Abstract
Background
Red blood cell (RBC) transfusions are essential in health care. The quality of recommendations included in clinical practice guidelines (CPG), regarding this intervention, has not been systematically evaluated. This paper systematically assessed CPGs for RBC‐transfusion, to appraise their methodological quality, to explore changes in quality over time, and to assess the consistency of the hemoglobin threshold (HT) recommendations.
Methods
We searched for CPGs that included recommendations of RBC‐transfusion in generic databases, compiler entities, registries, clearinghouses and guideline developers. Three reviewers extracted data on CPGs characteristics and HT recommendations, independently appraised the quality of the studies using AGREE II and resolved disagreements by consensus.
Results
We examined 16 CPGs. Mean scores (mean ± SD) were: scope and purpose (59.4% ± 19.8%), stakeholder involvement (43.2% ± 22.6%), rigor of development (50% ± 25%), clarity of presentation (74.4% ± 12.6%), applicability (19.4% ± 18.8%), and editorial independence (41% ± 30%). Seven CPGs recommended a restrictive strategy for RBC transfusion; four CPGs gave a guarded statement considering an HT of 7 g/dL, as safe to prescribe an RBC transfusion. Eight CPGs did not provide an HT stating that RBC transfusions should not be prescribed by HT alone.
Conclusions
Only 3 out of the 16 evaluated CPGs were “recommended” by the independent evaluators. Four domains “stakeholder involvement,” “rigor of development,” applicability,” and “editorial independence” had serious shortcomings. Recommendations about the use of an HT for RBC‐transfusion were heterogeneous among guidelines. Greater efforts are needed to provide high‐quality CPGs in the RBC‐transfusion practice.
Keywords: blood transfusion, clinical practice guidelines, red blood cells, systematic review
1. INTRODUCTION
Blood transfusion is the infusion of both soluble and cell‐associated forms like RBCs, white blood cells, and platelets into a recipient.1 A blood transfusion is an acute intervention, implemented to solve life and health‐threatening conditions on a short‐term basis.2, 3 However, RBCs and other blood components therapies have been associated with several adverse clinical events, and require physicians to be fully informed of the risks and benefits.4, 5 Several strategies for preventing adverse events caused by RBC‐transfusions have been studied; however, their clinical effectiveness has not yet sufficiently demonstrated.2, 3, 6, 7, 8, 9, 10, 11
About 85 million people are transfused annually, with considerable variation in the use of RBC‐transfusion practices worldwide.6 In spite of the efforts to standardize transfusion practice, as the publication of clinical practice guidelines, this variability in transfusion practices has persisted. For instance, while some CPGs have included recommendations focused on hemoglobin concentration to guide RBC‐transfusion, other CPGs emphasize that transfusions should be provided in the presence of anemia symptoms and should not be based on hemoglobin concentration only.2, 5, 8, 9
CPGs are defined as systematically developed statements to assist practitioner and patient decisions about appropriate health care.12 International organizations have introduced and promote standards for the development of CPG, such as the Institute of Medicine (IOM),13 World Health Organization (WHO),14 National Institute for Health and Clinical Excellence (NICE),15 Scottish Intercollegiate Guideline Network (SIGN),16 and Guidelines International Network (G‐I‐N).17 All these efforts provide resources to assist guideline developers in producing high‐quality recommendations. Despite these initiatives, the quality of the CPGs and the adherence to methodological guidelines has been improved only lightly in the last decade.18, 19, 20, 21
In the field of RBC‐transfusion, a large body of clinical evidence has been generated; resulting in the publication of many CPGs.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 These CPGs face with inconsistent recommendations that potentially result in confusion among clinicians, and the quality of the guidelines could be put to question. For these reasons, there is a need to assess the methodological quality of the CPGs in this field, to explain the variability of the recommendations. We conducted a systematic assessment of CPGs for RBC‐transfusion, to appraisal their methodological quality using AGREE II tool, and to explore changes in quality over time, and to evaluate the consistency of hemoglobin concentration recommendations to guide transfusion.
2. METHODS
2.1. Data search
We searched for CPGs that included recommendations of RBC‐transfusion in generic databases, compiler entities, registries, clearinghouses and guideline developers. We used free terms such as red blood cell transfusion, blood transfusion, anemia, and erythrocyte cells for these searches. For the MEDLINE search, via PubMed, we combined MeSH terms (“blood transfusion,” “erythrocytes,” “Erythrocyte Transfusion,” “blood component transfusion,” “anemia”) and free terms (transfus* [tiab], transfusion requirements, RBC, RBCs, transfusion strategy, blood loss, blood conservation, transfusion of RBCs, red cell transfusion, management of anemia). Additionally, we used a series of terms related to guidelines as: “practice guideline,” “consensus,” “development conference,” and “guideline.” The search strategy and sources are listed in Table 1.
Table 1.
