Abstract
Combination immune checkpoint blockade with concurrent administration of the anti-ctla4 antibody ipilimumab and the anti–PD-1 antibody nivolumab has demonstrated impressive responses in patients with advanced melanoma and other diseases. That combination has also been associated with increased toxicity, including rare immune-related adverse events. Here we describe a case of fatal steroid-refractory myocarditis and panmyositis associated with the use of this combination in a patient with metastatic melanoma. Correlative studies indicated increased levels of serum interleukin 6 in this patient at the onset of toxicity, suggesting a possible role for anti–interleukin 6 receptor antibodies in the treatment of subsequent cases of this rare, but fatal, toxicity.
Keywords: Melanoma, checkpoint blockade, myocarditis
INTRODUCTION
Compared with either agent alone, combined administration of the anti-ctla4 antibody ipilimumab (ipi) and the anti–PD-1 antibody nivolumab (nivo) has been associated with improved response rates in patients with advanced melanoma (reviewed in Carlino and Long1). However, the increased efficacy of the combination is accompanied by increased toxicity. Approximately 55% of patients treated with combined ipi–nivo in clinical trials experience grade 3 or 4 toxicities2. Most are immune-related adverse events (iraes).
The well-characterized iraes experienced by treated patients include colitis, hepatitis, thyroiditis, and various cutaneous reactions. As more patients are treated with the ipi–nivo regime, additional rare iraes are being encountered. Recently, 2 cases of lethal myocarditis with associated myositis were reported after a single dose of combination ipi–nivo3. Here, we describe an additional case of fulminant and fatal combined myocarditis and myositis after a single dose of ipi–nivo in a patient with metastatic melanoma.
CASE DESCRIPTION
The patient was a 67-year-old man with BRAF wild-type metastatic melanoma. This patient’s past history was significant for hypercholesterolemia and hypertension, both of which were controlled. He had initially presented with stage iiic melanoma 1 year earlier and had been treated with resection and adjuvant radiation to the left axilla. At 9 months after his initial surgery, a left axillary nodal recurrence was re-treated with resection. Two months later, he developed left anterior chest wall subcutaneous lesions, confirmed to be melanoma, and new pulmonary nodules suspicious for metastases. He was then started on combined ipi–nivo.
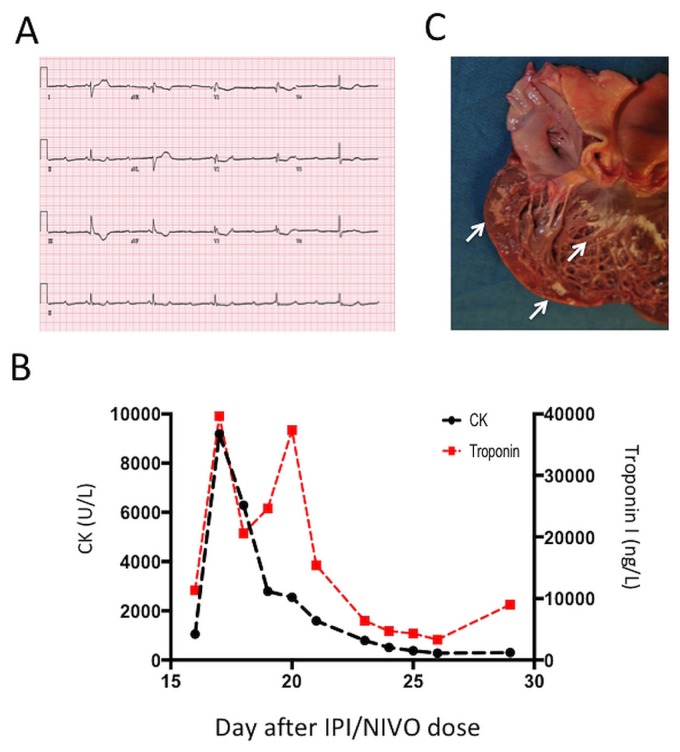
At 16 days after his first dose of ipi–nivo, the patient presented to the emergency room with a 3-day history of increasing fatigue, weakness, and dyspnea. On presentation, he also complained of feeling pre-syncopal. In the emergency room, he was bradycardic (heart rate: 32 bpm), and an electrocardiogram demonstrated 3rd-degree block [Figure 1(A)]. Troponin i and creatinine kinase were markedly elevated [Figure 1(B)]. An echocardiogram demonstrated normal biventricular function, and no significant arterial occlusion was observed on coronary angiography. After cardiac catheterization, myocarditis was diagnosed, and high-dose methylprednisone was started (200 mg on day 1, then 1000 mg daily for 3 days).
