Abstract
Background
Women and their health care providers need a reliable answer to this important question: If a woman chooses to participate in regular mammography screening, then how much will this choice improve her chances of avoiding a death from breast cancer compared with women who choose not to participate?
Methods
To answer this question, we used comprehensive registries for population, screening history, breast cancer incidence, and disease‐specific death data in a defined population in Dalarna County, Sweden. The annual incidence of breast cancer was calculated along with the annual incidence of breast cancers that were fatal within 10 and within 11 to 20 years of diagnosis among women aged 40 to 69 years who either did or did not participate in mammography screening during a 39‐year period (1977‐2015). For an additional comparison, corresponding data are presented from 19 years of the prescreening period (1958‐1976). All patients received stage‐specific therapy according to the latest national guidelines, irrespective of the mode of detection.
Results
The benefit for women who chose to participate in an organized breast cancer screening program was a 60% lower risk of dying from breast cancer within 10 years after diagnosis (relative risk, 0.40; 95% confidence interval, 0.34‐0.48) and a 47% lower risk of dying from breast cancer within 20 years after diagnosis (relative risk, 0.53; 95% confidence interval, 0.44‐0.63) compared with the corresponding risks for nonparticipants.
Conclusions
Although all patients with breast cancer stand to benefit from advances in breast cancer therapy, the current results demonstrate that women who have participated in mammography screening obtain a significantly greater benefit from the therapy available at the time of diagnosis than do those who have not participated.
Keywords: breast cancer, fatality, mammography, mortality, screening
Short abstract
After 20 years of follow‐up, women who participate in mammography screening have a 47% lower risk of dying from breast cancer. Although all patients with breast cancer potentially can benefit from advances in breast cancer therapy, women who participate in mammography screening obtain a significantly greater benefit from the therapy available at the time of diagnosis than those who do not participate.
Introduction
Breast cancer mortality data include women who were diagnosed over the previous years, often over decades. Conversely, breast cancer incidence data include women who may die of breast cancer over the coming years, often over decades. Thus, incidence and mortality data for a given year are based on different cancer cases, requiring adjustments for changes in incidence and therapy over time. In the context of breast cancer screening and its effect on breast cancer mortality, the issue is that exposure or not to screening pertains to the time of diagnosis, whereas the endpoint, death from breast cancer, takes place years later.
To overcome this disparity, we have used a new methodology to improve the evaluation of the impact of organized mammography screening on death from breast cancer; we have calculated the annual incidence of breast cancers fatal within 10 years and within 11 to 20 years after breast cancer diagnosis. This novel indicator, the incidence of fatal breast cancer, provides a direct measure of the impact of earlier diagnosis through mammography screening upon women who participate in screening (screen‐detected and interval cancer cases combined) compared with women who do not participate in screening. Using the incidence of fatal cancer with long‐term follow‐up virtually eliminates length bias and greatly reduces the influence of lead‐time bias. Jonsson et al demonstrated that lead time is longest in cancers that do not prove fatal and is very short in fatal cancers.1, 2 A 20‐year follow‐up period can be expected to give a reliable indication of the eventual fate of most patients with breast cancer, because it has been demonstrated that approximately 95% of deaths from breast cancer occur within 20 years of diagnosis.3, 4, 5 We studied women aged 40 to 69 years and used their person‐years at the time of diagnosis as the denominator for breast cancers that were fatal at 10 and 20 years. The incidence of fatal breast cancer avoids the biases affecting survival analysis and case fatality calculations, for which the denominators are time to death and cancer cases, respectively. Overdiagnosis is not an issue when studying fatal cancers, because an overdiagnosed breast cancer, by definition, cannot ever be fatal.
Materials And Methods
The population we studied includes all women aged 40 to 69 years in the county of Dalarna, Sweden, during 39 years of the screening era (1977‐2015). In addition, we present the results from 19 years of the prescreening period (1958‐1976) for additional comparison with observations in the screening era. This enables both contemporaneous and historical comparisons of the rate of death from breast cancer among women before the onset of the screening programs (1958‐1976) and starting in 1977 among those who did and did not participate in mammography screening. Dalarna County had a stable population of women who were aged 40 to 69 years during the 6 decades of this study (mean, 52,438 women aged 40‐69 years).
The Swedish Cancer Registry was founded in 1958 and provides data about all women diagnosed with breast cancer. Having obtained permission from the Ethics Committee of Uppsala University, Sweden (Ups dnr 03‐671), data on all breast cancer cases diagnosed at ages 40 to 69 years in Dalarna County, Sweden, were extracted. The cause and date of death of each patient with breast cancer who died were collected from the National Death Registry of the Swedish National Board of Health and Welfare. Breast cancer deaths and all other causes of death are included in the reports from this registry using codes according to the International Classification of Diseases. Vital statistics on the mid‐year population of women aged 40 to 69 years residing in Dalarna County during each of the years from 1958 to 2015 were obtained from the National Population Registry to calculate the denominator of the corresponding annual incidence of fatal breast cancer. Follow‐up was complete through December 31, 2015.
