Abstract
Background
Literature suggests that factors such as rural residence and low socioeconomic status (ses) might contribute to disparities in survival for Canadian cancer patients because of inequities in access to care. However, evidence specific to brain cancer is limited. The present research estimates the effects of rural or urban residence and ses on survival for Canadian patients diagnosed with brain cancer.
Methods
Adults diagnosed with primary malignant brain tumours during 1996–2008 were identified through the Canadian Cancer Registry. Brain tumours were classified using International Classification of Diseases for Oncology (3rd edition) site and histology codes. Hazard ratios (hrs) and 95% confidence intervals (cis) were estimated using Cox proportional hazards models. Events were restricted to individuals whose underlying cause of death was cancer-related. Postal codes were used to match patient records with Statistics Canada data for rural or urban residence and neighbourhood income as a surrogate measure of ses.
Results
Of 25,700 patients included in the analysis, 78% died during the study period, 21% lived in rural areas, and 19% were in the lowest income group. A modest variation in survival by rural compared with urban residence was observed for patients with glioblastoma (first 5 weeks after diagnosis hr: 0.86; 95% ci: 0.79 to 0.99) and oligoastrocytoma (first 3 years after diagnosis hr: 1.41; 95% ci: 1.03 to 1.93). Small effects of low compared with high income were seen for patients with glioblastoma (first 1.5 years after diagnosis hr: 1.15; 95% ci: 1.08 to 1.22) and diffuse astrocytoma (first 6 months after diagnosis hr: 1.17; 95% ci: 1.00 to 1.36).
Conclusions
Our analysis did not yield evidence of strong effects of rural compared with urban residence or ses strata on survival in brain cancer. However, some variation in survival for patients with specific histologies warrants further research into the mechanisms by which rural or urban residence and income stratum influences survival.
Keywords: Primary malignant brain tumours, survival, socioeconomic factors, rural residence
INTRODUCTION
Current literature suggests that factors such as socioeconomic status (ses) and rural or urban residence might explain disparities in survival for patients with a variety of cancers1–3. Specifically, social deprivation and rural residence have been investigated as mediators of access to screening, diagnosis, and treatment1–3. The exact mechanisms by which social deprivation influences access to care and, subsequently, survival for patients with various types of cancer are complex2. Rural residence is thought to be associated with cancer survival because it is a surrogate for geographic distance from cancer treatment centres, which are predominantly located in urban areas4. Disparities in survival rates associated with ses strata and rural or urban residence are evident within Canada1. However, research investigating the extent to which those factors influence survival for specific patient groups in Canada is limited.
Evidence compiled by the Canadian Partnership Against Cancer highlights variation in survival rates by income level, characterized by higher mortality for patients residing in low-income urban neighbourhoods relative to those residing in higher-income areas1. The same Canadian Partnership Against Cancer report showed higher age-standardized mortality rates for rural residents with prostate, lung, and colorectal cancers relative to their urban counterparts1. However, disparities in survival by rural or urban residence were not evident for other cancer types in the report1.
A systematic review of Canadian literature found that Canadians living a greater distance from a cancer centre have poorer access to systemic therapy and are more likely to experience a cancer-related death within the first 6 months after diagnosis5. With approximately 19% of Canada’s population living in rural or remote locations6, it is important to determine whether rural populations experience inequities in survival. Such inequities would indicate targets for clinical and policy interventions by health care practitioners, management, and policymakers to improve access by rural Canadians to cancer care5.
Some efforts have already been made to improve cancer care in rural Canada, including programs to improve access to cancer screening, to train rural family physicians in the administration of chemotherapy in community hospitals, and to increase the number of radiation therapy–capable facilities4,7. Significant strides have been made in rural areas for the detection through screening of some cancers, including breast, colorectal, and cervical cancers7. Brain cancers pose a unique challenge because there is no screening for this group of cancers, and tumour location and rarity necessitate highly specialized treatment strategies that limit treatment delivery to major metropolitan centres. The unique challenges in the diagnosis and treatment of primary malignant brain tumours might result in survival disparities for patients of lower ses or rural residence. Published research examining the effect of ses or geographic residence on survival in primary malignant brain tumours in Canada is limited. The objective of the present research was to compare the survival experience of Canadian patients with brain tumours based on rural compared with urban residence and on various ses strata.
METHODS
Data Source
Data for this analysis were obtained from the Canadian Cancer Registry (ccr). The ccr is an administrative population-based dataset that contains information about cancer incidence for all usual residents of Canada in all 13 provinces and territories. The ccr is a collaborative effort between Statistics Canada and each provincial and territorial cancer registry. Each provincial or territorial cancer registry is responsible for ensuring the completeness and accuracy of every record submitted to Statistics Canada. Primary cancer diagnoses are coded using the International Classification of Diseases for Oncology, 3rd edition. The ccr contains separate variables for anatomic site, histologic features, and behaviour. Patient mortality data are obtained from vital statistics databases that are linked to the cancer registry by each province. Data about the demographic characteristics of each patient include age at diagnosis, sex, and residence (province, postal code, and census tract).
