ABSTRACT
The complete division of the atrial cavity by a septum, resulting in a left and right atrium, is found in many amphibians and all amniotes (reptiles, birds, and mammals). Surprisingly, it is only in eutherian, or placental, mammals that full atrial septation necessitates addition from a second septum. The high incidence of incomplete closure of the atrial septum in human, so‐called probe patency, suggests this manner of closure is inefficient. We review the evolution and development of the atrial septum to understand the peculiar means of forming the atrial septum in eutherian mammals. The most primitive atrial septum is found in lungfishes and comprises a myocardial component with a mesenchymal cap on its leading edge, reminiscent to the primary atrial septum of embryonic mammals before closure of the primary foramen. In reptiles, birds, and mammals, the primary foramen is closed by the mesenchymal tissues of the atrioventricular cushions, the dorsal mesenchymal protrusion, and the mesenchymal cap. These tissues are also found in lungfishes. The closure of the primary foramen is preceded by the development of secondary perforations in the septal myocardium. In all amniotes, with the exception of eutherian mammals, the secondary perforations do not coalesce to a secondary foramen. Instead, the secondary perforations persist and are sealed by myocardial and endocardial growth after birth or hatching. We suggest that the error‐prone secondary foramen allows large volumes of oxygen‐rich blood to reach the cardiac left side, needed to sustain the growth of the extraordinary large offspring that characterizes eutherian mammals. Anat Rec, 302:32–48, 2019. © 2018 The Authors. The Anatomical Record published by Wiley Periodicals, Inc. on behalf of American Association of Anatomists.
Keywords: atrial septum, evolution, development, congenital malformations
Septation of the atrium is an extraordinarily long and complicated process during the ontogenetic development of the human heart. Septation starts already in the fourth week of gestation, but is not completed until after birth when the secondary septum merges with the primary septum (Anderson et al., 2014). Ventricular septation, in contrast, is completed within the second month of gestation (Van Mierop and Kutsche, 1985; Sizarov et al., 2011a). The primary atrial septum is a flimsy sheet formed almost 2 months before the secondary septum. The need for the secondary atrial septum, stems from the development of a large hole, the secondary foramen, in the primary atrial septum (Anderson et al., 2014). Remarkably, the postnatal merger of the primary and secondary septum fails to complete in approximately a quarter of the human population, resulting in a persistent foramen ovale, where a probe can be advanced from the right atrium to the left atrium (Fig. 1) (Hagen et al., 1984; Calvert et al., 2011). The incidence of a persistent foramen ovale is much higher than the low incidence of ventricular septal defects or patent ductus arteriosus (around 0.36% and 0.08%, respectively) (Hoffman and Kaplan, 2002). Although the setting of persistent foramen ovale is rarely a clinical concern, it may associate with cryptogenic stroke and an increased risk of developing decompression illness when diving (Calvert et al., 2011; Nakanishi et al., 2017). Thus, “if an oral or subcutaneous vaccination for PFO [persistent foramen ovale] closure was available, it would be used generously” (Meier and Nietlispach, 2017). Human atrial septation is, therefore, a surprisingly protracted, complicated, and inefficient process.
Figure 1.

The human atrial septum, in the setting of persistent foramen ovale, seen from the cavity of the right (A) and left atrium (B). A. The fossa ovalis is delimited by the so‐called secondary septum which comprises a superior (cranial) fold (S) of the atrial roof and its inferior (caudal) buttress (I) derived from embryonic mesenchymal tissues. While the atrial septum appears fully formed, a probe can be advanced to the left atrial cavity. B. In the left atrial cavity, the probe emerges from beneath the primary septum, or flap valve (1st septum), and through the persistent foramen ovale. CS, mouth of the coronary sinus; LV, left ventricle; RV, right ventricle; SCV, superior caval vein.
In every minute of fetal life, oxygen‐enriched blood pass through the foramen ovale to reach the left side of the heart and subsequently the coronary and cephalic circulation (Kiserud, 2005) (Fig. 2). Both heart and brain are metabolically highly active organs and may have a greater dependency on oxygen‐enriched blood than most other fetal organs (Battaglia and Meschia, 1978; Fisher et al., 1980; Feit et al., 1991). The magnitude of the shunt across the foramen ovale is 20–30% of total cardiac output and equals hundreds of milliliters in late fetal life (Kiserud, 2005). Indeed, a small foramen ovale associates with fetal growth retardation (Sutton et al., 1994; Kiserud et al., 2004), although this association is often in a setting of multiple abnormalities (Feit et al., 1991; Uzun et al., 2014). The fetus takes up oxygen in the placenta from where the oxygen‐enriched blood is returned to the right atrium via the umbilical vein, the ductus venosus, and the inferior (or posterior) caval vein (Morton and Brodsky, 2016) (Fig. 2). The inferior caval vein is also a conduit for the substantial amounts of oxygen‐poor blood coming from the abdomen. Nonetheless, the ductus venosus and inferior caval vein are angled with regards to the right atrium, such that oxygen‐enriched blood preferentially streams through the foramen ovale (Fig. 2) (Dawes et al., 1955; Edelstone and Rudolph, 1979; Anderson et al., 1981; Kiserud et al., 1992). The probe shown in Figure 1 approximates the direction of the stream of the oxygen‐enriched blood in the fetal circulation. After birth, the placental circulation regresses, the pulmonary circulation more than doubles, and a relatively high left atrial pressure presses the primary septum against the secondary septum which closes the foramen ovale (Dawes et al., 1955; Morton and Brodsky, 2016). The role of the foramen ovale therefore changes dramatically around birth from being of paramount importance for normal growth to being of no consequence in postnatal life in many and a potential risk for well‐being in a few.
Figure 2.
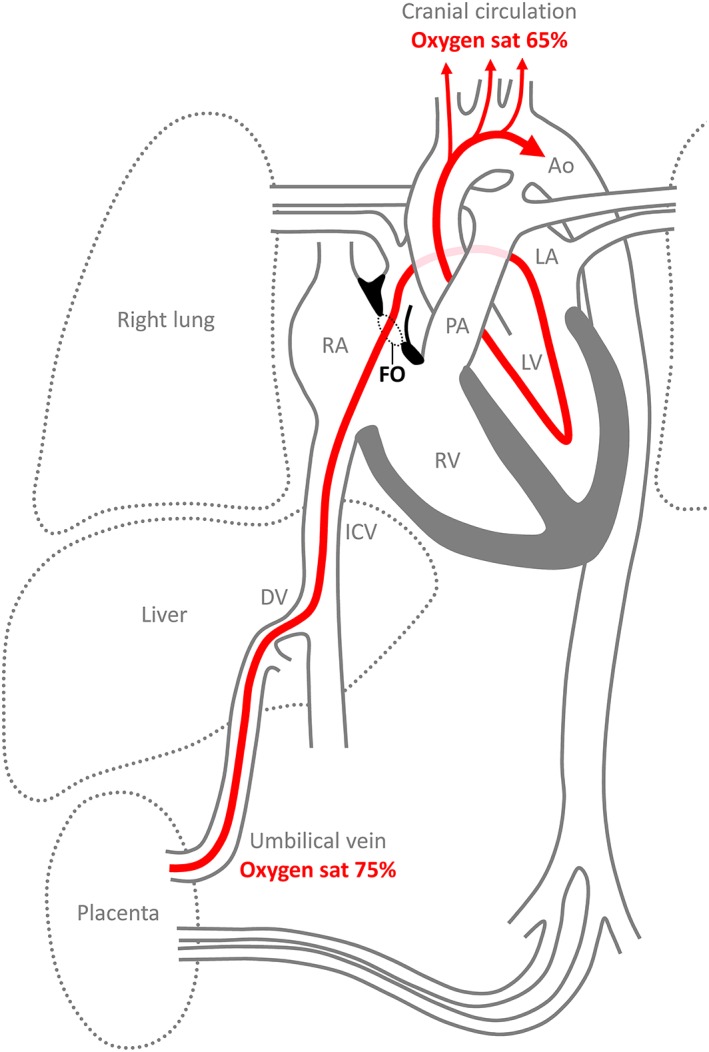
Overview of the fetal circulation, emphasizing the route of oxygen‐enriched blood (red) from the placenta, through the foramen ovale (FO), to the cranial circulation. This route entails two shunts, through the ductus venosus (DV) and the foramen ovale. The third fetal shunt is from the pulmonary artery (PA) through the ductus arteriosus to the aorta (Ao). The oxygen saturation (sat) only drops from ~75–65% from the placenta to the cranial arterial circulation. This suggests that the stream of oxygen‐enriched blood avoids mixing with much of the oxygen‐poor blood coming from the inferior caval vein (ICV) and other systemic veins. It also shows that a large proportion of the left ventricular output stems from the blood shunted across the foramen ovale, a flow that equals ~20% of cardiac output, with the remainder of the output stemming from the pulmonary veins. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
To better understand the peculiar traits of atrial septation, we first summarize the key structures and events of human atrial septation. The most conspicuous feature of the formed atrial septum is the fossa ovalis, a circular depression approximately in the center of the atrial septum. Our survey of the literature reveals that the fossa ovalis is a feature of the atrial septum of eutherian mammals only. We then show that the key mesenchymal and myocardial elements of the primary atrial septum can be traced back in evolution to lungfishes and that these tissues form the basis for incomplete and complete atrial septation in all tetrapods (amphibians, reptiles, birds, and mammals). Only eutherian mammals, not marsupial or monotreme mammals, develop a large secondary foramen in the primary septum and subsequently require a secondary septum to close it. Finally, we suggest the secondary foramen is an adaptation required to deliver sufficient amounts of oxygen to the cardiac and cephalic circulations of the large eutherian mammal fetuses during the exceptionally long gestation periods.
THE ANATOMY OF THE ATRIAL SEPTUM OF EUTHERIAN MAMMALS
As Seen from the Right Atrium
The atrial septum divides the cavities of the right and left atrium (Rowlatt, 1990). It has a dorsal position and a smooth surface, when inspected from the right atrial cavity, and is thereby distinct from the right atrial appendage, which is dominated by pectinate muscles (Fig. 1A). Cranial to the atrial septum (superior in human), is always the entrance of the right cranial caval vein, and caudal to it (inferior in human) is always the mouths of the caudal caval vein and the left cranial caval vein, or coronary sinus (Rowlatt, 1990). There is a deep depression on the right atrial face of the septum, the fossa ovalis (Fig. 1A). Between species, the position of the fossa ovalis can differ and is, for instance, more cranially positioned in pigs compared to humans (Crick et al., 1998). The bottom of the fossa ovalis is the thinnest part of the atrial septum and is the only part of the primary atrial septum that can be seen from the right atrium. The roundish rim of the fossa ovalis is commonly called the secondary septum, or limbus (Rowlatt, 1990), because of its distinct and later development than the primary septum. (Fig. 1A) (Jensen et al., 2017). Muscular and mesenchymal lineages give rise to the secondary septum, and it can be considered to have a distinct dorso‐cranial and ventro‐caudal part accordingly (Fig. 1A).
