Abstract
Background
Commonly used first-line (1L) chemotherapies for patients with advanced squamous-cell lung cancer (scc) include gemcitabine–platinum (gp), nab-paclitaxel–carboplatin (nabpc), and sb-paclitaxel–carboplatin (sbpc) regimens. However, no head-to-head trials have compared those treatments. In the present study, we compared the efficacy of 1L gp, nabpc, and sbpc in patients with scc and in patients with scc who subsequently received second-line (2L) immunotherapy.
Methods
Medical records of patients who initiated the 1L treatments of interest between June 2014 and October 2015 were reviewed by 132 participating physicians. Kaplan–Meier curves were used to evaluate overall survival (os), progression-free survival (pfs), and treatment discontinuation (td), and then Cox proportional hazards regression was used to compare the results between the cohorts.
Results
Medical records of 458 patients with scc receiving gp (n = 139), nabpc (n = 159), or sbpc (n = 160) as 1L therapy were reviewed. Median os was longer with nabpc (23.9 months) than with gp (16.9 months; adjusted hazard ratio vs. nabpc: 1.55; p < 0.05) and with sbpc (18.3 months; adjusted hazard ratio: 1.42; p = 0.10). No differences were observed in pfs (median pfs: 8.8, 8.0, and 7.6 months for gp, nabpc, and sbpc respectively; log-rank p = 0.76) or in td (median td: 5.5, 5.7, and 4.6 months respectively; p = 0.65). For patients who subsequently received 2L immunotherapy, no differences in os were observed (median os: 27.3, 25.0, and 23.0 months respectively; p = 0.59).
Conclusions
In a nationwide sample of scc patients, longer median os was associated with 1L nabpc than with gp and sbpc. Median os for all 1L agents considered was similar in the subgroup of patients who sequenced to a 2L immunotherapy.
Keywords: Non-small-cell lung cancer, squamous cell lung cancer, immunotherapy, platinum doublet chemotherapy, comparative analyses, chart reviews
BACKGROUND
Since about 2010, unprecedented scientific advances in treatments for non-small-cell lung cancer (nsclc) have translated into meaningful improvements in clinical outcomes. Many of those gains have been predicated on a better understanding of tumour biology, including the identification of driver mutations that allow for patients to receive novel genotype-driven therapies. In addition, firmer insight into tumour immunology and tumour escape mechanisms has led to the development of immune checkpoint inhibitors (antibodies against PD-1 and its ligand PD-L1)1–3. Targeted and immunotherapy strategies have both been routinely incorporated into the frontline management of patients with advanced adenocarcinoma, but to date, patients with squamous cell lung cancer (scc) have not shared in many of those advances.
Currently, platinum doublet chemotherapy remains the standard-of-care first-line (1L) treatment for patients with advanced scc whose PD-L1 tumour proportion score is less than 50%4–6. Trials evaluating various platinum regimens in advanced nsclc have led to competing chemotherapeutic strategies for scc. Gemcitabine plus a platinum agent (gp) is a commonly used regimen, and its efficacy in the treatment of scc has been demonstrated in several randomized trials7–9. Additionally, evidence from a phase iii trial showed that the objective response rate in patients with scc improves significantly with nab-paclitaxel–carboplatin (nabpc) compared with sb-paclitaxel–carboplatin (sbpc)10,11. Notably, the trial found no statistically significant difference in overall survival (os) between the two regimens.
Unlike the situation in adenocarcinoma, immunotherapy agents in scc are currently restricted to second-line (2L) use and have had dramatic effects on patient outcomes2. Specifically, single-agent nivolumab was found to be superior to docetaxel in terms of response rate, progression-free survival (pfs), and os in patients with scc who had progressed on platinum-based chemotherapy12. Other trials evaluating pembrolizumab13 and atezolizumab14,15 have demonstrated similar improvements in outcomes. A recent study found that, compared with 1L chemotherapy alone, therapy with pembrolizumab in combination with chemotherapy (nabpc or sbpc) was associated with significantly longer os and pfs in patients with metastatic squamous nsclc16.
Notwithstanding the aforementioned trials, head-to-head trials and prospective data comparing the efficacy and safety of the various 1L chemotherapy options for patients with scc—and the subsequent effects on outcomes for patients who go on to receive 2L immunotherapy—are lacking. Real-world data for outcomes associated with immunotherapy after platinum-based therapies in patients with scc are also limited, with only two recent small studies evaluating outcomes for those patients17,18.