Study Population
From the ccr, we identified adults diagnosed with a first-ever primary malignant brain tumour between 1 June 1996 and 31 December 2008. Brain tumours were defined as those occurring at International Classification of Diseases for Oncology site codes C700–C729, C751, and C753. International Classification of Diseases for Oncology histology codes were then grouped into major histologic categories as defined by the Central Brain Tumor Registry of the United States8, yielding these groupings: glioblastoma, diffuse astrocytoma, glioma not otherwise specified, oligodendroglioma, anaplastic astrocytoma, oligoastrocytic tumours, anaplastic oligodendroglioma, and “all others.” Because of differences in treatment between adults and children, the analysis was restricted to patients diagnosed at 21 years of age or older.
Outcome Ascertainment
Events were restricted to individuals whose underlying cause of death was cancer-related, as determined by the appropriate International Classification of Diseases code (revision 9 for those with a diagnosis date before 2000, and revision 10 for those with a diagnosis date in 2000 and onward). Unrelated causes of death were treated as censoring events. Time-to-event was determined using the survival interval, which was estimated using the date of diagnosis and the date of death or the date last known to be alive (censoring date).
Definitions of Rural and Urban Residence
Rural and urban residence were classified according to the definition of “rural and small town” developed by du Plessis et al.9. That definition is recommended for use in studies examining access to care. It classifies regions with a population less than 10,000 as rural9; however, although that definition is recommended for use in research into general health care services access, it might not be the most appropriate measure for brain cancers because of the centralization of treatment to major metropolitan centres. All analyses were therefore repeated using an alternative “non-metropolitan” definition of rural, in which all regions outside census metropolitan areas (areas with 100,000 people or more and at least 50% in an urban core) are classified as “rural”9. Postal codes were used to match patient records with community size variables from Statistics Canada’s Postal Code Conversion File (PCCF+)10. Community size variables representing the “rural and small town” definition were missing for patients diagnosed between 1992–1996, and variables representing the “non-metropolitan” definition were missing for patients diagnosed before 200110. Each analysis was restricted to patients with complete data.
Definition of SES
In this analysis, neighbourhood income was used as a proxy for each patient’s income and as a surrogate measure of ses. Data for neighbourhood income were obtained from the PCCF+ dataset and were matched to each patient based on their place of residence at the time of diagnosis. In the PCCF+ dataset, the average neighbourhood income is divided into quintiles based on the income distribution within each census metropolitan area or census agglomeration. Because of similarities in trends between income quintiles, only 3 categories were used: “lowest income” included income quintile 1, “middle income” included income quintiles 2 and 3, and “highest income” included income quintiles 4 and 5.
Statistical Methods
Data were accessed through the Research Data Centre at the University of Alberta and analyzed using the SAS statistical software application (version 9.4: SAS Institute, Cary, NC, U.S.A.). All frequencies were rounded in accordance with Statistics Canada’s requirements. Crude Kaplan–Meier survival estimates (1-, 2-, and 5-year) were calculated for each major histology type, stratified by rural or urban residence and by income group. Individuals who were alive at the end of study (31 December 2008) were right-censored. Kaplan–Meier curves for each histology were created and smoothed according to Statistics Canada’s requirements.
Hazard ratios (hrs) and 95% confidence intervals (cis) were estimated using Cox proportional hazards regression models. Separate models were built for each histology, with sex, region, income, and rural or urban residence as covariates. Models were stratified by age group to accommodate non-proportional hazards by age. The extent to which the proportional hazards assumptions held across time periods for each histology was tested. If the assumption was violated for any histology, the hrs were split at the point where the survival functions for rural and urban crossed. Hypothesized interactions were tested using the likelihood ratio test. Interactions that did not improve model fit were not included in the final model and are not discussed further.
RESULTS
Between 1 June 1996 and 31 December 2008, 25,700 people in Canada who met the inclusion criteria were diagnosed with a primary brain cancer and had complete data for the characteristics of interest. Of those 25,700 people, 78.1% died within the study period. Patients were predominantly men (55.8%). Patients 65 years of age and older constituted 41.4% of the study population. The number of patients varied by geographic region, but were proportional to the population of each province. The largest proportion of cases came from Ontario (38.4%), followed by Quebec (26.3%) and British Columbia (12.5%). The most common histology was glioblastoma (42.7%), followed by diffuse astrocytoma (13.1%).
Table I summarizes the distribution of participant characteristics by rural or urban residence and income quintile. The proportion of patients with brain cancer residing in rural areas was 21.3%, which is consistent with the proportion of Canadians living in rural areas according to the 2006 census (19.9%)6. The proportions of patients residing in rural areas in each province were also consistent with the proportions identified by the Canadian census6. No variation in the distribution of income was observed for the categories of rural or urban residence. The proportion of patients with brain cancer in the lowest income group was 19.2%.
TABLE I.
