Abstract
Introduction
Cardiovascular disease is the 2nd leading cause of long-term morbidity and mortality in cancer survivors. Cardio-oncology clinics (cocs) have emerged to address the issue; however, there is a paucity of data about the demographics and clinical outcomes of patients seen in the coc setting.
Methods
Cancer patients referred to The Ottawa Hospital coc were included in this retrospective observational study. Data collected were patient demographics, cancer type and stage, reason for referral, cardiac risk factors, cardiac assessments and treatment, and clinical outcomes.
Results
Between 2008 and 2015, 779 patients (516 women, 66%; 263 men, 34%) were referred to the coc. Median age of the patients at cancer diagnosis was 60 years (range: 18–90 years). The most frequent reasons for referral were decreased left ventricular ejection fraction (33%), pre-chemotherapy assessment (14%), and arrhythmia (14%). Treatment with cardiac medication was given in 322 patients (41%), 181 (56%) of whom received more than 2 cardiac medications, with 57 (18%) receiving an angiotensin-converting enzyme inhibitor (acei), 46 (14%) receiving an acei and a beta-blocker, and 38 (12%) receiving a beta-blocker. Of 163 breast cancer patients, 129 (79%) were able to complete targeted therapy with coc co-management. Most of the 779 patients (n = 643, 83%) were alive at the time of the last data collection.
Conclusions
This cohort study is one of the largest to report characteristics and clinical outcomes of patients referred to a coc. Collaboration between oncologists and cardiologists resulted in completion of cancer therapy in most patients. Ongoing analysis of referral patterns, management plans, and patient outcomes will help to guide the cardiac care of oncology patients, ultimately optimizing cancer and cardiac outcomes alike.
Keywords: Cardio-oncology, cardiotoxicity, targeted therapy, lvef, cardiac outcomes
INTRODUCTION
Since the start of the 2000s, advances in cancer diagnosis and treatment have led to a significant increase in the number of cancer survivors, with an estimated 15.5 million in the United States alone as of January 20161. With fewer patients dying of cancer, long-term toxicity related to cancer therapy has become a growing concern2. Cancer and cardiovascular disease are the two leading causes of mortality in Canada and the United States3. Multiple factors— including age, genetics, lifestyle factors, and increasingly, the cardiotoxic effects of cancer therapies—place cancer patients and survivors at risk of heart disease4–6.
The cardiotoxicity of traditional cytotoxic chemotherapy agents, such as anthracyclines, has been well established7. The improved understanding of cancer biology has led to an increasing number of targeted therapies being introduced into clinical practice, including small-molecule tyrosine kinase inhibitors and monoclonal antibodies that target various oncogenic drivers. Although clinically effective, those cancer treatments have been associated with cardiovascular toxicity8. Trastuzumab, a monoclonal antibody that targets her2 (the human epidermal growth factor receptor 2), has shown significant clinical benefit in her2-positive early and advanced breast cancer. However, a meta-analysis of adjuvant trastuzumab trials demonstrated a 2.5%–4% incidence of heart failure9. Inhibitors of vascular endothelial growth factor receptor (for example, sunitinib, sorafenib, bevacizumab), commonly used in gastrointestinal and genitourinary cancers, are associated with hypertension8. Immunotherapy (for example, pembrolizumab) has greatly improved outcomes in cancers that have traditionally been difficult to treat, such as metastatic melanoma and advanced non-small-cell lung cancer10,11. However, when given in combination (for example, nivolumab–ipilimumab), they have, in rare cases, led to fulminant myocarditis12.
In recognition of the potential cardiotoxic effects of known and novel cancer therapies, cardiologists, oncologists, and other allied health care professionals are now working together toward the goal of optimizing cancer care while maintaining cardiovascular health13,14. Dedicated cardio-oncology clinics (cocs) have emerged across North America and internationally to manage patients who are at risk for, or who have developed, cardiotoxicity while receiving cancer therapy. A recent publication by Lancelloti et al.15 provides guidance to those interested in starting a cardio-oncology program, including objectives, organization, and implementation. But despite the growing interest in this emerging field, there is a paucity of data about the impact of such clinics on patient care and clinical outcomes.
