Abstract
Background
Cervical cancer is the 2nd most common malignant tumour in women worldwide. Previous research studies have given little attention to its prognostic factors in the rapidly growing Asian American population. In the present study, we explored prognostic factors in Asian and white American patients with cervical cancer, considering competing risks.
Methods
The study included 58,780 patients with cervical cancer, of whom 54,827 were white and 3953 were Asian American, and for all of whom complete clinical information was available in the U.S. Surveillance, Epidemiology, and End Results database. Death from cervical cancer was considered to be the event of interest, and deaths from other causes were defined as competing risks. The cumulative incidence function and the Fine–Gray method were applied for univariate and multivariate analysis respectively.
Results
We found that, for all patients (white and Asian American combined), the cumulative incidence function was associated with several factors, such as age at diagnosis, figo (Fédération internationale de Gynécologie et d’Obstétrique) stage, registry area, and lymph node metastasis. Similar results were found when considering white patients only. However, for Asian American patients, registry area was not associated with the cumulative incidence function, but the other factors (for example, figo stage) remained statistically significant. Similarly, in multivariate analyses, we found that age at diagnosis, figo stage, lymph node metastasis, tumour histology, treatment method, and race were all associated with prognosis.
Conclusions
Survival status differs for white and Asian American patients with cervical cancer. Our results could guide the treatment of, and facilitate prognostic judgments about, white and Asian American patients with cervical cancer.
Keywords: Cervical neoplasms, prognosis, cause of death, competing risks
INTRODUCTION
Cervical cancer is the 2nd most common malignant tumour in women worldwide and ranks 3rd among the causes of mortality in women1. There are 500,000 new cases of cervical cancer and 290,000 deaths from the disease worldwide each year, posing a serious threat to the health of women1. Epidemiology studies have determined that the incidence of and mortality from cervical cancer differ significantly by region and race2,3. In the United States, which has the largest population of the developed countries, approximately 4000 women die of cervical cancer annually. For example, in 2011, 4290 women died of cervical cancer, and 12,710 women were diagnosed. The age-adjusted incidence of cervical cancer was 8.1 per 100,000 women, which is lower than the incidence in Latina and African American women (11.1 per 100,000 and 10.0 per 100,000 respectively). The incidence rate for Vietnamese American women was almost 5 times that for white American women, and the incidence rates of cervical cancer in African American, Latina, white, and Asian American women were 4.4, 3.1, 2.2, and 2.1 per 100,000 respectively3. Previous studies showed that factors such as race, age at diagnosis, figo (Fédération internationale de Gynécologie et d’Obstétrique) stage, tumour diameter, pathology type, lymph node metastasis, tumour cell differentiation, new adjuvant chemotherapy, surgery, radiation therapy, parametrial involvement, and vascular involvement affect patient survival and prognosis4–8. However, studies of cervical cancer have focused mostly on white, African American, or general populations of women, with little attention given to prognostic factors in the rapidly growing Asian American population. Asian American individuals account for a small proportion of the total American population (approximately 4%), but the growth rate in that population in 2013 was 4 times the average growth rate (2.9% vs. 0.73%). Moreover, when considering the survival status of patients with cervical cancer, study subjects might survive, be lost to follow-up, or die from cervical cancer or another cause (for example, heart disease, other cancers, circulatory system disease, respiratory disease, or an unknown disease). However, in most studies considering the survival status of patients with cervical cancer, only a single endpoint (death from cervical cancer) or a combination of endpoints (several different causes of mortality) was considered, although competing risks were presented4,9.
Given that background, we compared the demographics, pathophysiologic states, and treatments for patients with cervical cancer who were white (including Latinas and non-Latinas)—the largest population group in America—and Asian American, examining their survival status in the presence of competing risks, and we explored factors that might affect prognosis. Asian American patients were also divided into racial subgroups for the survival analysis.
METHODS
Data were obtained from the U.S. Surveillance, Epidemiology, and End Results database, whose registration covers 28% of the U.S. population10. The study began on 1 January 1988 and ended on 31 December 2011. We excluded patients whose records showed a number of primary cancers greater than 1 (10,237 cases), patients for whom the diagnosis was confirmed by autopsy (160 cases), patients whose follow-up duration was 0 or unknown (2305 cases), and patients with a case-specific survival status of “N/A not first tumour” (3539 cases)11,12. After the exclusions, the number of patients with cervical cancer was 71,066 (International Classification of Diseases for Oncology, 3rd edition, codes C53.0–C53.9). Of those patients, 54,827 were white, and 3953 were Asian American (supplemental Figure 1). The variables of age at diagnosis, figo stage, lymph node metastasis, registry area, marital status, primary site, cell differentiation, histology, tumour diameter, radiation, and surgery were obtained for the study. For the race subgroup analysis, the Asian American patients were further classified into Chinese, Japanese, South Korean, Vietnamese, Indian or Pakistani, and Other groups. We considered two outcomes: death from cervical cancer (the event of interest) and death from causes other than cervical cancer (competing events). Data were censored on loss to follow-up or the death of the patient.
Data are presented as medians and ranges for continuous variables and as frequencies and percentages for categorical variables. Chi-square tests for demographic data, pathophysiologic condition, and treatment were used to compare the white and Asian American groups. The initial univariate analysis used a cumulative risk model with Gray test to investigate patient characteristics at 5-year follow-up (white patients) and end of follow-up (Asian American patients)13. The cumulative incidence function (details given in supplemental Method 1), which quantifies the probability of experiencing death from cervical cancer (the event of interest) in the presence of death from causes other than cervical cancer (competing events), was also determined. To further investigate the cumulative incidence rate of cervical cancer (the event of interest) adjusted for potential confounders, a multivariate analysis based on the Fine–Gray model14 was performed. In our analysis, categorical variables (for example, age at diagnosis, figo stage, lymph node metastasis, histology, and treatment) were converted into dichotomous dummy variables, and the Bayesian information criteria for competing risks were adopted for screening variables15. All statistical analyses were performed using the R software application (version 3.1.1: The R Foundation, Vienna, Austria). A two-sided p value less than 0.05 was considered statistically significant.