Patient characteristics stratified by residence and income category, from the Canadian Cancer Registry, 1996–2008
| Characteristic | Residence [n (%)] | Neighbourhood income groupa [n (%)] | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Urban | Rural | Total | Lowest | Middle | Highest | Total | |
| Sex | |||||||
| Women | 8860 (79.0) | 2360 (21.0) | 11220 | 2240 (20.2) | 4540 (40.9) | 4330 (39.0) | 11110 |
| Men | 11130 (78.2) | 3110 (21.8) | 14240 | 2590 (18.4) | 5670 (40.2) | 5830 (41.4) | 14090 |
|
| |||||||
| Age group | |||||||
| 21–44 Years | 4290 (81.3) | 990 (18.8) | 5280 | 1050 (20.0) | 2150 (41.0) | 2040 (38.9) | 5240 |
| 45–64 Years | 7560 (78.2) | 2110 (21.8) | 9670 | 1660 (17.3) | 3710 (38.7) | 4210 (43.9) | 9580 |
| ≥65 Years | 8140 (77.5) | 2370 (22.5) | 10510 | 2120 (20.4) | 4350 (41.9) | 3910 (37.7) | 10380 |
|
| |||||||
| Histology | |||||||
| All others | 5150 (79.1) | 1360 (20.9) | 6510 | 1390 (21.6) | 2650 (41.1) | 2400 (37.3) | 6440 |
| Anaplastic astrocytoma | 610 (80.3) | 150 (19.7) | 760 | 130 (17.3) | 310 (41.3) | 310 (41.3) | 750 |
| Anaplastic oligodendroglioma | 580 (79.5) | 150 (20.5) | 730 | 130 (17.8) | 280 (38.4) | 320 (43.8) | 730 |
| Diffuse astrocytoma | 2640 (78.8) | 710 (21.2) | 3350 | 630 (18.9) | 1380 (41.4) | 1320 (39.6) | 3330 |
| Glioblastoma | 8470 (77.4) | 2480 (22.6) | 10950 | 1960 (18.1) | 4340 (40.0) | 4540 (41.9) | 10840 |
| Glioma, malignant, NOS | 850 (80.2) | 210 (19.8) | 1060 | 230 (21.9) | 430 (41.0) | 390 (37.1) | 1050 |
| Oligoastrocytic tumours | 680 (80.0) | 170 (20.0) | 850 | 130 (15.7) | 350 (42.2) | 350 (42.2) | 830 |
| Oligodendroglioma | 1010 (81.5) | 230 (18.5) | 1240 | 230 (18.7) | 480 (39.0) | 520 (42.3) | 1230 |
|
| |||||||
| Regionb | |||||||
| AB | 1580 (74.2) | 550 (25.8) | 2130 | 410 (19.4) | 870 (41.2) | 830 (39.3) | 2110 |
| BC | 2680 (84.3) | 500 (15.7) | 3180 | 640 (20.4) | 1260 (40.3) | 1230 (39.3) | 3130 |
| ON | 8290 (85.5) | 1410 (14.5) | 9700 | 1670 (17.4) | 3860 (40.1) | 4090 (42.5) | 9620 |
| PEI–NB–NL–NS | 1140 (55.3) | 920 (44.7) | 2060 | 410 (20.2) | 860 (42.4) | 760 (37.4) | 2030 |
| QC | 5220 (77.6) | 1510 (22.4) | 6730 | 1400 (20.9) | 2730 (40.7) | 2580 (38.5) | 6710 |
| SK–MB | 1080 (65.5) | 570 (34.5) | 1650 | 300 (18.8) | 640 (40.0) | 660 (41.3) | 1600 |
|
| |||||||
| TOTAL | 19990 (78.5) | 5460 (21.5) | 25450 | 4830 (19.2) | 10220 (40.6) | 10150 (40.3) | 25200 |
Lowest = income quintile 1; Middle = income quintiles 2 and 3; Highest = income quintiles 4 and 5.
Region was defined as the province or territory of diagnosis. Because of small numbers, some geographically adjacent provinces were combined to achieve a sufficient sample size in each region. Territories were excluded from the analysis because of extremely low case numbers.
Slight variations in category totals within and between rural or urban residence and income are a result of the random rounding procedures required by Statistics Canada
NOS = not otherwise specified.
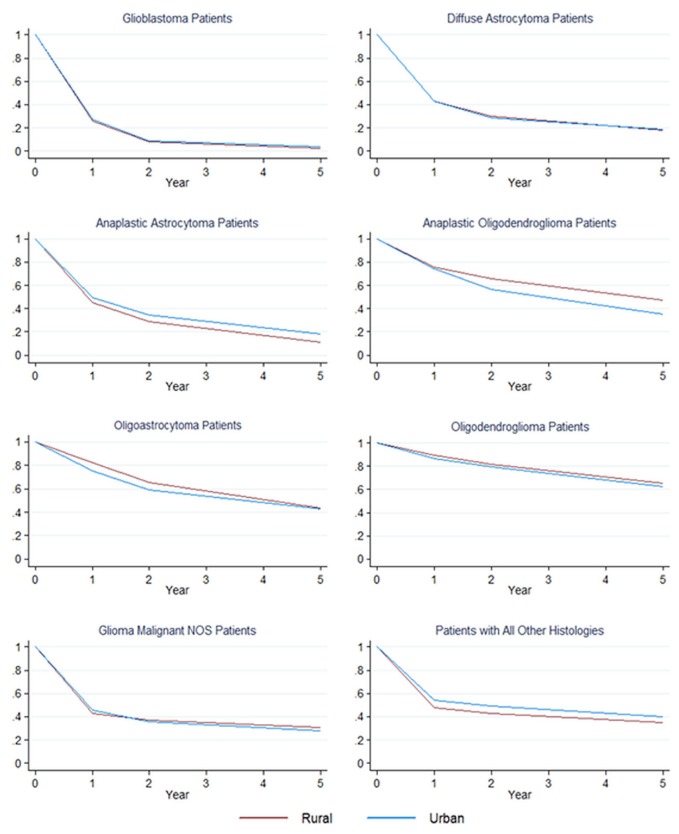
Figure 1 shows crude Kaplan–Meier 1-, 2-, and 5-year survival curves for each histology, stratified by rural or urban residence. Patients with oligodendroglioma experienced the best survival, independent of residence. Patients with glioblastoma experienced the worst survival overall, with patients residing in rural areas experiencing worse 5-year survival (2.4%; 95% ci: 1.8% to 3.2%) relative to their urban counterparts (3.7%; 95% ci: 3.3% to 4.2%; Figure 1, supplementary Table 1). The estimated 5-year survival rate was lower for rural-residing patients than for urban-residing patients with anaplastic astrocytoma [10.9% (95% ci: 5.9% to 17.5%) vs. 18.4% (95% ci: 15.0% to 22.0%)] and with the “all others” histology [34.8% (95% ci: 32.2% to 37.5%) vs. 40.3% (95% ci: 38.9% to 41.7%)]. Conversely, there was evidence of better 5-year survival for rural-residing patients with anaplastic oligodendroglioma (47.6%; 95% ci: 38.9% to 55.9%) than for their urban-residing counterparts (35.0%; 95% ci: 30.7% to 39.4%). Survival for patients with other histologies did not vary by rural or urban residence (Figure 1, supplementary Table 1).