Searched sites for the identification of CPGs
| Generic databases | Websites |
|---|---|
| MEDLINE (PubMed) | http://www.ncbi.nlm.nih.gov/pubmed/ |
| TRIP database | http://www.tripdatabase.com |
| Excelencia Clínica | http://www.excelenciaclinica.net/ |
| Compiler Entities, Registries, or Clearinghouses | Websites |
| National Guidelines Clearinghouse | http://www.guideline.gov/ |
| Agency for Healthcare Research and Quality | http://www.ahrq.gov/ |
| Biblioteca de Guías de Práctica Clínica del Sistema Nacional de Salud | http://www.guiasalud.es |
| Canadian Medical Association Infobase: Clinical Practice Guidelines | http://www.cma.ca |
| Guidelines Developers | Websites |
| National Institute for Health and Care Excellence | http://www.nice.org.uk |
| Scottish Intercollegiate Guidelines Network | http://www.sign.ac.uk |
| New Zealand Guidelines Group | http://www.nzgg.org.nz |
| Institute for Clinical Systems Improvement | http://www.icsi.org |
| American College of Physicians | http://www.acponline.org |
| International Society of Blood Transfusion | http://www.isbtweb.org/ |
| Asian Association of Transfusion Medicine (AATM) | http://saatm.org/ |
| Australian and New Zeland Society of Blood Transfusion | http://www.anzsbt.org.au/ |
| British Blood Transfusion Society | https://www.bbts.org.uk/ |
| American Red Cross | http://www.redcross.org/ |
2.2. Inclusion and exclusion criteria
We included (1) CPGs with recommendations related to hemoglobin concentration to guide the RBC‐transfusion; (2) CPGs that performed a search in at least one database; and (3) CPGs published from 2006 until October 2017, in English or Spanish. We excluded (1) secondary publications like systematic reviews or meta‐analyses and (2) CPGs with recommendations about pediatric patients (<15 years) and neonates.
2.3. Data collection
Two reviewers independently screened abstracts using the inclusion criteria stated above. If the inclusion criteria met, we retrieved the full‐text article and screened it to determine their eligibility. Two reviewers independently extracted the following data from each CPG: title, year, organization that developed the guideline, country of origin, and source of funding. In the case of disagreement, a third reviewer was consulted. One reviewer extracted the recommendation about hemoglobin threshold to guide transfusion, and the individual studies used to support the recommendation.
2.4. Quality assessment
We used the AGREE instrument to evaluate the quality of the included CPGs.41, 42, 43, 44 This was developed primarily for guideline developers and researchers, to outline and measure the core elements of guideline development and implementation. The AGREE instrument (initially AGREE I, now AGREE II) contains 23 items,41 spread over six domains: scope and purpose, stakeholder involvement, rigor of development, clarity and presentation, applicability, and editorial independence, in addition to a final general item that evaluates the extent to which the guideline can be recommended for use in practice. To evaluate the items within the six domains, a 7‐point Likert scale was used, ranging from “strongly disagree” to “strongly agree.” For the overall assessment, we used a 3‐point scale ranging from “not recommended” to “strongly recommended.” Three independent reviewers, with experience in CGs assessment, applied the AGREE II instrument. In the case of disagreement, an agreement was reached by consensus. In the event of persistent disagreement, a fourth evaluator was consulted.
2.5. Statistical analysis
We performed a descriptive analysis of the CPGs according to the country of origin, the type of organization that developed them, the year of publication and the language of the CPGs. To establish the quality of each CG, the standardized score was calculated as a percentage; this was obtained by adding all the individual points from the items of a domain, and standardizing the total as a percentage of the maximum possible score from that area: (score obtained − minimum possible score)/(maximum possible score − minimum possible score) × 100. Once the quality of each CG was established, it was compared to the aforementioned descriptive variables. The degree of agreement between the reviewers was assessed using an intraclass correlation coefficient (ICC) with a 95% confidence interval (CI). Student's t‐test compared the scores between different variables (date of publication and restrictive recommendations). For the analysis of the change in the global score over time, the date of publication was categorized into two periods (2006–2011 and 2012–2015). We used the statistical package IBM SPSS (version 22).
3. RESULTS
3.1. Guideline characteristics
The search strategy provided 615 references after eliminating duplicates. A review of the titles and abstracts identified 47 potentially eligible CPGs. From the 47 examined CPGs, only 16 fulfilled the eligibility criteria and were included (Table 2).22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 One of these guidelines included four chapters that give the hemoglobin threshold recommendation for different settings.34, 35, 36, 37 Included guidelines were published from 2008 to 2016. Six CPGs were from the United States,23, 28, 29, 33, 40 four from the United Kingdom,22, 31, 38, 39 one with four chapters from Australia,34, 35, 36, 37 one from Canada,24 Finland,27 the Netherlands,26 Singapore,25 and Spain.30 Twelve documents were developed by scientific societies,22, 23, 24, 27, 28, 29, 30, 31, 32, 33, 39, 40 and seven CPGs were developed by government agencies.25, 26, 34, 35, 36, 37, 38 Five of 16 included CPGs, focused solely on RBC‐transfusion,22, 23, 24, 31, 40 while there remaining 11 gave recommendations on blood products in general.25, 26, 27, 28, 29, 30, 32, 33, 34, 35, 36, 37, 38, 39
Table 2.