In 2008, we established the first dedicated multidisciplinary coc in Canada at The Ottawa Hospital16. The clinic, staffed by 4 cardiologists on a rotating basis, operates 1 afternoon each week. The clinic offers expedited access for cancer patients to specialized cardiac care. Referral criteria include pre-chemotherapy cardiac optimization, change in left ventricular ejection fraction (lvef) during cancer therapy or suspected cardiac dysfunction related to cancer treatment, and cardiovascular complications after cancer treatment. Monthly cardio-oncology rounds, during which cardiologists, oncologists, and allied health care providers meet to discuss challenging cases for shared learning, complement the clinic. Here, we report on the demographics and clinical outcomes of cancer patients referred to The Ottawa Hospital coc.
METHODS
All cancer patients referred to the cocbetween October 2008 and October 2015 were included in this retrospective observational study. Data including patient demographics, cancer type, stage at diagnosis, biomarkers (for example, estrogen receptor status), cancer therapy (including anthracycline and targeted therapy exposure), and reason for referral were collected from electronic charts held in The Ottawa Hospital electronic medical record system. Cardiac risk factors collected included body mass index, history of smoking, dyslipidemia, hypertension, history of coronary artery disease, family history of early cardiovascular death, and past medical history of heart conditions. Results of left ventricular function assessments (by echocardiography or multigated acquisition imaging) before and during cancer treatments, and cardiac medications and interventions were recorded. Clinical outcomes measured included completion of prescribed cancer therapy, recovery of left ventricular (lv) function, and overall survival. Changes in lvef were calculated based on percentage differences from the baseline assessment. Per institution policy, most patients underwent lvef assessment by multigated acquisition imaging or the Simpson biplane method for echocardiography before commencing cancer therapy. Additional cardiac investigations were performed at the discretion of the treating physician or every 3–4 months for patients receiving trastuzumab-based therapy for early-stage breast cancer. Radiation therapy data were not complete in our patient population and so were excluded from the present study. Descriptive statistics are used to report patient characteristics and outcomes as means with standard deviation or medians with interquartile range.
RESULTS
Patient Demographics and Baseline Data
Between October 2008 and October 2015, 779 patients (516 women, 66%; 263 men, 34%) were referred to The Ottawa Hospital coc. Table I shows baseline patient characteristics, demographics, and the most common reasons for referral to the coc. Median age of the patients at the time of cancer diagnosis was 60 years (range: 18–90 years). The most common malignancy was breast cancer (n = 408, 52%), followed by gastrointestinal cancer (n = 131, 17%), genitourinary cancer (n = 90, 12%), hematologic cancer (n = 55, 7%), lung cancer (n = 40, 5%), and other less common tumour types (n = 55, 7%). Most patients (n = 544, 70%) had stage i–iii disease; 211 patients (27%) had metastatic disease. In 24 patients (3%), disease stage was unknown at diagnosis. Median baseline lvef in the study population was 58.7% (range: 20.0%–80.0%).
TABLE I.
Patient demographics and reason for referral to the cardio-oncology clinic
| Variable | Value |
|---|---|
| Patients (n) | 779 |
|
| |
| Age at diagnosis (years) | |
| Median | 60 |
| Range | 18–90 |
|
| |
| Sex [n (%)] | |
| Women | 516 (66) |
| Men | 263 (34) |
|
| |
| Primary tumour type [n (%)] | |
| Breast | 408 (52) |
| Gastrointestinal | 131 (17) |
| Genitourinary | 90 (12) |
| Hematological | 55 (7) |
| Lung | 40 (5) |
| Othera | 55 (7) |
|
| |
| Cardiac risk factors (n) | |
| Median | 2 |
| Range | 0–10 |
|
| |
| Risk factor types [n (%)] | |
| Smoking | 350 (45) |
| Hypertension | 337 (43) |
| Obesity (BMI > 30) | 218 (28) |
| Hypercholesterolemia | 208 (27) |
| Diabetes | 131 (17) |
| Coronary artery disease | 50 (6) |
|
| |
| Reasons for referral [n (%)] | |
| Decreased LVEF | 255 (33) |
| Pre-therapy assessment | 106 (14) |
| Arrhythmia | 106 (14) |
| Coronary artery disease | 74 (9) |
| Congestive heart failure | 56 (7) |
| Hypertension | 35 (4) |
| Otherb | 147 (19) |
Amyloidosis, gynecologic, musculoskeletal, neurologic, sarcoma, skin, thyroid.