FIGURE 1.
Kaplan–Meier survival curves for brain cancer patients by histology and rural or urban residence (Canadian Cancer Registry, 1996–2008). NOS = not otherwise specified.
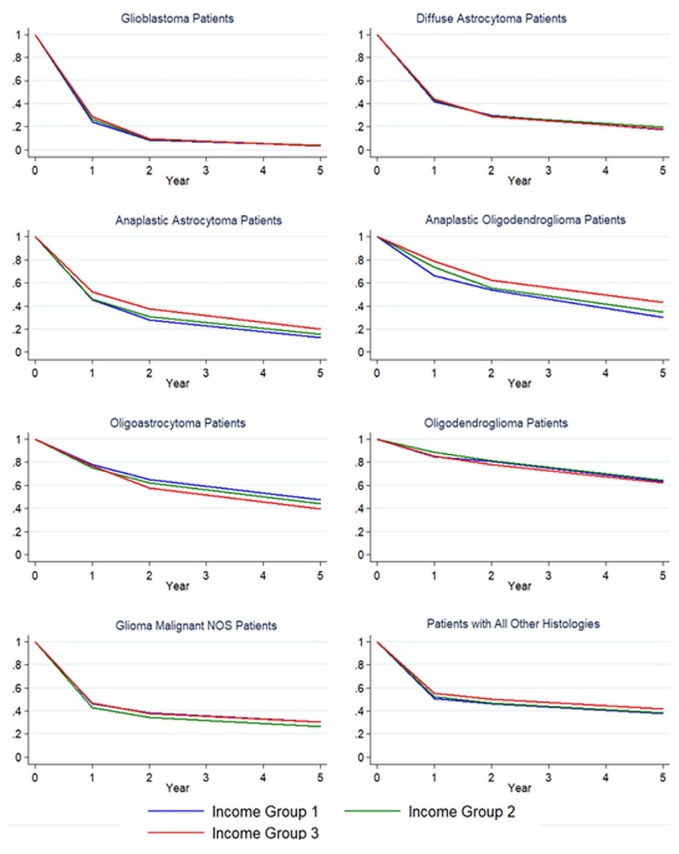
Figure 2 shows crude Kaplan–Meier 1-, 2-, and 5-year survival curves for each histology group, stratified by income category. At 1 year after diagnosis, survival was worse for patients in the lowest income group relative to their counterparts in the highest income group when they had glioblastoma [24.1% (95% ci: 22.2% to 26.1%) vs. 28.9% (95% ci: 27.6% to 30.2%)], anaplastic oligodendroglioma [66.5% (95% ci: 57.6% to 74.0%) vs. 78.8% (95% ci: 73.8% to 82.9%)], and “all others” tumours [51.0% (95% ci: 48.3% to 53.6%) vs. 55.3% (95% ci: 53.2% to 57.3%)], but there was no evidence of a difference in survival by year 2 or thereafter. The relationship between income and survival did not follow expected patterns for patients in the remaining histology groups. Relative to their counterparts in lower income categories, patients in the highest income category who had oligoastrocytic tumours experienced the lowest survival rate at all time points (Figure 2, supplementary Table 2). Similarly, compared with both the lowest and the highest income groups, the group in the middle income category who had glioma not otherwise specified experienced worse survival at all time points after diagnosis (Figure 2, supplementary Table 2).
FIGURE 2.
Kaplan–Meier survival curves for brain cancer patients by histology and income group (Canadian Cancer Registry, 1996–2008). NOS = not otherwise specified.
