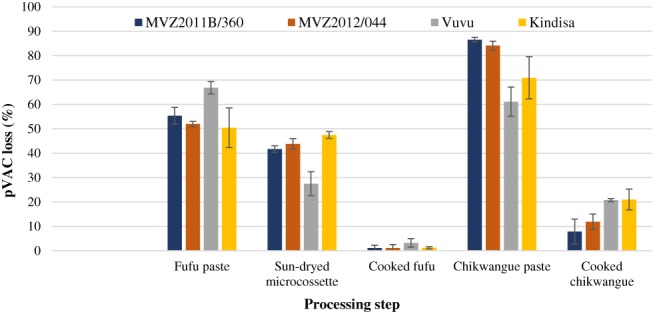
Loss of pVACs (%) in each processing step of three cassava products (boiled, fufu, and chikwangue) made with four yellow biofortified cassava varieties.
In DRC and the Republic of Congo, fermented cassava to prepare fufu is generally dried as large chips (cossettes), which are large pieces of roots. To minimize product losses due to molding during drying, the use of smaller chips (microcossettes) to give fast drying is widely promoted. Given the low pVACs retention observed in this study using microcossettes, it will be important to study the rate of carotenoid loss when sun‐drying large pieces of cassava and determine if higher carotenoid retention could be obtained compared with the currently suggested method using microcossettes.
Carotenoid retention in cooked chikwangue
Large variability in pVACs TR between genotypes was observed when producing chikwangues. The TR values of carotenoids in cooked chikwangue prepared with freshly fermented cassava were 4.0–18.1%. Genotype Vuvu had the highest TR, but its AR was not significantly different to the other genotypes. The pVACs in cooked chikwangue was 0.52–1.75 µg g−1 FW and 1.39–6.73 µg g−1 DW (Table 2). Significant carotenoids loss occurred in each processing step for chikwangue. On average, the greatest loss of pVACs was during the production of the fine paste (75.7%), whereas during cooking cassava paste into chikwangue the loss was 15.4% (Fig. 1).
Higher pVACs loss was found during production of chikwangue paste than with fufu paste, except for the genotype Vuvu. This difference could be attributed to the higher dry matter loss when producing paste for chikwangue (70.5%) compared with fufu (36.7%). The higher dry matter loss in chikwangue paste was due to the additional sieving step in which 16.8–40.6% of dry matter was removed as fine residue. The pVACs in the residue removed during sieving represented 3.8–21.6% of the initial pVACs and significantly increased the pVACs loss in chikwangue paste (p < 0.05). The amount of residue removed was positively correlated to the total amount of pVACs retained in the residue (r 2 = 0.95), but the proportion of pVACs loss was lower than the proportion of solids loss.
In general, pVACs TR in chikwangue was lower than boiled cassava but higher than fufu (Fig. 1). Despite the low pVACs TR of chikwangue (8.9%), its AR was high (28.1%) compared with fufu (3.2%). Cooked chikwangue could deliver a higher amount of pVACs than cooked fufu when considering daily consumption of final cooked product only, without accounting for dry matter losses during processing. Considering the significant carotenoid losses during sieving, biofortified cassava varieties that produce lower residue during chikwangue preparation should be developed.
Relationship of carotenoids by HPLC and spectrophotometry
In fresh roots, TCs values measured by spectrophotometry and pVACs values by HPLC had a high correlation (r 2 = 0.97). The correlation was also high for boiled roots (r 2 = 0.97), cooked fufu (r 2 = 0.95), and cooked chikwangue (r 2 = 0.91) (Fig. 2). The high correlation for raw cassava roots is in concordance with Sánchez et al.,23 who suggested that spectrophotometry is a reliable alternative to measure TCs for fast genotyping cassava in breeding programs, instead of HPLC. Given that no major changes in proportions of 13‐cis‐βC and 9‐cis‐βC were found between raw and cooked cassava, the high correlation found for raw cassava was also expected for cooked products. The use of spectrophotometry to estimate pVACs content in processed products should be considered as a tool to select material during the breeding process, not only to analyze raw and boiled cassava, but also for processed products such as fufu and chikwangue. The significant genotypic effect on carotenoid retention during processing and limited availability of equipment (such as for HPLC) in countries where biofortified breeding programs exist are reasons to consider the use of spectrophotometry to estimate pVACs content in raw and processed roots in breeding programs, where large numbers of genotypes are evaluated.