As Seen from the Left Atrium
The left atrial surface of the atrial septum is also smooth, but without any structure resembling the fossa ovalis (Rowlatt, 1990) (Fig. 1B). The wall of the left atrium is also smooth, except the small appendage, and there is no distinct border between the primary septum and the body of the left atrium (Rowlatt, 1990) (Fig. 1B). Most of the left atrial face of the septum is the primary septum, commonly called the “flap valve” in medical literature, which has two horns, or cornua (Franklin et al., 1942), extending cranially (Jensen et al., 2017). Between the horns is a depression that is the postnatal remnant of the embryonic secondary foramen, which is probe patent in the human heart as shown in Figure 1B. Even in the setting of persistent foramen ovale, there is no overlap between the secondary foramen and fossa ovalis due to the dorso‐cranial rim (Fig. 1A,B) (Calvert et al., 2011).
Common Defects of the Atrial Septum
The presence of a fossa ovalis indicates that the atrial septum was formed from the merger of the primary and secondary septum. The incidence of failed merger, persistent foramen ovale, is only well established in humans, but is probably as prevalent in rats (LekanneDeprez et al., 1998; Michaëlsson and Ho, 2000). In one relatively comprehensive study, however, only one of the 36 investigated hearts of the harbor porpoise (Phocoena phocoena) had persistent foramen ovale (Rowlatt and Gaskin, 1975). This incidence is much lower than in humans, but still much greater than the incidence of ventricular septal defects and patent ductus arteriosus in human and domesticated mammals (Michaëlsson and Ho, 2000). The data from human and eutherian mammals, therefore, strongly suggest that the formation of a full atrial septum from two septa is an inefficient process. Inefficiency is selected against in evolution. We propose, however, that the eutherian mammalian manner of forming the atrial septum also allows for particularly long gestation and large offspring and this has a greater positive impact on reproductive success than the limited detrimental impact of inefficient atrial septation.
In addition to a persistent foramen ovale, the human atrial septum can be afflicted by multiple types of other congenital malformations (Patten, 1938; Driscoll, 2016; Jensen et al., 2017). In the setting of the septum secundum defect, a relatively common congenital malformation in human and other eutherian mammals, the secondary foramen or other perforations may be so extensive that they are not covered by the dorso‐cranial rim of the secondary septum (Patten, 1938; Michaëlsson and Ho, 2000). Principally, the dorso‐cranial rim may also be feebly developed, leading to interatrial communication despite a normal secondary foramen (Patten, 1938; Michaëlsson and Ho, 2000). There are recent and comprehensive reviews on genes associated with atrial septal defects in human (Khan and Jay, 2016) and on experimental models of atrial septal defects (Jay et al., 2016).
The Atrial Septum in Other Mammals
Extending back to at least the 19th century, it was occasionally observed that the fossa ovalis can be found in eutherian mammals only, and not in marsupial mammals, such as kangaroos, or monotreme mammals (Martin, 1836; Röse, 1890; Franklin et al., 1942; Ottaway, 1944; Rowlatt, 1990; Runciman et al., 1992). Developmental studies further showed that the secondary foramen is particular to eutherian mammals (Röse, 1890; Franklin et al., 1942). In marsupials, for instance, the primary septum does develop secondary perforations, but these will not coalesce to a secondary foramen. Already Röse (1890) proposed the secondary foramen was an adaptation to particular conditions of gestation and Runciman and colleagues (Runciman et al., 1995) suggested a role for the great duration of the eutherian mammal gestation. Marsupials almost invariably have shorter gestational period (12–38 days) than eutherians (16–660 days) (Ferner et al., 2017). Some reptiles have a gestation period of several months (Gregorovicova et al., 2012), but the work of the heart, cardiac output and metabolic rate of mammals is several times greater than in similarly sized reptiles (Jensen et al., 2014). To the best of our knowledge, however, the secondary foramen of eutherian mammals remains a curious feature that has not been ascribed any advantageous function over the secondary perforations from which it develops. In the next two sections, we first summarize the key features of the normal development of the atrial septum in eutherian mammals and then attempt to establish their evolution. Last, we synthesize the preceding sections to propose an advantageous function of the oval foramen in the final section.
NORMAL DEVELOPMENT OF THE ATRIAL SEPTUM IN EUTHERIAN MAMMALS
The Primary Septum
In the diagrams of Figure 3, we summarize the time course and key events and structures in the formation of the full atrial septum in human, much of which has already been described (Patten, 1938). The morphogenesis and lineages of the mesenchymal and myocardial tissues of the primary septum are well described in both human and mouse, whereas the formation of the components of the secondary septum is less understood (Webb et al., 2001; Briggs et al., 2012; Anderson et al., 2014; Sylva et al., 2014; Jay et al., 2016).
Figure 3.

Structures and processes of normal atrial septation in human in four‐chamber view. (A) The primary septum (1) initiates from a mesenchymal cap in the atrial roof (2) beneath which is the primary foramen (3) and the cushions of the atrioventricular canal (4). Approximately week 4, Carnegie stage 12. (B) The septum grows (5) toward the atrioventricular cushions. Approximately week 4, Carnegie stage 14. (B’) Here illustrated in the sagittal view, the dorsal mesocardium (d) contributes the dorsal mesenchymal protrusion (D) to the atrial cavity. (C) Formation of secondary perforations (6) just precedes the merger (7) of the mesenchymal cap (2), dorsal mesenchymal protrusion (D), and the atrioventricular cushion (4) to close the primary foramen. Approximately week 5, Carnegie stage 16. (D) The secondary perforations expand and coalesce to form the secondary foramen (8). Approximately week 5, Carnegie stage 16. (E) The atrial roof folds inward on the right atrial side (9) and mesenchyme of the mesenchymal cap and dorsal mesenchymal protrusion differentiates into myocardium and grows (11) and collectively constitutes the rim of the foramen ovale (10). Approximately week 8, around embryo‐fetal transition. (F) After birth, the primary septum is pressed against the posterior‐superior (dorso‐cranial) fold and merges with it (12). The foramen ovale is now called the fossa ovalis (12) and is delimited by the posterior‐superior fold (9), which is buttressed anterior‐inferiorly (ventro‐caudally) by the myocardialized mesenchyme of the mesenchymal cap (2) and dorsal mesenchymal protrusion (D). These myocardial structures comprise the secondary septum. (F′). Persistent foramen ovale (PFO) results from incomplete merger (12). Timeline based on (Webb et al., 2001; Sizarov et al., 2011b; Anderson et al., 2014; Jensen et al., 2017).
In early development, the tubular heart is attached to the pharyngeal mesenchyme by a dorsal mesocardium (Wessels et al., 2000; Sizarov et al., 2011b). As the heart tube loops and chambers start to form in the fourth week of gestation, the middle part of the tubular heart breaks free from the dorsal mesocardium. Concomitantly, cardiac jelly of the atria retracts such that the endocardium is nestled against myocardium, except in a ridge of mesenchyme that spans the atrial roof from the ventral part of the atrioventricular cushions to the dorsal mesocardium. The myocardial primary septum will grow from beneath this mesenchymal ridge, which will then be carried forward as a cap on the leading edge of the myocardial primary septum (Fig. 3A,B). Similar to the forming ventricular septum, the leading edge of the atrial septum expresses the transcription factor Tbx3 (Aanhaanen et al., 2010), which is involved in establishing chamber boundaries among all vertebrates (Kokubo et al., 2010; Jensen et al., 2012). The lumen between the atrioventricular cushions and the mesenchymal cap is called the primary foramen, and this lumen is progressively diminished by the growth of the septum (Patten, 1938; Wessels et al., 2000) (Fig. 3B). Around this time, the fourth week of gestation, dorsal mesenchymal cells protrude into the atrial lumen forming the dorsal mesenchymal protrusion, or vestibular spine (Tasaka et al., 1996; Briggs et al., 2012; Anderson et al., 2014). On its left, is the single pulmonary vein (Fig. 3B’). The dorsal mesenchymal protrusion can be identified by expression of the transcription factor Isl1 just like the pharyngeal mesoderm it derives from (Mommersteeg et al., 2006; Snarr et al., 2007; Sizarov et al., 2010; Sizarov et al., 2011b). Within the fifth week of gestation, growth of the septum has brought its mesenchymal cap in contact with the atrioventricular cushions and these mesenchymal tissues, together with the dorsal mesenchymal protrusion, merge and thereby close the primary foramen (Fig. 3C) (Briggs et al., 2012). Accordingly, distinct types of the atrial septal defects can be ascribed to defects in the vestibular spine, mesenchymal cap, and the atrioventricular cushions (Jensen et al., 2017).
Formation of the Secondary Foramen
In week 5 of human gestation and before the primary foramen closes, the cranial part of the primary atrial septum develops multiple perforations (Fig. 3C). This enables continued shunting, where the oxygen‐rich blood that returns to the right atrium can enter directly into the left atrium without having to flow through the pulmonary circulation prior to reaching the left side of the heart. At least in the fetal setting, the shunting to the left atrium is very substantial and amounts to some 20–30% of total cardiac output (Kiserud, 2005). Some 10–20% of cardiac output returns to the left atrium from the pulmonary circulation and the left ventricle pumps ~40% of total cardiac output (Kiserud, 2005) (Fig. 2). Shortly after the secondary perforations have formed, they will coalesce to form a single hole, the secondary foramen (Fig. 3D). The presence of the secondary perforation, but not yet a secondary septum, remains until around week 12. The molecular mechanisms of formation of the perforations and the secondary foramen are not clear. In chicken, the secondary perforations are always lined with endothelium suggesting they are not created by destructive forces or programmes (Morse and Hendrix, 1980). Apoptosis does occur in developing hearts, but is not considered part of the formative process for the atrial septum (Icardo, 1996; Poelmann et al., 2000; Cheng et al., 2002).
Closure of the Secondary Foramen
In the last stages of embryonic development, approximately weeks 6 through 8, the mesenchyme that closed the primary foramen will start to differentiate into myocardium (Wessels et al., 2000; Snarr et al., 2007; Briggs et al., 2012; Anderson et al., 2014). This is the beginning of the formation of the inferior‐anterior rim of the secondary septum (Figs. 1A and 3E). Later, around week 12, an infolding will start in the right atrial roof between the primary septum and the mouth of the cranial caval vein (Webb et al., 2001) (Fig. 3E). In humans, the infolding is pronounced, whereas in mouse it is more of a solid ridge (Jay et al., 2016; Wessels, 2016). This is the beginning of the formation of the dorso‐cranial rim of the secondary septum, and while further development will take place, the principal components to the secondary septum are now in place. In mice, the infolding of the atrial roof develops in the days just before birth (Anderson et al., 2014). The duration of human gestation is much longer than mouse. During the rapid growth of the last 20 weeks of human gestation (Kiserud et al., 2017), the gap between the primary and secondary septal parts, that is, the foramen ovale, remains approximately constant relative to the size of the right atrium (Kiserud et al., 2004). Presumably, all components of the atrial septum follow the overall rapid growth of the heart. In the fetal setting of hypoplastic left or right ventricle, the foramen ovale is proportionally small or large respectively, suggesting hemodynamics may shape the orifice (Feit et al., 1991). At these stages, the primary septum is commonly referred to as the flap valve of the developing oval foramen, and echocardiography reveals that the foramen ovale is closed by the flap valve for ~20% of the cardiac cycle during atrial systole (Schmidt et al., 1996). During the temporary closure, presumably, left atrial pressure exceeds that of the right atrium, and the left atrial pressure in preterm lamb is slightly higher than the right atrial pressure (Anderson et al., 1985). After birth, left atrial diastolic pressure exceeds right atrial pressure and ensures that the primary septum is pressed against the dorso‐cranial rim of the fossa ovalis, such that the two structures merge (Fig. 3F). In smaller mammals, such as mouse and rat, closure of the foramen ovale occurs in the first week, whereas it takes months in cattle (Murakami et al., 1990; Cole‐Jeffrey et al., 2012; Elliott et al., 2014). The closure in rat is due to fibrosis mediated by transition of the endocardium into mesenchyme of the primary and secondary septum (Elliott et al., 2014). Failed or incomplete merger results in persistent foramen ovale (Fig. 3F’) (Calvert et al., 2011).