RESULTS
The study included 58,780 patients with cervical cancer, of whom 54,827 (93%) were white and 3953 (7%) were Asian American. Table I shows demographic data, pathophysiologic data, and treatment for the white and Asian American patient groups.
TABLE I.
Statistical analysis of baseline data
| Characteristic | Patient group | p Valuea | ||
|---|---|---|---|---|
|
| ||||
| Overall | White | Asian American | ||
| Patients (n) | 58,780 | 54,827 | 3,953 | |
|
| ||||
| Age at diagnosis (years) | <0.001 | |||
| Median | 34 | 33 | 42 | |
| Range | 6–104 | 6–104 | 13–97 | |
|
| ||||
| Age group at diagnosis [n (%)] | ||||
| <25 Years | 8770 (14.9) | 8573 (15.6) | 197 (5.0) | |
| 25–45 Years | 36195 (61.6) | 34194 (62.4) | 2001 (50.6) | |
| 45–60 Years | 8554 (14.6) | 7506 (13.7) | 1048 (26.5) | |
| >60 Years | 5260 (8.9) | 4554 (8.3) | 706 (17.9) | |
| Unknown | 1 (0.0) | 0 (0.0) | 1 (0.0) | |
|
| ||||
| FIGO stage (1994) [n (%)] | <0.001 | |||
| In situ | 40860 (69.6) | 38985 (71.1) | 1875 (47.4) | |
| I | 11122 (18.9) | 9908 (18.0) | 1214 (30.7) | |
| II | 3078 (5.2) | 2620 (4.8) | 458 (11.6) | |
| III | 1954 (3.3) | 1683 (3.1) | 271 (6.9) | |
| IV | 1006 (1.7) | 925 (1.7) | 81 (2.0) | |
| Unknown | 760 (1.3) | 706 (1.3) | 54 (1.40) | |
|
| ||||
| Lymph node metastasis [n (%)] | <0.001 | |||
| None | 52310 (89.0) | 49041 (89.5) | 3269 (82.7) | |
| Regional | 2091 (3.5) | 1821 (3.3) | 270 (6.8) | |
| Distant | 507 (0.9) | 444 (0.8) | 63 (1.6) | |
| Unknown | 3872 (6.6) | 3521 (6.4) | 351 (8.9) | |
|
| ||||
| Registry area [n (%)] | <0.001 | |||
| Middle | 20263 (34.5) | 20034 (36.6) | 229 (5.8) | |
| Eastern | 16514 (28.1) | 16239 (29.6) | 275 (6.9) | |
| Western | 22003 (37.4) | 18554 (33.8) | 3449 (87.3) | |
|
| ||||
| Marital status [n (%)] | <0.001 | |||
| Single or other | 14323 (24.4) | 13651 (24.9) | 672 (17.0) | |
| Married | 35552 (60.5) | 32616 (59.5) | 2936 (74.3) | |
| Unknown | 8905 (15.1) | 8560 (15.6) | 345 (8.7) | |
|
| ||||
| Primary site [n (%)] | <0.001 | |||
| Endocervix | 4742 (8.1) | 4321 (7.8) | 421 (10.7) | |
| Exocervix | 3103 (5.3) | 2992 (5.5) | 111 (2.8) | |
| Overlapping lesions | 1610 (2.7) | 1538 (2.8) | 72 (1.8) | |
| Cervix uteri | 49325 (83.9) | 45976 (83.9) | 3349 (84.7) | |
|
| ||||
| Grade [n (%)] | 0.132 | |||
| Well differentiated | 1764 (3.0) | 1579 (2.8) | 185 (4.7) | |
| Moderately differentiated | 4930 (8.4) | 4309 (7.9) | 621 (15.7) | |
| Poorly differentiated | 4856 (8.2) | 4258 (7.8) | 598 (15.1) | |
| Undifferentiated | 500 (0.9) | 439 (0.8) | 61 (1.5) | |
| Unknown | 46730 (79.5) | 44242 (80.7) | 2488 (63.0) | |
|
| ||||
| Histology [n (%)] | <0.001 | |||
| Squamous cell | 38960 (66.3) | 36415 (66.5) | 2545 (64.4) | |
| Adenocarcinoma | 4506 (7.7) | 4026 (7.3) | 480 (12.1) | |
| Adenosquamous | 794 (1.3) | 712 (1.3) | 82 (2.1) | |
| Others | 140 (24.7) | 13674 (24.9) | 846 (21.4) | |
|
| ||||
| Tumour diameter [n (%)] | <0.001 | |||
| <7 mm | 8579 (14.6) | 8165 (14.9) | 414 (10.5) | |
| 7 mm–4 cm | 3850 (6.5) | 3300 (6.0) | 550 (13.9) | |
| >4 cm | 3319 (5.6) | 2885 (5.3) | 434 (11.0) | |
| Unknown | 43032 (73.3) | 40477 (73.8) | 2555 (64.6) | |
|
| ||||
| Radiation [n (%)] | <0.001 | |||
| No | 49970 (85.0) | 47152 (86.0) | 2818 (71.3) | |
| Yes | 8646 (14.7) | 7528 (13.7) | 1118 (28.3) | |
| Unknown | 164 (0.3) | 147 (0.3) | 17 (0.4) | |
|
| ||||
| Surgery [n (%)] | <0.001 | |||
| No | 10282 (17.5) | 9382 (17.1) | 900 (22.8) | |
| Yes | 48436 (82.4) | 45390 (82.8) | 3046 (77.0) | |
| Unknown | 62 (0.1) | 55 (0.1) | 7 (0.2) | |
By chi-square test; does not include the “Unknown” category.