Figure 2.
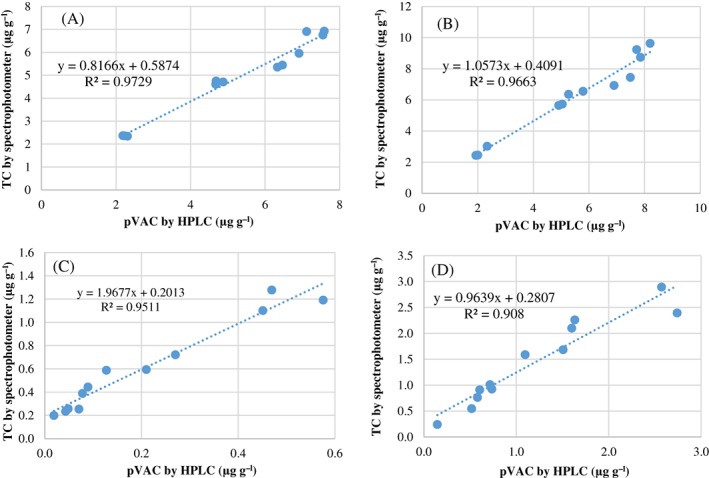
Correlation between carotenoids determined by HPLC and spectrophotometry for (A) fresh peeled roots, (B) boiled roots, (C) cooked fufu, and (D) cooked chikwangue prepared with cassava roots.
Estimation of degradation kinetics of carotenoids in cassava paste and microcossettes
Considering that fermented cassava microcossettes and paste are commonly used for cooking up to 1 month after processing, an estimation of the TC degradation kinetics during common storage for such products was determined based on measurements of retention values at different timepoints. TC retention in microcossettes was 62 ± 17% after 20 days of storage and 65 ± 10% for paste stored for 30 days. A first‐order degradation was observed for TC in both products for biofortified Kindisa variety processed in seven locations in Kongo Central province. The degradation rate constant k (day−1) was higher in fermented cassava microcossettes than in cassava paste, with averages of 0.0282 (r 2 = 0.73) and 0.0144 (r 2 = 0.89) respectively (p < 0.01). The k values for TC in cassava microcossettes were similar to values reported for βC in dry cassava granules stored at 26–33 °C (k = 0.0144–0.0271) by Bechoff et al.24 Retention values after 30 days at such degradation rates represented 65.0% for fermented chikwangue paste and 43.0% for fermented cassava microcossettes, at 24–27 °C. It is known that the rate of degradation of pVACs is relatively high in dry products, especially when the particle size of the product is small, due to its high surface area exposing the carotenoids to oxygen.25, 26, 27 Lower k values for fermented cassava paste suggest that carotenoids in an aqueous matrix are more stable than carotenoids exposed to oxygen in a dry matrix such as cassava microcossettes. These results are in concordance with previous studies showing that the main factor influencing degradation of carotenoids during storage of biofortified foods is oxygen availability.28, 29 Assuming that products are consumed in similar proportion during a 30 day storage period, the resulting average TCs retention for fufu and chikwangue could be 68% and 82% respectively.