CPG characteristics and hemoglobin threshold recommendations
| Guideline | Organization | Year | Hemoglobin threshold recommendation | Country and language | Methods used to assess the quality and strength of the evidence |
|---|---|---|---|---|---|
| Blood transfusion and the anesthetists. Red cell transfusion22 | The Association of Anaesthetists of Great Britain and Ireland | 2008 | “The decision to transfuse should always be made on an individual patient basis. Patients should not normally be transfused if the hemoglobin concentration is >10 g/dL. A strong indication for transfusion is a hemoglobin concentration <7 g/dL″ (Perioperative patients) | UK/IR, English | Grading system not stated |
| Clinical practice guideline: red blood cell transfusion in adult trauma and critical care23 | The Eastern Association for Surgery of Trauma Practice Management Workgroup | 2009 | “The use of only Hb level as a ‘trigger’ for transfusion should be avoided. A ‘restrictive’ strategy of RBC transfusion (transfuse when Hb 7 g/dL) is as effective as a “liberal” transfusion strategy (transfusion when Hb 10 g/dL) in critically ill patients with hemodynamically stable anemia, except possibly in patients with acute myocardial ischemia” (Critically ill patients) | USA, English | Canadian and US Preventative Task Force grading system |
| Guidelines for red blood cell and plasma transfusion for adults and children. updated24 | Guidelines for Canadian Clinical Practice Guidelines. Expert Panel | 2009 | “Red blood cell transfusion should not be dictated by a single hemoglobin trigger but should be based on a complete evaluation of the patient including volume status, tissue perfusion and comorbid disease” (General medical patients) | Canada, English | Modified version of the Canadian Task Force on the Periodic Health Examination grading system |
| Clinical blood transfusion25 | Singapore Ministry of Health | 2011 | “When hemoglobin >10 g/dL, there is usually very little indication for red cell transfusion. When hemoglobin <7 g/dL, red cells transfusion may be beneficial particularly in symptomatic patients or ongoing blood loss is expected” (General medical patients) | Singapore, English | Own rating scheme used to assess the quality of the evidence |
| Blood transfusion guideline26 | Dutch Institute for Healthcare Improvement | 2011 | “The indication for administering erythrocytes is based on medical factors and is aimed at treating or preventing the symptoms of a lack of oxygen transport capacity by the blood. Consider a transfusion if the following occurs at a Hb < 4 mmol/L: acute blood loss in a healthy individual (ASA I) < 60 years, normovolemic, blood loss at 1 location” (General medical patients) | The Netherlands, English‐Dutch | Own rating scheme used to assess the quality of the evidence |
| Blood transfusion: indications, administration, and adverse reactions24 | Finnish Medical Society Duodecim | 2011 | “It is not possible to give single hemoglobin (Hb) value as a trigger for red cell transfusion since the requirement for a transfusion is based on anemia symptoms, the patient's age, and the underlying diseases (chronic or slowly developing anemia)” (General medical patients) | Finland, English | Rating scheme modified of GRADE 2011 by the EBM Guidelines Editorial Team |
| 2011 update to The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists Blood Conservation Clinical Practice Guidelines28 | The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists | 2011 | “With hemoglobin levels below 6 g/dL, red blood cell transfusion is reasonable since this can be life‐saving. Transfusion is reasonable in most postoperative patients whose hemoglobin is less than 7 g/dL, but no high‐level evidence supports this recommendation” (Perioperative patients) | USA, English | The assessment was conducted according to the level of evidence recommended by the AHA/ACCF Task Force on Practice Guidelines |
| KDIGO Clinical Practice Guideline for anemia in chronic kidney disease29 | Kidney Disease: Improving Global Outcomes (KDIGO) | 2012 | “We suggest that the decision to transfuse a CKD patient with nonacute anemia should not be based on any arbitrary Hb threshold, but should be determined by the occurrence of symptoms caused by anemia” (Chronic kidney diseases patients) | USA, English | KDIGO grading system |
| The “Seville” document on consensus on the alternatives to allogenic blood transfusion30 | The Spanish Societies of Anesthesiology, Critical Care Medicine and Coronary Units, Hematology and Hemotherapy, Blood Transfusion and Thrombosis and Hemostasis | 2013 | “The majority of trauma, critical and surgical patients can tolerate hemoglobin levels of 70 g/L. However, if they present acute cardiological and/or central nervous system involvement, hemoglobin levels of at least 80 g/L may be required. In any case, the decision to transfuse should be individualized for each patient” (General medical patients) | Spain, Spanish | GRADE 2008 |
| Guidelines on the management of anemia and red cell transfusion in adult critically ill patients31 | British Committee for Standards in Haematology | 2013 | “A transfusion threshold of 70 g/L or below, with a target Hb range of 70–90 g/L, should be the default for all critically ill patients, unless specific comorbidities or acute illness‐related factors modify clinical decision‐making” (Critically ill patients) | UK, English | GRADE 2008 |
| Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians32 | American College of Physicians | 2013 | “ACP recommends using a restrictive red blood cell transfusion strategy (trigger hemoglobin threshold of 7 to 8 g/dL compared with higher hemoglobin levels) in hospitalized patients with coronary heart disease”(Patients with heart disease) | USA, English | ACP's clinical practice guidelines grading system |
| Practice guidelines for perioperative blood management—an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management33 | American Society of Anesthesiologists | 2014 | “A restrictive red blood cell transfusion strategy may be safely used to reduce transfusion administration” (Perioperative patients) | USA, English | ASA grading system |