Variation in the Primary Septum and the Secondary Foramen Among Eutherian Mammals
In postnatal mouse and rat hearts, there is a fairly extensive primary septum as compared to the fossa ovalis, at least in contracted atria, and the foramen ovale is closed within the first week after birth (Cole‐Jeffrey et al., 2012; Elliott et al., 2014). Possibly, the secondary foramen is relatively small in rodents, but the configuration in rodents (order Rodentia) resembles those of human and other primates (Franklin et al., 1942; Anderson et al., 2014). In carnivores (order Carnivora), there is a single large secondary foramen, but this may come at the end of a short sleeve‐like extension of the primary septum, which projects into the left atrial lumen (Franklin et al., 1942; Macdonald et al., 1995; Macdonald and Johnstone, 1995). Among odd‐toed ungulates (order Perissodactyla, e.g., horse) and even‐toed ungulates (order Artiodactyla, e.g., sheep and whales), the setting can become extreme (Fig. 4). Here, the fetuses of some species develop a substantial “tunnel” rather than a short sleeve as in Carnivora. Further, the secondary foramen, which is at the distal end of the tunnel, may comprise multiple large perforations rather than a single orifice (Franklin et al., 1942; Anderson et al., 1985; Macdonald et al., 1988, 2007) (Fig. 4). After birth, the extensive primary septum of horse and cow merges into the wall of the left atrium and is hardly distinguishable by 6 weeks (Ottaway, 1944). While the settings shown in Figure 4 are surely extreme, it can be envisioned that these are the outcomes of an expansion of the primary atrial septum along with its secondary perforations driven by the long exposure to incremental blood flows during a long gestation.
Figure 4.
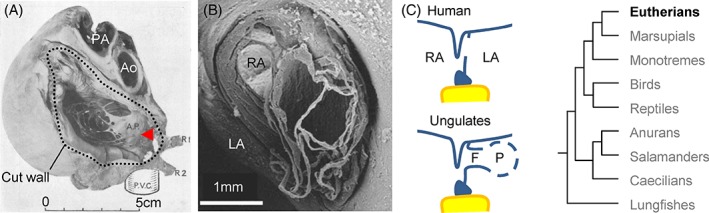
The secondary foramen in fetal specimens of horse and humpback whale. (A) The roof of the left atrium has been cut away in this fetal horse heart to illustrate the extensive primary septum (A.P.) which ends in a meshwork of numerous fine strings. Franklin and colleagues (Franklin et al., 1942), from whom the figure is adapted, likened the appearance of the septum to a “worn‐out finger stall”. (B) In the fetal humpback whale, the primary septum projects into the left atrial cavity (LA) and ends in a meshwork of numerous fine strings. The right atrial cavity (RA) can be seen through the prospective fossa ovalis. Adapted from (Macdonald et al., 2007). (C) In contrast to the human setting, in ungulates and cetaceans, the primary septum becomes extensive as it projects deep into the left atrial cavity, comprising a proximal funnel (F) which ends in numerous perforations (P). Ao, aorta; PA, pulmonary artery; P.V.C., posterior caval vein.
Elephants belong to an early branch of eutherian mammals (Song et al., 2012) and it is possible that the extensive atrial septum with a perforated distal part in the fetal heart of the African elephant represents the ancestral condition (Amoroso et al., 1941). In agreement with this notion, a secondary foramen of multiple perforations, rather than a single foramen, is common among eutherian mammals. When surveying eutherian mammals, it appears the secondary perforations can either coalesce or expand and the magnitude of either process is partly given by phylogeny. Based on an extensive analysis, Franklin and colleagues (Franklin et al., 1942) concluded there can be considerable intraspecific differences in the structures that surround the secondary foramen, but the differences between mammalian orders are nonetheless much larger. The secondary foramen expands as the fetus grows, but it is not clear how its configuration is affected by hemodynamics. The evolutionary ancestral condition of the secondary foramen may be little more than a simple expansion of the primary atrial septum with numerous secondary perforations.
EVOLUTION OF THE ATRIAL SEPTUM
Components of the Most Primitive Atrial Septum
Fossilized hearts have only been described for fish without any cardiac septation (Maldanis et al., 2016). Rather than relying on fossils, we can compare hearts of extant vertebrates with known phylogenetic relations to infer evolutionary trends (Burggren et al., 2014). All fish have a single atrium, and lungfishes (Dipnoi) were the first vertebrate group to develop an atrial septum as the evolutionary emergence of lungs provided a functional advantage by reducing the mixing of the oxygen‐poor and oxygen‐rich blood flows returning to the heart. Lungfishes, are a sister group to the tetrapods, which comprise amphibians, reptiles, birds, and mammals (Perry et al., 2001; Amemiya et al., 2013). Given their phylogenetic position, their poorly developed atrial septum, particularly in the Australian lungfish, is probably also the most primitive state of atrial septation (Klitgaard, 1978; de Bakker et al., 2015). Only in the Hynobidae and Plethodontidae salamanders is the atrial septum less developed, but this is a derived state related to the subsequent evolutionary loss of lungs (Lewis and Hanken, 2017).
In the fully formed lungfish, the atrial septum is a flimsy meshwork of fine trabeculations, but systemic and pulmonary venous blood streams are well separated in the partially divided atrium. This may be surprising given the meager appearance of the atrial septum and laminar flow behavior of the blood could play a substantial role (Fig. 5) (Burggren and Johansen, 1986). At the earliest stages of lungfish heart development, the atrial septum initiates as a ridge of mesenchyme, as in eutherian mammals (Robertson, 1913; Sizarov et al., 2010) (Fig. 6A,B). In later stages, this ridge, which we deem to be homologous to the mesenchymal cap of the primary septum (Fig. 3A), serves as anchorage for the meshwork of trabeculations that constitute the atrial septum (Fig. 5) (Robertson, 1913). At this point, the atrial septum of the lungfish resembles the perforated primary septum of the human heart prior to the closure of the primary foramen (Fig. 3B,C) and atrial septation is considered similar in lungfishes and salamanders (Robertson, 1913). Text‐Figure 13 of Robertson (Robertson, 1913) shows the developing atrial septum of the South American lungfish as an unperforated sheet. However, it is not clear whether secondary perforations develop in lungfishes, or the later growth is by trabeculated myocardium (as the remainder of the atrial wall) whereby the atrial septum acquires the appearance of a meshwork. In the formed lungfishes, the mesenchymal cap is recognized as the so‐called pulmonary fold that directs the pulmonary venous return from the single pulmonary vein toward the left side of the atrial cavity (Robertson, 1913; Klitgaard, 1978; Icardo et al., 2005; Icardo, 2017). Part of the atrial mesenchyme of the lungfish derives from the dorsal mesocardium, which harbors the solitary lung vein on its left in both lungfishes and mammals (Fig. 6C) (Robertson, 1913). We consider this dorsal mesenchymal protrusion homologous to the one of mammals and it will likely be without sarcomeric protein and express Isl1, but this remains to be clarified. To conclude on the atrial septum of lungfishes, its earliest development builds on the same principal components as in eutherian mammals (cf., Fig. 3A,B’), but its later development and final appearance is different from any stages seen in eutherian mammals.
Figure 5.
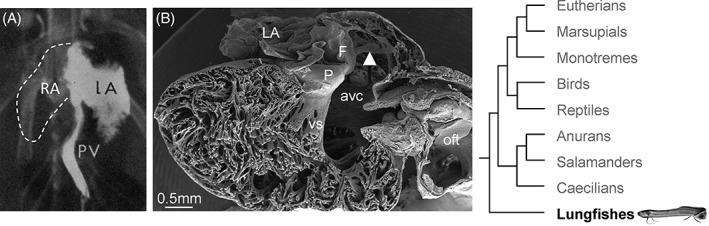
Blood flow and septation in the atrium of the African lungfish. (A) In the atria of the African lungfish pulmonary venous return is remarkably well separated from the systemic venous return of the right atrium (RA), here visualized by X‐ray contrast. (B) The atrial septum is a sheet of mesenchymal tissue associated with the ventricular base, the so‐called pulmonary fold (F), to which several prominent trabeculations attach (arrowhead). We consider this the most primitive state of the myocardial primary septum among extant vertebrates. In this preparation, the atrial cavity is quite contracted. Avc, atrioventricular canal; LA, left atrium; oft, outflow tract; P, the atrioventricular plug (valve); vs, ventricular septum. A, adapted from (Johansen and Hol, 1968), B adapted from (Icardo et al., 2015).
Figure 6.
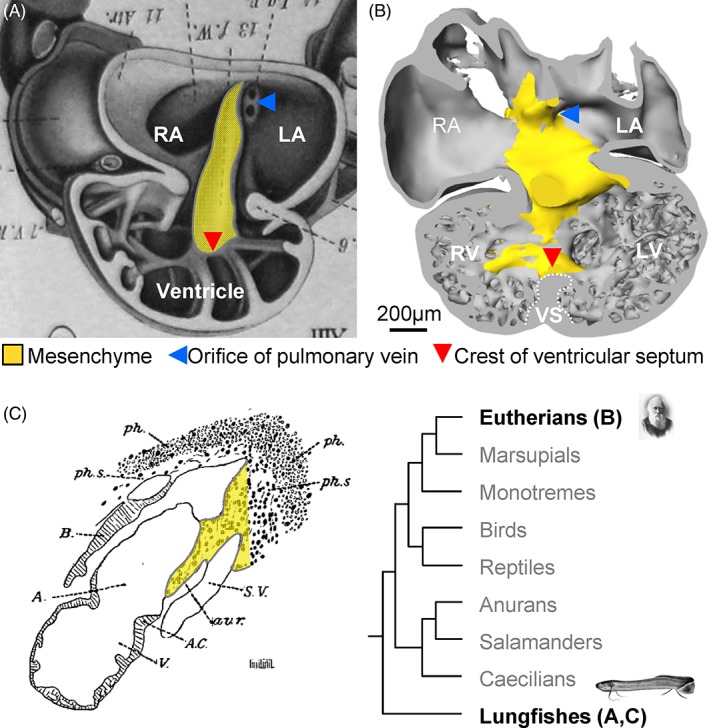
Cardiac mesenchyme (yellow) functions as a “guide” (“Leitbahn” in German (Benninghoff, 1933)) to atrial and ventricular septation in both embryonic Australian lungfish (A) and human (B, Carnegie stage 14). Adapted from (Greil, 1913; Sizarov et al., 2011a). (C) In the South American lungfish, a dorsal mesenchymal protrusion (in yellow) contributes to the mesenchymal “auriculo‐ventricular ridge” (av.r.) and thereby establishes a bridge between the atrial cavity and the pulmonary vascular plexus. This section is sagittal and is perpendicular to the image plane of A. Adapted from (Robertson, 1913).
Figure 13.