Contribution of biofortified cassava to improve vitamin A intake
The contribution of biofortified cassava to vitamin A intake in populations with high cassava consumption as fufu and chikwangue was estimated for all genotypes. Cassava processed as fufu had significantly higher pVACs loss than boiled cassava for each genotype, whereas chikwangue had less pVACs loss than fufu but more than boiled cassava. These differences in TR have an impact on the vitamin A EAR contribution from cassava based on type of product eaten. Contribution to the EAR was calculated considering an EAR of 275 µg for children aged 4–6 years and 500 µg for women of child‐bearing age, as suggested by the Institute of Medicine.30 Average TC storage retention, 68% for fufu and 82% for chikwangue, was used based on the TC storage retention obtained for Kindisa variety. Intake of 300 g of peeled cassava for children and 500 g for women was used based on cassava intake from Nigeria and production in DRC.1, 31 For children aged 4–6 years, biofortified cassava would contribute 117%, 8%, or 2% of the vitamin A requirement when respectively consuming boiled cassava, chikwangue, or fermented fufu prepared with 300 g of peeled cassava roots (Table 3). For women of child‐bearing age, biofortified cassava would contribute 107%, 8%, or 2% of the EAR when 500 g of peeled cassava is eaten as boiled, chikwangue, or fufu respectively (Table 3). The potential contribution to the EAR (280–305%) by consuming only boiled cassava prepared with a variety containing the pVACs target level (15 µg g−1) may not be reached in countries where fufu and chikwangue are the main cassava‐based products. However, with a partial substitution of 10%, the contribution of biofortified boiled cassava to the EAR could be up to 31% for children 4–6 years old and 28% for women, higher than when chikwangue and fufu are consumed as the only source of cassava product (Table 3). Using 17% and 18% of biofortified cassava with the full pVACs target content to prepare boiled cassava could provide enough pVACs to reach >50% of the vitamin A EAR for children 4–6 years old and women respectively (Table 3). Cyanide values reported for biofortified cassava are not always lower than recommended for boiled cassava.23 To promote consumption of biofortified cassava as boiled, breeders need to develop biofortified cassava varieties with low levels of cyanide to reduce the risk of toxicity, and farmers should be trained on proper boiling methods to reduce cyanide content.22
Table 3.
Estimation of the contribution of four cassava genotypes and target variety (15 µg g−1 pVACs) to vitamin A estimated average requirement (EAR) for children aged 4–6 years and women when cassava prepared as boiled, chikwangue, or fufu at different usage levels
| Product | Usage (%) | Population | EAR (%)(a , b) | ||||
|---|---|---|---|---|---|---|---|
| Vuvu | MVZ2012/044 | Kindisa | MVZ2011B/360 | Target | |||
| Boiled | 100 | Children 4–6 | 37 | 87 | 117 | 140 | 305 |
| 100 | Women | 34 | 80 | 107 | 129 | 280 | |
| 10 | Children 4–6 | 4 | 9 | 12 | 14 | 31 | |
| 10 | Women | 3 | 8 | 11 | 13 | 28 | |
| 17 | Children 4–6 | 6 | 15 | 20 | 24 | 52 | |
| 18 | Women | 6 | 14 | 19 | 23 | 50 | |
| Chikwangue | 100 | Children 4–6 | 6 | 3 | 8 | 7 | 11 |
| 100 | Women | 5 | 3 | 8 | 6 | 10 | |
| 20 | Children 4–6 | 1 | 1 | 2 | 1 | 2 | |
| 20 | Women | 1 | 1 | 2 | 1 | 2 | |
| Fufu | 100 | Children 4–6 | 1 | 2 | 1 | 2 | 2 |
| 100 | Women | 1 | 2 | 1 | 2 | 2 | |
| 70 | Children 4–6 | 0 | 1 | 1 | 1 | 1 | |
| 70 | Women | 0 | 1 | 0 | 1 | 1 | |
EAR, estimated average requirement. EAR for children 4–6 = 275 µg retinol equivalents (RE) and for women = 500 µg RE.
Calculated based on the lowest retention for each type of product within the 4 genotypes tested.
In general, the TR of carotenoids was high in boiled cassava, low in chikwangue, and very low in fufu using current processing methods in DRC and the Republic of Congo. Promoting the consumption of chikwangue and boiled cassava instead of fufu could be a good strategy, especially if considering only the concentration of pVACs in the final product (apparent retention) without accounting for the losses of dry matter during processing. Boiled cassava has the potential to achieve the 50% EAR of vitamin A target level for biofortified crops; however, consuming biofortified cassava only as fufu and chikwangue may not provide the vitamin A intake target level. Since consumers in rural areas of DRC and the Republic of Congo eat less chikwangue than fufu, farmers could produce chikwangue with biofortified yellow cassava and fufu with white cassava. The consumption of boiled products in areas where high deficiency of vitamin A exists should be promoted only if the cassava roots used have low levels of cyanide. Meanwhile, fufu and chikwangue processing should be improved to minimize carotenoid loss, not only in African countries, but in any country where the processing includes extensive loss of dry matter and sun‐drying. Considering the genotypic variation for pVACs retention during processing, it is recommended to evaluate the carotenoid retention for chikwangue and fufu made with advanced biofortified breeding material before they are released as new varieties. Spectrophotometry or HPLC methods could be used in such breeding programs to test raw cassava and fufu and chikwangue.