Compared with the Asian American patients, the white patients were much younger (median: 33 years vs. 42 years; p < 0.001). The difference in figo stage was also significantly different for the white and Asian American patients (p < 0.001), and the rate of carcinoma in situ was much higher in white patients than in Asian American patients (71.1% vs. 47.4%). Compared with white patients, Asian American patients were more likely to have lymph node metastasis (regional: 6.8% vs. 3.3%; distant: 4.6% vs. 0.8%; both p < 0.001). In addition, the distribution of patient registry locations was different in the two groups. Most Asian American patients (87.3%) were registered in the western region; the white patients tended to be evenly distributed across the central, eastern, and western regions (33.6%, 29.6%, and 33.8% respectively). The rates of married status were 59.5% and 74.3% for white and Asian American patients respectively (p < 0.001).
The primary sites of cancer included the endocervix, exocervix, overlapping lesions, and cervix uteri. Primary sites differed between the white and Asian American patients (p < 0.001), but the grade of tumour differentiation showed no significant difference (p = 0.132). Approximately 7.3% of the white patients and 12.1% of the Asian American patients had adenocarcinoma (p < 0.001). Of Asian American patients, 11% had a tumour of relatively large diameter (that is, >4 cm); however, only 5.3% of white patients had a tumour with a diameter exceeding 4 cm (p < 0.001).
Differences in treatment methods were evident between Asian American and white patients: 28.3% of Asian American patients and 13.7% of white patients received radiation therapy (p < 0.001), and 77.0% of Asian American patients and 82.8% of white patients underwent surgical treatment (p < 0.001).
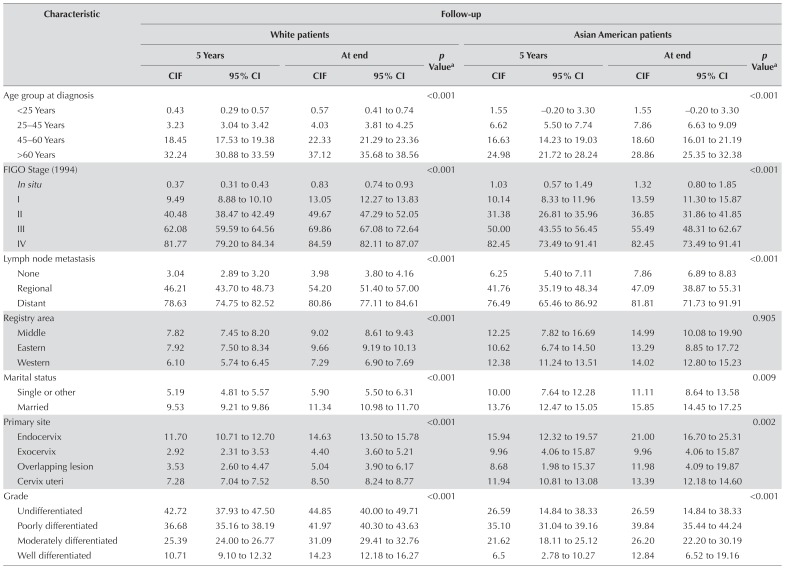
Table II compares the cumulative incidences of the events of interest for all 58,780 patients. Differences in cervical cancer mortality were associated with different levels of various factors (p < 0.001), and the cumulative incidence function differed with age at diagnosis. The cumulative incidence of the event of interest was compared for the Asian American and white groups [Table III and supplemental Figure 2(A–K)]. The cumulative incidence of the event of interest differed for the white patients depending on age at diagnosis, figo stage, lymph node metastasis, registry area, marital status, primary site, grade of tumour differentiation, histology, tumour diameter, and treatment method. In Asian American patients, no difference in the cumulative incidence of the event of interest was observed for registry area (p = 0.905); however, differences were observed for other factors, such as figo stage and primary site. Supplemental Table 1 shows cumulative incidences for the various factors in the various Asian American race subgroups. We observed no difference in the cumulative incidence of the event of interest by race group (Vietnamese, Filipina, Chinese, South Korean, Indian or Pakistani, and Other; p = 0.132); however, Filipina, Chinese, and Japanese patients accounted for large proportions of the Asian American group (21.5%, 17.2%, and 15.5% respectively).
TABLE II.