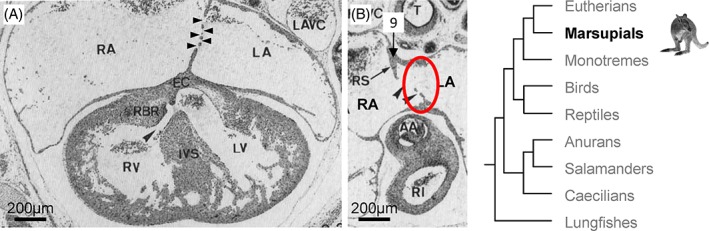
Atrial septation in the peri‐natal the marsupial tammar wallaby. (A) Four‐chamber view, showing one atrial septum only with multiple secondary perforations (black arrowheads). (B) The roof of the right atrium (RA) has a crest (9), or “rudimentary septum” (RS), beneath a groove in the atrial roof and next to the secondary perforations (within the red circle) and therefore in a position comparable to the dorso‐cranial fold of the eutherian mammal heart (Fig. 2E,F, structure 9). Compare also to Figure 11B, structure 9. The secondary perforations in the tammar wallaby vary in diameter from ~50–200 μm (Runciman et al., 1995). Adapted from (Runciman et al., 1995). LA, left atrium; RA, right atrium.
Atrial Septation in Amphibians
The earliest tetrapods were amphibians. Present‐day amphibians are grouped in three orders (Kamei et al., 2012; San Mauro et al., 2014). The most recently evolved order is the Anura (frogs and toads). To infer the ancestral condition of amphibians, one can then compare the Urodela (salamanders) and Gymnophiona (caecilians), which are snake‐like amphibians. Recent publications have surveyed the atrial septum across multiple families of caecilians and salamanders (de Bakker et al., 2015; Lewis and Hanken, 2017).
In all caecilians and salamanders there is always a gap between the leading edge of the atrial septum and the atrioventricular valve (Davies and Francis, 1941; Ramaswami, 1944; Putnam and Kelly, 1978; de Bakker et al., 2015; Lewis and Hanken, 2017). From a mammalian stance, this resembles persistent primary foramen (Fig. 3B). Compared to lungfishes, the myocardial part of the septum is much more extensive and is essentially a sheet with a great number of perforations (Fig. 7A, A’). The perforations may be secondarily formed (Schilling, 1935; Terhal, 1942) and at least in the early development of the caecilian Idiocranium, the atrial septum starts as an unperforated sheet (Fig. 7B). The dorsal base of the atrial septum of adult caecilians and salamanders consists of connective tissue and is without myocardium (Fig. 7A). This part has also been called the “Atrial Septum 2” (Lewis and Hanken, 2017) and “ib” (Putnam and Parkerson, 1985). Presumably, the connective tissue part is derived from the dorsal mesocardium, but this has not been demonstrated (de Bakker et al., 2015). In the Xenopus frog, the pharyngeal mesoderm expresses Isl1 and contributes to the arterial pole as in other vertebrates, but it has not been investigated whether it adds dorsal mesenchyme in the form of a dorsal mesenchymal protrusion (Brade et al., 2007). Architecturally, the atrial septum of caecilians and salamanders resembles an expanded state of the perforated primary atrial septum before closure of the primary foramen (Fig. 3B,B’). In the Xenopus frog, the atrial septum initiates from a ridge of mesenchyme and grows as a single unperforated sheet (Jahr and Manner, 2011) and thus resembles the early stages of the mammalian primary septum (Fig. 3B). In fully formed frogs and toads, there is a full atrial septum without any indication of rim‐like structures to delimit a fossa ovalis (Fig. 8). Given the state of the atrial septum in caecilians and salamanders, the full atrial septum of anurans is probably a derived condition.
Figure 7.
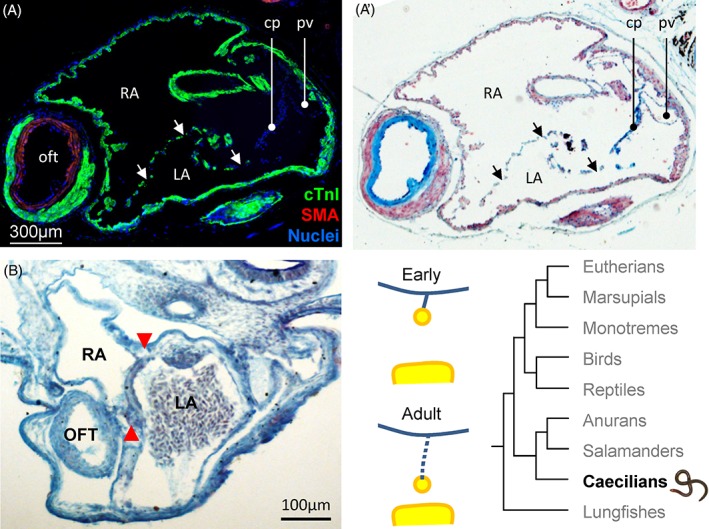
The atrial septum of the caecilian amphibian, Idiocranium. (A–A’). Transverse section of the atrial cavity, showing an atrial septum with a myocardial component with multiple perforations (arrows) and a smaller connective tissue component (cp) dorsally. (in A staining is by fluorescent immunohistochemistry, in A' the same section was subsequently stained with alcian blue for connective tissue). Adapted from (de Bakker et al., 2015). (B) The atrial septum (red arrowheads) of a developing Idiocranium. In this early stage, the atrial septum appears as a single unperforated sheet. The photo is from specimen AMP‐121 of the Hill collection. cTnI, cardiac troponin I; LA, left atrium; oft, outflow tract; pv, pulmonary vein; RA, right atrium; SMA, smooth muscle actin.
Figure 8.
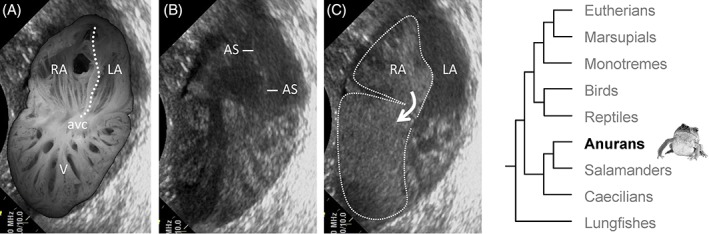
Blood flows in the toad heart. (A) Dorsal halve of the fixed heart of a cane toad (Rhinella marina) projected onto a copied echocardiography screen shot, similar to the one shown in B. Note the highly trabeculated ventricle (V). The atrial septum is indicated by the dashed line and fully separates the cavities of the right (RA) and left atrium (LA) from the atrial roof to the atrioventricular canal (avc). (B) Echocardiography in the horizontal plane in an anesthetized cane toad at late atrial diastole. The atrial septum (AS) is the echogenic substrate in the center of the atrial cavity. (C) Same specimen, 8 s later, also late atrial diastole, where physiological saline containing micro‐bubbles had been injected on the cardiac right side via the postcaval vein. Blood of the right atrium (RA) is entirely separated from blood of the left atrium (LA) due to the full atrial septum. Note the preferential distribution of micro‐bubbles on the ventricular right side, despite the absence of a ventricular septum.
Atrial Septation in Reptiles and Birds
Reptiles, birds, and mammals develop with extraembryonic membranes, including the amnion, not to be found in fishes and amphibians (Alfoldi et al., 2011). Accordingly, they are grouped together as amniotes. Birds and crocodilians evolved from basal reptiles and are grouped in the archosaur clade, whereas mammals evolved from an even earlier branch of reptiles (Green et al., 2014). Concerning the heart, the typical reptile heart likely represents the ancestral condition of reptiles and mammals.1 In all amniotes, the atrial septum is complete (Jensen et al., 2014).
Following the building plan of lungfishes and amphibians, the atrial septum initiates as a mesenchymal ridge in the atrial roof (Greil, 1903; Benninghoff, 1933; Jensen et al., 2013). In chicken, the mesenchymal ridge is subtle around Hamburger‐Hamilton stages 17–18 when the myocardial component starts to grow (Chang, 1931; Quiring, 1933; Männer and Merkel, 2007). The subsequent growth of the primary septum, the closure of the primary foramen, and the formation of secondary perforations in the cranial part of the septum is like in eutherian mammals (Figs. 3A–C, 9)(Hendrix and Morse, 1977; Kutsche and Van Mierop, 1988; Jensen et al., 2013; Cook et al., 2017). We show here, as has previously been shown for mammals (Fedorow, 1910), that dorsal mesenchyme expressing Isl1 contributes a dorsal mesenchymal protrusion to the mesenchymal tissues that close the primary foramen (Figs. 3B’, 10). In contrast to the setting of eutherian mammals, the secondary perforations do not coalesce to a secondary foramen.2 Around birth or hatching, the secondary perforations simply close by growth of myocardial and endothelial tissues around the margins of each perforation (Röse, 1890; Hendrix and Morse, 1977; Jensen et al., 2013). The differentiation from mesenchyme into myocardium is a key process in the formation of the ventro‐caudal margin of the fossa ovalis (Figs. 1A, 3E) and it is noteworthy that atrial mesenchymal tissues in reptiles also exhibit some capacity to differentiate into myocardium (Fig. 10). This capacity may be lacking in amphibians as the connective tissue part of the atrial septum is without myocardium (Fig. 7). In chicken, and presumably birds in general, much atrial mesenchyme differentiates into myocardium, including at the base of the atrial septum (de Groot et al., 1987; van den Hoff et al., 2001; van den Hoff et al., 2004).
Figure 9.
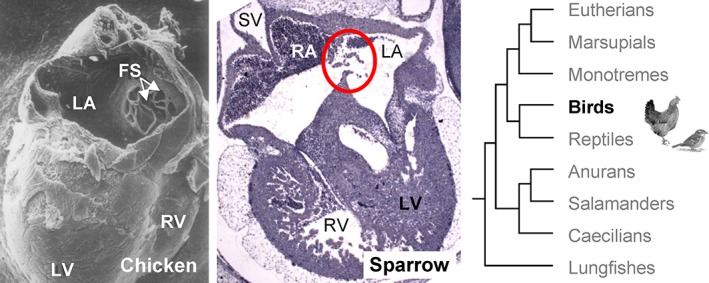
The developing atrial septum of birds. In the 7‐day‐old chicken embryo, the roof of the left atrium has been cut away to show that the primary atrial septum has developed multiple perforations (FS) and the strings surrounding these are pushed into the left atrial cavity. Adapted from (Hendrix and Morse, 1977). In the embryonic house sparrow (Passer domesticus), a passerine bird that is phylogenetically distant from chicken (Zhang et al., 2014), the atrial septum (red circle) is perforated like in chicken. The photo is from specimen A153 of the Hill collection. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; SV, sinus venosus.
Figure 10.

Dorsal mesenchymal protrusion in a lizard. (A) In the embryonic green anole lizard (Anolis carolinensis, Sanger (Sanger et al., 2008) stage 7), Ils1 is expressed in the dorsal mesenchymal protrusion (negative for the sarcomeric protein tnnt2, save a few noticeable specks) on the left of which is the pulmonary vein (pv) as in mammals. (B) Schematized sagittal section of the heart with the same principal components as in eutherian mammals (Fig. 2B’). 1, primary septum; 2, mesenchymal cap; 4, atrioventricular cushions; d, dorsal mesocardium; D, dorsal mesenchymal protrusion; LA, left atrium; RA, right atrium; V, ventricle.