| The National Blood Authority's Patient Blood Management Guideline: Module 2—Perioperative34 | National Blood Authority Australia | 2012 | “RBC transfusion should not be dictated by a hemoglobin ‘trigger’ alone but should be based on an assessment of the patient's clinical status” (Perioperative patients) | Australia, English | National Blood Authority scheme |
| The National Blood Authority's Patient Blood Management Guideline: Module 3—Medical35 | National Blood Authority Australia | 2012 | “RBC transfusion should not be dictated by a Hb concentration alone, but should also be based on an assessment of the patient's clinical status. Direct evidence is not available in general medical patients” (General medical patients) | Australia, English | National Blood Authority scheme |
| The National Blood Authority's Patient Blood Management Guideline: Module 4—Critical Care36 | National Blood Authority Australia | 2012 | “In critically ill patients, a restrictive transfusion strategy should be employed” (Critically ill patients) | Australia, English | National Blood Authority scheme |
| The National Blood Authority's Patient Blood Management Guideline: Module 5—Obstetrics and Maternity37 | National Blood Authority Australia | 2015 | “In maternity patients who are not actively bleeding, RBC transfusion should not be dictated by a Hb concentration alone, but should also be based on an assessment of the patient's clinical status (eg, the risk of further hemorrhage)” (Obstetric patients) | Australia, English | National Blood Authority scheme |
| Blood Transfusion NICE guideline38 | National Institute for Health and Care Excellence | 2015 | “Use restrictive red blood cell transfusion thresholds for patients who need red blood cell transfusions and who do not: have major hemorrhage, or have the acute coronary syndrome, or need regular blood transfusions for chronic anemia” (General medical patients) | UK, English | GRADE |
| Blood transfusion in obstetrics39 | Royal College of Obstetricians & Gynaecologists | 2015 | “There are no firm criteria for initiating red cell transfusion. The decision to provide blood transfusion should be made on clinical and hematological grounds” (Obstetric patients) | UK, English | Scheme using Royal College of Obstetricians and Gynaecologists for grading recommendations |
| Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage40 | American Association of Blood Banks | 2016 | “The AABB recommends a restrictive RBC transfusion threshold in which the transfusion is not indicated until the hemoglobin level is 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients, rather than a liberal threshold when the hemoglobin level is 10 g/dL” (General medical patients) | USA, English | GRADE |
Eight guidelines gave recommendations for general medical patients.24, 25, 26, 27, 30, 35, 38, 40 The other 11 CPGs focused on specific populations: four on perioperative patients22, 28, 33, 34; three on critically ill patients23, 31, 36; two on obstetric patients37, 39; one on patients with heart disease32; and one on chronic kidney diseases patients.29
For the analysis of recommendations, each chapter of one of the CPGs included,34, 35, 36, 37 were considered separately due to differences in hemoglobin threshold recommendation. Seven guidelines recommended a restrictive strategy for RBC transfusion,30, 31, 32, 33, 36, 38, 40 defined as the administration of blood transfusion when the hemoglobin level falls below 7 g/dL. Four CPGs had a guarded statement considering a hemoglobin threshold of 7 g/dL, as safe to indicate RBC transfusion.22, 25, 26, 28 The remaining eight CPGs avoid giving a hemoglobin threshold23, 24, 27, 29, 34, 35, 37, 39 and state that RBC transfusion should not be dictated by hemoglobin concentration alone (Table 2).
Overall, 39 clinical trials supported these recommendations. The references of included studies to base the recommendations were not possible to obtain in two guidelines.24, 27 Another two CPGs supported their recommendations in previously published guidelines37, 39 (see supplementary material for information about the evidence supporting recommendations). Only four guidelines used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to evaluate the quality of evidence and to grade the strength of recommendations.30, 31, 38, 40 One guideline used a modified version of GRADE,27 13 guidelines applied other methods to determine the quality of the evidence,23, 24, 25, 26, 28, 29, 32, 33, 34, 35, 36, 37, 39 and 1 guideline did not explain the methodology used to assess the quality of evidence.22
3.2. Quality assessment
The agreement between the three reviewers was high, with an ICC of 0.90 (95% CI: 0.81‐0.96). Table 2 shows the standardized score of the AGREE II tool by domain and by guideline, as well as the overall evaluation. Additionally, Figure 1 shows the statistical summarized analysis of the total standardized score by domain.
Figure 1.
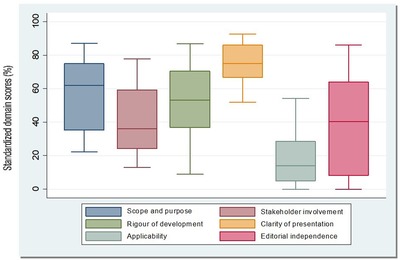
Distribution of the standardized domain scores for 16 CPGs. The top and bottom of the box represent the 75th (Q3) and 25th percentile (Q1), respectively, and the band near the middle of the box indicates the 50th percentile (median). The upper and lower ends of the whisker represent Q3 + 1.5× (interquartile range), and Q1‐1.5× (interquartile range), respectively
3.2.1. Domain 1: scope and purpose
This domain focuses on the general goal of the CPGs, considering the health condition, and the specific population for applying the guideline. The average score was 59.4% (median = 62% and a range from 22.2% to 87%; Figure 1). Five CPGs (31%) scored above 70%.23, 30, 32, 34, 38 See Table 2 for details about Domain 1.
3.2.2. Domain 2: stakeholder involvement
This domain assesses the working group that developed the CPGs, the involvement of stakeholders, and potential users. The average score was 43.2% (median = 40% and a range from 13% to 78%; Figure 1). Only three CPGs (18.7%) scored more than 70 on this domain.26, 29, 38 See Table 3 for details about Domain 2.
Table 3.