Pericardial disease, valvular heart disease, cardiomyopathy.
LVEF = left ventricular ejection fraction.
Reasons for Referral
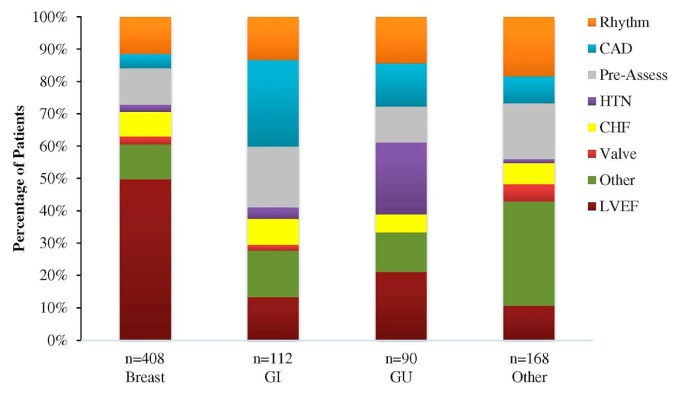
The most common reasons for referral were decreased lvef (n = 255, 33%), pre-chemotherapy cardiac risk assessment (106, 14%), and arrhythmias (106, 14%). Less-common reasons included coronary artery disease, heart failure, and hypertension. Patients had a median of 2 cardiovascular risk factors (range: 0–10 risk factors) at the time of referral to the coc, the most common risk factors being smoking (n = 350, 45%), hypertension (n = 337, 43% ), obesity (n = 218, 28%), and hypercholesterolemia (n = 208, 27%). As seen in Figure 1, most of the 408 breast cancer patients (n = 203, 50%) were referred because of a decrease in lvef. The most common reason for referral in gastrointestinal cancer patients was coronary artery disease (n = 30, 27%); in genitourinary patients, it was hypertension (n = 20, 22%).
FIGURE 1.
Reason for referral of patients to the cardio-oncology clinic based on their cancer type and expressed as a percentage of the total number of patients within their cancer type group. GI = gastrointestinal; GU = genitourinary; CAD = coronary artery disease; HTN = hypertension; CHF = congestive heart failure; LVEF = left ventricular ejection fraction.
Systemic Therapy
Most patients—430 in the first line, and 253 in the second line—received systemic therapy (Table II). First-line therapy included chemotherapy alone (n = 186, 43%), targeted therapy alone [monoclonal antibodies or tyrosine kinase inhibitors (n = 35, 8%)], and combined therapy (chemotherapy and targeted therapy, n = 209, 49%). In the second line, 253 patients received either or both of chemotherapy and targeted therapy. Of the 186 patients who received chemotherapy alone or in combination as first-line therapy, 92 (49%) were exposed to anthracycline-based regimens. The median dose of epirubicin was 280 mg/m2, and the median dose of doxorubicin was 231 mg/m2. For patients with advanced cancer, the median number of first-line cancer therapy cycles (including targeted therapy and chemotherapy) was 6 (range: 0–59 cycles); for second-line cancer therapy, the median was 6 cycles (range: 0–70 cycles).
TABLE II.