FIGURE 1.
Evidence of cardiac pathology. (A) Electrocardiogram at time of the patient’s presentation to the emergency room. (B) Serial serum creatine kinase (CK) and troponin I values during the patient’s admission to hospital. “Days” are relative to the first dose of combination ipilimumab (IPI) and nivolumab (NIVO). (C) The opened left ventricle of the heart, with regions of endocardial and myocardial pallor (arrows).
The patient appeared to stabilize with steroid treatment. However, 3 days later, he developed increased respiratory distress and hypotension progressing to hypercapnic respiratory failure requiring inotropes and mechanical ventilation. He was given 1 dose of infliximab (5 mg/kg) and 2 doses of intravenous immunoglobulin. His condition worsened, requiring dialysis for acute kidney injury. He remained intubated and on dialysis for 7 more days. A lack of diaphragmatic activity was then confirmed. On day 18 of his admission, he became febrile and hypotensive and expired within 24 hours.
At autopsy, the cause of death was found to be multiple organ failure in the context of rhabdomyositis and myocarditis. Features of acute biventricular heart failure, including dilatation of all chambers, pulmonary edema, pleural effusions, and severe congestive hepatopathy were also noted. Grossly, the myocardium demonstrated patchy areas of pallor in the left ventricular wall, not corresponding to any vascular territory [Figure 1(C)]. Histology revealed numerous areas of biventricular cardiomyolysis.
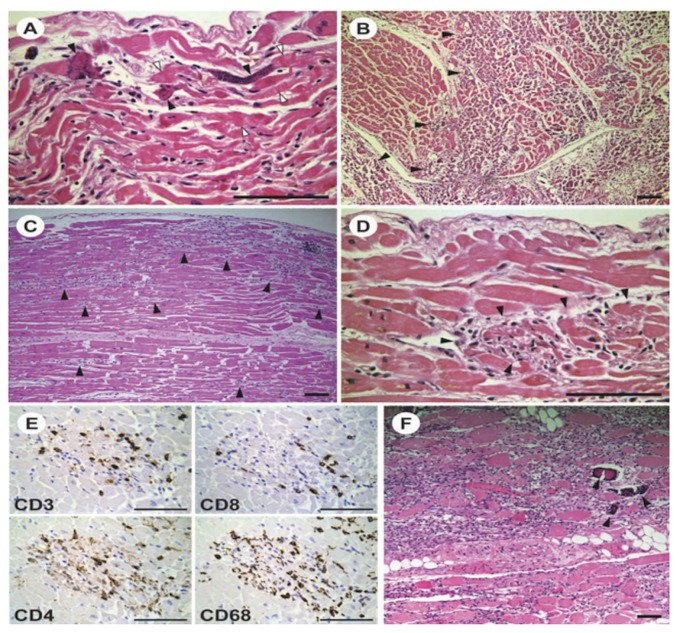
Two histologic patterns were observed. The predominant pattern in the left ventricle was myocyte calcification with a minimal inflammatory response [Figure 2(A,B)]. The second pattern, present mainly in the right ventricle, was myocyte lysis [Figure 2(C,D)] with associated inflammatory and healing response. Immunostaining revealed accumulation of CD68+ myeloid cells and CD4+ and CD8+ T lymphocytes in the areas of injury [Figure 2(E)]. Staining of the myocardium for CD20 was negative (data not shown). A similar mononuclear leucocyte infiltrate was observed in skeletal muscle, including the biceps, thigh, and calf muscles and the diaphragm. Again, those cells were predominantly CD4+ and CD8+ T cells and CD68+ myeloid cells. The immune infiltrates were associated with rhabdomyolysis, muscle fibre necrosis, and focal calcification that was particularly severe in the diaphragm [Figure 2(F)]. The severe diaphragmatic involvement was felt to have caused the patient’s respiratory failure.
FIGURE 2.
Histologic evidence of autoimmune pathology. (A–D) Representative images of histology sections of the left ventricular myocardium (hematoxylin and eosin stain). (A) Area of wavy myocyte fibres, interstitial edema, and contraction band necrosis (white arrowheads), with some fibres showing calcification (black arrowheads). (B) Area of extensive myocyte calcification (arrowheads) without an inflammatory response. (C,D) Intermediate- and high-magnification images of regions showing patchy myocytolysis with an associated mononuclear leucocyte infiltrate (arrowheads). (E) Immunoperoxidase staining of serial sections showing a region with focal myocytolysis and inflammation similar to the image in panel D. Occasional CD8-positive T cells are located primarily at the periphery; CD4-positive cells and abundant CD68-positive myeloid cells are seen throughout the lesion. (F) Representative image of the diaphragm, showing intense rhabdomyolysis, with robust reactive inflammatory mononuclear leucocyte infiltrate and focal calcification (arrowheads). Magnification bars in all panels represent 100 μm.