The population‐based, organized mammography screening program began examining women in Dalarna County, Sweden, on October 3, 1977, first with a randomized trial, in which approximately two‐thirds of the population aged 40 to 69 years were regularly invited to screening,6 followed by the service screening program, in which all women aged 40 to 69 years were regularly invited from 1986 onward. In the randomized controlled trial (RCT), women aged 40 to 74 years were invited to attend single‐view mammography screening at intervals of 24 months for women aged 40 to 49 years and 33 months for those aged 50 to 74 years.3 In the service screening program, women aged 40 to 54 years are invited to mammography screening every 18 months, and those aged 55 to 69 years are invited to mammography screening every 24 months. The screening protocol is 2‐view mammography.7 These personal invitations to mammography screening were sent by authority of the County Council using data from the population registry. The dates of all screening mammography examinations for each participating woman in the county were and continue to be recorded in the archives of the Department of Mammography at the Falun Central Hospital. The scheduled date of the next screening examination was (and continues to be) allocated prospectively by the screening authority for each invited woman in the county regardless of whether or not she participated in the previous screening examination. The date of invitation to and participation in screening as well as the date of the next scheduled invitation were entered prospectively into the database and were used to determine the status of screening participation for all women aged 40 to 69 years. These dates also were used to determine the detection mode of each breast cancer case during the 39‐year period that corresponds to the screening era; in the 19‐year prescreening period, no women were invited to screening, nor was there any mammography equipment in the county.
The population that participates in screening, determined separately for each year, includes all women who participated in their most recent scheduled mammography screening examination; all others were classified for that year as not participating in screening. Women who were diagnosed with breast cancer through 2005 all had 10 years of follow‐up, and those who were diagnosed through 1995 all had 20 years of follow‐up using the calculation of person‐years mentioned above.
The detection mode of each breast cancer case was determined as follows: 1) breast cancers diagnosed in women who had participated in their most recent screening examination, including interval cancers, were classified as cancers in women participating in screening; and 2) breast cancers diagnosed in the prescreening era and in women who were invited but did not participate in their most recent scheduled screening examination were classified as breast cancers in women who had not participated in screening. The numbers of cases of breast cancer that were fatal within 10 years and within 11 to 20 years of diagnosis and the corresponding person‐years, stratified by screening participation in the screening period, were determined from the sources listed above.
The annual incidence (per 100,000 women aged 40‐69 years at diagnosis) of breast cancer and of breast cancer that was fatal within 10 years and within 11 to 20 years of diagnosis, respectively, were calculated for all women in the 19‐year prescreening period and separately for women who did or did not participate in screening during the 39‐year screening period. Five‐year moving averages were calculated for both the prescreening and screening periods, which involved truncating the data for nonparticipants and participants by 2 years at each end.
It could be argued that the relative risk (RR) estimates associated with screening are affected by self‐selection bias, because cancers diagnosed among nonparticipants after 1989 were among women who chose not to attend screening. Therefore, we adjusted the RR of the incidence of cancers that were fatal within 20 years of diagnosis for self‐selection bias using the method described by Duffy et al.8 Details are provided in the Supporting Materials.
The Swedish National Treatment Guidelines for breast cancer have stage‐specific protocols, which are independent of detection mode and must be adhered to closely by therapeutic teams.9, 10 Tailoring therapy to the advantage or disadvantage of any woman according to whether or not she attended screening would be contrary to accepted medical practice. Furthermore, over the 58‐year period of this study, all women residing in Dalarna County received treatment in the Falun Central Hospital.
Results
Figure 1 illustrates the population of women aged 40 to 69 years in Dalarna County according to nonparticipation and participation in mammography screening from 1958 to 2015. The mean number of women aged 40 to 69 years over the period of our study was 52,438. During the period from 1986 to 2015, when all women aged 40 to 69 years were regularly invited to an organized, population‐based screening program, the women who chose to undergo mammography screening comprised 85.1% (n = 1,351,708 of 1,588,784 women) of the invited population.
Figure 1.