AGREE II domain‐standardized scores
| Guideline | Scope and purpose | Stakeholder involvement | Rigor of development | Clarity of presentation | Applicability | Editorial independence | Overall recommendation |
|---|---|---|---|---|---|---|---|
| Blood transfusion and the anesthetists. Red cell transfusion22 | 35.2 | 24.1 | 9 | 53.7 | 8.3 | 0 | Not recommended |
| Clinical practice guideline: red blood cell transfusion in adult trauma and critical care23 | 83.3 | 33.3 | 54.2 | 92.6 | 11.1 | 55.6 | Recommended with modifications |
| Guidelines for red blood cell and plasma transfusion for adults and children. updated24 | 22.2 | 13 | 9 | 51.9 | 1.4 | 5.6 | Not recommended |
| Clinical blood transfusion25 | 55.6 | 42.6 | 36.8 | 87 | 22.2 | 0 | Not recommended |
| Blood transfusion guideline26 | 64.8 | 77.8 | 79.2 | 75.9 | 52.8 | 36.1 | Recommended with modifications |
| Blood transfusion: indications, administration and adverse reactions27 | 35.2 | 14.8 | 16 | 68.5 | 0 | 8.3 | Not recommended |
| 2011 update to The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists Blood Conservation Clinical Practice guidelines28 | 27.8 | 50 | 45.1 | 64.8 | 9.7 | 33.3 | Recommended with modifications |
| KDIGO Clinical Practice Guideline for anemia in chronic kidney disease29 | 66.7 | 74.1 | 80.6 | 87 | 34.7 | 72.2 | Recommended |
| The “Seville” document on consensus on the alternatives to allogenic blood transfusion30 | 72.2 | 37 | 37.5 | 75.9 | 13.9 | 41.7 | Recommended with modifications |
| Guidelines on the management of anemia and red cell transfusion in adult critically ill patients31 | 61.1 | 24.1 | 36.8 | 74.1 | 18.1 | 8.3 | Not recommended |
| Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians32 | 77.8 | 35.2 | 61.8 | 92.6 | 0 | 75 | Recommended with modifications |
| Practice guidelines for perioperative blood management—an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management33 | 63 | 48.1 | 52.1 | 68.5 | 1.4 | 55.6 | Not recommended |
| The National Blood Authority's patient blood management guideline34 – 37 | 79.6 | 31.1 | 81.9 | 81.5 | 54.2 | 86.1 | Recommended |
| Blood transfusion NICE guideline38 | 87 | 74.1 | 86.8 | 85.2 | 51.4 | 83.3 | Recommended |
| Blood transfusion in obstetrics39 | 59.3 | 13 | 54.2 | 63 | 18.1 | 38.9 | Not recommended |
| Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage40 | 31.1 | 68.5 | 59 | 68.5 | 13.9 | 55.6 | Recommended with modifications |
| Mean score (SD) | 59.5 (±19.9) | 43.1 (±22) | 50 (±25.1) | 74.4 (±12.7) | 19.4 (±18.8) | 41(±30) | |
| Median (range) | 62 (22.2–87) | 39.8 (13–77.8) | 53 (9–86.8) | 75 (52–92.6) | 13.8 (0–54.2) | 40.2 (0–86.1) |
3.2.3. Domain 3: rigor of development
This domain addresses the process used to identify and summarize the evidence, the methodology to formulate recommendations, and their updates. The average score was 50% (median = 53% and a range from 9% to 87%; Figure 1). Four CPGs (25%) scored above 70% on this domain.26, 29, 34, 38 See Table 3 for details about Domain 3.
3.2.4. Domain 4: clarity and presentation
This domain focuses on the wording, the structure, and the general format of the CPGs. The average score was 74.4% (median = 75% and a range from 51% to 92.6%; Figure 1). Nine CPGs (56.2%) scored above 70% on this domain.23, 25, 26, 29, 30, 31, 32, 34, 38 This domain scored the highest among the six domains included in the AGREE II instrument. See Table 3 for details about Domain 4.
3.2.5. Domain 5: applicability
This domain considers the barriers and facilitators for the implementation of the CPGs, including aspects of resources and adherence to the recommendations. The average score was 19.4% (median = 14% and a range from 0% to 54.2%; Figure 1). This was the lowest evaluated domain for all the CPGs, and none of the included CPGs scored above 70% on this domain. See Table 3 for details about Domain 5.
3.2.6. Domain 6: editorial independence
This domain assesses if funding sources influenced recommendations. The average score was 41% (median = 40% and a range from 0% to 86%; Figure 1). Four CPGs (25%) scored above 70% on this domain.29, 32, 34, 38 See Table 3 for details about Domain 6.
3.2.7. Overall assessment
Three out of the 16 evaluated CPGs (18.7%) were “recommended” by the independent evaluators,29, 34, 38 6 CPGs (37.5%) were “recommended with modifications,”23,26,28,30,32,40 and 7 CPGs (43.7%) were “not recommended” (see Table 3).22, 24, 25, 27, 31, 33, 39 The three “recommended” CPGs scored ≥ 70% in the “rigor of development” domain. The seven CPGs (18.7%) “not recommended”22,24,25,27,31,33,39 by evaluators had scores below 70% in five of the six reported domains (see Table 3).
We did not find statistically significant differences in the AGREE II global score between CPGs published in 2006‐2011 and those published in the period 2012–2015 (P = 0.49). Additionally, those CPGs recommending restrictive strategies scored similarly in the rigor of development domain, as those that did not recommend a specific threshold (P = 0.92).