Given the paucity of data addressing the optimal platinum doublet in patients with scc, we collected real-world data for patients with scc treated in the United States in community and academic practices alike. We characterized treatment patterns and compared os, pfs, and treatment discontinuation (td) in patients with scc who initiated treatment with 1L gp, nabpc, or sbpc. Importantly, we compared the os associated with various treatment sequences in the subset of patients who were sequenced to 2L immunotherapy regimens. To our knowledge, this work represents one of the largest retrospective analyses performed in the United States to compare various standard-of-care chemotherapies in patients with scc, and it is the first to compare the os associated with treatment sequences.
METHODS
Study Design
In a retrospective cohort design, data were collected from the medical records of patients with scc, thus representing real-world clinical practice—that is, care not driven by a study protocol.
Physician Recruitment
Medical oncologists were recruited from a nationwide panel of U.S. physicians with verified credentials who had pre-registered to be invited for participation in retrospective chart reviews. This address-based panel was maintained by Schlesinger Associates. To maintain the objectivity of patient sampling and data entry, participating physicians were not informed of the research objectives or the identity of the study sponsor. Physicians were instructed to identify all charts meeting the selection criteria (Table I) and to extract data from up to 5 randomly selected charts. Physicians were characterized in terms of their geographic location, medical specialty, and practice setting.
TABLE I.
Study inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
Patient Inclusion and Exclusion Criteria
To be eligible for retrospective collection of data from their medical records, patients had to meet the criteria in Table I. Data for included patients were extracted by the participating physicians and were entered into a secure electronic case report form. Data collected in the electronic case report form were personally non-identifiable. The electronic case report form and study synopsis were reviewed by the New England Institutional Review Board, which granted the current study an exemption from a full review given that it was a retrospective non-interventional study of non-identifiable data.
Sample Selection
Patients were categorized into 3 study cohorts: patients who started on gp, nabpc, or sbpc as 1L therapy from 1 June 2014 to 31 October 2015. This treatment initiation period was designed to ensure a minimum follow-up time of 1 year after initiation of 1L therapy and also opportunities for patients to receive immunotherapies in the 2L setting after U.S. Food and Drug Administration approvals. Because atezolizumab was approved for 2L use in 2016, no data were collected for that agent.
The study used a sequential approach to data collection, with two waves of patients obtained from two waves of invitations sent to non-overlapping sets of physicians. Wave 1 (43.9% of the entire sample) included patients in the 3 cohorts already defined, regardless of 2L treatment. The sample in this first wave therefore served to estimate the real-world frequencies of various treatment sequences. Wave 2 included only patients who received 2L immunotherapies (nivolumab or pembrolizumab). The goal of that sampling design was to enrich the sample size of patients receiving those immunotherapies in 2L treatment to better assess the outcomes associated with those specific treatment sequences. When analyzing the data, the proportions of patients receiving various treatment sequences in wave 1 were used to derive sampling weights to adjust for the imposed oversampling of patients receiving 2L immunotherapy in wave 2.
Baseline patient characteristics assessed before the initiation of 1L therapy included demographics, comorbidities, metastatic sites, performance status, laboratory measures, and prior treatment. The main outcomes of interest were os, defined as the time from 1L initiation to death from any cause, and pfs, measured as the time from 1L initiation to first clinical progression (including death). To capture the os information, physicians were asked to review charts and to provide a date of death, if such information was available. If physicians did not know a patient’s survival status at the time of chart abstraction, they were instructed to enter “unknown” for that question. To collect the pfs information, physicians were asked for the date of clinical progression based on their evaluation, if any, after 1L initiation, and the clinical evidence used to define progression (for example, radiographic evidence, Response Evaluation Criteria in Solid Tumors version 1.1 progressive disease criteria, cancer-related symptoms). Treatment discontinuation was defined as the time from 1L initiation to the date of 1L discontinuation (discontinuation was defined as cessation of both agents in the regimen together), including discontinuation because of death. Patients with “unknown” or missing death or progression data were excluded from the os and pfs analyses.
Statistical Analyses
Charts describing the percentages of patients receiving each possible 2-line treatment sequence are presented. Percentages incorporate the sampling weights obtained from wave 1 to recover the true real-world distribution of treatment sequences.
