Abbreviations
- ADA‐SCID
severe combined immunodeficiency caused by adenosine deaminase deficiency
- ALT
alanine aminotransferase
- EMA
European Medicines Agency
- GT
gene therapy
- HCV
hepatitis C virus
- HSC
hematopoietic stem cells
- PEG‐ADA
polyethylene‐glycol‐conjugated bovine ADA
- SVR
sustained virological response
Patients with inborn error diseases can be candidates for autologous hematopoietic stem cells (HSC) gene therapies (GT) but may require negative viral screening, including hepatitis C virus (HCV), to allow HSC manipulation in Good Manufacturing Practices areas. In the case of HCV positivity, patients might be excluded from life‐saving treatments. As HCV antibodies could be negative in young infant immunodeficient patients due to their immature/impaired immune system, or positive due to maternal–fetal antibody transmission, the risk is usually also evaluated on the basis of the HCV‐RNA. HCV clearance can now be achieved through direct‐acting antivirals. The fixed‐dose combination of ledipasvir/sofosbuvir (Harvoni; Gilead Sciences, Foster City, CA) in children ages 12 to 17 years was approved in 2017 for HCV genotypes 1, 4, 5, and 6.1, 2 For younger patients, a phase II study (3‐17 years) with Harvoni is ongoing (ClinicalTrials.gov identifier: NCT02249182) and an anecdotal report of viral clearance has been published in a 4‐year‐old boy with HCV genotype 1b infection.3 Growing experience on direct‐acting antivirals in children, who are more often treatment naïvely and less likely to show hepatic cirrhosis, suggest that HCV can be eradicated in a short time, thus allowing potential eligibility to GT for subjects in need of urgent treatment.
Presentation of the Case
We report the experience in a male Egyptian infant affected by severe combined immunodeficiency caused by adenosine deaminase deficiency (ADA‐SCID) with HCV 4a genotype infection. The patient was not eligible for GT due to the infectious risk of bone marrow cells used as starting material for the manufacture of Strimvelis, based on the current EU Cell and Tissue Directive. Harvoni was administered off‐label to the patient and HCV clearance allowed the patient to be treated with autologous HSC‐GT with Strimvelis for the correction of his immunodeficiency.4, 5
The patient was born from consanguineous parents and delivered by way of caesarean section. His older brother died due to ADA‐SCID. Our patient was soon diagnosed with ADA‐SCID and started on polyethylene‐glycol‐conjugated bovine adenosine deaminase (PEG‐ADA). In the absence of a human leukocyte antigen–identical sibling donor, the patient was referred at 4 months of age to our institution for HSC‐GT.5 At screening (Table 1), we diagnosed HCV 4a genotype infection and suspended the GT treatment program.
Table 1.
Clinical Data and Significant Laboratory Findings at the Start of Harvoni
| Age (months) | 5 |
| Weight (kg) (percentile) | 6.5 (50th) |
| Length (cm) (percentile) | 58.7 (3rd) |
| White blood cell (×109/L) (normal value, 6.7‐14×109/L) | 3.8 |
| Lymphocytes (×109/L) (normal value, 3.9‐9.0) | 0.8 |
| CD3+ (cells/μL) (normal value, (2500‐5600) | 16 |
| CD3+CD4+ (cells/μL) (normal value, 1800‐4000) | 13 |
| CD3+CD8+ (cells/μL) (normal value, 590‐1600) | 0.4 |
| CD4+ naïve (cells/μL) (normal value, 1300‐3600) | 1.2 |
| ALT (IU/L) (normal value, 6‐59 IU/L) | 135 |
| AST (IU/L) (normal value, 5‐35 IU/L) | 85 |
| Total bilirubin (mg/dL) (normal value 0.1‐1.0) | 0.19 |
| Direct bilirubin (mg/dL) (normal value 0.01‐0.25) | 0.14 |
| GGT (IU/L) (normal value 11‐68) | 31 |
| HCV antibody | Negative* |
| Liver ultrasound | Normal |
The child was on immunoglobulin supplementation, given to the known impaired antibody production in ADA SCID patients.