ACKNOWLEDGEMENTS
To HarvestPlus for providing funding. To Marceline Limpete and the staff of the breeding program at INERA Mvuazi station in Kongo Central, DRC, for all their support during processing of the products, and to Daniel Mbogo at the Food and Nutritional Evaluation Laboratory CIP in Nairobi, for sample analysis.
REFERENCES
- 1. Food and Agriculture Organization of the United Nations , FAOSTAT (2018). Available http://www.fao.org/faostat/en/#data/QC [20 January 2018].
- 2. WHO , Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency. World Health Organization, Geneva: (2009). [Google Scholar]
- 3. Saltzman A, Andersson MS, Asare‐Marfo D, Lividini K, De Moura FF, Moursi M et al, Biofortification techniques to improve food security, in Reference Module in Food Science, Vol. 1, pp. 1–9 (2016). [Google Scholar]
- 4. Saltzman A, Birol E, Bouis HE, Boy E, De Moura F, Islam Y et al, Biofortification: progress toward a more nourishing future. Glob Food Sec 2:9–17 (2013). [Google Scholar]
- 5. Andersson ME, Saltzman A, Virk PS and Pfeiffer WH, Progress update: crop development of biofortified staple food crops under HarvestPlus. Afr J Food Agric Nutr Dev 17:11905–11935 (2017). [Google Scholar]
- 6. Chávez AL, Sánchez T, Ceballos H, Rodriguez‐Amaya DB, Nestel P, Tohme J et al, Retention of carotenoids in cassava roots submitted to different processing methods. J Sci Food Agric 87:388–393 (2007). [Google Scholar]
- 7. De Moura FF, Miloff A and Boy E, Retention of provitamin A carotenoids in staple crops targeted for biofortification in Africa: cassava, maize and sweet potato. Crit Rev Food Sci Nutr 55:1246–1269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Talsma EF, Brouwe ID, Verhoef H, Mbera GNK, Mwangi AM, Demir AY et al, Biofortified yellow cassava and vitamin A status of Kenyan children: a randomized controlled trail. Am J Clin Nutr 103:258–267 (2016). [DOI] [PubMed] [Google Scholar]
- 9. Nweke FI, Lutete D, Dixon AGO, Ugwu BO, Ajobo O, Kalombo N et al, Cassava Production and Processing in the Democratic Republic of Congo Collaborative Study of Cassava in Africa (COSCA) Working Paper no. 22. International Institute of Tropical Agriculture, Ibadan, Nigeria. (2000).
- 10. Treche S, Legros O, Avouampo E, Muchnik J and Massamba J, Fabrication de Chikwangue au Congo, L'Institut Français de Recherche Scientifique pour le Développement en Cooperation, Paris: (1993). [Google Scholar]
- 11. Avouampo E, Gallon G and Treche S, Influence de la variété et de l'ordre de réalization de l'épluchage et du rouissage sur l'aptitude à la transformation des raciness de manioc, in Transformation Alimentaire du Manioc, ed. by Egbe TA, Brauman A, Griffon D. and Treche S. ORSTOM Editions, Paris, pp. 429–447 (1995). [Google Scholar]
- 12. Tollens E, Maize Commodity Assessment and Workplan Related to Maize, Report no. 42. US Agency for International Development, Washington, DC, (2011) Available http://pdf.usaid.gov/pdf_docs/pnady675.pdf [20 January 2018]. [Google Scholar]
- 13. Humpal D, Musangu B and Tunieka M, Cassava Value Chain Assessment: Bas‐Congo, Kinshasa, and Bandundu Provinces. US Agency for International Development, Washington, DC, (2012). Available http://pdf.usaid.gov/pdf_docs/pnady673.pdf [20 January 2018]. [Google Scholar]
- 14. Omodamiro RM, Oti E, Etudaiye HA, Egesi C, Olasanmi B and Ukpabi UJ, Production of fufu from yellow cassava roots using the odourless flour technique and the traditional method: evaluation of carotenoids retention in the fufu. Adv Appl Sci Res 3:2566–2572 (2012). [Google Scholar]