Univariate analysis for the patients overall, by selected characteristics
| Characteristic | Follow-up | p Valuea | |||
|---|---|---|---|---|---|
|
| |||||
| 5 Years | At end | ||||
|
|
|
||||
| CIF | 95% CI | CIF | 95% CI | ||
| Race | <0.001 | ||||
| White | 7.27 | 6.98 to 7.49 | 8.63 | 8.15 to 9.06 | |
| Asian American | 12.25 | 11.57 to 13.14 | 14.02 | 13.26 to 14.75 | |
|
| |||||
| Age group at diagnosis | <0.001 | ||||
| <25 Years | 0.46 | 0.31 to 0.60 | 0.60 | 0.43 to 0.76 | |
| 25–45 Years | 3.41 | 3.22 to 3.60 | 4.24 | 4.02 to 4.45 | |
| 45–60 Years | 18.23 | 17.37 to 19.09 | 21.89 | 20.93 to 22.86 | |
| >60 Years | 31.29 | 30.04 to 32.54 | 36.07 | 34.73 to 37.40 | |
|
| |||||
| FIGO stage (1994) | <0.001 | ||||
| In situ | 0.40 | 0.34 to 0.46 | 0.86 | 0.76 to 0.95 | |
| I | 9.56 | 8.99 to 10.14 | 13.11 | 12.38 to 13.85 | |
| II | 39.16 | 37.32 to 41.01 | 47.85 | 45.67 to 50.03 | |
| III | 60.42 | 58.09 to 62.74 | 67.86 | 65.27 to 70.45 | |
| IV | 81.85 | 79.39 to 84.32 | 84.76 | 82.38 to 87.14 | |
|
| |||||
| Lymph node metastasis | <0.001 | ||||
| None | 3.24 | 3.08 to 3.39 | 4.22 | 4.04 to 4.40 | |
| Regional | 45.66 | 43.31 to 48.02 | 53.36 | 50.71 to 56.01 | |
| Distant | 78.33 | 74.68 to 81.98 | 80.95 | 77.46 to 84.45 | |
|
| |||||
| Registry area | <0.001 | ||||
| Middle | 7.87 | 7.49 to 8.25 | 9.09 | 8.68 to 9.49 | |
| Eastern | 7.96 | 7.54 to 8.38 | 9.71 | 9.24 to 10.19 | |
| Western | 7.07 | 6.72 to 7.41 | 8.33 | 7.95 to 8.71 | |
|
| |||||
| Marital status | <0.001 | ||||
| Single or other | 5.41 | 5.03 to 5.79 | 6.14 | 5.74 to 6.55 | |
| Married | 9.87 | 9.56 to 10.19 | 11.70 | 11.36 to 12.05 | |
|
| |||||
| Primary site | <0.001 | ||||
| Endocervix | 12.08 | 11.12 to 13.05 | 15.18 | 14.08 to 16.29 | |
| Exocervix | 3.16 | 2.53 to 3.78 | 4.60 | 3.79 to 5.40 | |
| Overlapping lesion | 3.76 | 2.82 to 4.70 | 5.33 | 4.20 to 6.48 | |
| Cervix uteri | 7.59 | 7.35 to 7.83 | 8.82 | 8.57 to 9.09 | |
|
| |||||
| Grade | <0.001 | ||||
| Undifferentiated | 10.27 | 8.77 to 11.76 | 14.06 | 12.12 to 16.01 | |
| Poorly differentiated | 24.92 | 23.64 to 26.21 | 30.51 | 28.95 to 32.06 | |
| Moderately differentiated | 36.28 | 35.07 to 37.90 | 41.74 | 40.18 to 43.30 | |
| Well differentiated | 40.81 | 36.35 to 45.27 | 42.73 | 38.19 to 47.27 | |
|
| |||||
| Histology | <0.001 | ||||
| Squamous cell | 7.65 | 7.38 to 7.92 | 9.07 | 8.77 to 9.37 | |
| Adenocarcinoma | 17.63 | 16.46 to 18.79 | 21.79 | 20.45 to 23.13 | |
| Adenosquamous | 31.22 | 27.88 to 34.57 | 35.23 | 31.68 to 38.77 | |
| Others | 3.29 | 3.00 to 3.59 | 3.92 | 3.60 to 4.24 | |
|
| |||||
| Tumour diameter | <0.001 | ||||
| <7 mm | 0.50 | 0.35 to 0.65 | 1.05 | 0.81 to 1.28 | |
| 7 mm–4 cm | 18.45 | 17.12 to 19.78 | 24.21 | 22.52 to 25.89 | |
| >4 cm | 46.04 | 44.21 to 478.87 | 52.78 | 50.68 to 54.88 | |
|
| |||||
| Radiation | <0.001 | ||||
| Yes | 41.53 | 40.43 to 42.63 | 49.31 | 48.06 to 50.57 | |
| No | 2.01 | 1.89 to 2.14 | 2.73 | 2.58 to 2.88 | |
|
| |||||
| Surgery | <0.001 | ||||
| Yes | 2.96 | 2.81 to 3.12 | 4.00 | 3.82 to 4.19 | |
| No | 29.99 | 29.07 to 30.90 | 33.23 | 32.27 to 34.19 | |
By the Gray test.
CIF = cumulative incidence function; CI = confidence interval; FIGO = Fédération Internationale de Gynécologie et d’Obstétrique.
TABLE III.
Univariate analysis by ethnicity and selected characteristics
| Characteristic | Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| White patients | Asian American patients | |||||||||
|
|
|
|||||||||
| 5 Years | At end | p Valuea | 5 Years | At end | p Valuea | |||||
|
|
|
|
|
|||||||
| CIF | 95% CI | CIF | 95% CI | CIF | 95% CI | CIF | 95% CI | |||
| Age group at diagnosis | <0.001 | <0.001 | ||||||||
| <25 Years | 0.43 | 0.29 to 0.57 | 0.57 | 0.41 to 0.74 | 1.55 | −0.20 to 3.30 | 1.55 | −0.20 to 3.30 | ||
| 25–45 Years | 3.23 | 3.04 to 3.42 | 4.03 | 3.81 to 4.25 | 6.62 | 5.50 to 7.74 | 7.86 | 6.63 to 9.09 | ||
| 45–60 Years | 18.45 | 17.53 to 19.38 | 22.33 | 21.29 to 23.36 | 16.63 | 14.23 to 19.03 | 18.60 | 16.01 to 21.19 | ||
| >60 Years | 32.24 | 30.88 to 33.59 | 37.12 | 35.68 to 38.56 | 24.98 | 21.72 to 28.24 | 28.86 | 25.35 to 32.38 | ||
|
| ||||||||||