Treatment details for 430 patients receiving at least one line of systemic therapy
| Variable | Value |
|---|---|
| First-line therapy [n (%)] | |
| CTx alone | 186 (43) |
| Targeted therapya alone | 35 (8) |
| CTx and targeted therapy | 209 (49) |
|
| |
| Second-line therapy (n) | |
| CTx or targeted therapy, or both | 253 |
|
| |
| First-line CTx alone [n (%)] | 186 |
| Anthracycline-based | 92 (49) |
| Median anthracycline dose (mg/m2) | |
| Epirubicin | 280 |
| Doxorubicin | 231 |
|
| |
| Median cycles of cancer therapy in advanced cancer (n) | |
| First-line | |
| Median | 6 |
| Range | 0–59 |
| Second-line | |
| Median | 6 |
| Range | 0–70 |
For example, trastuzumab, sunitinib.
CTx = chemotherapy.
Rates of Cancer Therapy Completion
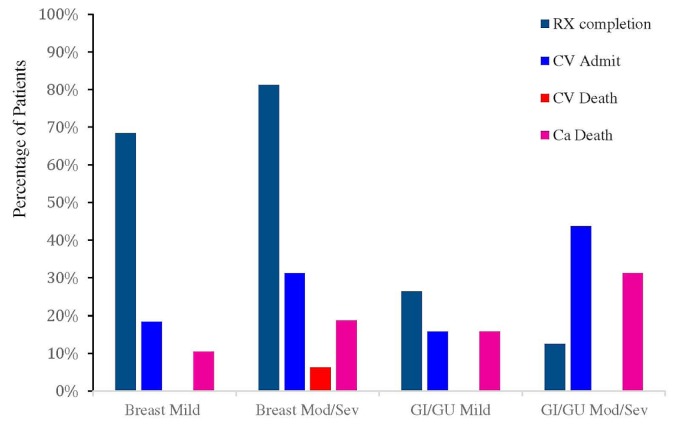
At October 2015, most patients who were prescribed chemotherapy (n = 611, 78%) had successfully completed their cancer therapy (369, 60%, Figure 2, Table III). Treatment was ongoing in 50 patients (8%), and 192 patients (31%) had discontinued their chemotherapy for various reasons, the most common being change in clinical status (for example, disease progression). Most breast cancer patients with lv dysfunction (n = 39, 72%), whether mild (lvef 40%–49.9%), moderate (lvef 30%–39.9%), or severe (lvef <30%), were able to complete their cancer treatment. In patients with gastrointestinal and genitourinary cancers and moderate or severe lv dysfunction, the most common reason for hospital admission was cardiovascular dysfunction (n = 10, 29%). One cardiac death occurred in the breast cancer group (2%), in a patient with severe lv dysfunction. With respect to rates of targeted therapy completion, 172 patients (45%) completed their first targeted therapy as prescribed, and 52 (14%) were receiving ongoing therapy (Table III). Another 150 (39%) discontinued their targeted therapy, most for cardiac causes (n = 64, 43%), followed by cancer progression or death (n = 48, 32%).
FIGURE 2.
Cardiovascular and cancer outcomes for patients with either a breast or a gastrointestinal (GI) or genitourinary (GU) cancer and either mild or moderate (Mod) or severe (Sev) left ventricular dysfunction. RX = treatment; CV = cardiovascular; Ca = cancer.
TABLE III.