Table II shows estimated hrs and 95% cis for the effect of rural compared with urban residence on survival adjusted for age group, sex, region, and income. The hrs for rural compared with urban residence changed over time for patients with glioblastoma, oligodendroglioma, and oligoastrocytoma. The hrs for those histology groups were therefore split at the point where the survival functions for rural and urban residence crossed. Overall, more than 90% of glioblastoma patients had died by year 3 after diagnosis. Rural residents with glioblastoma experienced a lower hazard for death in the first 5 weeks after diagnosis (Table II). However, from 5 weeks to 3 years after diagnosis, we observed no evidence of a difference in the hazard for death between rural- and urban-residing patients and evidence of a higher hazard for death for rural-residing patients after 3 years. Rural residents with oligoastrocytoma showed no difference in the hazard for death in the first 3 years after diagnosis, but showed a greater hazard for death after year 3. No variation in the hazard for death by rural or urban residence was evident for patients with the remaining histologies. Repeating the analyses using the “non-metropolitan” definition of “rural” yielded findings that were consistent with those from analyses using the “rural and small town” definition (data not shown).
TABLE II.
Histology-specific brain cancer survival estimates comparing rural with urban residence, adjusted for age group, sex, region, and socioeconomic status
| Histology | Intervala | HRb | 95% CI | p Value |
|---|---|---|---|---|
| Glioblastoma | <5 Weeks | 0.88 | 0.78 to 0.99 | 0.031 |
| 5 Weeks to 3 years | 1.03 | 0.98 to 1.09 | 0.207 | |
| >3 Years | 1.41 | 1.03, 1.93 | 0.034 | |
|
| ||||
| Anaplastic astrocytoma | Overall | 0.95 | 0.77 to 1.16 | 0.591 |
|
| ||||
| Diffuse astrocytoma | Overall | 1.01 | 0.92 to 1.11 | 0.813 |
|
| ||||
| Anaplastic oligodendroglioma | Overall | 1.16 | 0.91 to 1.47 | 0.236 |
|
| ||||
| Oligoastrocytic tumours | <3 Years | 1.01 | 0.76 to 1.35 | 0.935 |
| >3 Years | 1.98 | 1.30 to 3.02 | 0.001 | |
|
| ||||
| Oligodendroglioma | <6 Years | 0.83 | 0.64 to 1.07 | 0.144 |
| >6 Years | 1.49 | 0.97 to 2.31 | 0.070 | |
|
| ||||
| Glioma, malignant, NOS | Overall | 1.00 | 0.83 to 1.21 | 0.984 |
|
| ||||
| All others | Overall | 0.98 | 0.91 to 1.06 | 0.651 |
Rural compared with urban residence.
Split by time if the proportional hazards assumption did not hold, indicating that the hazard changed over time for the associated histology.
HR = hazard ratio; CI = confidence interval; NOS = not otherwise specified.
Table III shows the hrs and 95% cis for the effects of lowest or middle income compared with highest income on survival, adjusted for age group, sex, region, and rural or urban residence. The proportional hazards assumption was not met for the effect of income on survival for patients with glioblastoma or diffuse astrocytoma. Compared with their counterparts in the highest income group, patients in the lowest income group with glioblastoma experienced a significantly higher hazard for death in the first 1.5 years after diagnosis. Conversely, compared with patients in the highest income group, those in the lowest income group had a lower hazard for death from 1.5 years to 5 years after diagnosis. The same pattern was also evident for patients in the middle income group, relative to those with the highest income (Table III). Compared with patients in the highest income group, those in the lowest income group with diffuse astrocytoma experienced a higher hazard for death in the first 6 months, but a lower hazard for death from 6 months to 2 years (Table III). The hazard for death did not differ between the lowest- and highest-income groups after 2 years (Table III). The middle-income group also had a higher hazard for death in the first 6 months. There was evidence of a lower hazard for death in that patient group from 6 months to 2 years after diagnosis; however, the ci associated with that estimate suggests some uncertainty about the direction and magnitude of the effect. Patients with anaplastic oligodendroglioma and tumours in the “all others” category experienced worse overall survival in both the lowest-income and the middle-income groups relative to the highest-income group. The hazard for death did not vary by income in patients with other histologies.
TABLE III.