- 15. Maziya‐Dixon B, Awoyale W and Dixon A, Effect of processing on the retention of total carotenoid, iron and zinc contents of yellow‐fleshed cassava roots. J Food Nutr Res 3:483–488 (2015). [Google Scholar]
- 16. Rodriguez‐Amaya DB, Food Carotenoids: Chemistry, Biology, and Technology. John Wiley & Sons, Ltd, Chichester: (2015). [Google Scholar]
- 17. Kurilich AC and Juvik JA, Quantification of carotenoid and tocopherol antioxidants in Zea mays . J Agric Food Chem 47:1948–1955 (1999). [DOI] [PubMed] [Google Scholar]
- 18. La Frano MR, Woodhouse LR, Burnett DJ and Burri BJ, Biofortified cassava increases β‐carotene and vitamin A concentrations in the TAG‐rich plasma layer of American women. Br J Nutr 110:310–320 (2013). [DOI] [PubMed] [Google Scholar]
- 19. Thakkar SK, Huo T, Maziya‐Dixon B and Failla ML, Impact of style of processing on retention and bioaccessibility of β‐carotene in cassava (Manihot esculenta, Crantz). J Agric Food Chem 57:1344–1348 (2009). [DOI] [PubMed] [Google Scholar]
- 20. Ceballos H, Luna J, Escobar AF, Ortiz D, Pérez JC, Sánchez T et al, Spatial distribution of dry matter in yellow fleshed cassava roots and its influence on carotenoid retention upon boiling. Food Res Int 45:52–59 (2012). [Google Scholar]
- 21. Deming DM, Baker DH and Erdman JW, The relative vitamin A value of 9‐cis β‐carotene is less and that of 13‐cis β‐carotene may be greater than the accepted 50% that of all‐trans β‐carotene in gerbils. J Nutr 132:2709–2712 (2002). [DOI] [PubMed] [Google Scholar]
- 22. Montagnac JA, Davis CR and Tanumihardjo SA, Techniques to reduce toxicity and antinutrients of cassava for use as a staple food. Compr Rev Food Sci Food Saf 8:17–27 (2009). [DOI] [PubMed] [Google Scholar]
- 23. Sánchez T, Ceballos H, Dufour D, Ortiz D, Morante N, Calle F et al, Prediction of carotenoid, cyanide and dry matter contents in fresh cassava root using NIRS and Hunter color techniques. Food Chem 151:444–451 (2014). [DOI] [PubMed] [Google Scholar]
- 24. Bechoff A, Chijioke U, Tomlins KI, Govinden P, Ilona P, Westby A et al, Carotenoid stability during storage of gari made from yellow cassava or with palm oil. J Food Compos Anal 44:36–44 (2015). [Google Scholar]
- 25. Alves RMV, Ito D, Carvalho JLV, Melo WF and Godoy RLO, Estabilidade de farinha de batata‐doce biofortificada. Braz J Food Technol 15:59–71 (2012). [Google Scholar]
- 26. Júnior LM, Ito D, Ribeiro SML, da Silva MG and Alves RMV, Stability of β‐carotene rich sweet potato chips packed in different packaging systems. LWT – Food Sci Technol 92:442–450 (2018). Available 10.1016/j.lwt.2018.02.066. [DOI] [Google Scholar]
- 27. Taleon V, Mugode L, Cabrera‐Soto L and Palacios‐Rojas N, Carotenoid retention in biofortified maize using different post‐harvest storage and packaging methods. Food Chem 232:60–66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bechoff A, Dhuique‐Mayer C, Dornier M, Tomlins KI, Boulanger R, Dufour D et al, Relationship between the kinetics of β‐carotene degradation and formation of norisoprenoids in the storage of dried sweet potato chips. Food Chem 121:348–357 (2010). [Google Scholar]
- 29. Rodriguez‐Amaya DB, Food Carotenoids: Chemistry, Biology, and Technology. John Wiley & Sons, Ltd, Chichester: (2015). [Google Scholar]
- 30. Institute of Medicine (IOM) , Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press, Washington, DC: (2001). [PubMed] [Google Scholar]
- 31. De Moura FF, Moursi M, Lubowa A, Ha B, Boy E, Oguntona B et al, Cassava intake and vitamin A status among women and preschool children in Akwa‐Ibom, Nigeria. PLoS One 10:e0129436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