Reptiles have a large groove in the atrial roof containing the truncus arteriosus, the common trunk of the pulmonary artery and the two aortae (Jensen et al., 2014). Beneath this groove is a prominent crest of trabeculations, which is immediately to the right of the primary septum and then in the same position as the dorso‐cranial rim of the fossa ovalis (Figs. 3E,F, 11). During ventricular diastole, the atrial septum is pressed against this crest in a manner that strongly resembles the setting of persistent foramen ovale in human, where the primary septum, or flap valve, is pressed against the dorso‐cranial rim of the fossa ovalis (Fig. 11A,A’). In reptiles, it is the cranial part of the atrial septum that develops secondary perforations, as in eutherian mammals, and that is pressed against the crest in the atrial roof (Fig. 11B). It is not clear whether the bird heart has a similar crest. Instead, the atrial roof is dominated by a large transverse arch (Benninghoff, 1933; Smith et al., 2000). Concluding on reptiles and birds, they have a primary atrial septum like in mammals (cf., Fig. 3C), but their secondary perforations do not coalesce to a secondary foramen. The mesenchyme that will form the ventro‐caudal rim of the fossa ovalis of eutherian mammals, can differentiate into myocardium (cf., Fig. 3E, structure 11), but does not form a rim. At least reptiles appear to have a structure comparable to the dorso‐cranial rim of the fossa ovalis (cf., Fig. 3E, structure 10), but a rim and fossa ovalis does not form, because the primary septum and the putative rim do not merge.
Figure 11.

Cranial fold in the atrial roof of reptiles. (A) Echocardiography of a 6 kg yellow anaconda (Eunectes notaeus), showing the atrial septum (red arrowheads). Numbers on the scalebar are centimeters. The roof of the right atrium has a crest (9) in a position comparable to the dorso‐cranial fold of the eutherian mammal heart (Fig. 2E,F, structure 9). (A’) During ventricular filling, the cranial part of the atrial septum is pressed against the crest, and thus resembles the apposition of the primary septum (flap valve) against the dorso‐cranial rim of the fossa ovalis in a setting of persistent foramen ovale. Adapted from (Jensen et al., 2014). (B) The heart of a brown anole lizard, just before hatching (Anolis sagrei, Sanger (et al., 2008) stage 17), stained for atrial myosin (Myh6). The crest (9) is recognizable by particularly prominent trabeculations beneath a fold in the atrial roof. Note the secondary perforations (within the red circle) immediately next to the crest. LA, left atrium; RA, right atrium; V, ventricle.
Atrial Septation in Monotreme and Marsupial Mammals
Mammals of the order Monotremata (meaning single hole), like the Australian platypus, have a cloaca like reptiles and birds, rather than separate orifices for feces and offspring, they lay eggs, and belong to an early branch within mammals (Warren et al., 2008). They also have fur and secrete milk from pores on the belly. Marsupials, like kangaroos, give birth to very immature offspring that complete most of the development in the mother's pouch (Ferner et al., 2017; Guernsey et al., 2017). Marsupials are considered a sister group to eutherian mammals (Song et al., 2012). Very little is known of the formation of the atrial septum in monotremes, but the existing literature on marsupials allows for some anatomical comparison to other vertebrates.
In monotremes the atrial septum initiates as a mesenchymal ridge in the atrial roof (Fig. 12) Around the time of hatching a single thin atrial septum has formed that has a few perforations cranially (Fig. 12). Presumably, the development of the monotreme atrial septum follows the sequence and events summarized in Figure 3A–C. In the formed heart, the atrial septum is unperforated, but without a fossa ovalis (Rowlatt, 1990). The perforations are presumably sealed by growth of the endothelium and myocardium after hatching. In marsupials, just before birth, there is, like in the monotremes, a single thin atrial septum. There can be multiple perforations, some may be a few hundred micrometers in diameter while other perforations remain much smaller, but they do not coalesce to form a single secondary foramen (Fig. 13A) (Röse, 1890; Franklin et al., 1942; Runciman et al., 1995). Runciman et al. (1995) studied perinatal hearts of eight species of marsupials and in one, the Eastern native cat (Dasyurus viverrinus), there was a single and large secondary perforation resembling the foramen ovale in three out of four studied hearts, but this is likely a derived condition given the phylogenetic position of Dasyurus in the marsupial clade (Kealy and Beck, 2017). After birth, the perforations are sealed by growth of endothelium and probably myocardium as well (Runciman et al., 1995). The formed atrial septum is without a fossa ovalis (Rowlatt, 1990; Runciman et al., 1992).
Figure 12.

In the embryonic echidna (Tachyglossus sp), at the earliest stage of cardiac chamber formation, the prospective atrial septum is identified by a ridge of cardiac jelly in the process of becoming mesenchyme. The insert schematizes the ridge of cardiac jelly in yellow and myocardium in blue. The photo is from specimen MOF‐161 of the Hill collection. In the hatchling of the echidna, the single atrial septum only has few secondary perforations (black arrows). The photo is from specimen M‐44 of the Hill collection. LA, left atrium; OFT, outflow tract; RA, right atrium; V, ventricle.
Based on the available literature, it is not possible to come to an exact conclusion on the differences between the multiple secondary perforations of the marsupial primary septum and the likely primitive state of the foramen ovale in eutherian mammals comprising multiple perforations. The greater duration of gestation in eutherian mammals may explain most of these differences.
The right atrium of fully‐formed monotremes and marsupials always has a so‐called “torus of Lower” (Rowlatt, 1990). The torus, or tubercle, of Lower is considered to be the dorso‐cranial rim of the fossa ovalis (Loukas et al., 2012) (cf., Fig. 3E, structure 9). Although present, it is poorly developed, forming “a flat band,” when compared to eutherian mammals (Rowlatt, 1990). The dorso‐cranial rim can also be recognized in embryonic marsupials of some species as a “rudimentary septum,” but, if present, it does not “overlap the fenestrated septum to any extend” and is in a less developed state than in eutherian mammals (Fig. 13B structure 9, also compare to Fig. 11B structure 9) (Runciman et al., 1995). It remains unclear whether the atrial mesenchyme of monotremes and marsupials can differentiate into myocardium (cf., Fig. 3E, structure 11). A structure like the ventro‐caudal rim of the fossa ovalis appears absent (Röse, 1890; Franklin et al., 1942; Rowlatt, 1990; Runciman et al., 1995).
SYNTHESIS
Evolution of the Atrial Septum
In vertebrates that form an atrial septum, atrial septation initiates around the time of chamber formation and begins as a ridge of cardiac jelly or mesenchyme. Presumably, this ridge of mesenchyme is the ancestral setting (Fig. 14). Amphibians, reptiles, birds, and mammals share with lungfishes the principal components for forming a full primary atrial septum. The full atrial septum by the primary septum only, always requires the closure of the primary foramen by the merger of the mesenchymal tissues of the atrioventricular cushions, the mesenchymal cap on the leading edge of the septum, and the dorsal mesenchymal protrusion. The relative importance of the three tissues cannot be assessed yet. Full septation further requires the closure of perforations by endothelial and myocardial growth. This is the setting of reptiles, birds, and mammals of Monotremata and Marsupialia, and possibly also in anuran amphibians. Only in eutherian mammals will a fossa ovalis form, which reflects firstly an unparalleled growth of the myocardialized mesenchyme at the atrial septal base, secondly an enhanced development of an atrial fold, or secondary atrial septum, and associated myocardium in the atrial roof, and thirdly the merger of the primary septum and the dorso‐cranial rim of the fossa ovalis (Fig. 14).
Figure 14.
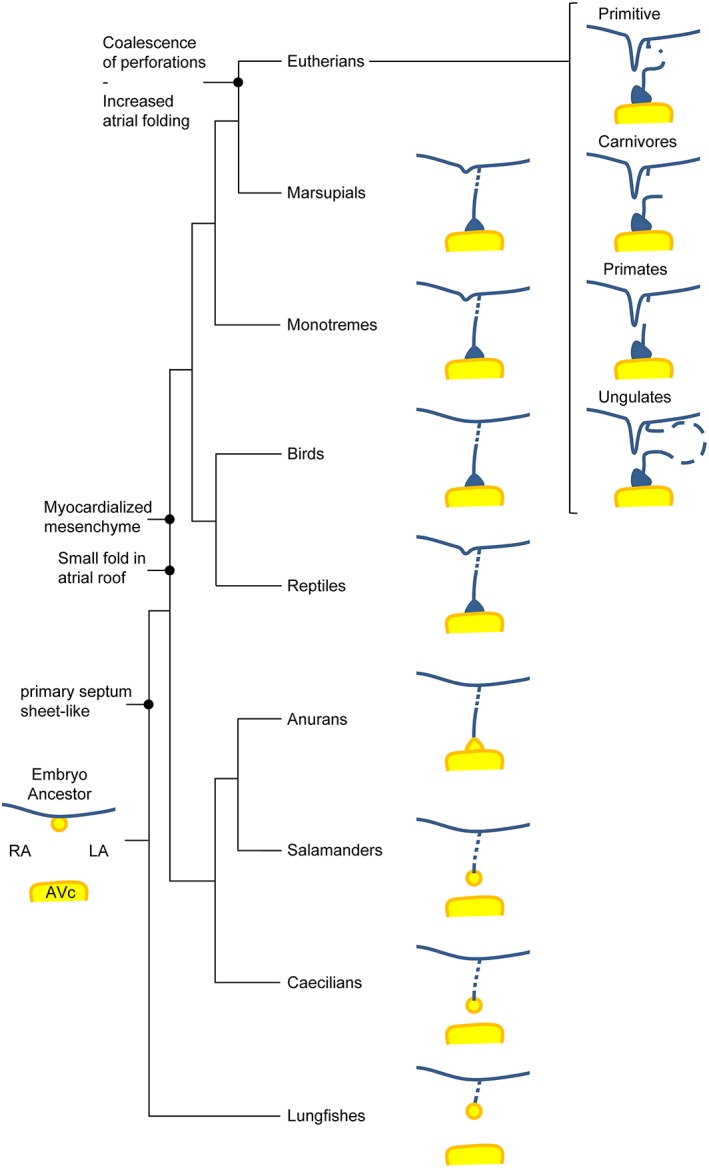
Evolution of the atrial septum. The schematized atrial septa represent a late developmental state to illustrate the variation in primary foramen closure and secondary perforations to the primary septum. Within eutherian mammals, the variation in architecture of the primary septum is under the influence of phylogeny. AVc, atrioventricular cushion (or valve); LA, left atrium; RA, right atrium.
A Full Septum Evolved Earlier in the Atrium Than in the Ventricle
It is noteworthy that many species without ventricular septation have either a substantial atrial septum (Gymnophiona and Urodela) or a full atrial septum (non‐crocodilian reptiles). This suggests that the evolution of atrial septation had a greater impact on the separation of the systemic venous and pulmonary venous blood streams than ventricular septation. Septation of the outflow tract, either partial as seen in amphibians or full as seen in reptiles, also evolved earlier than full septation of the ventricle (Jensen et al., 2014; Poelmann et al., 2017). Indeed, the highly trabeculated ventricles of amphibians and reptilians are capable of pronounced blood flow separation presumably because inflowing blood is lodged within the innumerous intertrabecular lumina (exemplified in the marine toad in Fig. 8) (Johansen and Burggren, 1980; Hicks and Wang, 2012; Jensen et al., 2014).