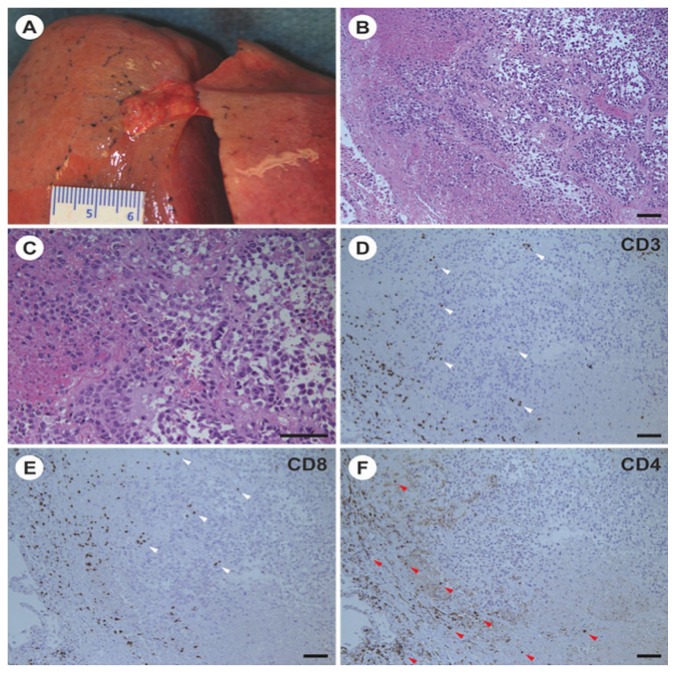
Post-mortem examination of the lung revealed 2 remaining foci of melanoma [Figure 3(A)]. Histology demonstrated extensive tumour necrosis, with small remaining nodules of viable tumour surrounded by a brisk mononuclear infiltrate of CD4+ and CD8+ T lymphocytes and CD68+ myeloid cells [Figure 3(B–F)]. Interestingly, melanoma was not identified in skin biopsies from the chest and back. A site of previously biopsy-proven melanoma overlying the left clavicle showed no residual disease. Thus, it was apparent from the post-mortem examination that the patient was simultaneously experiencing both a dramatic reduction in tumour burden in response to ipi–nivo and extensive autoimmune pathology.
FIGURE 3.
Pathology evidence of antitumour response. (A) Small subpleural nodule of residual tumour in the left upper lobe of the lung. Similar nodules were found in the right upper and lower lobes. (B,C) Representative images of histology sections of the tumour nodule in the left upper lobe showing morphologic features of an epithelioid cell type melanoma (hematoxylin and eosin stain). The morphology of the tumour was similar in a premortem biopsy, when the tumour immunophenotype was determined. (D–F) Immunoperoxidase staining of serial sections of the left upper lobe tumour. T Cells positive for CD8 are located at the periphery and within the tumour nodule (white arrowheads); CD4-positive T cells are found at the periphery (red arrowheads). Magnification bars in all panels represent 100 μm.
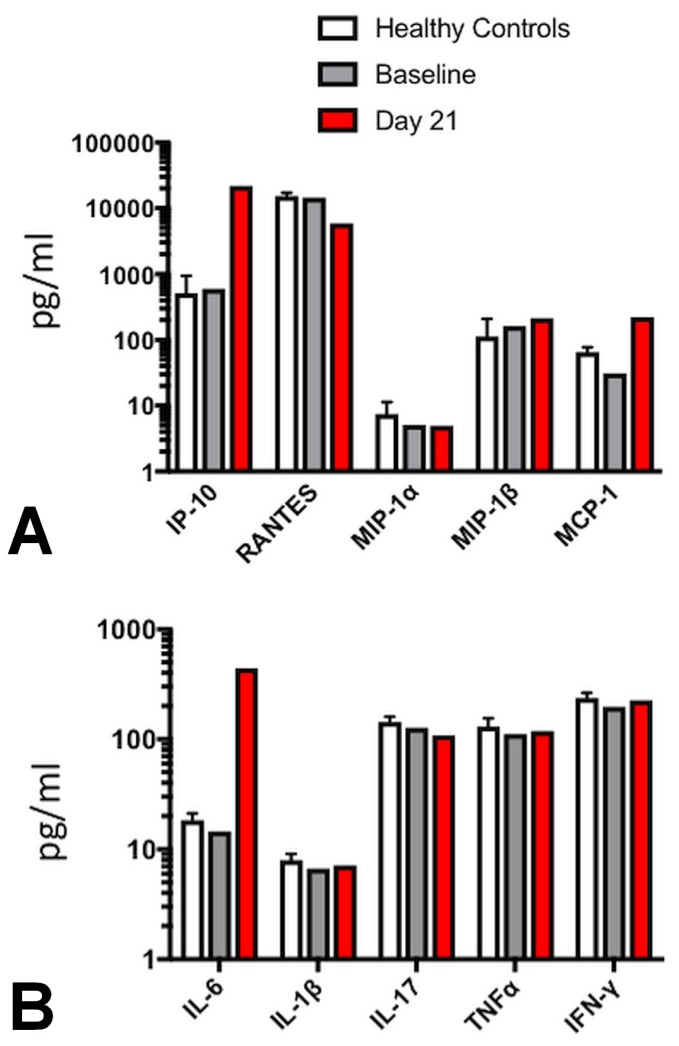
Serum chemokine and cytokine levels were assessed before treatment with ipi–nivo and 21 days after the first dose. Consistent with previous reports4, treatment with ipi–nivo resulted in a marked increase in serum levels of the chemokine interferon-γ–inducible protein 10 kDa (also called cxcl10) [Figure 4(A)]. Of the cytokines tested, interleukin 6 (il-6) was observed to be markedly elevated after treatment, but no changes in serum levels of other inflammatory cytokines such as interleukin 1β, tumour necrosis α, or interferon γ were detected [Figure 4(B)].
FIGURE 4.
Evidence of elevated serum CXCL10 (interferon γ–induced protein 10) and interleukin 6. (A) Chemokine levels from the patient’s serum the day before treatment (baseline) and 21 days after the first dose of combined ipilimumab and nivolumab. Average level from healthy control patients (n = 10) are shown for reference. MIP, RANTES = macrophage inflammatory proteins; MCP = monocyte chemoattractant protein. (B) Cytokine levels from the patient’s serum the day before treatment (baseline) and 21 days after the first dose of combined ipilimumab and nivolumab. Average levels from healthy control patients (n = 10) are shown for reference. IL = interleukin; TNF = tumour necrosis factor; IFN = interferon. Serum concentrations of chemokines and cytokines were measured using the Bio-Plex Pro Human Cytokine 27-plex immunoassay (Bio-Rad Laboratories, Hercules, CA, U.S.A.) per the manufacturer’s instructions. Serum samples obtained from 10 healthy donors were also tested to establish a normal range for each cytokine. Samples were run and analyzed using the Bio-Plex Magpix Multiplex Reader (Bio-Rad Laboratories).