| FIGO Stage (1994) | <0.001 | <0.001 | ||||||||
| In situ | 0.37 | 0.31 to 0.43 | 0.83 | 0.74 to 0.93 | 1.03 | 0.57 to 1.49 | 1.32 | 0.80 to 1.85 | ||
| I | 9.49 | 8.88 to 10.10 | 13.05 | 12.27 to 13.83 | 10.14 | 8.33 to 11.96 | 13.59 | 11.30 to 15.87 | ||
| II | 40.48 | 38.47 to 42.49 | 49.67 | 47.29 to 52.05 | 31.38 | 26.81 to 35.96 | 36.85 | 31.86 to 41.85 | ||
| III | 62.08 | 59.59 to 64.56 | 69.86 | 67.08 to 72.64 | 50.00 | 43.55 to 56.45 | 55.49 | 48.31 to 62.67 | ||
| IV | 81.77 | 79.20 to 84.34 | 84.59 | 82.11 to 87.07 | 82.45 | 73.49 to 91.41 | 82.45 | 73.49 to 91.41 | ||
|
| ||||||||||
| Lymph node metastasis | <0.001 | <0.001 | ||||||||
| None | 3.04 | 2.89 to 3.20 | 3.98 | 3.80 to 4.16 | 6.25 | 5.40 to 7.11 | 7.86 | 6.89 to 8.83 | ||
| Regional | 46.21 | 43.70 to 48.73 | 54.20 | 51.40 to 57.00 | 41.76 | 35.19 to 48.34 | 47.09 | 38.87 to 55.31 | ||
| Distant | 78.63 | 74.75 to 82.52 | 80.86 | 77.11 to 84.61 | 76.49 | 65.46 to 86.92 | 81.81 | 71.73 to 91.91 | ||
|
| ||||||||||
| Registry area | <0.001 | 0.905 | ||||||||
| Middle | 7.82 | 7.45 to 8.20 | 9.02 | 8.61 to 9.43 | 12.25 | 7.82 to 16.69 | 14.99 | 10.08 to 19.90 | ||
| Eastern | 7.92 | 7.50 to 8.34 | 9.66 | 9.19 to 10.13 | 10.62 | 6.74 to 14.50 | 13.29 | 8.85 to 17.72 | ||
| Western | 6.10 | 5.74 to 6.45 | 7.29 | 6.90 to 7.69 | 12.38 | 11.24 to 13.51 | 14.02 | 12.80 to 15.23 | ||
|
| ||||||||||
| Marital status | <0.001 | 0.009 | ||||||||
| Single or other | 5.19 | 4.81 to 5.57 | 5.90 | 5.50 to 6.31 | 10.00 | 7.64 to 12.28 | 11.11 | 8.64 to 13.58 | ||
| Married | 9.53 | 9.21 to 9.86 | 11.34 | 10.98 to 11.70 | 13.76 | 12.47 to 15.05 | 15.85 | 14.45 to 17.25 | ||
|
| ||||||||||
| Primary site | <0.001 | 0.002 | ||||||||
| Endocervix | 11.70 | 10.71 to 12.70 | 14.63 | 13.50 to 15.78 | 15.94 | 12.32 to 19.57 | 21.00 | 16.70 to 25.31 | ||
| Exocervix | 2.92 | 2.31 to 3.53 | 4.40 | 3.60 to 5.21 | 9.96 | 4.06 to 15.87 | 9.96 | 4.06 to 15.87 | ||
| Overlapping lesion | 3.53 | 2.60 to 4.47 | 5.04 | 3.90 to 6.17 | 8.68 | 1.98 to 15.37 | 11.98 | 4.09 to 19.87 | ||
| Cervix uteri | 7.28 | 7.04 to 7.52 | 8.50 | 8.24 to 8.77 | 11.94 | 10.81 to 13.08 | 13.39 | 12.18 to 14.60 | ||
|
| ||||||||||
| Grade | <0.001 | <0.001 | ||||||||
| Undifferentiated | 42.72 | 37.93 to 47.50 | 44.85 | 40.00 to 49.71 | 26.59 | 14.84 to 38.33 | 26.59 | 14.84 to 38.33 | ||
| Poorly differentiated | 36.68 | 35.16 to 38.19 | 41.97 | 40.30 to 43.63 | 35.10 | 31.04 to 39.16 | 39.84 | 35.44 to 44.24 | ||
| Moderately differentiated | 25.39 | 24.00 to 26.77 | 31.09 | 29.41 to 32.76 | 21.62 | 18.11 to 25.12 | 26.20 | 22.20 to 30.19 | ||
| Well differentiated | 10.71 | 9.10 to 12.32 | 14.23 | 12.18 to 16.27 | 6.5 | 2.78 to 10.27 | 12.84 | 6.52 to 19.16 | ||
|
| ||||||||||
| Histology | <0.001 | <0.001 | ||||||||
| Squamous cell | 7.37 | 7.10 to 7.64 | 8.77 | 8.45 to 9.07 | 11.75 | 10.45 to 13.04 | 13.48 | 12.08 to 14.88 | ||
| Adenocarcinoma | 17.02 | 15.80 to 18.24 | 21.18 | 19.77 to 22.59 | 22.72 | −1.31 to 1.77 | 26.90 | 22.56 to 31.25 | ||
| Adenosquamous | 31.25 | 27.77 to 34.77 | 35.14 | 31.44 to 38.84 | 30.95 | 19.96 to 41.93 | 37.46 | 23.65 to 51.27 | ||
| Others | 3.10 | 2.80 to 3.40 | 3.72 | 3.40 to 4.05 | 6.45 | 4.78 to 8.14 | 7.15 | 5.37 to 8.93 | ||
|
| ||||||||||
| Tumour diameter | <0.001 | <0.001 | ||||||||
| <7 mm | 0.47 | 0.32 to 0.62 | 1.04 | 0.80 to 1.28 | 1.10 | 0.03 to 2.17 | 1.10 | 0.03 to 2.17 | ||
| 7 mm–4 cm | 18.63 | 17.19 to 20.07 | 24.23 | 22.42 to 26.04 | 17.34 | 13.90 to 20.79 | 24.03 | 19.47 to 28.60 | ||
| >4 cm | 46.64 | 44.68 to 48.60 | 53.44 | 51.19 to 55.69 | 42.00 | 36.89 to 47.09 | 48.13 | 42.38 to 53.87 | ||
|
| ||||||||||
| Radiation | <0.001 | <0.001 | ||||||||
| Yes | 42.47 | 41.28 to 43.65 | 50.46 | 49.12 to 51.81 | 35.06 | 32.07 to 38.06 | 41.06 | 37.68 to 44.43 | ||
| No | 1.91 | 1.79 to 2.04 | 2.63 | 2.47 to 2.78 | 3.93 | 3.02 to 4.45 | 4.50 | 3.70 to 5.30 | ||
|
| ||||||||||
| Surgery | <0.001 | <0.001 | ||||||||
| Yes | 2.81 | 2.66 to 2.97 | 3.85 | 3.67 to 4.03 | 5.24 | 4.42 to 6.06 | 6.40 | 5.49 to 7.32 | ||
| No | 29.35 | 28.40 to 30.30 | 32.51 | 31.51 to 33.51 | 36.93 | 33.59 to 40.27 | 41.51 | 37.79 to 45.10 | ||
By the Gray test.