4. DISCUSSION
4.1. Summary of the main finding
In our review, we found 16 CPGs that met the eligibility criteria.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 In the overall CPGs’ assessment, only 3 out of the 16 evaluated CPGs (18.7%) were “recommended” by the independent evaluators,29, 34, 38 6 CPGs (37.5%) were “recommended with modifications,”23, 26, 28, 30, 32, 40 and 7 CPGs (43.7%) were “not recommended.”22,24,25,27,31,33,39
Most of the CPGs did not describe the literature search and selection methods, and they were ambiguous regarding how the evidence was appraized and whether or not the recommendations were truly evidence‐based. The domains with the highest scores were “clarity and presentation” and “scope and purpose,” and the domains with the lowest scores were “applicability” and “editorial independence” (see Table 3 and Figure 1). Only four CGs26, 29, 34, 38 scored ≥ 70% in the domain “rigor of development,” which was considered one of the most critical domains, as it refers to methodological aspects concerning how the recommendations were developed.
In the analyzed CPGs, the use of a hemoglobin threshold for RBC‐transfusion was variable. Some guidelines recommended restrictive strategies, and other CPGs avoided using a hemoglobin threshold, on the basis that RBC‐transfusion should not be dictated by hemoglobin concentration alone. However, when the score in the rigor of development domain, of the CPGs recommending restrictive strategies, as compared with the CPGs that avoid giving a hemoglobin threshold, we did not find statistically significant differences. Therefore, the variability in recommendations cannot be explained by differences in this domain (P = 0.92).
Finally, our study could not demonstrate statistical differences over time in the global score of CPGs quality (published in 2006–2011 versus 2012‐2016; P = 0.49). However, we believe that the low number of included CPGs did not allow an adequate evaluation of the variability in the quality of RBC‐transfusion CGs over time.
4.2. The context of this review with other literature
This review represents the first systematic assessment of the quality of clinical practice guidelines focused on red blood cell transfusion recommendations. Consistently with previous CPG evaluations in other clinical areas,45, 46, 47, 48 the domains with the highest scores were “clarity of presentation” and “scope and purpose,” whereas the domains with the lowest scores were “stakeholder involvement,” “editorial independence,” and “applicability.” The lowest scores related to the “applicability” domain can be related to the belief that the activity of formulating recommendations was separated from the implementation processes. Our results for the domains were similar to those of previous systematic assessment done by our group, that included the evaluation of 626 CPGs.19 Specifically, in the “rigor of development” domain our review found low quality, with an average of 46.3% compared to 68% in other similar reviews.19
4.3. Strengths and limitations
Our systematic assessment has some limitations. First, although a robust set of search criteria was formulated and tested prior to full guideline identification, some CPGs might not have been adequately indexed as they were only used for institutional purposes, so we failed in their identification. We think that the quality of the CPGs not indexed in biomedical databases is probably lower compared to those indexed. Second, there is also a potential risk of selection bias because we included only studies that had been published in English or Spanish. To this extent, our assessment could be overestimating the quality of CPGs in RBC‐transfusion. Third, the AGREE II41, 42, 43, 44 instrument has undergone some revisions since the development of the original AGREE instrument.41 A 7‐point scale is used instead of a 4‐point scale for evaluating the items in the domains. This may have been a limitation in assessing the quality of the CPGs because the only well‐defined points in the scale are 1 and 7. We found that the evaluators had difficulty in distinguishing between 3, 4, and 5 Likert values, which may have introduced a potential risk of reporting bias. However, the agreement among reviewers using the AGREE II instrument was high, with an ICC of 0.97.
On the other hand, we recognize some strengths of this systematic assessment. First, we are the first to assess the quality of development of clinical practice guidelines focused on red blood cell transfusion recommendations using methodological instruments that are widely recognized and accepted. Second, the uses of extensive search strategies, covering both indexed and gray literature and the use of expert appraisers who completed training and calibration to assess the quality of CPGs.
In conclusion, our findings show that much remains to be done to reach excellence in the area of CPGs on RBC‐transfusion. Only three out of the 16 evaluated CPGs were “recommended” by the independent evaluators. Four domains (“stakeholder involvement,” “rigor of development,” “applicability,” and “editorial independence”) had serious shortcomings. The domains: “scope and purpose” and “clarity of presentation” were the more precisely reported.
Moreover, our study could not demonstrate statistical differences over time in the global score of CPGs quality (published in 2006‐2011 versus 2012‐2016; P = 0.49). Also, the recommendations about hemoglobin threshold for RBC‐transfusion was variable among the CPGs analyzed.
Clinical practice guidelines users should be aware of the low quality reported in this study. Meanwhile, developers should adhere to rigorous methods, like those provided in handbooks from institutions, such as the Scottish Intercollegiate Guidelines Network (SIGN) or the National Institute for Health and Care Excellence (NICE).15 Additionally, guideline developers should use checklists to optimize methods for the development and reporting of CPG, such as the GIN‐McMaster Guideline Development Checklist (GDC)49, 50 and AGREE II,42, 43, 44 respectively. Moreover, to improve communication among users and developers, GRADE methodology is highly recommended, as it is a widely implemented rigorous system.
Regarding research strategies, additional efforts should be made to develop and consolidate networks, to improve the evaluation and synthesis of the available evidence in the RBC‐transfusion field. Researchers, who wish to identify knowledge gaps, and policy‐makers, looking to endorse adequate CPG development, should work together to ensure the adherence to recommendations related to RBC‐transfusion, and minimize the heterogeneity in clinical practice.