Histology-specific brain cancer survival estimates by income category, adjusted for age group, sex, region, and residence
| Histology | Income groupa | Intervalb | HR | 95% CI | p Value |
|---|---|---|---|---|---|
| Glioblastoma | Lowest | <1.5 Years | 1.15 | 1.08, 1.22 | 0.000 |
| 1.5 to 5 Years | 0.84 | 0.70, 1.01 | 0.065 | ||
| >5 Years | 1.07 | 0.54, 2.12 | 0.851 | ||
| Middle | <1.5 Years | 1.06 | 1.02, 1.11 | 0.008 | |
| 1.5 to 5 Years | 0.87 | 0.76, 0.99 | 0.039 | ||
| >5 Years | 0.85 | 0.48, 1.49 | 0.565 | ||
| Highest | 1.0 | — | — | ||
|
| |||||
| Anaplastic astrocytoma | Lowest | Overall | 1.16 | 0.92, 1.46 | 0.206 |
| Middle | Overall | 1.08 | 0.90, 1.29 | 0.422 | |
| Highest | — | 1.0 | — | — | |
|
| |||||
| Diffuse astrocytoma | Lowest | <0.5 Years | 1.17 | 1.00, 1.36 | 0.044 |
| 0.5 to 2 Years | 0.82 | 0.69, 0.98 | 0.031 | ||
| >2 Years | 1.03 | 0.79, 1.34 | 0.811 | ||
| Middle | <0.5 Years | 1.21 | 1.07, 1.37 | 0.002 | |
| 0.5 to 2 Years | 0.85 | 0.68, 1.07 | 0.174 | ||
| >2 Years | 1.07 | 0.79, 1.44 | 0.658 | ||
| Highest | — | 1.0 | |||
|
| |||||
| Anaplastic oligodendroglioma | Lowest | Overall | 1.35 | 1.04, 1.74 | 0.022 |
| Middle | Overall | 1.25 | 1.02, 1.54 | 0.036 | |
| Highest | — | 1.0 | — | — | |
|
| |||||
| Oligoastrocytic tumours | Lowest | Overall | 0.96 | 0.73, 1.28 | 0.800 |
| Middle | Overall | 1.03 | 0.84, 1.26 | 0.771 | |
| Highest | — | 1.0 | |||
|
| |||||
| Oligodendroglioma | Lowest | Overall | 0.92 | 0.72, 1.18 | 0.52 |
| Middle | Overall | 0.89 | 0.74, 1.08 | 0.241 | |
| Highest | — | 1.0 | — | — | |
|
| |||||
| Glioma NOS | Lowest | Overall | 0.96 | 0.78, 1.17 | 0.657 |
| Middle | Overall | 1.001 | 0.85, 1.18 | 0.9883 | |
| Highest | — | 1.0 | |||
|
| |||||
| All othersc | Lowest | Overall | 1.12 | 1.03, 1.22 | 0.009 |
| Middle | Overall | 1.08 | 1.00, 1.16 | 0.047 | |
| Highest | — | 1.0 | — | — | |
Lowest = income quintile 1; Middle = income quintiles 2 and 3; Highest = income quintiles 4 and 5.
Split by time if the proportional hazards assumption did not hold, indicating that the hazard changed over time for the associated histology.
Adjusted for microscopic confirmation.
HR = hazard ratio; CI = confidence interval; NOS = not otherwise specified.
We observed no evidence for an effect of sex on survival in patients with any brain tumour type except for tumours in the “all others” category (hr: 0.89; 95% ci: 0.83 to 0.95).
Survival varied by province for all brain tumour categories except for anaplastic astrocytoma and oligodendroglioma. Patients with glioblastoma had a higher hazard for death in British Columbia relative to Ontario (reference province): 1.43 (95% ci: 1.28 to 1.60). Similarly, patients with diffuse astrocytoma had the highest hazard for death in British Columbia relative to Ontario (hr: 1.93; 95% ci: 1.69 to 2.20). Patients diagnosed with glioma not otherwise specified had a higher hazard for death in Alberta and British Columbia compared with Ontario, with hrs of 1.49 (95% ci: 1.17 to 1.90) and 1.81 (95% ci: 1.43 to 2.28) respectively. Patients with oligoastrocytoma had the lowest hazard for death in the Maritime provinces compared with Ontario (hr: 0.60; 95% ci: 0.39 to 0.93), and those in British Columbia had the highest hazard for death (hr: 1.58; 95% ci: 1.24 to 2.02). Patients with anaplastic oligodendroglioma had the lowest hazard for death in Alberta (hr: 0.621; 95% ci: 0.41 to 0.93). Patients with tumours in the “all other” category had the highest hazard for death in Saskatchewan and Manitoba compared with Ontario (hr: 1.73; 95% ci: 1.51 to 1.97).
DISCUSSION
Our data suggest that disparities in survival for all brain cancer subtypes by rural or urban residence are not generally apparent at diagnosis, but might develop for certain tumours over time. Rural-residing patients with oligoastrocytoma who survived longer than 3 years had a hazard for death almost double that of urban-residing patients, which corresponds to a worse survival experience. Similarly, at 6 years after diagnosis, rural-residing patients with oligodendroglioma and glioblastoma experienced a hazard for death approximately 1.5 times the hazard for urban-residing patients with glioblastoma. However, the absolute difference in survival between rural- and urban-residing patients with glioma at 5 years was small.
The data also suggest that income group affects survival for some brain tumour subgroups, particularly anaplastic oligodendroglioma, diffuse astrocytoma, and glioblastoma. However, the negative effect of income on survival for glioblastoma and diffuse astrocytoma was limited to the first 1.5 years after diagnosis.
Two main factors might explain the general absence, in this study, of disparities in survival for patients with brain cancer depending on rural or urban residence, or on varying levels of ses. First, access to treatment and subsequent care for rural and low-income Canadians with brain cancer might be more equitable than it is for other cancer types. It is important to note that, because of disease severity, identification of any primary malignant brain tumour initiates an urgent response. Patients tend to receive quick referrals to specialized cancer centres that are capable of appropriately managing their cancer treatment. That quick referral likely has the effect of minimizing the delay in treatment for all patients, regardless of their residence or income level. In that context, the absence of a strong effect of rural or urban residence or income on survival might therefore suggest equity in access to care. Second, access to treatment might not have a significant effect on prognosis for patients with brain cancer, given the absence of effective treatment modalities for specific histologies, particularly glioblastoma. For patients with tumours of more aggressive histology, we therefore cannot be sure whether the general absence of disparities in survival between rural- and urban-residing patients and between those with varying levels of income is attributable to equity in access to care or because of a lack of a meaningful effect of treatment on survival. Differences in the survival rates for patients with tumours of less-aggressive histology in the low-income group suggest that inequities in access to care might exist for tumour subtypes in which treatment is more effective or the prognosis is generally better.