Means and standard deviations were calculated for continuous variables; counts and percentages were calculated for categorical variables. Weighted analyses of variance (for continuous variables) and weighted chi-square tests (for categorical variables) were used to compare baseline characteristics in the 1L study cohorts. Unadjusted comparisons of time-to-event outcomes between the 1L study cohorts were summarized using survival curves, with time-to-event censored at last contact. The survival curves corresponding to each 1L therapy were based on the weighted Nelson–Aalen estimator; survival curves for os by treatment sequence were based on the Kaplan–Meier estimator. By definition, all patients in the sequencing analyses received 2L immunotherapy, and therefore the outcomes associated with those sequences do not need adjustment using the sampling weights described earlier. Adjusted comparisons were based on multivariable Cox proportional hazards models, with adjustment for baseline characteristics that are important prognostic factors for outcomes, including age at 1L therapy initiation, sex, race, number of comorbidities, number of metastatic sites, duration of scc at 1L therapy initiation (how long patients had been diagnosed before initiation of 1L therapy), and Eastern Cooperative Oncology Group score. The proportional hazards assumption was tested for the models. All statistical analyses of the 1L cohorts incorporated sampling weights derived from wave 1 to recover the real-world distribution of treatment sequences. A subgroup analysis of os was performed for patients who received an immunotherapy as 2L treatment. All analyses were conducted using the R statistical software application (version 3.3: The R Foundation, Vienna, Austria). A 2-tailed α of 0.05 was used to assess statistical significance.
RESULTS
Baseline Characteristics
The 132 oncologists who participated in the study had an average practice experience of 15 years. Most (65.9%) were in community practice as opposed to academic practice (25.0%) or a hospital setting (9.1%). Charts were reviewed for 458 patients receiving either gp, nabpc, or sbpc as 1L therapy for scc.
Table II describes baseline patient characteristics. Average age at initiation of 1L treatment was 64 years, with an average follow-up time of 15 months. The demographics, performance score, and number of comorbidities were balanced between the 3 cohorts. Patients receiving 1L nabpc were less likely to have multiple metastatic sites and more likely to have autoimmune diseases. Patients receiving 1L gp were more likely to have cardiovascular diseases, and patients receiving 1L sbpc were more likely to have cerebrovascular diseases. The differences between the cohorts in the proportion of patients with brain or central nervous system metastasis were not statistically significant. Similarly, the proportion of patients who had ever smoked (patients who had smoked for at least 1 year in total) was not significantly different between the cohorts.
TABLE II.
Comparison of baseline characteristics for first-line cohorts with squamous non-small-cell lung cancer
| Characteristic | Treatment cohort | p Valuea | |||
|---|---|---|---|---|---|
|
|
|||||
| Overall | GP | nabPC | sbPC | ||
| Patients (n) | 458 | 139 | 159 | 160 | |
|
| |||||
| Mean age (years)b | 64±9 | 64±9 | 63±10 | 64±8 | 0.52 |
|
| |||||
| Follow-up time (months) | 15±8 | 15±7 | 15±7 | 16±8 | 0.55 |
|
| |||||
| Duration of SCC (months)b | 2±5 | 2±4 | 1±3 | 2±6 | 0.69 |
|
| |||||
| Sex (%) | |||||
| Women | 35 | 28 | 35 | 41 | 0.19 |
| Men | 65 | 72 | 65 | 59 | |
|
| |||||
| Ethnicity (%) | |||||
| White | 68 | 66 | 74 | 64 | |
| Black or African American | 27 | 28 | 22 | 31 | 0.57 |
| Asian or other | 5 | 5 | 4 | 5 | |
|
| |||||
| Metastases | |||||
| Mean number of sites (n) | 3±3 | 3±3 | 3±2 | 4±4 | 0.43 |
| Multiple sites (%) | 83 | 88 | 77 | 86 | <0.05 |
| Single site (%) | 17 | 12 | 23 | 14 | |
| Brain or CNS (%) | 8 | 8 | 7 | 11 | 0.56 |
|
| |||||
| ECOG PS (%) | |||||
| 0 | 16 | 16 | 17 | 15 | |
| 1 | 59 | 53 | 61 | 61 | 0.58 |
| >2 | 25 | 31 | 21 | 23 | |
|
| |||||
| Mean number of comorbidities (n) | 2±1 | 2±1 | 1±1 | 2±2 | 0.45 |
| Autoimmune (%) | 2 | 1 | 4 | 0 | <0.01 |
| Cardiovascular (%) | 22 | 31 | 13 | 25 | <0.01 |
| Cerebrovascular (%) | 10 | 4 | 8 | 16 | <0.05 |
|
| |||||
| Ever-smoker | 44 | 43 | 40 | 48 | 0.52 |
Boldface type indicates significance.
At first-line therapy.
GP = gemcitabine–platinum agent; nabPC = nab-paclitaxel–carboplatin; sbPC = sb-paclitaxel–carboplatin; SCC = squamous cell carcinoma; CNS = central nervous system; ECOG PS = Eastern cooperative Oncology Group performance status.