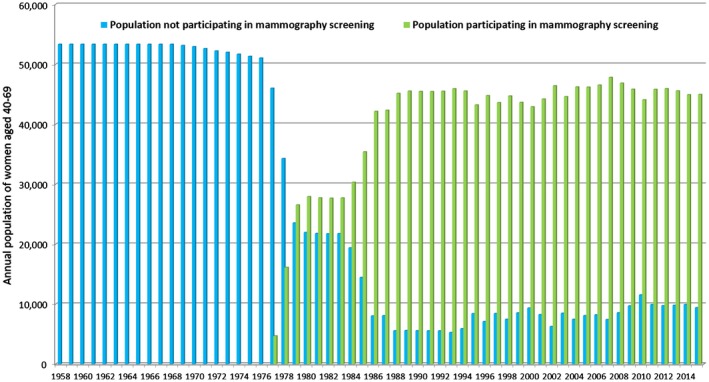
Annual population of women ages 40 to 69 years who did and did not participate in mammography screening (data from statistics of Dalarna County, Sweden, from 1958 to 2015).
During the 58‐year period of our study, 4513 women were diagnosed with breast cancer; of these, 3231 women (1225 in the prescreening period and 2006 in the screening period through 1995) could be followed for 20 years after diagnosis. Another 1281 women who were diagnosed with breast cancer in the years 1996 to 2005 could be followed for 10 years after diagnosis. Altogether, 1061 women died from breast cancer within 10 years of diagnosis (552 in the prescreening period and 509 in the screening period), and another 213 women (87 in the prescreening period and 126 in the screening period) died from breast cancer 11 to 20 years after diagnosis.
Tables 1 and 2 detail the incidence of breast cancer (invasive and in situ combined) and the incidence of breast cancer that proved fatal within 10, 11 to 20, and 20 years of diagnosis during the prescreening period and for both nonparticipating and participating women during the screening period. Table 1 includes all women with breast cancer who had a minimum of 10 years of follow‐up (detected through 2005), and Table 2 includes all women with breast cancer who had a minimum of 20 years of follow‐up (detected through 1995).
Table 1.
Incidence of Breast Cancer and Breast Cancer Fatal Within 10 Years of Diagnosis for All Breast Cancers Diagnosed Through 2005 in the Prescreening and Screening Eras According to Mammography Screening Participation Among Women With 10 Years of Follow‐Up
| Screening Participation | Years of Diagnosis | Incidence of Breast Cancer per 100,000 Women Aged 40 to 69 Years (No./Total No.) | Incidence of Breast Cancer Fatal Within 10 Years (No./Total No.) |
|---|---|---|---|
| No (Pre‐screening) | 1958‐1976 | 122.1 (1225/1,003,673) | 55.0 (552/1,003,673) |
| No (During screening) | 1977‐2005 | 197.7 (727/367,721) | 62.3 (229/367,721) |
| Yes (During screening) | 1977‐2005 | 229.2 (2561/1,117,483) | 25.1 (280/1,117,483) |
Table 2.
Incidence of Breast Cancer and Breast Cancer Fatal Within 10 Years, 11 to 20 Years, and 20 Years of Diagnosis for All Breast Cancers Diagnosed Through 1995 in the Prescreening and Screening Eras According to Mammography Screening Participation Among Women With 20 Years of Follow‐Up
| Incidence of Fatal Breast Cancer (No./Total No.) | |||||
|---|---|---|---|---|---|
| Screening Participation | Years of Diagnosis | Incidence of Breast Cancer per 100,000 Women Aged 40 to 69 Years (No./Total No.) | Within 10 Years | Within 11 to 20 Years | Within 20 Years |
| No (Pre‐screening) | 1958‐1976 | 122.1 (1225/1,003,673) | 55.0 (552/1,003,673) | 8.7 (87/1,003,673) | 63.7 (639/1,003,673) |
| No (During screening) | 1977‐1995 | 181.7 (524/288,329) | 65.9 (190/288,329) | 13.2 (38/288,329) | 79.1 (228/288,329) |
| Yes (During screening) | 1977‐1995 | 221.1 (1482/670,265) | 29.5 (198/670,265) | 12.1 (81/670,265) | 41.6 (279/670,265) |
The data in Figure 2A‐C are presented as 5‐year moving averages. Figure 2A illustrates the annual incidence per 100,000 women aged 40 to 69 years for all breast cancers (invasive and in situ) combined, according to their status of participation in screening. Breast cancer incidence gradually increased over the 6 decades, both among women who participated in screening and among those who did not. There was a slightly higher incidence of breast cancer (RR, 1.16; 95% confidence interval [CI], 1.07‐1.26) in participants versus nonparticipants during the screening period (Table 3). The overall breast cancer incidence was higher during the screening period both for participating women (88%; RR, 1.88; 95% CI, 1.75‐2.01) and for nonparticipating women (62%; RR, 1.62; 95% CI, 1.48‐1.78) compared with the prescreening period (Table 4).
Figure 2.