Abbreviations: AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase.
Vertical transmission from the mother was ruled out, as testing resulted in HCV‐RNA and antibody being negative. Transfusions were never administered to the child and infection from substitutive immunoglobulins was considered highly unlikely. The patient’s contagion during caesarean section or blood withdrawal in his home country, where HCV genotype 4 is endemic, was postulated.
Because of the patient’s profound lymphopenia and reduced T‐cell functions, despite the ongoing enzyme replacement therapy with PEG‐ADA, spontaneous clearance of HCV was considered unlikely and the patient was judged at risk of poor control of HCV infection. Moreover, the risk/benefit of early eradication of the HCV infection was favorable because of the urgency of treatment with Strimvelis.4 Sofosbuvir/ledipasvir was started at 5 months of age at 100/22.5 mg (15/3.4 mg/kg/day) daily dose and continued for 12 weeks. The dose was extrapolated from that used in adults and children over 6 years of age1 and from treatment duration time for genotype 4a.2 Tablets were pulverized by San Raffaele Hospital pharmacy and the required daily dose was packed in single sachets. The powder was dissolved into milk and regularly administered by parents, after appropriate training. Approval of the local Ethical Committee and from the hospital management board for off‐label use and written informed consent from the parents were obtained prior to treatment.
A diffuse maculo‐papular skin rash appeared after 1 week of Harvoni, but did not contraindicate the continuation of therapy and resolved in 1 month. Rapid viral clearance was observed (Fig. 1). Following consultation with European Medicines Agency (EMA), a risk‐assessment procedure was formulated for the manipulation of HSC from a patient with previous HCV infection and shared with the Competent Authority for Cells and Tissues (Centro Nazionale Trapianti). Based on the evidence that sustained virological response at week 4 (SVR4) is 95% to 100% predictive of SVR24,1 the risk assessment was favorable, and the patient underwent treatment with Strimvelis (Fig. 1) after conditioning with a low dose of busulfan.4
Figure 1.
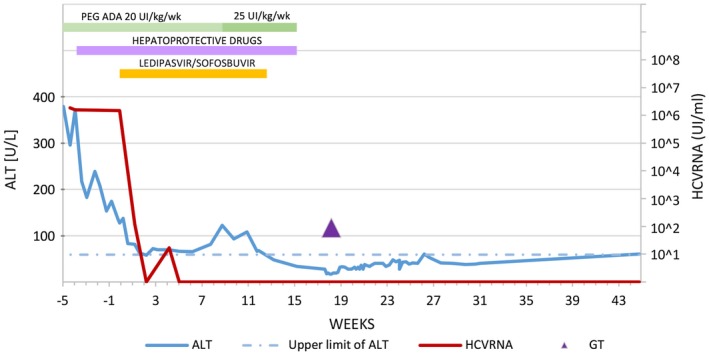
HCV‐RNA and alanine aminotransferase (ALT) during treatment with Harvoni. HCV‐RNA levels persisted high for 1 month before starting Harvoni. Plasmatic viral copies were 1.85 × 106 UI/mL at diagnosis and 1.5 × 106 UI/mL in more than one evaluation when Harvoni was started. After 2 weeks of treatment, the viral load resulted in being negative, but on the following plasma evaluation, the HCV‐RNA was 19 copies/mL, probably due to a slight initial fluctuation around the lower threshold of PCR method and returned persistently negative in all of the following evaluations. Although the family did not report episodes of vomiting or lack of compliance, we cannot exclude a transient issue of administration in the first weeks of treatments. From week 5 from the start of Harvoni onward, HCV‐RNA was undetectable, allowing its suspension at week 12. ALT values remarkably decreased during treatment. A transient ALT increase with persistent HCV‐RNA negativity was detected from weeks 3 to 11 from the start of Harvoni and ascribed to insufficient detoxification; it resolved after PEG‐ADA dose adjustment. Hepatoprotective drugs (silymarin 6 mg/kg/day and ursodeoxycholic acid 20 mg/kg/day, oral formulations) were administered from week 4, according to our local protocol for hepatic prophylaxis in the context of hematopoietic stem cell transplantation. This may be more relevant for ADA‐SCID patients, who are known to experience also hepatic toxic damage due to accumulation of adenosine metabolites.6 PEG‐ADA was stopped 3 weeks before GT, as foreseen in the treatment schedule. The post‐GT slight ALT increase may be ascribed to busulfan administration and the PEG‐ADA suspension carryover effect. The threshold of less than 12 IU/mL HCV‐RNA represents our rate of undetectable HCV‐RNA levels in real‐time PCR.