First-Line Outcomes
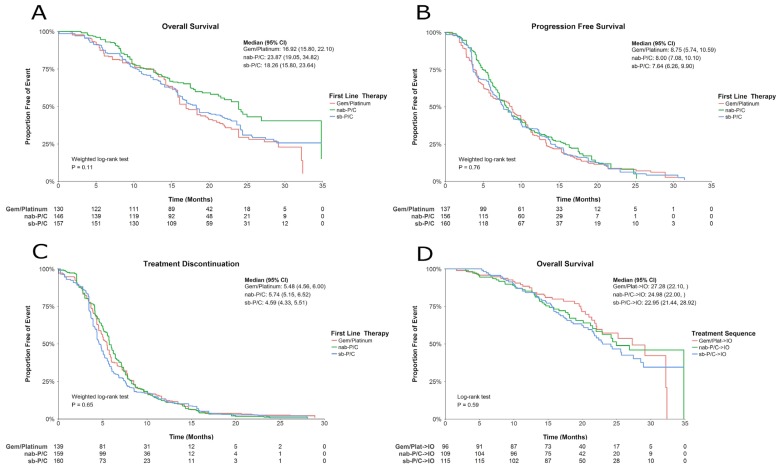
In unadjusted analyses, nabpc, compared with gp and sbpc, was associated with a sizable and borderline statistically significant reduction in risk of death [Figure 1(A)]. The unadjusted hazard ratios (hrs) for gp compared with nabpc and for sbpc compared with nabpc were 1.49 (p = 0.05) and 1.38 (p = 0.11) respectively. Median os durations were 16.9 months [95% confidence interval (ci): 15.8 months to 22.1 months], 23.9 months (95% ci: 19.1 months to 34.8 months), and 18.3 months (95% ci: 15.8 months to 23.6 months) for patients in the gp, nabpc, and sbpc cohorts respectively. In the analyses adjusting for baseline covariates, presented in Table III, the hr for gp compared with nabpc was significant (hr: 1.55; p < 0.05), and the hr for sbpc compared with nabpc was nonsignificant (hr: 1.42; p = 0.10).
FIGURE 1.
Survival curves (A) for overall survival by first-line therapy; (B) for progression-free survival by first-line therapy; (C) for treatment discontinuation by first-line therapy; and (D) for overall survival by treatment sequence. Gem/Platinum = gemcitabine plus a platinum agent; nab-P/C = nab-paclitaxel–carboplatin; sb-P/C = sb-paclitaxel–carboplatin; CI = confidence interval; IO = immunotherapy (nivolumab or pembrolizumab).
TABLE III.
Adjusted analysis for selected outcomes in first-line therapy for squamous non-small-cell lung cancer
| Outcome variable | Comparator | HR | 95% CI | p Valuea |
|---|---|---|---|---|
| Overall survival | ||||
| nabPC | GP | 1.55 | 1.01 to 2.40 | <0.05 |
| sbPC | 1.42 | 0.93 to 2.16 | 0.10 | |
| Age (years)b | 1.04 | 1.02 to 1.06 | <0.001 | |
| Male sex | Female sex | 0.80 | 0.56 to 1.13 | 0.20 |
| White ethnicity | Black or African American | 1.32 | 0.91 to 1.91 | 0.14 |
| Asian or other | 0.83 | 0.30 to 2.30 | 0.72 | |
| Duration of SCC (months)b | 0.87 | 0.79 to 0.97 | <0.01 | |
| Metastases (sites) | 1.04 | 1.00 to 1.08 | 0.05 | |
| ECOG performance status 0 | 1 | 1.72 | 1.00 to 2.97 | 0.05 |
| 2–4 | 3.31 | 1.79 to 6.11 | <0.001 | |
| Comorbidities (n) | 1.14 | 1.00 to 1.29 | <0.05 | |
|
| ||||
| Progression-free survival | ||||
| nabPC | Gem/platinum | 1.05 | 0.76 to 1.44 | 0.78 |
| sbPC | 1.06 | 0.81 to 1.40 | 0.67 | |
| Age (years)b | 1.01 | 1.00 to 1.03 | 0.11 | |
| Male sex | Female sex | 0.87 | 0.67 to 1.12 | 0.28 |
| White ethnicity | Black or African American | 1.29 | 0.96 to 1.73 | 0.09 |
| Asian or other | 1.34 | 0.89 to 2.03 | 0.16 | |
| Duration of SCC (months)b | 0.97 | 0.93 to 1.00 | <0.05 | |
| Metastases (sites) | 1.01 | 0.99 to 1.04 | 0.25 | |
| ECOG performance status 0 | 1 | 1.29 | 0.87 to 1.91 | 0.21 |