CONFLICTS OF INTEREST
None
AUTHOR CONTRIBUTIONS
D S‐R, XB, and P A‐C conceived the idea for this research and designed the study. D S‐R and IS searched the literature. D S‐R, NM, I A‐R, and RB reviewed the published work and participated in data extraction. IG and D S‐R conducted the statistical analysis. All authors participated in data interpretation. D S‐R, NM, I A‐R, and RB wrote the first draft of the report. P A‐C and XB contributed to the review and approved the final manuscript. All other authors commented on the draft and approved the final version.
FUNDING SOURCES
This work was supported by Universidad UTE. Facultad de Ciencias de la Salud Eugenio Espejo. PA‐C was supported by a Miguel Servet investigator contract from the Instituto de Salud Carlos III (CPII15/0034).
ACKNOWLEDGMENTS
Daniel Simancas‐Racines is a PhD student at the Department of Pediatrics, Obstetrics and Gynecology, and Preventive Medicine of Universitat Autònoma de Barcelona. The authors acknowledge Maria Victoria Leo Rosas, Andrea Cervera and Susana Hidalgo for their assistance with the edition of this paper. The authors acknowledge Iván Solà for reviewing the Search strategy.
Simancas‐Racines D, Montero‐Oleas N, Vernooij RWM, et al. Quality of clinical practice guidelines about red blood cell transfusion. J Evid Based Med. 2019;12:113–124. 10.1111/jebm.12330
REFERENCES
- 1. Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion‐associated immunomodulation: fact or fiction. Blood. 2001;97:1180–1195. [DOI] [PubMed] [Google Scholar]
- 2. Vicent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. [DOI] [PubMed] [Google Scholar]
- 3. Rohde JM, Dimcheff DE, Blumberg N, et al. Health care associated infection after red blood cell transfusion: a systematic review and meta‐analysis. JAMA. 2014;311:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies . Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. [DOI] [PubMed] [Google Scholar]
- 5. Blood Transfusion and the Anaesthetist: Red Cell Transfusion 2 . London, U.K.: The Association of Anaesthetists of Great Britain and Ireland, https://www.aagbi.org/sites/default/files/bloodtransfusion06.pdf [accessed 15.11.01].
- 6. Takei T, Amin NA, Schmid G, Dhingra‐Kumar N, Rugg D. Progress in global blood safety for HIV. J Acquir Immune Defic Syndr. 2009;52:S127–31. [DOI] [PubMed] [Google Scholar]
- 7. Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta‐analysis and systematic review. Am J Med. 2014;127:124–131. [DOI] [PubMed] [Google Scholar]
- 8. Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curley GF, Shehata N, Mazer CD, et al. Transfusion triggers for guiding RBC transfusion for cardiovascular surgery: a systematic review and meta‐analysis. Crit Care Med. 2014;42:2611–2624. [DOI] [PubMed] [Google Scholar]
- 10. Simancas‐Racines D, Osorio D, Martí‐Carvajal AJ, Arevalo‐Rodriguez I. Leukoreduction for the prevention of adverse reactions from allogeneic blood transfusion. Cochrane Database Syst Rev. 2015;12:CD009745 10.1002/14651858.CD009745.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martí‐Carvajal AJ, Simancas‐Racines D, Peña‐González BS. Prolonged storage of packed red blood cells for blood transfusion. Cochrane Database Syst Rev. 2015;7:CD009330 10.1002/14651858.CD009330.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lohr KN, Field MJ, editors. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990. [PubMed] [Google Scholar]
- 13. Greenfield S, Steinberg EP, Auerbach A, Avorn J, Galvin R, Gibbons R. Clinical Practice Guidelines We Can Trust. Washington, DC: Institute of Medicine; 2011. [Google Scholar]
- 14. World Health Organization . Guidelines for WHO Guidelines, Global Programme on Evidence for Health Policy. World Health Organization, Geneva: 2003. http://whqlibdoc.who.int/hq/2003/EIP_GPE_EQC_2003_1.pdf [Google Scholar]
- 15. National Institute for Health and Clinical Excellence . The Guidelines Manual (PMG6), https://www.nice.org.uk/guidance/pmg6/resources/the-guidelines-manual-pdf-2007970804933; 2012. [accessed 15.12.01].
- 16. Scottish Intercollegiate Guidelines Network . SIGN 50: A Guideline Developer's Handbook. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network, http://www.sign.ac.uk/assets/sign50_2015.pdf; 2015. [accessed 15.12.01]. [Google Scholar]
- 17. Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Internal Med. 2012;156:525–531. [DOI] [PubMed] [Google Scholar]
- 18. Kung J, Miller R, Mackowiak P. Failure of clinical practice guidelines to meet Institute of Medicine standards: two more decades of little, if any, progress. Arch Internal Med. 2012;172:1628–1633. [DOI] [PubMed] [Google Scholar]
- 19. Alonso‐Coello P, Irfan A, Solà I. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care. 2010;19:e58. [DOI] [PubMed] [Google Scholar]
- 20. Esandi ME, Ortiz Z, Chapman E, Dieguez MG, Mejía R, Bernztein R. Production and quality of clinical practice guidelines in Argentina (1994–2004): a cross‐sectional study. Implement Sci. 2008;3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318:527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Association of Anaesthetists of Great Britain and Ireland . Blood transfusion and the anaesthetist: red cell transfusion 2. Association of Anaesthetists of Great Britain and Ireland; 2008, https://www.aagbi.org/sites/default/files/red_cell_08.pdf [Google Scholar]
- 23. Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Critical Care Medicine. 2009;37:3124–3157. [DOI] [PubMed] [Google Scholar]
- 24. Group ExpertWorkin. Guidelines for red blood cell and plasma transfusion for adults and children. Update from Expert Working Group: Guidelines for Red Blood Cell and Plasma Transfusion for Adults and Children CMAJ. 1997;156. Special Supplement (S1‐S24). Updated at 2009. [Google Scholar]
- 25. Singapore Ministry of Health. Clinical blood transfusion . Singapore: Singapore Ministry of Health; 2011.