Completion rates of systemic therapy
| Variable | Value |
|---|---|
| Chemotherapy [n (%)] | 611 |
| Completed course | 369 (60) |
| Before cardiac therapy start | 230 (62) |
| During cardiac therapy | 95 (26) |
| After cardiac therapy completion | 44 (12) |
| Resumed or ongoing | 50 (8) |
| Discontinued | 192 (31) |
|
| |
| Targeted therapy, 1st agent [n (%)] | 382 |
| Completed course | 172 (45) |
| Ongoing | 52 (14) |
| Discontinued | 150 (39) |
| For cardiac causes | 64 (43) |
| For cancer progression or death | 48 (32) |
| For other reasons | 38 (25) |
| Unknown | 8 (2) |
|
| |
| Targeted therapy, 2nd agent | 88 |
| Completed course | 7 (8) |
| Ongoing | 19 (22) |
| Discontinued | 58 (66) |
| For cardiac causes | 6 (10) |
| For cancer progression or death | 41 (71) |
| For other reasons | 11 (19) |
| Unknown | 4 (5) |
Outcomes
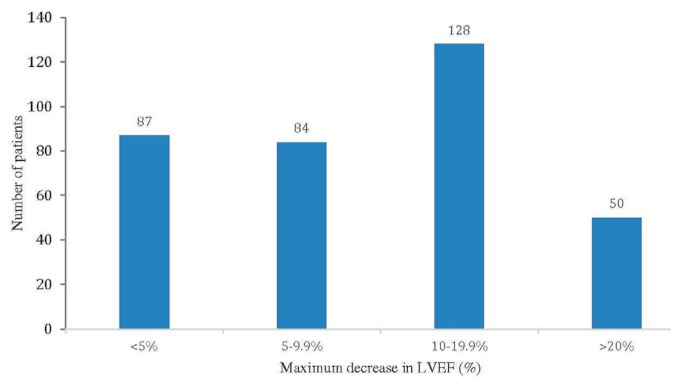
Table IV presents clinical outcomes. Most patients (n = 436, 56%), before commencing cancer treatment, underwent baseline cardiac imaging (echocardiography: 281 patients, 64%; multigated acquisition imaging: 140 patients, 32%; other or combined modalities: 15 patients, 3%). Subsequently, cardiac imaging was performed at the discretion of the treating physician, except in patients who were taking adjuvant trastuzumab, who underwent mandated cardiac assessments every 3–4 months per the product monograph. Median pre-chemotherapy lvef was 61% (range: 30%–88.3%). A large proportion of the 502 patients who experienced some change in lvef experienced at least 1 episode of decrease in lvef from their baseline (n = 349, 70%). In 128 of those patients (37%), the decrease was in the 10%–19.9% range, and in 50 patients (14%), the decrease in lvef was 20% or more (Figure 3, Table IV). Considering all lvef results at any point in time (excluding baseline lv function), 176 patients (23%) had a measured lvef less than 50%, and 122 patients (16%) had a measured lvef less than 45%. Of the 349 patients experiencing any decline in lvef, 139 experienced recovery of lvef to baseline (40%), and a further 41 experienced partial recovery (12%). Further decline in lvef occurred in 56 patients (16%), and 113 patients (32%) achieved a stable lvef.
TABLE IV.
Cardiac outcomes
| Variable | Value |
|---|---|
| Pre-CTx LVEF assessment [n (%)] | 436 |
| Echocardiography | 281 (64) |
| Multigated acquisition imaging | 140 (32) |
| Other or combined modalities | 15 (3) |
|
| |
| Pre-CTx LVEF (%) | |
| Median | 61 |
| Range | 30–88.3 |
|
| |
| Change in LVEF [n (%)] | 502 |
| No significant decline | 153 (30) |
| Any decline | 349 (70) |
| <5% | 87 (25) |
| 5%–9.9% | 84 (24) |
| 10%–19.9% | 128 (37) |
| ≥20% | 50 (14) |
|
| |
| LVEF outcome [n (%)] | 349 |
| Full recovery | 139 (40) |
| Partial recovery | 41 (12) |
| Stable | 113 (32) |
| Progressive decline | 56 (16) |
|
| |
| Cardiac medications initiated [n (%)] | 322 |
| ACE inhibitor | 57 (18) |
| Beta-blocker | 38 (12) |
| ACE inhibitor and beta-blocker | 46 (14) |
| Multiple | 181 (56) |
CTx = chemotherapy; LVEF = left ventricular ejection fraction; ACE = angiotensin-converting enzyme.
FIGURE 3.
Maximum decrease in left ventricular ejection fraction (LVEF) since initial echocardiography in patients referred to the cardiooncology clinic.