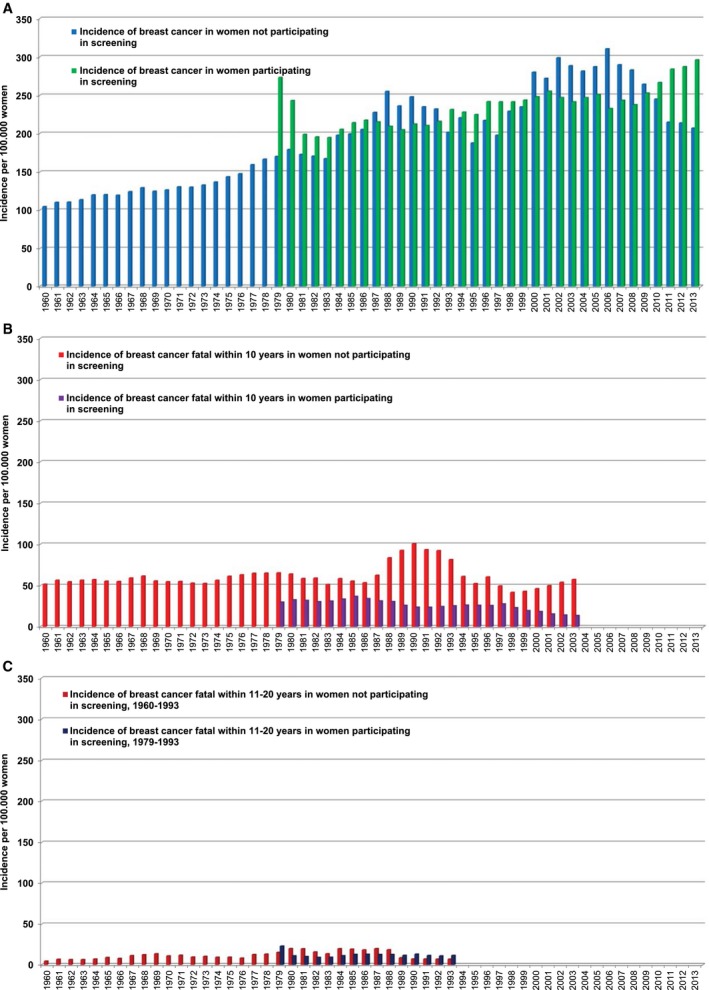
The incidence of (A) breast cancer and of (B) breast cancer fatal within 10 years and (C) breast cancer fatal within 11‐20 years among women who did and did not participate in mammography screening is illustrated. All values indicate 5‐year moving averages for women ages 40 to 69 years (data from statistics of Dalarna County, Sweden, from 1958 to 2015)
Table 3.
Relative Risk of Incidence (Participants/Nonparticipants) in the Screening Era for Breast Cancer and Breast Cancer Fatal Within 10 Years, 11 to 20 Years, and 20 Years of Diagnosis
| RR (95% CI) | RR (95% CI) | ||||
|---|---|---|---|---|---|
| Years of Diagnosis | Incidence of Breast Cancer per 100,000 Women Aged 40 to 69 Years | Incidence of Breast Cancer Fatal Within 10 Years | Years of Diagnosis | Incidence of Breast Cancer Fatal Within 11 to 20 Years | Incidence of Breast Cancer Fatal Within 20 Years |
| 1977‐2005 | 1.16 (1.07‐1.26) | 0.40 (0.34‐0.48) | 1977‐1995 | 0.92 (0.62‐1.35) | 0.53 (0.44‐0.63) |
Abbreviations: CI, confidence interval; RR, relative risk.
Table 4.
Relative Risk of Incidence in the Screening Era (1977‐2005) Compared With the Prescreening Era (1958‐1976) for Breast Cancer and for Breast Cancer Fatal Within 10 Years of Diagnosis Among Women Who Did and Did Not Participate in Screening During the Screening Era
| RR (95% CI) | ||
|---|---|---|
| Screening Participation | Incidence of Breast Cancer in Women Aged 40 to 69 Years | Incidence of Breast Cancer Fatal Within 10 Years in Women Aged 40 to 69 Years at Diagnosis |
| Yes | 1.88 (1.75‐2.01) | 0.46 (0.39‐0.53) |
| No | 1.62 (1.48‐1.78) | 1.13 (0.97‐1.32) |
Abbreviations: CI, confidence interval; RR, relative risk.
Figure 2B presents the annual incidence of breast cancers that were fatal within 10 years of diagnosis per 100,000 women, demonstrating the effect of participation in screening upon breast cancer deaths. Figure 2C presents the annual incidence of breast cancers that were fatal within 11 to 20 years of diagnosis per 100,000 women, demonstrating a similar incidence of breast cancer death among participants and nonparticipants alike.