| 2–4 | 1.74 | 1.07 to 2.83 | <0.05 | |
| Comorbidities (n) | 1.17 | 1.06 to 1.30 | <0.01 | |
|
| ||||
| Treatment discontinuation | ||||
| nabPC | GP | 1.02 | 0.78 to 1.34 | 0.88 |
| sbPC | 1.16 | 0.88 to 1.53 | 0.30 | |
| Age (years)b | 1.01 | 1.00 to 1.03 | <0.05 | |
| Male sex | Female sex | 0.78 | 0.62 to 0.99 | <0.05 |
| White ethnicity | Black or African American | 1.19 | 0.94 to 1.51 | 0.14 |
| Asian or other | 1.15 | 0.70 to 1.88 | 0.58 | |
| Duration of SCC (months)b | 0.93 | 0.90 to 0.96 | <0.001 | |
| Metastases (sites) | 1.02 | 0.99 to 1.05 | 0.18 | |
| ECOG performance status 0 | 1 | 1.01 | 0.75 to 1.36 | 0.94 |
| 2–4 | 1.24 | 0.87 to 1.78 | 0.23 | |
| Comorbidities (n) | 1.09 | 0.99 to 1.20 | 0.08 | |
Boldface type indicates significance.
At first-line therapy.
HR = hazard ratio; CI = confidence interval; nabPC = nab-paclitaxel–carboplatin; GP = gemcitabine–platinum agent; sbPC = sb-paclitaxel–carboplatin; SCC = squamous cell carcinoma; ECOG = Eastern cooperative Oncology Group.
A survival analysis was also conducted for the subgroup of patients who did not receive 2L immunotherapy. The results indicated that 1L nabpc was associated with an improvement in median os duration (approximately 25 months vs. 15 months in the other two cohorts, data not shown), which is in line with the results in the overall population [Figure 1(A)]. Similarly, an analysis of the subgroup of patients who did not receive any active 2L therapy suggested that, compared with the other two 1L therapies, 1L nabpc was again associated with an improvement in median os.
Median pfs durations were 8.8 months (95% ci: 5.7 months to 10.6 months), 8.0 months (95% ci: 7.1 months to 10.1 months), and 7.6 months (95% ci: 6.3 months to 9.9 months) for patients treated with 1L gp, nabpc, and sbpc respectively. Based on the unadjusted analyses, the treatment groups showed no statistically significant pfs differences [Figure 1(B)], with unadjusted hrs of 1.12 (p = 0.48) and 1.09 (p = 0.57) for gp compared with nabpc and sbpc compared with nabpc respectively. Similar results were obtained in the adjusted analyses presented in Table III.
Median tds were 5.5 months (95% ci: 4.6 months to 6.0 months), 5.7 months (95% ci: 5.2 months to 6.5 months), and 4.6 months (95% ci: 4.3 months to 5.5 months) in the gp, nabpc, and sbpc cohorts respectively. In unadjusted analyses, no statistically significant differences in td were observed between the treatment groups [Figure 1(C)], with unadjusted hrs of 1.00 (p = 1.00) and 1.13 (p = 0.37) for gp compared with nabpc and for sbpc compared with nabpc respectively. Results in the adjusted analyses were similar (Table III).