Is the Secondary Foramen Particular to Eutherian Mammals?
Only in eutherian mammals are the components of the second septum so extensive that a fossa ovalis appears (Rowlatt, 1990). As the second septum is the closing mechanism of the secondary holes, or hole, in the primary septum, then, presumably, there is something excessive about the secondary holes as well. The meshwork appearance of the secondary foramen in cetaceans and ungulates, and possibly elephants, could be a simple expansion of the multiple secondary perforations seen in the embryo and other amniotes. In contrast, a single hole of the secondary foramen, like in carnivores, rodents, and primates, appears to be a derived condition among eutherian mammals only.
The carnivorous bears have long gestation, almost as long as in similar sized ungulates, but a single secondary foramen like in other carnivores. This suggests phylogeny is a major determinant of the architecture of the secondary foramen, presumably mediated by clade‐specific morphogenetic programs. Humans have a single secondary foramen, small primary septum in the fetal stages, and a high frequency of patent foramen ovale. Conceivably, a perforated and extensive primary septum, like in the harbor porpoise (Macdonald et al., 2007), could deliver more tissue to close the foramen ovale and harbor porpoises appear to have a much lower incidence of persistent foramen ovale than human (Rowlatt and Gaskin, 1975). The risk of decompression sickness is greater in the setting of persistent foramen ovale (Calvert et al., 2011; Nakanishi et al., 2017) and the extensive primary septum and relative efficient closure of the foramen ovale of the harbor porpoise may be an adaptation to diving. The extensive primary septum, however, is a character of ungulates (Fig. 4), including the harbor porpoise and its fellow cetaceans, and it was likely present before the evolution of diving behavior in ungulates.
What is the Evolutionary Advantage to a Secondary Foramen?
The secondary foramen is probably an adaptation to the developmental conditions during the long gestation of eutherian mammals (Röse, 1890; Runciman et al., 1995). The large fetus resulting from a long gestation is enabled by the presence of the diaphragm, which prevents the large growing offspring from displacing the mother's abdominal organs into her thoracic cavity and thus restricting the lung volume (Perry et al., 2010; Ferner et al., 2017). During human gestation, fetal cardiac output increases exponentially to exceed 1 L min−1 and the secondary foramen accommodates ~20–30% of this flow (Teitel et al., 1987; Sutton et al., 1994; Mielke and Benda, 2001; Kiserud et al., 2006). This flow is driven by a miniscule pressure head; in late fetal lambs, the right and left atrial pressures are low and almost similar, ~3 mmHg (Assali et al., 1965; Anderson et al., 1981; Anderson et al., 1985). The oxygen‐enriched blood at the intake of the ductus venosus flows at more than 0.5 m s−1 and kinetic energy may drive much of the shunt across the foramen ovale (Kiserud, 2005). The hemodynamic forces, however, are small and the secondary foramen must offer little resistance to flow. In humans, the secondary foramen and the foramen ovale expand in proportion to atrial growth and, before birth, will have a cross sectional area of approximately 0.5 cm2 or some fourfold greater than the corresponding area of the brachial artery of the arm of adult males (Kiserud and Rasmussen, 2001; Tomiyama et al., 2015). By expansion of the numerous secondary perforations, or coalescence, the secondary foramen acquires a greater cross sectional area and will then have a lower resistance to flow.3 We, therefore, propose the advantage of the secondary foramen is its low resistance to shunts to the systemic circulation. Indeed, gestational growth retardation in human has been associated with a proportionally small foramen ovale, suggesting that the absolute size of the secondary foramen can be a bottleneck for fetal growth (Kiserud et al., 2004).
CONCLUSION
The secondary foramen is likely an adaptation to accommodate the large right‐to‐left shunts associated with the prolonged gestation of eutherian mammals. The secondary septum is a compensating adaptation to the large and secondarily formed holes in the primary atrial septum. It develops from structures that are present in a less developed state in other mammals and reptiles. In evolution, the incidence of persistent foramen ovale and the associated risks, were likely benign compared to the benefits associated with prolonging gestation and hence delaying birth to enable more mature offspring with increased survival capacities immediately after birth. The human predicament of ubiquitous persistence of the foramen ovale is not necessarily a trait that is shared with most other eutherian mammals and may reflect the much‐reduced state of the primary septum in humans.
ACKNOWLEDGEMENTS
The specimens of the Hill collection were kindly made available by Dr. Peter Giere of the Museum für Naturkunde in Berlin (Germany). It was a collaboration with Robert H. Anderson that spurred us to review most of the topics presented here. Jan M. Nielsen performed the echocardiography of Figure 8.
Endnotes
The pre‐endotherm mammalian ancestors can be assumed to have had a heart quite like those of squamate reptiles based on the following observations: (1) Reptile hearts have a valve between the sinus venosus and the right atrium, so do monotreme mammals and many birds (Jensen et al., 2017); (2) Reptile hearts have a primary atrial septum only, so do monotreme and marsupial mammals and birds. Only eutherian mammals have a primary and secondary atrial septum; (3) All amniotes incorporate the myocardial outflow tract during development, but amphibians do not. In this process, the muscular ridge/horizontal septum/folding septum is formed (Jensen et al., 2013); (4) All ectotherms have a highly trabeculated ventricle (Burggren et al., 2014; Jensen et al., 2016). It seems a reasonable assumption that the pre‐endotherm mammalian ancestors had features 1–4, and the presence of all four characters only occur in the formed reptile hearts.
In a case report on the Komodo dragon, the defect atrial septum had a single large hole resembling the foramen ovale, but we deem this resemblance to be coincidental (Pizzi et al., 2009).
In rigid tubes, the Hagen‐Poiseuille equation gives the resistance to flow which is negatively related to the radius of the tube raised to the power of 4. The remaining four determinants of resistance are two constants (8 and π), the viscosity of the fluid (positive relation, raised to the power of 1), and length of the tube (positive relation, raised to the power of 1). In a setting of changing variables, radius is major determinant of resistance. The secondary perforations are not rigid tubes and flow across them may not be adequately be described by the Hagen‐Poiseuille equation. We do, nonetheless, consider it likely that the radius of the secondary perforations, or secondary foramen, is the weighty component in the resistance to flow of oxygen‐rich blood to the left atrium and systemic circulation.
Literature Cited
- Aanhaanen WT, Mommersteeg MT, Norden J, Wakker V, de Gier‐de VC, Anderson RH, Kispert A, Moorman AF, Christoffels VM. 2010. Developmental origin, growth, and three‐dimensional architecture of the atrioventricular conduction axis of the mouse heart. Circ Res 107:728–736. [DOI] [PubMed] [Google Scholar]
- Alfoldi J, Di PF, Grabherr M, Williams C, Kong L, Mauceli E, Russell P, Lowe CB, Glor RE, Jaffe JD, et al. 2011. The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 477:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemiya CT, Alfoldi J, Lee AP, Fan S, Philippe H, Maccallum I, Braasch I, Manousaki T, Schneider I, Rohner N, et al. 2013. The African coelacanth genome provides insights into tetrapod evolution. Nature 496:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso EC, Franklin KJ, Prichard MM. 1941. Observations on the cardio‐vascular system and lungs of an African elephant foetus. J Anat 76:100–111. [PMC free article] [PubMed] [Google Scholar]
- Anderson DF, Bissonnette JM, Faber JJ, Thornburg KL. 1981. Central shunt flows and pressures in the mature fetal lamb. Am J Physiol 241:H60–H66. [DOI] [PubMed] [Google Scholar]
- Anderson DF, Faber JJ, Morton MJ, Parks CM, Pinson CW, Thornburg KL. 1985. Flow through the foramen ovale of the fetal and new‐born lamb. J Physiol 365:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RH, Spicer DE, Brown NA, Mohun TJ. 2014. The development of septation in the four‐chambered heart. Anat Rec (Hoboken) 297:1414–1429. [DOI] [PubMed] [Google Scholar]
- Assali NS, Morris JA, Beck R. 1965. Cardiovascular hemodynamics in the fetal lamb before and after lung expansion. Am J Physiol 208:122–129. [DOI] [PubMed] [Google Scholar]
- Battaglia FC, Meschia G. 1978. Principal substrates of fetal metabolism. Physiol Rev 58:499–527. [DOI] [PubMed] [Google Scholar]
- Benninghoff A. 1933. Das Herz In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der vergleichende Anatomie der Wirbeltiere. Berlin: Urban & Schwarzenberg; p 467–555. [Google Scholar]
- Brade T, Gessert S, Kuhl M, Pandur P. 2007. The amphibian second heart field: Xenopus islet‐1 is required for cardiovascular development. Dev Biol 311:297–310. [DOI] [PubMed] [Google Scholar]
- Briggs LE, Kakarla J, Wessels A. 2012. The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion. Differentiation 84:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren WW, Christoffels VM, Crossley DA, Enok S, Farrell AP, Hedrick MS, Hicks JW, Jensen B, Moorman AFM, Mueller CA, et al. 2014. Comparative cardiovascular physiology: Future trends, opportunities and challenges. Acta Physiol 210:257–276. [DOI] [PubMed] [Google Scholar]
- Burggren WW, Johansen K. 1986. Circulation and respiration in lungfishes (Dipnoi). J Morph 1:217–236. [Google Scholar]
- Calvert PA, Rana BS, Kydd AC, Shapiro LM. 2011. Patent foramen ovale: Anatomy, outcomes, and closure. Nat Rev Cardiol 8:148–160. [DOI] [PubMed] [Google Scholar]
- Chang C. 1931. The formation of the interatrial septum in chick embryos. Anat Rec 50:9–22. [Google Scholar]
- Cheng G, Wessels A, Gourdie RG, Thompson RP. 2002. Spatiotemporal and tissue specific distribution of apoptosis in the developing chick heart. Dev Dyn 223:119–133. [DOI] [PubMed] [Google Scholar]
- Cole‐Jeffrey CT, Terada R, Neth MR, Wessels A, Kasahara H. 2012. Progressive anatomical closure of foramen ovale in normal neonatal mouse hearts. Anat Rec (Hoboken) 295:764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AC, Tran VH, Spicer DE, Rob JMH, Sridharan S, Taylor A, Anderson RH, Jensen B. 2017. Sequential segmental analysis of the crocodilian heart. J Anat 231:484–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH. 1998. Anatomy of the pig heart: Comparisons with normal human cardiac structure. J Anat 193(Part 1):105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies F, Francis ETB. 1941. The Heart of the Salamander (Salamandra salamandra, L.), with special reference to the conducting (connecting) system and its bearing on the phylogeny of the conducting systems of mammalian and avian hearts. Philos Trans R Soc Lond Ser B. Biol Sci 231:99–130. [Google Scholar]