The contemporaneous comparison in Table 3 indicates that the incidence of breast cancers that were fatal within 10 years of diagnosis per 100,000 women aged 40 to 69 years during the screening period was significantly lower (60%) among participants compared with nonparticipants (RR, 0.40; 95% CI, 0.34‐0.48). For cancers that were fatal within 20 years of diagnosis, the corresponding figure is a reduction of 47% (RR, 0.53; 95% CI, 0.44‐0.63) in the risk of dying from breast cancer among screening participants. After adjustment for potential self‐selection bias, there remained a significant 45% reduction in the risk of dying from breast cancer within 20 years of diagnosis in association with screening participation (RR, 0.55; 95% CI, 0.45‐0.67).
Table 4 lists incidence ratios in the screening era compared with the prescreening era for breast cancers that were fatal within 10 years of diagnosis among women who did and did not participate in screening. During the screening era, the incidence of breast cancers that were fatal within 10 years of diagnosis was significantly lower (54%) among women who participated in screening (RR, 0.46; 95% CI, 0.39‐0.53) than it was in the prescreening period, but it was slightly greater (13%) among those who did not participate in screening (RR, 1.13; 95% CI, 0.97‐1.32).
Discussion
The conventional judgment of the effectiveness of organized breast cancer screening still relies heavily on meta‐analyses of intention‐to‐treat estimates from RCTs to determine whether sending women invitations to participate in mammography screening actually reduces breast cancer mortality.11, 12 Although the demonstrated benefit of these invitations was the basis for shaping health care policy, summary estimates from meta‐analyses underestimate the benefit of attending mammography screening. In addition, variability among RCTs in the attendance rates and in the sensitivity of protocols further underestimates the benefit of screening.13 Every woman and her health care provider need to know how much her chance of avoiding a premature death from breast cancer will improve if she chooses to participate in regular screening.
Here, we report the annual incidence of breast cancers that became fatal within 10 or 20 years after breast cancer diagnosis. This novel method provides a direct measure of the beneficial impact of participating in mammography screening compared with not participating. Using the incidence rates of fatal cancers allows us to directly compare cancers that were diagnosed during the study period among women who did and did not participate in mammography screening. This method considerably reduces the risk of lead‐time bias given the long duration of follow‐up and also, for all practical purposes, eliminates the potential influence of length bias, because length bias cases cannot reduce the incidence of fatal cancers when the denominator is the population at risk rather than cancers (case fatality).
The use of this method requires prospectively collected, reliable data on the date of diagnosis, the detection mode of each individual cancer case, and the date of disease‐specific death over several decades of follow‐up. In addition, it is necessary to have comprehensive data from a stable, defined population, including the screening history on an individual basis. National death registries should be used to identify breast cancer deaths, including those in women who have moved to other parts of the country. These stringent requirements account for the infrequent use of this valuable methodology. A recent publication used the incidence rates of prostate cancer fatal within 10 years to clarify the role of the prostate‐specific antigen test in the significant decrease in mortality from prostate cancer.14 The authors of that report noted that using 10 years of follow‐up to determine whether the disease is fatal may not be long enough to eliminate lead‐time bias completely or to nullify calendar period shifts in lead time attributable to changes in diagnostic practice.14 When we also applied this novel method to breast cancer with incidence data on cancers that proved fatal within years 11 to 20 after diagnosis, we observed similar results for participants and nonparticipants alike (Fig. 2C). Thus, it appears that an evaluation of the incidence of fatal breast cancer during the first 10 years after diagnosis will provide a reliable measure of the benefit of regular participation in mammography screening.
The increased incidence of breast cancer overall was 16% higher in participants than in nonparticipants, suggesting an element of lead time. However, this is unlikely to affect our estimate of the effect on incidence of fatal cancers for 2 reasons. First, Jonsson et al demonstrated that, on average, lead time in fatal cancers is very short (approximately 0.29 years). This is because fatal cancers are either not screen‐detected (ie, have zero lead time) or, despite screen detection, the advance in time of diagnosis is too short to affect the ultimate outcome (lead time is present but short). Second, there has been ample time for the nonparticipant group to “catch up” in terms of lead time, because most deaths from breast cancer occur within 20 years of diagnosis. When comparing the incidence of cancers that were fatal within 20 years between participants versus nonparticipants, there remains a 47% reduction in the risk of dying from breast cancer in the women participating in screening and a 45% difference after adjustment for selection bias. This indicates that lead time has little impact on these results.
The incidence of cancer among nonparticipating women during the screening era was 62% greater than in the prescreening era, whereas the incidence of cancer that was fatal within 10 years was only 13% greater, indicating a benefit attributable to improved treatment and management of the disease. If there had been no such benefit, then it might be expected that the increase in the incidence of fatal breast cancers would be closer to the increase in the incidence of breast cancer. It is worth noting that we have used a more conservative estimate of 17% in adjusting for self‐selection, so our adjusted estimates may slightly underestimate the benefit of screening. However, the substantially lower incidence of cancers that were fatal at either 10 years (60 %) or 20 years (47%) in the participating women compared with the nonparticipating women within the screening period indicates that a woman’s decision to participate in screening resulted in a greater reduction in death from breast cancer compared with women who did not participate. This notable difference is attributable to earlier detection and treatment at an earlier phase in the natural history of the disease among women who participated in mammography screening.7, 15, 16 Despite advances in treatment, women who participated in mammography screening had the added advantage of earlier detection and received a far greater benefit from less aggressive therapy than women who did not participate.