Sequencing Outcomes
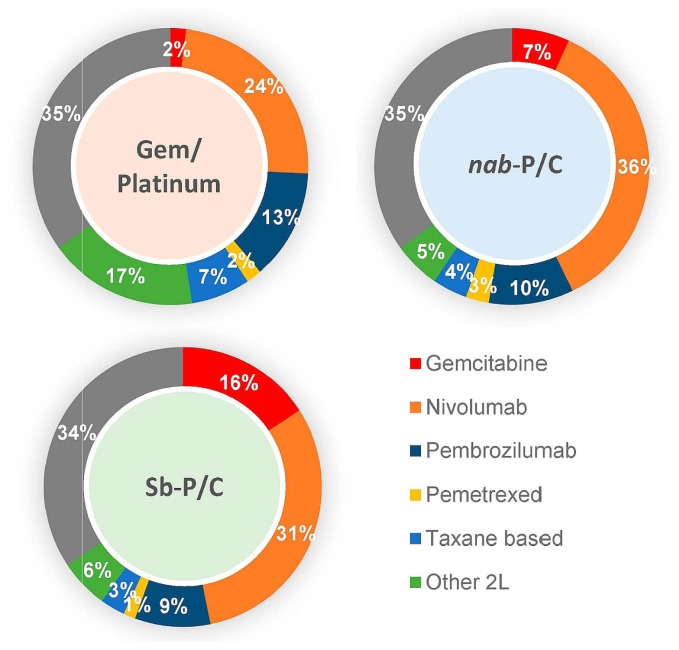
A checkpoint inhibitor was the most commonly used 2L treatment: 36.8%, 45.9%, and 39.8% respectively of patients in the gp, nabpc, and sbpc cohorts (p = 0.34) were treated with 2L immunotherapy. Other 2L treatments were mainly chemotherapy, including gemcitabine and pemetrexed (Figure 2). Based on the unadjusted analyses, no statistically significant differences in os were observed for patients who received both 1L chemotherapy and 2L immunotherapy [Figure 1(D)]. Median os durations were, respectively, 27.3 months (95% ci: 22.10 months to not reached), 25.0 months (95% ci: 22.00 months to not reached), and 23.0 months (95% ci: 21.4 months to 28.9 months) for patients receiving gp, nabpc, and sbpc who sequenced to immunotherapy. The unadjusted hrs were 0.90 (p = 0.63) and 1.11 (p = 0.59) for the gp cohort compared with the nabpc cohort and for the sbpc cohort compared with the nabpc cohort respectively. Similarly, the adjusted analyses suggested no significant difference between the groups (Table IV). Notably, the statistical powers for those comparisons are lower than those for the 1L cohorts, because the sample sizes for the 2L immunotherapy sequencing cohorts were approximately 60% smaller [sample sizes ranging from 96 to 115 patients, Figure 1(D)].
FIGURE 2.
Treatment sequences. The inner cycle depicts first-line treatment; the outer cycle depicts second-line treatment. Gem/Platinum = gemcitabine plus a platinum agent; Nab-P/C = nab-paclitaxel–carboplatin; Sb-P/C = sb-paclitaxel–carboplatin; Other = other (active) drug treatment; 2L = second line.
TABLE IV.
Adjusted analysis for overall survival in squamous non-small-cell lung cancer, by treatment sequence
| Characteristic | Comparator | HR | 95% CI | p Valuea |
|---|---|---|---|---|
| nabPC→IO | GP→IO | 0.98 | 0.63 to 1.55 | 0.95 |
| sbPC→IO | 1.08 | 0.72 to 1.62 | 0.72 | |
|
| ||||
| Age (years)b | 1.03 | 1.01 to 1.05 | <0.01 | |
|
| ||||
| Male sex | Female sex | 0.97 | 0.67 to 1.43 | 0.89 |
|
| ||||
| White ethnicity | Black or African American | 1.37 | 0.92 to 2.03 | 0.12 |
| Asian or other | 1.20 | 0.57 to 2.52 | 0.63 | |
|
| ||||
| Duration of SCC (months)b | 0.84 | 0.73 to 0.98 | <0.05 | |
|
| ||||
| Metastases (sites) | 1.00 | 0.89 to 1.12 | 0.99 | |
|
| ||||
| ECOG performance status 0 | 1 | 1.48 | 0.84 to 2.61 | 0.17 |
| 2–4 | 2.77 | 1.50 to 5.12 | <0.01 | |
|
| ||||
| Comorbidities (n) | 1.11 | 0.98 to 1.25 | 0.11 | |
Boldface type indicates significance.
At first-line therapy.
HR = hazard ratio; CI = confidence interval; nabPC = nab-paclitaxel–carboplatin; GP = gemcitabine–platinum agent; IO = immunotherapy (nivolumab or pembrolizumab); sbPC = sb-paclitaxel–carboplatin; SCC = squamous cell carcinoma.
DISCUSSION
Treatment patterns in advanced nsclc are rapidly evolving as novel immunotherapies become available. However, data evaluating the real-world effectiveness of 1L chemotherapy treatments followed by immunotherapy, specifically in scc patients, are lacking. In the present retrospective cohort study, we compared outcomes associated with various standard-of-care chemotherapy regimens in scc patients and in a subgroup of those patients who sequenced to immunotherapy. To our knowledge, this study represents the first real-world comparison of the treatment patterns and outcomes associated with the regimens of interest and subsequent immunotherapy in a large and representative sample of U.S. oncology practices.