- Dawes GS, Mott JC, Widdicombe JG. 1955. Closure of the foramen ovale in newborn lambs. J Physiol 128:384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bakker DM, Wilkinson M, Jensen B. 2015. Extreme variation in the atrial septation of caecilians (Amphibia: Gymnophiona). J Anat 226:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot I, Sanders E, Visser S, Lamers W, de Jong F, Los J, Moorman A. 1987. Isomyosin expression in developing chicken atria: A marker for the development of conductive tissue? Anat Embryol 176:515–523. [DOI] [PubMed] [Google Scholar]
- Driscoll DJ. 2016. Clinical presentation and therapy of atrial septal defect In: Rickert‐Sperling S, Kelly RG, Driscoll DJ, editors. Congenital heart diseases: The broken heart: Clinical features, human genetics and molecular pathways. Vienna: Springer Vienna; p 273–277. [Google Scholar]
- Edelstone DI, Rudolph AM. 1979. Preferential streaming of ductus venosus blood to the brain and heart in fetal lambs. Am J Physiol 237:H724–H729. [DOI] [PubMed] [Google Scholar]
- Elliott GC, Gurtu R, McCollum C, Newman WG, Wang T. 2014. Foramen ovale closure is a process of endothelial‐to‐mesenchymal transition leading to fibrosis. PLoS One 9:e107175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorow V. 1910. Über die Entwickelung der Lungenvene. Anat Embryol 40:529–607. [Google Scholar]
- Feit LR, Copel JA, Kleinman CS. 1991. Foramen ovale size in the normal and abnormal human fetal heart: An indicator of transatrial flow physiology. Ultrasound Obstet Gynecol 1:313–319. [DOI] [PubMed] [Google Scholar]
- Ferner K, Schultz JA, Zeller U. 2017. Comparative anatomy of neonates of the three major mammalian groups (monotremes, marsupials, placentals) and implications for the ancestral mammalian neonate morphotype. J Anat 231:798–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DJ, Heymann MA, Rudolph AM. 1980. Myocardial oxygen and carbohydrate consumption in fetal lambs in utero and in adult sheep. Am J Physiol 238:H399–H405. [DOI] [PubMed] [Google Scholar]
- Franklin KJ, Amoroso EC, Barclay AE, Prichard MML. 1942. The valve of the foramen ovale its relation to pulmonary vein entries. Vet J 98:29–41. [Google Scholar]
- Green RE, Braun EL, Armstrong J, Earl D, Nguyen N, Hickey G, Vandewege MW, St John JA, Capella‐Gutierrez S, Castoe TA, et al. 2014. Three crocodilian genomes reveal ancestral patterns of evolution among archosaurs. Science 346:1254449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorovicova M, Zahradnicek O, Tucker AS, Velensky P, Horacek I. 2012. Embryonic development of the monitor lizard, Varanus indicus . Amphibia‐Reptilia 33:451–468. [Google Scholar]
- Greil A. 1903. Beitrage zur vergelichenden anatomie und entwicklungsgeschichte des herzens und des trauncus arteriosus der wirbelthiere. MorphJahrbuch 31:123–310. [Google Scholar]
- Greil A. 1913. Entwickelungsgeschichte des Kopfes und des Blutgefässsystemes von Ceratodus forsteri. II. Die epigenetischen Erwerbungen während der Stadien 39‐48. Denkschriften der Medizinisch‐Naturwissenschaftlichen Gesellschaft zu Jena 9:935–1492. [Google Scholar]
- Guernsey MW, Chuong EB, Cornelis G, Renfree MB, Baker JC. 2017. Molecular conservation of marsupial and eutherian placentation and lactation. Elife 6 e27450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen PT, Scholz DG, Edwards WD. 1984. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin Proc 59:17–20. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Morse DE. 1977. Atrial septation. I. Scanning electron microscopy in the chick. Dev Biol 57:345–363. [DOI] [PubMed] [Google Scholar]
- Hicks JW, Wang T. 2012. The functional significance of the reptilian heart: New insights into an old question In: Sedmera D, Wang T, editors. Ontogeny and phylogeny of the vertebrate heart. New York: Springer Science+Business Media; p 207–227. [Google Scholar]
- Hoffman JI, Kaplan S. 2002. The incidence of congenital heart disease. J Am Coll Cardiol 39:1890–1900. [DOI] [PubMed] [Google Scholar]
- Icardo JM. 1996. Developmental biology of the vertebrate heart. J Exp Zool 275:144–161. [PubMed] [Google Scholar]
- Icardo JM. 2017. Heart morphology and anatomy. Fish Physiol 36:1–54. [Google Scholar]
- Icardo JM, Ojeda JL, Colvee E, Tota B, Wong WP, Ip YK. 2005. Heart inflow tract of the African lungfish Protopterus dolloi . J Morphol 263:30–38. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Tota B, Ip YK. 2015. Anatomy of the heart and circulation in lungfishes. Phylogeny Anat Physiol Ancient Fishes 133.
- Jahr M, Manner J. 2011. Development of the venous pole of the heart in the frog Xenopus laevis: A morphological study with special focus on the development of the venoatrial connections. Dev Dyn 240:1518–1527. [DOI] [PubMed] [Google Scholar]
- Jay PY, Degenhardt KR, Anderson RH. 2016. Molecular pathways and animal models of atrial septal defect In: Rickert‐Sperling S, Kelly GR, Driscoll JD, editors. Congenital heart diseases: The broken heart: Clinical features, human genetics and molecular pathways. Vienna: Springer Vienna; p 291–300. [Google Scholar]
- Jensen B, Agger P, de Boer BA, Oostra RJ, Pedersen M, van der Wal AC, Nils Planken R, Moorman AF. 2016. The hypertrabeculated (noncompacted) left ventricle is different from the ventricle of embryos and ectothermic vertebrates. Biochim Biophys Acta 1863:1696–1706. [DOI] [PubMed] [Google Scholar]
- Jensen B, Boukens BJ, Postma AV, Gunst QD, van den Hoff MJ, Moorman AF, Wang T, Christoffels VM. 2012. Identifying the evolutionary building blocks of the cardiac conduction system. PLoS One 7:e44231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Moorman AF, Wang T. 2014. Structure and function of the hearts of lizards and snakes. Biol Rev Camb Philos Soc 89:302–336. [DOI] [PubMed] [Google Scholar]
- Jensen B, Spicer DE, Sheppard MN, Anderson RH. 2017. Development of the atrial septum in relation to postnatal anatomy and interatrial communications. Heart 103:456–462. [DOI] [PubMed] [Google Scholar]
- Jensen B, van den Berg G, van den Doel R, Oostra RJ, Wang T, Moorman AF. 2013. Development of the hearts of lizards and snakes and perspectives to cardiac evolution. PLoS One 8:e63651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Vesterskov S, Boukens BJ, Nielsen JM, Moorman AFM, Christoffels VM, Wang T. 2017. Morpho‐functional characterization of the systemic venous pole of the reptile heart. Sci Rep 7:6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen K, Burggren WW. 1980. Cardiovasular function in the lower vertebrates. Hearts Heart‐like Organs 1:61–117. [Google Scholar]
- Johansen K, Hol R. 1968. A radiological study of the central circulation in the lungfish, Protopterus aethiopicus . J Morph 126(3):333–348. [DOI] [PubMed] [Google Scholar]
- Kamei RG, San Mauro D, Gower DJ, Van Bocxlaer I, Sherratt E, Thomas A, Babu S, Bossuyt F, Wilkinson M, Biju SD. 2012. Discovery of a new family of amphibians from northeast India with ancient links to Africa. Proc Biol Sci 279:2396–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealy S, Beck R. 2017. Total evidence phylogeny and evolutionary timescale for Australian faunivorous marsupials (Dasyuromorphia). BMC Evol Biol 17:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Jay PY. 2016. Human genetics of atrial septal defect In: Rickert‐Sperling S, Kelly GR, Driscoll JD, editors. Congenital heart diseases: The broken heart: Clinical features, human genetics and molecular pathways. Vienna: Springer Vienna; p 279–290. [Google Scholar]
- Kiserud T. 2005. Physiology of the fetal circulation. Semin Fetal Neonatal Med 10:493–503. [DOI] [PubMed] [Google Scholar]
- Kiserud T, Chedid G, Rasmussen S. 2004. Foramen ovale changes in growth‐restricted fetuses. Ultrasound Obstet Gynecol 24:141–146. [DOI] [PubMed] [Google Scholar]
- Kiserud T, Ebbing C, Kessler J, Rasmussen S. 2006. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet Gynecol 28:126–136. [DOI] [PubMed] [Google Scholar]
- Kiserud T, Eik‐Nes SH, Blaas HG, Hellevik LR. 1992. Foramen ovale: An ultrasonographic study of its relation to the inferior vena cava, ductus venosus and hepatic veins. Ultrasound Obstet Gynecol 2:389–396. [DOI] [PubMed] [Google Scholar]
- Kiserud T, Piaggio G, Carroli G, Widmer M, Carvalho J, Neerup Jensen L, Giordano D, Cecatti JG, Abdel Aleem H, Talegawkar SA, et al. 2017. The world health organization fetal growth charts: A multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med 14:e1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiserud T, Rasmussen S. 2001. Ultrasound assessment of the fetal foramen ovale. Ultrasound Obstet Gynecol 17:119–124. [DOI] [PubMed] [Google Scholar]
- Klitgaard T. 1978. Morphology and histology of the heart of the Australian lungfish, Neoceratodus‐Forsteri (Krefft). Acta Zool 59:187–198. [Google Scholar]
- Kokubo N, Matsuura M, Onimaru K, Tiecke E, Kuraku S, Kuratani S, Tanaka M. 2010. Mechanisms of heart development in the Japanese lamprey, Lethenteron japonicum . Evol Dev 12:34–44. [DOI] [PubMed] [Google Scholar]
- Kutsche LM, Van Mierop LHS. 1988. Development of the pulmonary vein in the American alligator (Alligator mississippiensis). Anat Rec 222:170–176. [DOI] [PubMed] [Google Scholar]
- LekanneDeprez RH, van den Hoff MJ, de Boer PA, Ruijter PM, Maas AA, Chamuleau RA, Lamers WH, Moorman AF. 1998. Changing patterns of gene expression in the pulmonary trunk‐banded rat heart. J Mol Cell Cardiol 30:1877–1888. [DOI] [PubMed] [Google Scholar]
- Lewis ZR, Hanken J. 2017. Convergent evolutionary reduction of atrial septation in lungless salamanders. J Anat 230:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas M, El‐Zammar D, Tubbs RS, Birungi J, Jacob J, Shoja MM, Anderson RH. 2012. Intervenous tubercle of lower: True tubercle or normal interatrial fold? Clin Anat 25:729–736. [DOI] [PubMed] [Google Scholar]
- Macdonald AA, Carr PA, Currie RJ. 2007. Comparative anatomy of the foramen ovale in the hearts of cetaceans. J Anat 211:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald AA, Dixon C, Boyd IL. 1995. Comparative anatomy of the cardiac foramen ovale in the Pinnipedia. Can J Zool 73:850–857. [Google Scholar]
- Macdonald AA, Fowden AL, Silver M, Ousey J, Rossdale PD. 1988. The foramen ovale of the foetal and neonatal foal. Equine Vet J 20:255–260. [DOI] [PubMed] [Google Scholar]
- Macdonald AA, Johnstone M. 1995. Comparative anatomy of the cardiac foramen ovale in cats (Felidae), dogs (Canidae), bears (Ursidae) and hyaenas (Hyaenidae). J Anat 186:235–243. [PMC free article] [PubMed] [Google Scholar]
- Maldanis L, Carvalho M, Almeida MR, Freitas FI, de Andrade JA, Nunes RS, Rochitte CE, Poppi RJ, Freitas RO, Rodrigues F, et al. 2016. Heart fossilization is possible and informs the evolution of cardiac outflow tract in vertebrates. Elife 5:e14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männer J, Merkel N. 2007. Early morphogenesis of the sinuatrial region of the chick heart: A contribution to the understanding of the pathogenesis of direct pulmonary venous connections to the right atrium and atrial septal defects in hearts with right isomerism of the atrial appendages. Anat Rec Adv Integr Anat Evol Biol 290:168–180. [DOI] [PubMed] [Google Scholar]
- Martin W. 1836. Notes on the anatomy of the koala (Phascolarctos fuscus). In: Proc Zool Soc Lond; p 109–113. [Google Scholar]
- Meier B, Nietlispach F. 2017. Editorial commentary: Closure of the patent foramen ovale viewed from a different angle. Trends Cardiovasc Med. 27:582–584. [DOI] [PubMed] [Google Scholar]
- Michaëlsson M, Ho SY. 2000. Congenital heart malformations in mammals. World Scientific Publishing Company.