CIF = cumulative incidence function; CI = confidence interval; FIGO = Fédération Internationale de Gynécologie et d’Obstétrique.
To further explore the factors affecting the survival status of patients with cervical cancer, Bayesian information criteria were used to screen variables for competing risks. Given the large number of women with an unknown tumour diameter and differentiation grade (80%), those particular variables were not included in the multivariate analysis in Table IV (supplemental Table 2 presents results that include tumour diameter and degree of tumour differentiation). Finally, using the Fine–Gray model, age at diagnosis, figo stage, lymph node metastasis, tumour histology, treatment method, and race group were all demonstrated to affect patient prognosis (Table IV). When examining the influence of treatment on patient prognosis, the hazard ratio of the event of interest for individuals who received radiation compared with those receiving no radiation was found to be relatively low (hazard ratio: 0.49; 95% confidence interval: 0.44 to 0.54). In contrast, surgery compared with no surgery had a hazard ratio of 1.06 (95% confidence interval: 1.03 to 1.09). Compared with Asian American patients, white patients had a higher risk of dying from cervical cancer (hazard ratio: 1.25; 95% confidence interval: 1.11 to 1.41).
TABLE IV.
Multivariable analysis, competing risks model
| Characteristic | HR | 95% CI | Wald p |
|---|---|---|---|
| Age group at diagnosis | |||
| <25 Years | 0.45 | 0.33 to 0.63 | <0.001 |
| 25–45 Years | 0.75 | 0.68 to 0.83 | <0.001 |
| 45–60 Years | Reference | ||
| >60 Years | 1.13 | 1.03 to 1.25 | 0.013 |
|
| |||
| FIGO stage (1994) | |||
| In situ | 0.06 | 0.05 to 0.08 | <0.001 |
| I | Reference | ||
| II | 2.48 | 2.21 to 2.78 | <0.001 |
| III | 3.45 | 3.00 to 3.97 | <0.001 |
| IV | 5.40 | 4.55 to 6.41 | <0.001 |
|
| |||
| Lymph node metastasis | |||
| No metastasis | 0.32 | 0.27 to 0.37 | <0.001 |
| Regional | 0.73 | 0.62 to 0.85 | <0.001 |
| Distant | Reference | ||
|
| |||
| Histology | |||
| Squamous cell | Reference | ||
| Adenocarcinoma | 1.30 | 1.18 to 1.44 | <0.001 |
| Adenosquamous | 1.66 | 1.41 to 1.94 | <0.001 |
| Others | 1.78 | 1.52 to 2.07 | <0.001 |
|
| |||
| Radiation | |||
| Yes | 1.12 | 1.07 to 1.16 | <0.001 |
| No | Reference | ||
|
| |||
| Surgery | |||
| Yes | 0.47 | 0.42 to 0.52 | <0.001 |
| No | Reference | ||
|
| |||
| Ethnicity | |||
| Asian American | Reference | ||
| White | 1.25 | 1.11 to 1.40 | <0.001 |
HR = hazard ratio; CI = confidence interval; FIGO = Fédération Internationale de Gynécologie et d’Obstétrique.
DISCUSSION
In clinical trials, survival analysis is a statistical method commonly used to study prognostic factors in patients with tumours. When dealing with a single-event outcome or endpoint (for example, death from cervical cancer) without competing risks, the Kaplan–Meier method, log-rank test, and Cox proportional hazards model are widely used as standard methods for nonparametric estimation, survival curve comparison, and semiparametric multivariate analysis respectively. However, in many clinical trials, the number of endpoints is not limited to one; they therefore have a “competing” trend. In the presence of competing risk events, events are generally perceived to be mutually exclusive—that is, the competing event cannot occur after the event of interest. In that case, statistical methods taking into account the competing risks are needed; otherwise, the results obtained might be incorrect16–19.
Previous studies have suggested that the survival status of patients with cervical cancer varies by racial group. People of Asian American descent are a rapidly growing group in the United States; however, few studies have investigated their survival status or the factors affecting their prognosis compared with all patients having cervical cancer. The 54,827 white patients and 3952 Asian American patients with cervical cancer who were diagnosed during 1988–2011 were studied here using a competing risks model to examine factors affecting their prognosis.
In cervical cancer, an age of onset less than 25 years is generally considered young. Turkistanli et al.20 reported that, in recent years, women with a reproductive tract tumour accounted for more than 20% of women with tumours. We found that 78% of white patients but only 55.6% of Asian American patients were less than 45 years of age and that the median age at diagnosis was older for the Asian American patients. The reasons for those observations might be related to the limited health care resources available to the Asian American patients (insurance coverage is 82% for Asian American populations and 88% for non-Hispanic white populations21). We know that screening is an important factor: under-screening can influence presentation at an older age. A study published in 2017 also revealed that 83.2% of white women underwent cervical cancer screening, but only 75.8% of Asian American women did22.