This large and significant reduction in the incidence of breast cancers that proved fatal within 10 years in association with screening is consistent with other observational studies for which individual data on screening participation were available. For a review of such studies in Europe, see Broeders et al17 and the recent update of the International Agency for Research on Cancer (IARC) Handbook on Breast Cancer Screening.7 Substantial reductions in breast cancer mortality with screening have been reported in North America,18, 19 Asia,20 Australasia,21 and elsewhere.7
Because the majority of women in the population attend some (but not all) of their screening invitations, we have reclassified the individual women in the population each year according to whether or not they participated in their latest screening mammography examination. The situation is different for the women who are diagnosed with breast cancer, because the classification of each cancer case is made only once, according to whether the woman participated in her latest screening examination. Thus, we are measuring the effect that the screening mammography examination has had on whether the patient dies of breast cancer.
Evidence from multiple RCTs, meta‐analyses, and numerous observational studies indicates that significant breast cancer mortality reductions are achieved through organized mammography screening programs.7 Despite this, artificial controversies still persist regarding the value of mammography, largely driven by conclusions drawn from study designs that are poorly suited to estimate the benefit of screening.22, 23 Among the hierarchy of study designs, ecological studies are generally accorded a lower status compared with RCTs or studies that use individual data, such as case‐control and cohort studies.7
Among the observational designs, perhaps the weakest is the utilization of trend analysis using registry data, for which drawing conclusions is complicated by changes in breast cancer incidence over time, contamination from breast cancer deaths in the screening era from cases diagnosed in the prescreening era, insufficient follow‐up periods, improvements in treatment over time, and (most important) the absence of data on exposure to screening.24, 25 Neglecting the results from RCTs and incidence‐based mortality studies with data on exposure to screening,7, 17, 26 investigators using trend analyses have concluded that modern mammography does not reduce the incidence of advanced breast cancers and only succeeds in detecting insignificant or slowly growing cancers that are not life threatening while missing more aggressive tumors that prove fatal.27, 28, 29 Instead, they argue, it is therapeutic advances that are principally or entirely responsible for the observed reductions in breast cancer mortality.29, 30
More credible evidence indicates otherwise. We have demonstrated that the risk of breast cancer death in the mammography RCTs was strongly correlated with the risk of being diagnosed with an advanced breast cancer (ie, the greater the reduction in risk of being diagnosed with an advanced breast cancer, the greater the observed mortality reduction).16 Furthermore, although it has been claimed that mammography screening can identify only insignificant or slowly growing cancers that are not life threatening, while missing more aggressive tumors that prove fatal, it has been demonstrated that the reverse is true.31 An incidence‐based mortality analysis of tumors diagnosed within the screening phase of the Swedish 2‐County Trial revealed that more deaths were prevented from grade 3 tumors compared with those prevented from grade 1 and 2 tumors, and that the proportions of tumors measuring ≥15 mm and lymph node‐positive tumors were reduced substantially in those who had grade 3 tumors from the invited group compared with the control group. It has also been claimed, using the same weak study designs, that therapeutic advances must be mostly or entirely responsible for the observed reductions in breast cancer mortality in nations with established mammography screening programs,27, 30 although existing data have not substantiated this claim.22 Cancer registries usually do not have information on the mode of detection, and the relative impact of screening versus treatment in reducing breast cancer mortality cannot be evaluated without knowing the mode of detection (participation vs nonparticipation) in each individual case. Furthermore, the relative contribution of screening versus treatment will depend on the screening uptake rate, the sensitivity of the protocol, and access to state‐of‐the‐art treatment. Likewise, exercises in modeling based on estimation of the short‐term effect of screening using aggregate data, without being able to distinguish between individual women who are or are not screened, also cannot measure the true impact of screening.32, 33 To date, no advance in therapy has been able to overcome the prognostic disadvantage of being diagnosed with a lymph node‐positive tumor. Currently, reducing deaths from breast cancer depends on the advantages of early detection and state‐of‐the‐art therapy.