- Mielke G, Benda N. 2001. Cardiac output and central distribution of blood flow in the human fetus. Circulation 103:1662–1668. [DOI] [PubMed] [Google Scholar]
- Mommersteeg MT, Soufan AT, de Lange FJ, van den Hoff MJ, Anderson RH, Christoffels VM, Moorman AF. 2006. Two distinct pools of mesenchyme contribute to the development of the atrial septum. Circ Res 99:351–353. [DOI] [PubMed] [Google Scholar]
- Morse DE, Hendrix MJ. 1980. Atrial septation. II. Formation of the foramina secunda in the chick. Dev Biol 78:25–35. [DOI] [PubMed] [Google Scholar]
- Morton SU, Brodsky D. 2016. Fetal physiology and the transition to extrauterine life. Clin Perinatol 43:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Hagio M, Nakai M. 1990. Anatomical closure of the foramen ovale in cattle. Adv Anim Cardiol 23:23–28. [Google Scholar]
- Nakanishi K, Yoshiyama M, Homma S. 2017. Patent foramen ovale and cryptogenic stroke. Trends Cardiovasc Med. 27:575–581. [DOI] [PubMed] [Google Scholar]
- Ottaway CW. 1944, 1900. The anatomical closure of the foramen ovale in the equine and bovine heart: A comparative study with observations on the fœtal and adult states**part of a thesis presented for the fellowship diploma of the royal college of veterinary surgeons.—I. Vet J 100:111–118. [Google Scholar]
- Patten BM. 1938. Developmental defects at the foramen ovale. Am J Pathol 14:135–162.139. [PMC free article] [PubMed] [Google Scholar]
- Perry SF, Similowski T, Klein W, Codd JR. 2010. The evolutionary origin of the mammalian diaphragm. Respir Physiol Neurobiol 171:1–16. [DOI] [PubMed] [Google Scholar]
- Perry SF, Wilson RJ, Straus C, Harris MB, Remmers JE. 2001. Which came first, the lung or the breath? Comp Biochem Physiol A Mol Integr Physiol 129:37–47. [DOI] [PubMed] [Google Scholar]
- Pizzi R, Pereira YM, Rambaud YF, Strike T, Flach E, Rendle M, Routh A. 2009. Secundum atrial septal defect in a Komodo dragon (Varanus komodoensis). Vet Rec 164:472–473. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Gittenberger‐de Groot AC, Biermans MWM, Dolfing AI, Jagessar A, van Hattum S, Hoogenboom A, Wisse LJ, Vicente‐Steijn R, de Bakker MAG, et al. 2017. Outflow tract septation and the aortic arch system in reptiles: Lessons for understanding the mammalian heart. Evodevo 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmann RE, Molin D, Wisse LJ, Gittenberger‐de Groot AC. 2000. Apoptosis in cardiac development. Cell Tissue Res 301:43–52. [DOI] [PubMed] [Google Scholar]
- Putnam JL, Kelly DL. 1978. A new interpretation of interatrial septation in the lungless salamander, Plethodon glutinosus . Copeia 1978(2):251–254. [Google Scholar]
- Putnam JL, Parkerson JB. 1985. Anatomy of the heart of the Amphibia II. Cryptobranchus alleganiensis Herpetologica 41:287–298. [Google Scholar]
- Quiring DP. 1933. The development of the sino‐atrial region of the chick heart. J Morphol 55:81–118. [Google Scholar]
- Ramaswami LS. 1944. An account of the heart and associated vessels in some genera of apoda (Amphibia). Proc Zool Soc Lond 114:117–139. [Google Scholar]
- Robertson JI. 1913. The development of the heart and vascular system of Lepidosiren paradoxa Quart J Micr Sci 53–132.
- Röse C. 1890. Beitrage zur vergleichenden Anatomie des Herzens der Wirbelthiere. Morphol Jahrb 16:27–96. [Google Scholar]
- Rowlatt U. 1990. Comparative anatomy of the heart of mammals. Zool J Linn Soc 98:73–110. [Google Scholar]
- Rowlatt U, Gaskin DE. 1975. Functional anatomy of the heart of the harbor porpoise, Phocaena phocaena. J Morphol 146:479–493. [DOI] [PubMed] [Google Scholar]
- Runciman SI, Gannon BJ, Baudinette RV. 1995. Central cardiovascular shunts in the perinatal marsupial. Anat Rec 243:71–83. [DOI] [PubMed] [Google Scholar]
- Runciman SIC, Baudinette RV, Gannon BJ. 1992. The anatomy of the adult marsupial heart—An historical review. Aust J Zool 40:21–34. [Google Scholar]
- San Mauro D, Gower DJ, Muller H, Loader SP, Zardoya R, Nussbaum RA, Wilkinson M. 2014. Life‐history evolution and mitogenomic phylogeny of caecilian amphibians. Mol Phylogenet Evol 73:177–189. [DOI] [PubMed] [Google Scholar]
- Sanger TJ, Losos JB, Gibson‐Brown JJ. 2008. A developmental staging series for the lizard genus Anolis: A new system for the integration of evolution, development, and ecology. J Morphol 269:129–137. [DOI] [PubMed] [Google Scholar]
- Schilling C. 1935. Das Herz von Hypogeophis und seine Entwicklung. Morphol J 76:52–91. [Google Scholar]
- Schmidt KG, Silverman NH, Rudolph AM. 1996. Assessment of flow events at the ductus venosus‐inferior vena cava junction and at the foramen ovale in fetal sheep by use of multimodal ultrasound. Circulation 93:826–833. [DOI] [PubMed] [Google Scholar]
- Sizarov A, Anderson RH, Christoffels VM, Moorman AF. 2010. Three‐dimensional and molecular analysis of the venous pole of the developing human heart. Circulation 122:798–807. [DOI] [PubMed] [Google Scholar]
- Sizarov A, Devalla HD, Anderson RH, Passier R, Christoffels VM, Moorman AF. 2011a. Molecular analysis of the patterning of the conduction tissues in the developing human heart. Circ Arrhythm Electrophysiol 4:532–542. [DOI] [PubMed] [Google Scholar]
- Sizarov A, Ya J, de Boer BA, Lamers WH, Christoffels VM, Moorman AF. 2011b. Formation of the building plan of the human heart: morphogenesis, growth, and differentiation. Circulation 123:1125–1135. [DOI] [PubMed] [Google Scholar]
- Smith FM, West NH, Jones DR. 2000. CHAPTER 9—The cardiovascular system A2. In: Whittow GC. Sturkie's avian physiology. 5th ed. San Diego: Academic Press. p 141–231.
- Snarr BS, O'Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, Wessels A. 2007. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res 101:971–974. [DOI] [PubMed] [Google Scholar]
- Song S, Liu L, Edwards SV, Wu S. 2012. Resolving conflict in eutherian mammal phylogeny using phylogenomics and the multispecies coalescent model. Proc Natl Acad Sci USA 109:14942–14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MS, Groves A, MacNeill A, Sharland G, Allan L. 1994. Assessment of changes in blood flow through the lungs and foramen ovale in the normal human fetus with gestational age: A prospective Doppler echocardiographic study. Br Heart J 71:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylva M, van den Hoff MJ, Moorman AF. 2014. Development of the human heart. Am J Med Genet A 164A:1347–1371. [DOI] [PubMed] [Google Scholar]
- Tasaka H, Krug EL, Markwald RR. 1996. Origin of the pulmonary venous orifice in the mouse and its relation to the morphogenesis of the sinus venosus, extracardiac mesenchyme (spina vestibuli), and atrium. Anat Rec 246:107–113. [DOI] [PubMed] [Google Scholar]
- Teitel DF, Iwamoto HS, Rudolph AM. 1987. Effects of birth‐related events on central blood flow patterns. Pediatr Res 22:557–566. [DOI] [PubMed] [Google Scholar]
- Terhal HJJ. 1942. On the heart and arterial arches of salamandra maculosa laur. and ambystoma mexicanum shaw during metamorphosis. Arch Néerland Zool 6:161–254. [Google Scholar]
- Tomiyama Y, Yoshinaga K, Fujii S, Ochi N, Inoue M, Nishida M, Aziki K, Horie T, Katoh C, Tamaki N. 2015. Accurate quantitative measurements of brachial artery cross‐sectional vascular area and vascular volume elastic modulus using automated oscillometric measurements: Comparison with brachial artery ultrasound. Hypertens Res 38:478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzun O, Babaoglu K, Ayhan YI, Moselhi M, Rushworth F, Morris S, Beattie B, Wiener J, Lewis MJ. 2014. Diagnostic ultrasound features and outcome of restrictive foramen ovale in fetuses with structurally normal hearts. Pediatr Cardiol 35:943–952. [DOI] [PubMed] [Google Scholar]
- van den Hoff MJ, Kruithof BP, Moorman AF. 2004. Making more heart muscle. Bioessays 26:248–261. [DOI] [PubMed] [Google Scholar]
- van den Hoff MJ, Kruithof BP, Moorman AF, Markwald RR, Wessels A. 2001. Formation of myocardium after the initial development of the linear heart tube. Dev Biol 240:61–76. [DOI] [PubMed] [Google Scholar]
- Van Mierop LHS, Kutsche LM. 1985. Development of the ventricular septum of the heart. Heart Vessels 1:114–119. [DOI] [PubMed] [Google Scholar]
- Warren WC, Hillier LW, Marshall Graves JA, Birney E, Ponting CP, Grutzner F, Belov K, Miller W, Clarke L, Chinwalla AT, et al. 2008. Genome analysis of the platypus reveals unique signatures of evolution. Nature 453:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S, Kanani M, Anderson RH, Richardson MK, Brown NA. 2001. Development of the human pulmonary vein and its incorporation in the morphologically left atrium. Cardiol Young 11:632–642. [DOI] [PubMed] [Google Scholar]
- Wessels A. 2016. Inflow tract development In: Rickert‐Sperling S, Kelly RG, Driscoll DJ, editors. Congenital heart diseases: The broken heart: Clinical features, human genetics and molecular pathways. Vienna: Springer Vienna; p 55–62. [Google Scholar]
- Wessels A, Anderson RH, Markwald RR, Webb S, Brown NA, Viragh S, Moorman AF, Lamers WH. 2000. Atrial development in the human heart: An immunohistochemical study with emphasis on the role of mesenchymal tissues. Anat Rec 259:288–300. [DOI] [PubMed] [Google Scholar]
- Zhang G, Li C, Li Q, Li B, Larkin DM, Lee C, Storz JF, Antunes A, Greenwold MJ, Meredith RW, et al. 2014. Comparative genomics reveals insights into avian genome evolution and adaptation. Science 346:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]