The body of evidence about the effect of income level—and more broadly ses—on survival is extremely limited. In an analysis of ses differences in cancer survival for Swedish children followed for 10 years from date of diagnosis until death or the end of follow-up in 2011, the estimated 5- and 10-year survivals for children with tumours of the nervous system were lowest for those whose parents had lower education relative to parents with higher education11. Evidence generated in our analysis did not consistently demonstrate a relationship between ses as measured using income and survival characterized as worse survival for those with indicators of lower ses. Disparities between our findings and those in the published literature might be attributable in part to the sole use of income to measure ses in our study, without accounting for other related dimensions of ses such as education.
Research investigating the effects of rural or urban residence on disparities in brain cancer survival is limited. Direct comparisons between findings from our analyses and those in the published literature are complicated by the various methods used to define rural or urban residence and to group or classify tumour types. In an analysis of U.S. Surveillance, Epidemiology, and End Results program data from 1973 to 2001, Deorah et al.12 classified patients into “metropolitan” and “non-metropolitan” groups as surrogates for rural and urban counties. Estimated incidence and survival rates were presented for all malignant brain tumour types combined. The 28-year risk for malignant brain tumours in residents of metropolitan areas was 1.35 times that in residents of non-metropolitan areas (95% ci not presented)12. However, the estimated 5-year survival rate was higher for affected patients residing in metropolitan areas (28%; 95% ci: 27.4 to 28.6) than for those residing in non-metropolitan areas (25.6%; 95% ci: 24.2 to 27.0)12. In an analysis of U.S. Surveillance, Epidemiology, and End Results data from 2011 to 2015, Henley et al.13 estimated age-adjusted rates of cancer deaths by site and by metropolitan compared with non-metropolitan residence. Non-metropolitan counties were further stratified by rural or urban areas, and metropolitan counties were further stratified by population greater than 1 million13. The annual age-adjusted mortality rates for patients with brain and other nervous system cancers were 4.6 and 4.3 per 100,000 people in rural and urban non-metropolitan counties respectively13. For patients with brain and other nervous system cancers residing in metropolitan areas, the age-adjusted annual mortality rates were 4.5 and 4.2 per 100,000 people for populations of less than 1 million and of 1 million or more respectively13.
Review of the literature indicates that rural and urban differences in survival are often observed only for tumours classified as mid-grade or having regional spread14–16, indicating that, compared with other cancers, mid-grade and regionally metastasized cancers are influenced to a greater degree by treatment factors and access to care. One explanation for that observation is that survival is good for low-grade tumours, but poor for high-grade tumours regardless of access to care and treatment received. Brain cancers do not metastasize, and so that characteristic does not explain those results. However, the grade of tumour at diagnosis, as identified by histology for gliomas, could be relevant.
Strengths and Limitations
Survival data to 31 December 2008 were available through the ccr, and so current patterns are not yet available. It is possible that recent advances in care17 might differentially affect brain cancer survival for patients diagnosed at a later time not captured in the present analysis. Because we used ccr data, our estimation was limited to variables collected and reported within the database. Data for modifying factors such as tumour characteristics and treatment types that have previously been reported to have a significant effect on cancer survival were unavailable for the present analysis16,18,19. Additionally, data about potential confounders were not available through the ccr.
The use of each patient’s home province and postal code at date of diagnosis to define place of residence might have contributed to misclassification error if, after diagnosis, patients tended to migrate to areas with cancer treatment facilities. If rural-residing patients tend to move to urban areas after diagnosis and the reason for migration is related to survival, the estimates might be biased toward finding urban or rural differences. A limitation of the income variable is that it is not patient-specific. If neighbourhood-level income is not an accurate proxy for individual-level ses, the effect of ses on the survival experience of patients with brain cancer might have been masked in our analysis. Additionally, methods used to ascertain rural or urban residence and neighbourhood income, and the time-varying nature of each variable, precluded adding an interaction term between those exposures to the model. The analysis was therefore unable to estimate the extent to which the effect of ses strata varied by rural or urban residence.
Although the data elements available in the ccr are limited, using that data source lends strength to our study. The quality of the data is high because, to detect errors, all data submitted to the ccr has undergone validation edits and correlation edits that verify the validity of each entry and check for appropriate relationships between patient and tumour data. Furthermore, because all reported cases of brain cancer in Canada for the defined age groups within the study period were analyzed, the results are representative of the Canadian population and are applicable to Canadian patients with brain cancer. Finally, use of the PCCF+ files allowed for accurate designation of rural or urban residence, with fewer than 1% of patients meeting the study inclusion criteria having an unknown rural or urban classification.
CONCLUSIONS
Research investigating the extent to which ses and rural or urban residence influence survival for specific patient groups in Canada is limited. Our analysis fills a gap in the existing literature by addressing the effects on survival of rural compared with urban residence and ses as measured by income level for Canadians diagnosed with brain cancer. Such inequities would suggest targets for clinical and policy interventions by health care practitioners, management, and policymakers to improve access to cancer care for rural-residing and low-income Canadians.