- 26. Haas FJ, van Rhenen DJ, de Vries RR. National users' board Sanquin blood supply. Blood transfusion guideline Chronic anaemia. Utrecht; 2011:108–165. http://www.isbtweb.org/fileadmin/user_upload/blood-transfusion-guideline.pdf [Google Scholar]
- 27. Finnish Medical Society Duodecim . Blood transfusion: indications, administration and adverse reaction, http://www.ebm-guidelines.com/go/ebm/ebm00332.html; 2014. [accessed 16.11.01].
- 28. Ferraris VA, Brown JR, Despotis GJ, et al. Update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. [DOI] [PubMed] [Google Scholar]
- 29. Kidney Disease Improving Global Outcomes . KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:311–316; 10.1038/kisup.2012.36 [DOI] [Google Scholar]
- 30. Leal‐Noval SR, Muñoz M, Asuero M, et al. The “Seville” document on consensus on the alternatives to allogenic blood transfusion. Update to the Seville document. Spanish Societies of Anaesthesiology (SEDAR), Haematology and Haemotherapy (SEHH), Hospital Pharmacy (SEFH), Critical Care Medicine (SEMICYUC), Thrombosis and Haemostasis (SETH) and Blood Transfusion (SETS). Farm Hosp. 2013;37:209–235. [DOI] [PubMed] [Google Scholar]
- 31. Retter A, Wyncoll D, Pearse R, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol. 2013;160:445–464. [DOI] [PubMed] [Google Scholar]
- 32. Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P. Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:770–779. [DOI] [PubMed] [Google Scholar]
- 33. Apfelbaum J, Nuttall G, Connis R, Harrison C, Miller R, Nickinovich D. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology. 2015;122:241–275. [DOI] [PubMed] [Google Scholar]
- 34. National Blood Authority (NBA) . Patient Blood Management Guidelines: Module 2. Perioperative. Canberra, National Blood Authority, https://www.blood.gov.au/pbm-module-2; 2012. [accessed 16.11.01]. [Google Scholar]
- 35. National Blood Authority (NBA) . Patient Blood Management Guidelines: Module 3. Medical. Canberra, National Blood Authority, https://www.blood.gov.au/pbm-module-3; 2012. [accessed 16.11.01]. [Google Scholar]
- 36. National Blood Authority (NBA) . Patient Blood Management Guidelines: Module 4. Critical Care. Canberra, National Blood Authority, https://www.blood.gov.au/pbm-module-4; 2012. [accessed 16.11.01]. [Google Scholar]
- 37. National Blood Authority (NBA) . Patient Blood Management Guidelines: Module 5—Obstetrics and Maternity. NBA, Canberra, Australia, https://www.blood.gov.au/pbm-module-5; 2015. [accessed 16.11.01].
- 38. National Institute for Health and Care Excellence (NICE) . Blood transfusion, https://www.nice.org.uk/guidance/ng24; 2015. [accessed 16.10.01]. [PubMed]
- 39. Royal College of Obstetricians and Gynaecologists . Blood transfusion in obstetrics. Green Top Guideline No. 47. London: Royal College of Obstetricians and Gynaecologists, 2015.
- 40. Carson J, Guyatt G, Heddle N, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316:2025–2035. [DOI] [PubMed] [Google Scholar]
- 41. AGREE Collaboration . Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003; 12:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ. 2010;182:E472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Delgado‐Noguera M, Tort S, Bonfill X, Gich I, Alonso‐Coello P. Quality assessment of clinical practice guidelines for the prevention and treatment of childhood overweight and obesity. Eur J Pediatr. 2009;168:789–799. [DOI] [PubMed] [Google Scholar]
- 46. Barajas‐Nava L, Solà I, Delgado‐Noguera M, et al. Quality assessment of clinical practice guidelines in perioperative care: a systematic appraisal. Qual Saf Health Care. 2010;19:e50. [DOI] [PubMed] [Google Scholar]
- 47. Acuña‐Izcaray A, Sánchez‐Angarita E, Plaza V, et al. Quality assessment of asthma clinical practice guidelines: a systematic appraisal. Chest. 2013;31:144:390–397. [DOI] [PubMed] [Google Scholar]
- 48. Serón P, Lanas F, Ríos E, Bonfill X, Alonso‐Coello P. Evaluation of the quality of clinical guidelines for cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2015;35:1–12. [DOI] [PubMed] [Google Scholar]
- 49. Mc Master University [Internet] . Ontario, Canada: GIN‐McMaster Guideline Development Checklist; https://cebgrade.mcmaster.ca/guidecheck.html; c2014 [accessed 16.02.01].
- 50. Schünemann HJ, Wiercioch W, Etxeandia I, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014;186:E123–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