An interesting point about the Surveillance, Epidemiology, and End Results database is that it includes carcinoma in situ, showing what a robust database it is for cervical cancer evaluation. We found that Asian American women did better in terms of mortality despite a greater proportion of metastatic disease, which speaks to other factors contributing to improved survival that might be making up for poorer screening rates and opportunities. In fact, carcinoma in situ should always be excluded from the evaluation of invasive cancer cases because it is not clinically considered cancer. Hence, we also considered the outcomes for women excluded because of carcinoma in situ, and simply compared their results with our other results. As shown in supplemental Table 3, age at diagnosis, figo stage, lymph node metastasis, tumour histology, treatment method, and race together affected patient prognosis, consistent with the results presented in Table IV. The cumulative incidence of cervical cancer during the period of interest was lower in white women than in Asian American women younger than 45 years, but for other age groups, a reverse relationship was evident. Most studies demonstrated differences in patient prognosis by racial group, which was associated with treatment methods, adjuvant treatment, and duration of follow-up23,24.
In a study of women diagnosed during 2000–2010, Nghiem et al.9 found that the survival rate for non-Hispanic white women was generally worse than that for Asian American women (hazard ratio: 1.29). When considering competing risks, we discovered that the hazard ratio for the event of interest in white women compared with Asian American women was 1.24. In their study of women with ovarian cancer, Fuh et al.10 asserted that patient deaths should be treated as an endpoint and, in a log-rank test of the survival function for different race groups, discovered a difference in the survival rates for Vietnamese, Filipina, Chinese, South Korean, Japanese, and Indian or Pakistani women (5-year disease-specific survival rates: 62%, 61%, 61%, 59%, and 55% respectively). Considering competing events and dividing our Asian American patients with cervical cancer into race subgroups for analysis, we observed no significant differences in the cumulative incidence rates of the event of interest for those subgroups.
In a study of 12,170 patients with cervical cancer, Rauh-Hain et al.6 combined deaths from cervical cancer and from other causes as a single outcome and used a Cox proportional hazards model to estimate the all-cause-specific hazard ratio. They found that a higher figo stage was associated with more severe lymph node metastasis and higher cervical cancer mortality. They found that the cervical cancer mortality rate for married patients was 0.94 of the rate for unmarried patients. However, we showed that, among Asian American and white patients with cervical cancer, the cumulative incidence rate of the event of interest was different depending on marital status; the rate for married white and Asian American patients was relatively high, probably because of more frequent penetrative sex25. The study indicated that surgery had a positive influence on the survival status of patients with cervical cancer, but that radiation was a risk factor, which might be interpreted to mean that surgery is used only for smaller tumours of lesser stage and that radiation is the default treatment for most cervical cancers at stages ii–iv. In addition, in a study of the influence of histologic type on prognosis in patients with cervical cancer in the presence of competing risks, Intaraphet et al.26 noted that a higher figo stage was associated with a higher mortality rate in patients with cervical cancer positive for lymph node metastasis. Generally, the more advanced the figo stage, the more likely it is that the tumour is bigger, that lymph node and other metastases are present, and that survival is poorer, which is consistent with the results of the present study with competing risks included.
We proposed the use of a competing-risks model to compare and analyze the survival status of white and Asian American patients with cervical cancer, and we discussed the influence of demographic characteristics, pathophysiologic data, and treatments on the prognosis of patients with cervical cancer from white and Asian American populations, which account for 28% of the American population. In looking at cause of death for patients with cervical cancer, we also divided the Asian American population into subgroups, the results of which might help in guiding the treatment of white and Asian American patients with cervical cancer and facilitate prognostic judgments.
However, this study has some limitations. It was retrospective, and thus had no strict experimental design; some potentially influential factors such as body mass index, smoking, chemotherapy, positivity for the human papillomavirus, and regional and lifestyle differences were not included in the analysis. Because the data came from patients diagnosed with cervical cancer during 1988–2011, the follow-up time for some patients was relatively short, which might have influenced the evaluation of cumulative incidence. Large-scale prospective clinical studies are therefore required to verify our findings.
CONCLUSIONS
Considering competing risks, we found a pronounced difference in cervical cancer mortality for white and Asian American women. Compared with white women, Asian American women with a cervical cancer diagnosis appeared to be older, to be classified with a higher figo stage, to have tumours larger in diameter, and to more often undergo radiation; however, after controlling for demographic and clinical factors, Asian American women still experienced better survival after cervical cancer. In women of both races, surgery was associated with a positive effect, and radiation appeared to be a risk factor. Response to treatment requires careful examination. The findings of our study will help to ensure that all women with cervical cancer receive adequate therapy based on appropriate assessment and might help to facilitate prognostic judgments in future studies.
Supplemental Materials
ACKNOWLEDGMENTS
This study used data obtained from the Surveillance, Epidemiology, and End Results program of the U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute.
This work was supported by the National Natural Science Foundation of China (nos. 81673268 and 81202288) and by the Natural Science Foundation of Guangdong Province, P.R.C. (nos. 2017A030313812 and 2018A030313849).