At the time of cardiac consultation in the coc, 432 patients (55%) were taking cardiac medications: 27 (6%), an angiotensin-converting enzyme inhibitor (acei) or its equivalent (an angiotensin ii receptor blocker) alone; 22 (5%), a beta-blocker alone; 9 (2%), a combination of an acei and a beta-blocker; and the remaining 374 (87%), various combinations of cardiac medications. The coc referral and consultation prompted the addition or initiation of 1 or more cardiac medications in 322 patients (41%): 57 (18%), an acei; 38 (12%), a beta-blocker; and 46 (14%), both an acei and a beta blocker. The remaining 181 patients (56%) were treated with various cardiac medications. Of all the patients referred to the coc, 703 (90%) were optimized on cardiac medications.
At October 2015, most patients (n = 643, 83%) were still alive, and 132 patients (17%) had died. Clinical outcome was unknown for 4 patients (0.5%) because of incomplete follow-up (Table V). Most deaths were attributable to cancer progression (n = 114, 86%); the rest were attributed to cardiac causes [for example, atrial fibrillation, pericardial effusion, diastolic heart failure (5%)], and other causes (9%).
TABLE V.
Outcomes in 779 patients
| Outcome | Value [n (%)] |
|---|---|
| Living | 643 (83) |
|
| |
| Deceased | 132 (17) |
| From disease progression | 114 (86) |
| From cardiac causes | 6 (5) |
| From other causes | 12 (9) |
|
| |
| Lost to follow-up | 4 (0.5) |
DISCUSSION
Since the early 2000s, cardio-oncology has evolved as a subspecialty in medicine, with the goal of coordinating the delivery of cancer therapy while simultaneously optimizing cardiovascular health. In 2008, a dedicated coc was established at The Ottawa Hospital to optimize the care of cancer patients at our centre without compromising short- and long-term cardiovascular health. Although breast cancer patients initially represented the greatest proportion of referrals to the clinic, the emergence of other therapies with the potential for cardiotoxicity has led to a more diverse referral population.
To minimize the short- and long-term sequelae of cancer treatment, it is essential to support the partnership between medical oncology, cardiology, and other health care professions tasked with caring for cancer patients who have, or who are at risk of, cancer therapy–related cardiac dysfunction. Despite growing interest in the subspecialty of cardio-oncology, most cancer patients in Canada and globally are not being screened for cardiovascular risk factors before initiation of potentially cardiotoxic cancer treatment.
There are many benefits to implementing a coc. Patients receive expedited access to a cardiologist who has knowledge of the impact of cancer drugs on cardiovascular health. Cardio-oncologists will risk-stratify, diagnose, and manage patients with cardiovascular disease—either underlying or secondary to cancer therapy. Growing interest in cardio-oncology has led to the emergence of clinics across the globe; however, there are currently no accepted standards defining the structure, goals, or outcome measures for those programs. Quality measures have not been clearly defined in the literature, but optimizing cardiac health while continuing anticancer therapy should be the primary goal. In our experience, most patients referred to our clinic were able to complete their prescribed cancer therapy either with temporary interruptions in cancer treatment or with appropriate interventions for the concurrent management of their cardiovascular disease (or both).
We readily acknowledge that the present study has limitations. The follow-up period is relatively short and a comparison group (not referred to a coc) is lacking. To include a comparison group would involve acquiring data from another centre, a large task that comes with its own limitations. Alternatively, a potential comparison cohort of cancer patients not referred to the coc at our own centre could have included breast cancer patients diagnosed before 2008. Using that group would have been challenging given the advances in cancer treatments since 2008; finding adequate controls treated with similar systemic therapies would be unlikely.
Our dataset has inherent bias, given that patients referred to the coc were more likely to have pre-existing cardiac dysfunction. However, we are presenting only descriptive data, and we do not believe that such bias negatively affects our results. We were unable to include data relating to left-sided and mediastinal radiation. Those data are being collected, but they were not robust enough to be included in the present analysis.
Cardiac imaging was limited to multigated acquisition imaging and echocardiography. Detection of cardiotoxicity has evolved in recent years to include echocardiography with myocardial strain imaging and serum biomarkers (brain natriuretic peptide and troponin); however, those tools require further standardization before they can be considered the standard of care17.