Although much attention has been devoted to the potential “harms” of participating in regular screening, little attention has been given to the harms of not participating in regular screening, the greatest harm being a significantly increased risk of death from breast cancer. In addition, women who choose not to participate in screening will experience a significantly higher rate of advanced breast cancers, a greater need for more extensive surgery, a greater risk of upper body impairments (including lymphedema), and more extensive radiotherapy and chemotherapy, for which many women experience significant, enduring adverse physical and cognitive effects. For each breast cancer death prevented by screening, a woman will be spared the terminal stages of this disease, and she will gain an average of 16.5 life‐years.16, 34, 35
Although some consider that advances in adjuvant therapy and chemotherapy mainly are responsible for improved breast cancer prognosis in screened populations in the modern era,28 we believe that it is time to focus attention on the combination of diagnosis and therapy, instead of viewing them as independent, or worse, as competing interests. Our results demonstrate that the benefit of therapy is significantly greater for women who have participated in mammography screening. Our results, from precise, individual‐based data covering 6 decades, should provide women and their physicians with reassurance that participating in regular, high‐quality mammography screening is the best way to reduce the risk of a premature death from breast cancer.
Funding Support
This work was supported by the American Cancer Society through a gift from the Longaberger Company's Horizon of Hope Campaign (Project NHPDCSGBR‐GBRLONG).
Conflict Of Interest Disclosures
Dr. Tabár reports personal fees from General Electric Healthcare for preparing automated breast ultrasound teaching cases, and honoraria and travel costs to General Electric Healthcare speaking engagements; personal fees from Mammography Education, Inc. in his capacity as president of the company, which organizes CME courses on breast imaging; and personal fees from Three Palm Software for consultation related to breast imaging interpretation, all outside the submitted work. The other authors made no disclosures.
Author Contributions
László Tabár: Conception and design; analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis); writing and or review of the article; administrative, technical, or material support (ie, reporting or organizing data, constructing databases); and study supervision. Peter B. Dean: Conception and design, analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis), writing and or review of the article, and study supervision. Tony Hsiu‐Hsi Chen: Conception and design, development of methodology and analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis), and writing and or review of the article. Amy Ming‐Fang Yen: Development of methodology, Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis) and writing and or review of the article. Sam Li‐Sheng Chen: Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis) and writing and or review of the article. Jean Ching‐Yuan Fann: Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis) and writing and or review of the article. Sherry Yueh‐Hsia Chiu: Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis); writing and or review of the article; and administrative, technical, or material support (ie, reporting or organizing data, constructing databases). May Mei‐Sheng Ku: Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis) and writing and or review of the article. Wendy Yi‐Ying Wu: Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis); writing and or review of the article; and administrative, technical, or material support (ie, reporting or organizing data, constructing databases). Chen‐Yang Hsu: Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis) and writing and or review of the article. Yu‐Ching Chen: Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis) and writing and or review of the article. Kerri Beckmann: Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis) and writing and or review of the article. Robert A. Smith: Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis); writing and or review of the article; and administrative, technical, or material support (ie, reporting or organizing data, constructing databases). Stephen W. Duffy: Development of methodology, analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis), and writing and or review of the article.
Supporting information
References
- 1. Jonsson H, Nystrom L, Tornberg S, Lenner P. Service screening with mammography of women aged 50–69 years in Sweden: effects on mortality from breast cancer. J Med Screen. 2001;8:152‐160. [DOI] [PubMed] [Google Scholar]
- 2. Jonsson H, Bordas P, Wallin H, Nystrom L, Lenner P. Service screening with mammography in northern Sweden: effects on breast cancer mortality—an update. J Med Screen. 2007;14:87‐93. [DOI] [PubMed] [Google Scholar]
- 3. Tabar L, Vitak B, Chen TH, et al. Swedish 2‐County Trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011;260:658‐663. [DOI] [PubMed] [Google Scholar]
- 4. Toikkanen SP, Kujari HP, Joensuu H. Factors predicting late mortality from breast cancer. Eur J Cancer. 1991;27:586‐591. [DOI] [PubMed] [Google Scholar]
- 5. Joensuu H, Pylkkanen L, Toikkanen S. Late mortality from pT1N0M0 breast carcinoma. Cancer. 1999;85:2183‐2189. [DOI] [PubMed] [Google Scholar]