DISCUSSION
We report a case of fulminant fatal myocarditis and myositis in a patient with metastatic melanoma treated with a single dose of combination ipi–nivo, at 3 mg/kg and 1 mg/kg respectively. At 16 days after treatment with the first ipi–nivo infusion, the patient presented with high-degree heart block and biochemical evidence of significant myocarditis and myositis. Despite treatment with high-dose intravenous steroids, an anti–tumour necrosis α agent, and intravenous immunoglobulin, the patient deteriorated progressively and succumbed to multiple organ failure.
This case is strikingly similar to two other recently described cases3, which together illustrate the presentation of a highly lethal irae with combination ipi–nivo. Thankfully, this irae appears to be extremely rare. Upon reviewing the Bristol–Myers Squibb corporate safety database, Johnson et al.3 found that, as of April 2016, myocarditis was reported in only 8 of 2974 patients treated with ipi–nivo (0.27%). That observation agrees with a data review from published phase iii studies of patients treated with either combined or single-agent checkpoint blockade, which found the total incidence of all adverse cardiovascular events to be only 0.19%5. Despite the rarity of myocarditis as an irae, 5 of the 8 reported cases of in the Bristol–Myers Squibb database were fatal, including all cases with concomitant myositis. Another case of fatal myocarditis was recently reported in a patient receiving PD-1 blockade after previous ipi–nivo6. No current biomarker is predictive for this rare toxicity, and no effective therapies to prevent its lethality have been found.
Increased understanding of the immunologic mechanisms underlying this fulminant myocarditis and myositis might identify both predictive biomarkers and novel therapeutic approaches. In agreement with the findings of Johnson et al.3, examination of myocardium and skeletal muscle revealed CD3+ mononuclear cell infiltration into areas associated with cardiomyolysis and rhabdomyolysis, suggesting a T cell–mediated immunopathology (Figure 2). We also found brisk T cell infiltration into the tumour and a striking response to therapy (Figure 3). Interestingly, increased serum levels of cxcl10, a chemokine known to recruit activated T cells both to sites of active inflammation and to the tumour microenvironment7, were also observed. It is tempting to speculate that cxcl10 plays a role in driving both the observed autoimmunity and the antitumour response. However, the utility of cxcl10 as a potential biomarker or therapeutic target seems limited. Elevated serum cxcl10 has previously been described in patients treated with combination ipi–nivo who did not develop severe autoimmunity4. Thus, although cxcl10 might have contributed to the autoimmune pathology in our patient, its elevation is not specific to patients who develop this rare irae.