We did not collect data about patient satisfaction from patients referred to our clinic. That measure should be one of the quality metrics for established and emerging cardio-oncology programs.
Interest in the development of multidisciplinary clinical programs that offer cardio-oncology services is growing. Pareek et al.18 recently reported their 5-year experience of patients referred to a coc in the United Kingdom. In their descriptive analysis, they found that implementation of standardized protocols can achieve optimization of the cardiovascular status of patients at high baseline risk or with established myocardial toxicity, leading to high levels of cancer treatment.
Although randomized evidence demonstrating the benefit of cocs is nonexistent, expert opinion supports their implementation. The Canadian Cardiovascular Society cardio-oncology guideline endorses the use of a multidisciplinary approach in this patient population14. Strategies for organizing and implementing cardio-oncology services was recently published by the European Society of Cardiology14. Around the world, cocs are becoming commonplace; however, no standardized quality indicators to evaluate their benefit have been developed. The data presented here are important in demonstrating merit for the implementation of cocs and their role in the multidisciplinary care of cancer patients.
CONCLUSIONS
Given the results of our research, the present work represents a call to action for established and emerging multidisciplinary cocs globally to report data for patient demographics, referral patterns, cardiac treatment and outcomes, and patient experiences. Such initiatives will further advance knowledge and understanding about how to provide optimal care for this patient population— especially in the context of the rapidly evolving field of novel antineoplastic agents.
ACKNOWLEDGMENTS
We acknowledge Freya Kelly for her contribution of data entry for the cardio-oncology clinic.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. 2014;89:1287–306. doi: 10.1016/j.mayocp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States, Department of Health and Human Services, Centers for Disease Control and Prevention (cdc) CDC National Health Report Highlights. Atlanta, GA: cdc; n.d. [Available online at: https://www.cdc.gov/healthreport/publications/compendium.pdf; cited 15 April 2018] [Google Scholar]
- 4.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:4–14. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–36. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anker MS, Lena A, Hadzibegovic S, et al. on behalf of the Heart Failure Association Cardio-Oncology Study Group of the European Society of Cardiology. Modern-day cardio-oncology: a report from the “Heart Failure and World Congress on Acute Heart Failure 2018”. ESC Heart Fail. 2018;5:1083–91. doi: 10.1002/ehf2.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong J, Chen H. Cardiotoxicity of anticancer therapeutics. Front Cardiovasc Med. 2018;5:9. doi: 10.3389/fcvm.2018.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–56. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 12.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–58. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 13.Lenihan DJ, Westcott G. Cardio-oncology: a tremendous opportunity to improve patient care. Future Oncol. 2015;11:2007–10. doi: 10.2217/fon.15.99. [DOI] [PubMed] [Google Scholar]
- 14.Okwuosa TM, Barac A. Burgeoning cardio-oncology programs: challenges and opportunities for early career cardiologists/faculty directors. J Am Coll Cardiol. 2015;66:1193–7. doi: 10.1016/j.jacc.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Lancellotti P, Suter TM, López-Fernández T, et al. Cardio-oncology services: rationale, organization, and implementation: a report from the esc Cardio-Oncology Council. Eur Heart J. 2018 doi: 10.1093/eurheartj/ehy453. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Sulpher J, Johnson C, Turek M, et al. Ottawa cardiac oncology program wins 2013 Cancer Quality Council of Ontario Innovation award. Curr Oncol. 2014;21:150. doi: 10.3747/co.21.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armenian SH, Lacchetti C, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline summary. J Oncol Pract. 2017;13:270–5. doi: 10.1200/JOP.2016.018770. [DOI] [PubMed] [Google Scholar]
- 18.Pareek N, Cevallos J, Moliner P, et al. Activity and outcomes of a cardio-oncology service in the United Kingdom—a five-year experience. Eur J Heart Fail. 2018;20:1721–31. doi: 10.1002/ejhf.1292. [DOI] [PubMed] [Google Scholar]