Results indicated that, compared with gp and sbpc as 1L treatment, nabpc was associated with a sizable reduction in the risk of death and that the reduction was statistically significant when napPC was compared with gp. No significant differences in pfs or td between the 3 1L cohorts were identified. The median os durations associated with gp, nabpc, and sbpc for all patients in the 1L cohorts were longer than those reported in prospective pivotal trials11,19. That finding might be explained by the fact that a substantial number of patients in each cohort received 2L immunotherapy (37%, 46%, and 40% respectively), a class of agents not commonly used when the pivotal trials were published. Given the impressive outcomes observed with checkpoint inhibitors in randomized clinical trials, it is not surprising that no survival differences were evident for patients in the 3 cohorts in our analysis who received both chemotherapy and immunotherapy. Additionally, the longer os durations could partly be the result of recall bias, given the retrospective approach to the sampling procedure (discussed in more detail shortly).
Two recent real-world studies also assessed the effectiveness of 1L nabpc compared with sbpc in metastatic nsclc20 and of 1L nabpc compared with gp in scc21, finding that nabpc was more effective than sbpc and gp in terms of os, although the difference was not statistically significant after adjustment for differences in baseline characteristics. Notably, the squamous populations in both studies were relatively small (less than half the size of the sample in the present study), which limits the conclusions that can be drawn from those efforts. Moreover, neither of the studies assessed the survival outcomes for patients who went on to 2L immunotherapy, underscoring the contributions of our study. Previous studies17,18 have also examined the real-world efficacy of immunotherapies as 2L treatment; however, it is difficult to draw comparisons between our results and the findings reported in the latter studies because those studies did not focus on the efficacy of treatment sequences, but rather on the efficacy of 2L treatment alone. Moreover, the patient populations were not restricted to those with scc.
Use of 2L immunotherapy was more frequent for patients in our nabpc cohort (46%) than for those in the gp (37%) and sbpc (40%) cohorts. Although those differences in proportion were not statistically significant (p = 0.34), it could be hypothesized that the os superiority observed in the nabpc cohort is the result of a higher proportion of patients receiving 2L immunotherapy rather than of the effects of the 1L regimen. To investigate that issue in more detail, we conducted a survival subgroup analysis for patients who did not receive 2L immunotherapy. The results indicated that 1L nabpc was still associated with an improvement in median os duration (approximately 25 months vs. 15 months in the other two cohorts), which is in line with the results in the overall population. Similarly, a subgroup analysis for patients who did not receive any active 2L therapy suggested that, compared with the other 2 1L therapies, 1L nabpc was again associated with an improvement in median os. Those subgroup analyses indicate that the superior os observed in the nabpc cohort is not fully attributable to a higher proportion of patients in that cohort having received 2L immunotherapy.
Given the retrospective nature of our study, it is subject to important limitations. Measured or unmeasured pre-treatment differences between patients receiving various treatments can confound comparisons of the outcomes associated with the treatments. Also, biases (for example, recall and selection biases) might have been present because of the retrospective approach to the sampling procedure. To minimize the potential for recall bias when collecting os data, physicians were asked to review patient charts and to provide a date of death, when available. If a patient chart had an unknown or missing death status, it was excluded from our analyses. However, the death dates were not verified using a second source. Although dates could possibly have been entered incorrectly or erroneously, such a scenario is less likely. To mitigate selection bias, we asked the physicians to randomly select eligible patients for chart abstraction; we adjusted for multiple prognostic factors in the comparative analyses; and the study design used a randomization algorithm for physician sampling of eligible patients. Nonrandom missing data could have also affected the results, but the effect, if any, would have been modest, given that outcome or covariate data were missing for only about 5% of the patients. Another limitation of our study is that it did not report data for the safety profiles associated with the treatments of interest, which is always important in making treatment decisions.
CONCLUSIONS
The present retrospective analysis is one of the largest to compare various standard-of-care chemotherapy regimens for patients with scc. In a U.S. nationwide real-world sample of such patients, median os duration was significantly longer with 1L nabpc than with 1L gp, and numerically longer than with 1L sbpc, although the latter difference was not statistically significant. Similar real-world effectiveness in terms of pfs and td was observed for the 3 1L cohorts. Median os for the subgroup of patients who sequenced to an immunotherapy after 1L therapy was similar in all 3 cohorts.
ACKNOWLEDGMENTS
Medical writing assistance was provided by Samuel Rochette, an employee of Analysis Group, Inc. This study was sponsored by Celgene Corporation. The study sponsor was involved in the design of the study; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: BPL has received consulting fees from AstraZeneca, Celgene, Eli Lilly, Bristol–Myers Squib, Genentech, and Takeda; JES, HY, OPL, and CQX are employees of Analysis Group, Inc., which received research grants from Celgene Corporation to conduct this study; MP is an employee of Celgene Corporation.