Footnotes
Supplemental material available at http://www.current-oncology.com
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Saleem A, Tristram A, Fiander A, Hibbitts S. Prophylactic hpv vaccination: a major breakthrough in the fight against cervical cancer? Minerva Med. 2009;100:503–23. [PubMed] [Google Scholar]
- 2.Underwood SM, Ramsay-Johnson E, Dean A, Russ J, Ivalis R. Expanding the scope of nursing research in low resource and middle resource countries, regions, and states focused on cervical cancer prevention, early detection, and control. J Natl Black Nurses Assoc. 2009;20:42–54. [PMC free article] [PubMed] [Google Scholar]
- 3.Glick SB, Clarke AR, Blanchard A, Whitaker AK. Cervical cancer screening, diagnosis and treatment interventions for racial and ethnic minorities: a systematic review. J Gen Intern Med. 2012;27:1016–32. doi: 10.1007/s11606-012-2052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu HC, Li X, Curtin JP, Goldberg JD, Schiff PB. Surveillance Epidemiology and End Results analysis demonstrates improvement in overall survival for cervical cancer patients treated in the era of concurrent chemoradiotherapy. Front Oncol. 2015;5:81. doi: 10.3389/fonc.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Ibrahimi S, Pinheiro P. No differences in cervical cancer stage at diagnosis for blacks and whites in the Mountain West. J Immigr Minor Health. 2015;17:632–7. doi: 10.1007/s10903-014-0149-x. [DOI] [PubMed] [Google Scholar]
- 6.Rauh-Hain JA, Clemmer JT, Bradford LS, et al. Racial disparities in cervical cancer survival over time. Cancer. 2013;119:3644–52. doi: 10.1002/cncr.28261. [DOI] [PubMed] [Google Scholar]
- 7.Yuan CC, Wang PH, Lai CR, Yen MS, Chen CY, Juang CM. Prognosis-predicting system based on factors related to survival of cervical carcinoma. Int J Gynaecol Obstet. 1998;63:163–7. doi: 10.1016/S0020-7292(98)00142-8. [DOI] [PubMed] [Google Scholar]
- 8.Howell EA, Chen YT, Concato J. Difference in cervical cancer mortality among black and white women. Obstet Gynecol. 1999;94:509–15. doi: 10.1016/s0029-7844(99)00334-8. [DOI] [PubMed] [Google Scholar]
- 9.Nghiem VT, Davies KR, Chan W, Mulla ZD, Cantor SB. Disparities in cervical cancer survival among Asian-American women. Ann Epidemiol. 2016;26:28–35. doi: 10.1016/j.annepidem.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuh KC, Shin JY, Kapp DS, et al. Survival differences of Asian and Caucasian epithelial ovarian cancer patients in the United States. Gynecol Oncol. 2015;136:491–7. doi: 10.1016/j.ygyno.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Montealegre JR, Zhou R, Amirian ES, Follen M, Scheurer ME. Nativity disparities in late-stage diagnosis and cause-specific survival among Hispanic women with invasive cervical cancer: an analysis of Surveillance, Epidemiology, and End Results data. Cancer Causes Control. 2013;24:1985–94. doi: 10.1007/s10552-013-0274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson JA, Rusthoven C, DeWitt PE, Davidson SA, Schefter TE, Fisher CM. Are we appropriately selecting therapy for patients with cervical cancer? Longitudinal patterns-of-care analysis for stage ib–iib cervical cancer. Int J Radiat Oncol Biol Phys. 2014;90:786–93. doi: 10.1016/j.ijrobp.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Haller B, Schmidt G, Ulm K. Applying competing risks regression models: an overview. Lifetime Data Anal. 2013;19:33–58. doi: 10.1007/s10985-012-9230-8. [DOI] [PubMed] [Google Scholar]
- 14.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18:2301–8. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuk D, Varadhan R. Model selection in competing risks regression. Stat Med. 2013;32:3077–88. doi: 10.1002/sim.5762. [DOI] [PubMed] [Google Scholar]
- 16.Lu ZH, Zhou LZ, Han D, Zhou YF, Chen Z. Statistical processing and application of competing risks data [Chinese] Mod Prev Med. 2013;40:804–7. [Google Scholar]
- 17.Southern DA, Faris PD, Brant R, et al. on behalf of the approach investigators. Kaplan–Meier methods yielded mis-leading results in competing risk scenarios. J Clin Epidemiol. 2006;59:1110–14. doi: 10.1016/j.jclinepi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: the importance of multistate models and competing risks analysis. Hepatology. 2015;62:292–302. doi: 10.1002/hep.27598. [DOI] [PubMed] [Google Scholar]
- 19.Wolkewitz M, Cooper BS, Bonten MJ, Barnett AG, Schumacher M. Interpreting and comparing risks in the presence of competing events. BMJ. 2014;349:g5060. doi: 10.1136/bmj.g5060. [DOI] [PubMed] [Google Scholar]
- 20.Turkistanli EC, Sogukpinar N, Saydam BK, Aydemir G. Cervical cancer prevention and early detection—the role of nurses and midwives. Asian Pac J Cancer Prev. 2003;4:15–21. [PubMed] [Google Scholar]
- 21.Eggleston KS, Coker AL, Williams M, Tortolero-Luna G, Martin JB, Tortolero SR. Cervical cancer survival by socioeconomic status, race/ethnicity, and place of residence in Texas, 1995–2001. J Womens Health (Larchmt) 2006;15:941–51. doi: 10.1089/jwh.2006.15.941. [DOI] [PubMed] [Google Scholar]
- 22.White A, Thompson TD, White MC, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:201–6. doi: 10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dignam JJ. Differences in breast cancer prognosis among African-American and Caucasian women. CA Cancer J Clin. 2000;50:50–64. doi: 10.3322/canjclin.50.1.50. [DOI] [PubMed] [Google Scholar]
- 24.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 25.Gulumser C, Tuncer A, Kuscu E, Ayhan A. Is colposcopic evaluation necessary in all women with postcoital bleeding? Eur J Obstet Gynecol Reprod Biol. 2015;193:83–7. doi: 10.1016/j.ejogrb.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Intaraphet S, Kasatpibal N, Søgaard M, et al. Histological type–specific prognostic factors of cervical small cell neuro-endocrine carcinoma, adenocarcinoma, and squamous cell carcinoma. Onco Targets Ther. 2014;7:1205–14. doi: 10.2147/OTT.S64714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.