Serum levels of the inflammatory cytokine il-6 were also increased in our patient. The role of il-6 in mediating the response to combination ipi–nivo has not been defined. In small patient cohorts treated with ipi alone, elevated serum il-6 was associated with reduced response rates and lower overall survival8,9. With respect to PD-1 blockade, elevated serum il-6 during treatment has been linked to cutaneous toxicity10. There is also a case report of concurrent administration of pembrolizumab (an anti–PD-1 antibody) and tocilizumab [an anti–il-6 receptor (il-6r) antibody] in a patient with metastatic melanoma and refractory Crohn disease11. A durable antitumour response and relative quiescence of the Crohn disease were observed. Further investigation into the role of il-6 signalling in driving autoimmunity in patients who develop ipi–nivo–induced myocarditis and myositis is clearly required. Additionally, we propose treating other patients that present with this rare irae with tocilizumab or other anti–il-6/il-6r agents. Based on the observation of elevated serum il-6 in cytokine release syndrome, tocilizumab has been successfully used to treat the immune-mediated cytokine release syndrome associated with the use of chimeric antigen receptor T cells to treat hematologic malignancy12.
SUMMARY
Our patient, and the 2 patients reported by Johnson et al.3, succumbed despite aggressive treatment with steroids and anti–tumour necrosis α agents. Clearly, additional therapies are required to prevent mortality from this rare, but lethal, autoimmune toxicity. We propose prompt treatment with tocilizumab in addition to corticosteroids for patients presenting with fulminant myocarditis and rhabdomyositis associated with ipi–nivo.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: MOB receives clinical trial support from Merck and Takara Bio, and participates on advisory boards for Merck, Bristol–Meyers Squibb, Novartis, GlaxoSmithKline, Immunocore, Immunovaccine, and emd Serono. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Carlino MS, Long GV. Ipilimumab combined with nivolumab: a standard of care for the treatment of advanced melanoma? Clin Cancer Res. 2016;22:3992–8. doi: 10.1158/1078-0432.CCR-15-2944. [DOI] [PubMed] [Google Scholar]
- 2.Sznol M, Ferrucci PF, Hogg D, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol. 2017;35:3815–22. doi: 10.1200/JCO.2016.72.1167. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–55. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das R, Verma R, Sznol M, et al. Combination therapy with anti–ctla-4 and anti–PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950–9. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ederhy S, Voisin AL, Champiat S. Myocarditis with immune checkpoint blockade. N Engl J Med. 2017;376:290–1. doi: 10.1056/NEJMc1615251. [DOI] [PubMed] [Google Scholar]
- 6.Tajmir-Riahi A, Bergmann T, Schmid M, Agaimy A, Schuler G, Heinzerling L. Life-threatening autoimmune cardiomyopathy reproducibly induced in a patient by checkpoint inhibitor therapy. J Immunother. 2018;41:35–8. doi: 10.1097/CJI.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Guo S, Stiles JK. The emerging role of cxcl10 in cancer (review) Oncol Lett. 2011;2:583–9. doi: 10.3892/ol.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjoern J, Juul Nitschke N, Zeeberg Iversen T, Schmidt H, Fode K, Svane IM. Immunological correlates of treatment and response in stage iv malignant melanoma patients treated with ipilimumab. Oncoimmunology. 2016;5:e1100788. doi: 10.1080/2162402X.2015.1100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damuzzo V, Solito S, Pinton L, et al. Clinical implication of tumor-associated and immunological parameters in melanoma patients treated with ipilimumab. Oncoimmunology. 2016;5:e1249559. doi: 10.1080/2162402X.2016.1249559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka R, Okiyama N, Okune M, et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor–alpha is a biomarker of nivolumab reactivity. J Dermatol Sci. 2017;86:71–3. doi: 10.1016/j.jdermsci.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Uemura M, Trinh VA, Haymaker C, et al. Selective inhibition of autoimmune exacerbation while preserving the antitumour clinical benefit using il-6 blockade in a patient with advanced melanoma and Crohn’s disease: a case report. J Hematol Oncol. 2016;9:81. doi: 10.1186/s13045-016-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]