REFERENCES
- 1.Derman BA, Mileham KF, Bonomi PD, Batus M, Fidler MJ. Treatment of advanced squamous cell carcinoma of the lung: a review. Transl Lung Cancer Res. 2015;4:524–32. doi: 10.3978/j.issn.2218-6751.2015.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji M, Liu Y, Li Q, et al. PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J Transl Med. 2015;13:5. doi: 10.1186/s12967-014-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malhotra J, Jabbour SK, Aisner J. Current state of immunotherapy for non–small cell lung cancer. Transl Lung Cancer Res. 2017;6:196–211. doi: 10.21037/tlcr.2017.03.01. [Erratum in: Transl Lung Cancer Res 2017;6:612] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Maio M, Chiodini P, Georgoulias V, et al. Meta-analysis of single-agent chemotherapy compared with combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:1836–43. doi: 10.1200/JCO.2008.17.5844. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger DS, Wood DE, Akerley W, et al. nccn guidelines insights: non–small cell lung cancer, version 4.2016. J Natl Compr Canc Netw. 2016;14:255–64. doi: 10.6004/jnccn.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna N, Johnson D, Temin S, et al. Systemic therapy for stage iv non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:3484–515. doi: 10.1200/JCO.2017.74.6065. [DOI] [PubMed] [Google Scholar]
- 7.Gandara DR, Edelman MJ, Lara PN, Lau DH. Gemcitabine in combination with new platinum compounds: an update. Oncology (Williston Park) 2001;15(suppl 6):13–17. [PubMed] [Google Scholar]
- 8.Le Chevalier T, Scagliotti G, Natale R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: a meta-analysis of survival outcomes. Lung Cancer. 2005;47:69–80. doi: 10.1016/j.lungcan.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Ye W, Yang Y, Wang J, Kadziola Z, Rajan N, Qin S. Prognostic factors for patients with advanced non–small cell lung cancer treated with gemcitabine–platinum as first-line therapy in an observational setting in China. Thorac Cancer. 2014;5:319–24. doi: 10.1111/1759-7714.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satouchi M, Okamoto I, Sakai H, et al. Efficacy and safety of weekly nab-paclitaxel plus carboplatin in patients with advanced non–small cell lung cancer. Lung Cancer. 2013;81:97–101. doi: 10.1016/j.lungcan.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase iii trial. J Clin Oncol. 2012;30:2055–62. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 12.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non-small-cell lung cancer (keynote-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 14.Fehrenbacher L, Spira A, Ballinger M, et al. on behalf of the poplar study group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (poplar): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 15.Rittmeyer A, Barlesi F, Waterkamp D, et al. on behalf of the oak study group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (oak): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paz-Ares L, Luft A, Vicente D, et al. on behalf of the keynote-407 investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–51. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 17.Areses MC, Campelo RG, González JG, et al. The real-world experience with nivolumab in previously treated patients with advanced non small cell lung cancer (nsclc): a Galician Lung Cancer Group clinical practice [abstract e20564] J Clin Oncol. 2017;35 doi: 10.1200/JCO.2017.35.15_suppl.e20564. [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.e20564; cited 18 March 2019] [DOI] [Google Scholar]
- 18.Brustugun OT, Sprauten M, Helland A. Real-world data on nivolumab treatment of non–small cell lung cancer. Acta Oncol. 2017;56:438–40. doi: 10.1080/0284186X.2016.1253865. [DOI] [PubMed] [Google Scholar]
- 19.Sandler AB, Nemunaitis J, Denham C, et al. Phase iii trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2000;18:122–30. doi: 10.1200/JCO.2000.18.1.122. [DOI] [PubMed] [Google Scholar]
- 20.Weiss J, Force RW, Pugmire BA, et al. Comparative effectiveness and resource usage in patients receiving first-line taxane-based chemotherapy for stage iv non-small-cell lung cancer in a US community oncology setting. Clin Lung Cancer. 2017;18:372–80. doi: 10.1016/j.cllc.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Mudad R, Patel MB, Margunato-Debay S, Garofalo D, Lal LS. Comparative effectiveness and safety of nab-paclitaxel plus carboplatin vs gemcitabine plus carboplatin in first-line treatment of advanced squamous cell non–small cell lung cancer in a US community oncology setting. Lung Cancer (Auckl) 2017;8:179–90. doi: 10.2147/LCTT.S139647. [DOI] [PMC free article] [PubMed] [Google Scholar]