- 6. Tabar L, Fagerberg CJ, Gad A, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;1:829‐832. [DOI] [PubMed] [Google Scholar]
- 7. IARC Working Group on the Evaluation of Cancer‐Preventive Strategies . Breast Cancer Screening. Vol 15 Lyon, France: IARC Press; 2016. [Google Scholar]
- 8. Duffy SW, Cuzick J, Tabar L, et al. Correcting for non‐compliance bias in case‐control studies to evaluate cancer screening programs. Appl Stat. 2002;51:234‐243. [Google Scholar]
- 9. Socialstyrelsen (National Board of Health and Welfare) . Nationella Riktlinjer for Brost‐, Kolorektal‐ och Prostatacancervard 2007. Stockholm, Sweden: Ansvarigt Regionalt Cancercentrum; 2007. [Google Scholar]
- 10. Brostcancer Nationellt Vardprogram . Brostcancer Nationellt Vardprogram: Landstingens och Regionernas Nationella Samverkansgrupp Inom Cancervarden. Stockholm, Sweden: Ansvarigt Regionalt Cancercentrum; 2014. [Google Scholar]
- 11. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta‐analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244‐255. [DOI] [PubMed] [Google Scholar]
- 12. Independent UK Panel on Breast Cancer Screening . The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778‐1786. [DOI] [PubMed] [Google Scholar]
- 13. Chen TH, Yen AM, Fann JC, et al. Clarifying the debate on population‐based screening for breast cancer with mammography: a systematic review of randomized controlled trials on mammography with Bayesian meta‐analysis and causal model [serial online]. Medicine (Baltimore). 2017;96:e5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly SP, Rosenberg PS, Anderson WF, et al. Trends in the incidence of fatal prostate cancer in the United States by race. Eur Urol. 2017;71:195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42:793‐806, v. [DOI] [PubMed] [Google Scholar]
- 16. Tabar L, Yen AM, Wu WY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13‐20. [DOI] [PubMed] [Google Scholar]
- 17. Broeders M, Moss S, Nystrom L, et al. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14‐25. [DOI] [PubMed] [Google Scholar]
- 18. Pocobelli G, Weiss NS. Breast cancer mortality in relation to receipt of screening mammography: a case‐control study in Saskatchewan, Canada. Cancer Causes Control. 2015;26:231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coldman A, Phillips N, Wilson C, et al. Pan‐Canadian study of mammography screening and mortality from breast cancer [serial online]. J Natl Cancer Inst. 2014;106:dju261. [DOI] [PubMed] [Google Scholar]
- 20. Yen AM, Tsau HS, Fann JC, et al. Population‐based breast cancer screening with risk‐based and universal mammography screening compared with clinical breast examination: a propensity score analysis of 1429890 Taiwanese women. JAMA Oncol. 2016;2:915‐921. [DOI] [PubMed] [Google Scholar]
- 21. Morrell S, Taylor R, Roder D, Robson B, Gregory M, Craig K. Mammography service screening and breast cancer mortality in New Zealand: a national cohort study 1999–2011. Br J Cancer. 2017;116:828‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duffy SW, Chen TH, Smith RA, Yen AM, Tabar L. Real and artificial controversies in breast cancer screening: a perspective article. Breast Cancer Manage. 2013;2:519‐528. [Google Scholar]
- 23. Kopans DB. The breast cancer screening “arcade” and the “whack‐a‐mole” efforts to reduce access to screening. Semin Ultrasound CT MR. 2018;39:2‐15. [DOI] [PubMed] [Google Scholar]
- 24. Moss SM, Nystrom L, Jonsson H, et al. The impact of mammographic screening on breast cancer mortality in Europe: a review of trend studies. J Med Screen. 2012;19(suppl 1):26‐32. [DOI] [PubMed] [Google Scholar]
- 25. Nystrom L. The Swedish randomised controlled trial on mammography screening has been properly designed, conducted and analysed. J R Soc Med. 2015;108:429‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Njor S, Nystrom L, Moss S, et al. Breast cancer mortality in mammographic screening in Europe: a review of incidence‐based mortality studies. J Med Screen. 2012;19(suppl 1):33‐41. [DOI] [PubMed] [Google Scholar]
- 27. Welch HG. Screening mammography—a long run for a short slide? N Engl J Med. 2010;363:1276‐1278. [DOI] [PubMed] [Google Scholar]
- 28. Autier P, Boniol M, Gavin A, Vatten LJ. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database [serial online]. BMJ. 2011;343:d4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast‐cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375:1438‐1447. [DOI] [PubMed] [Google Scholar]
- 30. Autier P, Boniol M. Mammography screening: a major issue in medicine. Eur J Cancer. 2018;90:34‐62. [DOI] [PubMed] [Google Scholar]
- 31. Tabar L, Chen TH, Yen AM, et al. effect of mammography screening on mortality by histological grade. Cancer Epidemiol Biomarkers Prev. 2018;27:154‐157. [DOI] [PubMed] [Google Scholar]
- 32. Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000–2012. JAMA. 2018;319:154‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen HH. Contributions of screening and treatment to mortality from breast cancer. JAMA. 2018;319:2335‐2336. [DOI] [PubMed] [Google Scholar]
- 34. Malmgren JA, Parikh J, Atwood MK, Kaplan HG. Impact of mammography detection on the course of breast cancer in women aged 40–49 years. Radiology. 2012;262:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Health Council of the Netherlands . Population Screening for Breast Cancer: Expectations and Developments. The Hague, The Netherlands: Health Council of the Netherlands; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