Our analysis did not yield evidence of strong effects of rural or urban residence or ses on survival for Canadians diagnosed with brain cancer during the study period. That finding might reflect equitable access to care for this patient population. There was some evidence of modest variation in survival over time by rural or urban residence for patients with tumours of specific histologies. That variation was more apparent for patients diagnosed with tumours generally known to have better prognoses. In contrast, associations between ses and brain tumour survival were more apparent for tumour subtypes generally known to have poor prognoses and for the early post-diagnosis period. Further research into the exact mechanisms by which rural or urban residence and ses influence survival is needed before targeted policy or program recommendations can be developed.
ACKNOWLEDGMENTS
This research was made possible through access to the Canadian Research Data Centre Network, which is supported by the Social Sciences and Humanities Research Council, the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, and Statistics Canada. This work was also supported through funding provided by the Brain Tumour Foundation of Canada. Although the research and analysis are based on data from Statistics Canada, the opinions expressed do not represent the views of Statistics Canada.
Footnotes
Supplemental material available at http://www.current-oncology.com
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Partnership Against Cancer (cpac) Examining Disparities in Cancer Control: A System Performance Special Focus Report. Toronto, ON: cpac; 2014. [Google Scholar]
- 2.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 3.Kogevinas M, Porta M. Differences in cancer survival: a review of the evidence. IARC Sci Publ. 1997:177–206. [PubMed] [Google Scholar]
- 4.Olson RA, Nichol A, Caron NR, et al. Effect of community population size on breast cancer screening, stage distribution, treatment use and outcomes. Can J Public Health. 2012;103:46–52. doi: 10.1007/BF03404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maddison AR, Asada Y, Urquhart R. Inequity in access to cancer care: a review of the Canadian literature. Cancer Causes Control. 2011;22:359–66. doi: 10.1007/s10552-010-9722-3. [DOI] [PubMed] [Google Scholar]
- 6.Statistics Canada. Canada’s Rural Population Since 1851: Population and Dwelling Counts, 2011 Census. Ottawa, ON: Statistics Canada; 2012. (Census in Brief series). [Google Scholar]
- 7.Kerner J, Liu J, Wang K, et al. Canadian cancer screening disparities: a recent historical perspective. Curr Oncol. 2015;22:156–63. doi: 10.3747/co.22.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolecek TA, Propp JM, Stroup NE, Kruchko C. cbtrus statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.du Plessis V, Beshiri R, Bollman RD, Clemenson H. Definitions of rural. Rural and Small Town Canada Analysis Bulletin. 2001;3:1–17. [Google Scholar]
- 10.Statistics Canada. Postal CodeOM Conversion File (PCCF) [digital file] Ottawa, ON: Statistics Canada; 2015. (Catalogue no. 92-154-X2015001). [Google Scholar]
- 11.Mogensen H, Modig K, Tettamanti G, Talbäck M, Feychting M. Socioeconomic differences in cancer survival among Swedish children. Br J Cancer. 2016;114:118–24. doi: 10.1038/bjc.2015.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. doi: 10.3171/foc.2006.20.4.E1. [DOI] [PubMed] [Google Scholar]
- 13.Henley SJ, Anderson RN, Thomas CC, Massetti GM, Peaker B, Richardson LC. Invasive cancer incidence, 2004–2013, and deaths, 2006–2015, in nonmetropolitan and metropolitan counties—United States. MMWR Surveill Summ. 2017;66:1–13. doi: 10.15585/mmwr.ss6614a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu XQ, Luo Q, Kahn C, O’Connell DL, Houssami N. Temporal trends show improved breast cancer survival in Australia but widening urban–rural differences. Breast. 2015;24:524–7. doi: 10.1016/j.breast.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Yu XQ, Luo Q, Smith DP, O’Connell DL, Baade PD. Geographic variation in prostate cancer survival in New South Wales. Med J Aust. 2014;200:586–90. doi: 10.5694/mja13.11134. [DOI] [PubMed] [Google Scholar]
- 16.Jong KE, Smith DP, Yu XQ, O’Connell DL, Goldstein D, Armstrong BK. Remoteness of residence and survival from cancer in New South Wales. Med J Aust. 2004;180:618–22. doi: 10.5694/j.1326-5377.2004.tb06123.x. [DOI] [PubMed] [Google Scholar]
- 17.Sherman JH, Hoes K, Marcus J, Komotar RJ, Brennan CW, Gutin PH. Neurosurgery for brain tumors: update on recent technical advances. Curr Neurol Neurosci Rep. 2011;11:313–19. doi: 10.1007/s11910-011-0188-9. [DOI] [PubMed] [Google Scholar]
- 18.Shugarman LR, Sorbero ME, Tian H, Jain AK, Ashwood JS. An exploration of urban and rural differences in lung cancer survival among Medicare beneficiaries. Am J Public Health. 2008;98:1280–7. doi: 10.2105/AJPH.2006.099416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell KJ, Fritschi L, Reid A, et al. Rural–urban differences in the presentation, management and survival of breast cancer in Western Australia. Breast. 2006;15:769–76. doi: 10.1016/j.breast.2006.04.001. [DOI] [PubMed] [Google Scholar]