HCV‐RNA negative testing persisted during GT and throughout the follow‐up. Currently, the patient is +11 months from GT, free from PEG‐ADA, and shows multilinear engraftment of transduced HSC in the bone marrow, increasing lymphocyte count, and restored ADA expression.
Discussion
A once‐daily single dose regimen of 12‐week treatment duration with sofosbuvir/ledipasvir had an acceptable safety profile and was efficacious in the clearance of HCV infection in an ADA‐SCID patient allowing GT with Strimvelis. Moreover, in this patient, SVR4 was predictive of SVR12, SVR24, and SVR48.
The occurrence of this case triggered a change in the control of procedures for Strimvelis, to allow autologous bone marrow to be used for manufacture if the HCV results from the patient are confirmed as negative by nucleic acid testing.
Additional data on a cohort of HCV‐infected children younger than 3 years are needed to provide further evidence of the safety and efficacy of this treatment. Our results support the need for the development of specific recommendations regarding the use of Harvoni off‐label, while waiting for a label extension, in infants who need life‐saving treatment with advanced therapies.
Potential conflict of interest
Nothing to report.
Patient’s treatment with Sofosbuvir/Ledipasvir was supported by Fondazione Telethon. Patient’s treatment with Strimvelis under exceptional circumstances was supported by Fondazione Telethon and Regione Lombardia. GSK donated Strimvelis under exceptional circumstances.
References
Author names in bold designate shared co‐first authorship.
- 1. Balistreri WF, Murray KF, Rosenthal P, Bansal S, Chuan‐Hao L, Kersey K, et al. The safety and effectiveness of ledipasvir‐sofosbuvir in adolescents 12 to 17 years old with hepatitis C virus genotype 1 infection. Hepatology 2017;66:371‐378. [DOI] [PubMed] [Google Scholar]
- 2. Indolfi G, Hierro L, Dezsofi A, Jahnel J, Debray D, Hadzic N, et al. Treatment of chronic hepatitis C virus infection in children: a position paper by the Hepatology Committee of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;66:505‐515. [DOI] [PubMed] [Google Scholar]
- 3. Einsiedel HG, Christiansen H, Wiegand J. Eight weeks treatment with sofosbuvir/ledipasvir in a 4‐year old child with chronic hepatitis C virus genotype 1 infection. Pediatr Infect Dis J 2016;35:1373. [DOI] [PubMed] [Google Scholar]
- 4. Cicalese MP, Ferrua F, Castagnaro L, Pajno R, Barzaghi F, Giannelli S, et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood 2016;128:45‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrua F, Aiuti A. Twenty‐five years of gene therapy for ADA‐SCID: from bubble babies to an approved drug. Hum Gene Ther 2017;28:972‐981. [DOI] [PubMed] [Google Scholar]
- 6. Bollinger ME, Arredondo‐Vega FX, Santisteban I, Schwarz K, Hershfield MS, Lederman HM. Brief report: hepatic dysfunction as a complication of adenosine deaminase deficiency. N Engl J Med 1996;334:1367‐1371. [DOI] [PubMed] [Google Scholar]


